Abstract
Adverse drug reactions, including those resulting from interactions between herbal medicines and conventional drugs, are a public health problem worldwide. The need for pharmacovigilance for herb-drug interactions (HDIs) is essential for the identification and assessment of risks of using herbal products (questionable safety, efficacy and quality), which are not always tested with rigor, or often not subject to approval by regulatory agencies.
Spontaneous and active surveillance conducted by national pharmacovigilance centres permits a rapid detection of potentially harmful combinations of products. The incidence and prevalence of HDIs are difficult to predict because of the underreporting of adverse effects. It is important for health professionals, consumers, regulatory authorities and suppliers of herbal medicines to be aware of the possible adverse effects and drug interactions caused when herbal medicines are co-administered with conventional drugs. National pharmacovigilance centres continue to play a significant role in increasing awareness of drug safety, in this case with HDIs.
The authors’ objective for this paper is to provide awareness among policy makers responsible for the design of appropriate pharmacovigilance practices and therefore to highlight the importance of pharmacovigilance in the safety monitoring of HDIs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
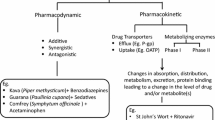
Herbal medicines are often used by consumers in combination with conventional drugs, raising the potential of pharmacokinetic and/or pharmacodynamic herb-drug interactions (HDIs). This is especially problematic with respect to drugs with a narrow therapeutic index.[1] Herbal medicines may affect absorption, metabolism, distribution and excretion mechanisms. When herbal medicines are coadministered with conventional drugs, there may be induction or inhibition of drug metabolizing enzymes. Altered drug protein binding and altered drug excretion may also occur, although the degree of susceptibility varies from individual to individual.[2,3] The modulation of drug transporters and metabolizing enzymes are also responsible for HDI. The prevalence of herbal medicines use in conjunction with conventional drugs in countries such as Canada, the US, Jamaica and some African countries is high.[4–7] This prevalence varies among specific patient populations.[8] For example, concomitant use of herbal medicines and psychotropic drugs for mental health indications has been shown to occur in 21.3% of patients studied.[4]
HDIs are a major safety concern[9] because herbal medicines contain numerous pharmacologically active constituents, including essential oils, tannins, coumarins, anthraquinones, saponins, glycosides, anthocyanins, alkaloids and flavonoids, all of which may potentially affect the pharmacokinetics and pharmacodynamics of drugs. In turn, this could lead to adverse reactions that may be life threatening. An example is the alkaloids obtained from the species of Ephedra (Ephedraceae). When administered as herbal medicines, or as products containing synthetically prepared ephedrine and pseudoephedrine, these alkaloids can cause adverse cardiovascular events associated with arrhythmias, palpitations, tachycardia, myocardial infarction and death.[10,11] Ephedrine raises blood pressure and induces peripheral vasoconstriction. Consumption of caffeine in Coffea arabica L. (Rubiaceae) or present in herbal medicines or drugs, and in association with ephedrine, increases cardiovascular risk.[12,13] The danger of using ephedrine-containing products is higher in patients who are sensitive to the effects of sympathomimetic agents (i.e. patients with hypertension, hyperthyroidism, diabetes mellitus, psychiatric conditions, glaucoma, prostate enlargement, seizure disorders and cardiovascular disease).[14] For all these reasons, HDIs should be evaluated by the usual methods of clinical pharmacology and pharmacovigilance adopted by the national pharmacovigilance programme in each country. It is important to consider the specific characteristics of herbal medicines in order to better assess HDIs.
Because of the universal rise in the popularity of herbal medicines, the danger resulting from concomitant use of both conventional drugs and herbal medicines is becoming an increasingly important public health issue. The objective of this paper is to provide awareness among policy makers responsible for the design of appropriate pharmacovigilance practices and therefore to highlight the importance of pharmacovigilance in the safety monitoring of HDIs.
1. The WHO International Drug Monitoring Programme
Adverse drug reactions (ADRs), including those resulting from HDIs, are a public health problem worldwide, and an important cause of death and hospitalization.[15] Under the WHO International Drug Monitoring Programme, national pharmacovigilance centres are responsible for the collection, processing and evaluation of case reports of suspected ADRs resulting from HDIs. As an immediate response to the need for pharmacovigilance for HDIs, the WHO promotes monitoring of HDIs. This programme will be described in a future WHO publication on the WHO Working Group meeting on interactions of herbal medicines with other medicines, which took place in Milan, Italy, 23–25 June 2011. The guideline will propose approaches for overcoming HDIs with the view that HDIs are included in the existing national pharmacovigilance systems (or their equivalent institutions) in various countries.
The WHO Collaborating Centre for International Drug Monitoring-Uppsala Monitoring Centre (WHO-UMC), located in Uppsala, Sweden, co-ordinates global ADR data and searches this data for signals of safety concerns raised by member countries. As a sub-category of drugs, the monitoring of herbal medicines and HDIs is captured in the programme. Indeed, the UMC identifies signals of new adverse reactions from cumulative risk assessments and notifies the national pharmacovigilance centres and others concerned with drug safety. The challenge in detecting such signals related to HDIs is not due to the technical process, but to the underreporting of such reactions. The reporting of HDIs should be encouraged from all WHO member countries. In a recent search using the following criteria, the number of HDI reports was 811, which represents 4.6% of all herbal reports (17 754) and 0.012 % of the total number of reports (7 017 658) in the WHO database:
-
Data source: VigiSearch™
-
Dataset date: 4 January 2012
-
Extraction date: 12 January 2012
-
Terminology: WHO-Adverse Reaction Terminology
-
Substance: Herbals, with Anatomical Therapeutic Chemical classification code V90 (unspecified herbal and traditional medicine)
-
Involvement: suspected/interacting; reactions (high-level term); drug interaction
-
Years: all
-
Countries: all
-
Other: reports with HDIs
The number of HDI reports, by country, is summarized in table I. One report can involve more than one herbal medicine, so the total number of reported herbal medicines involved in these interactions per country (877) is a little higher than the total number of reports (811). Table II shows the top 20 most common herbals involved.
2. Why Use Pharmacovigilance for Monitoring Herb-Drug Interactions (HDIs)?
Pharmacovigilance monitoring of HDIs is essential for the identification and assessment of risks. Many issues are related to herbal medicines, such as questionable safety, efficacy and quality. Herbal medicines are not tested with the scientific rigor required of conventional drugs, and they are often not subject to the approval process of regulatory agencies. They can be sold outside the country via the Internet, which facilitates their uncontrolled sale. The use of multiple medications, polypharmacy and self-medication increase the possibility of HDIs, particularly in elderly patients.[16] A variety of other causes of safety concern include adulteration, mistaken use of the wrong species or misidentification, incorrect dosing, errors in use, contamination (toxic metals, microbes, microbial toxins, environmental pollutants) and toxic constituents (for example, aristolochic acids[17]). In addition, herbal medicines can affect the pharmacokinetic and pharmacodynamic properties of conventional drugs and thus may cause HDIs.[9] For example, St John’s wort (Hypericum perforatum L., Hypericaceae) with its main constituents of hypericin, hyperforin and flavonoids, is one of the most commonly used herbal medicines for the treatment of mild to moderate depression in Western countries. It can have an important influence on the effectiveness, safety and outcome of a range of drug therapies. This is the case in relation to serotonin reuptake inhibitors and warfarin.[18,19] In this case, St John’s wort decreases the anticoagulant effect when taken with warfarin. The possible mechanism is hepatic enzyme induction. Warfarin is metabolized by cytochrome P450 (CYP) 1A2 in the liver, which is induced by St John’s wort;[20] St John’s wort also induces the human CYP enzymes CYP3A4and CYP2C9.[21–25] St John’s wort and ciclosporin co-administration after organ transplantation may result in ciclosporin therapeutic failure in transplant graft rejection,[23–25] and St John’s wort can increase the expression of P-glycoprotein, leading to potential drug interactions.[26–28] When HDIs appear, it is important that these are reported and analysed, and their significance communicated by existing pharmacovigilance systems.
3. Conducting Pharmacovigilance Activities in the Monitoring of HDIs
Evidence from the literature that herbal medicines have pharmacological effects and may lead to adverse interactions when co-administered with conventional drugs, has grown in recent years.[29–32] However, there is not enough information or adequate analysis to estimate the magnitude of the problem.[33,34] Pharmacosurveillance is required in order to identify the risk of HDIs. The inclusion of this kind of adverse reaction in pharmacovigilance systems is becoming increasingly important because HDIs may be life threatening and constitute, therefore, a major safety concern. Spontaneous and active surveillance conducted by national pharmacovigilance centres permits a rapid detection of potentially harmful combinations of products, which may facilitate regulatory control.
A single pharmacovigilance reporting form covering all health products, including herbal medicines and HDIs is recommended. This is important because, for those health professionals and herbal medicines providers already included in a national pharmacovigilance system, a single form will facilitate reporting. Spontaneous reports of adverse HDIs are the only practical way for general herb-drug safety evaluation in a postmarketing environment. However, and even if spontaneous reporting is still the best way to report adverse HDIs, the current international adverse event reporting system suffers from severe underreporting.[35,36] This is likely to be significant for herbal medicines, since users typically do not seek professional advice about their use of such products or report adverse effects. The incidence and prevalence of HDIs are consequently difficult to predict. There is no doubt that the monitoring of adverse HDIs has additional complexities related to the ways in which herbal medicines are named, perceived, sourced and utilized. Alternative methods such as epidemiology, registries and clinical trial studies ought to be considered in order to remedy this underreporting.
The importance of pharmacovigilance is increasing. With recent high-profile ADRs, regulatory agencies, media, consumers and others have become more aware about the benefits and risks of medicines and their potential interactions. At the global level, the WHO-UMC already collates some adverse HDI reports reported by national pharmacovigilance centres, but only from a few of them. Individual reporting countries still own, and can publish, their data, but the pooling of data on adverse HDIs from all member countries would add value to ongoing studies. HDI reactions should be captured by the national reporting system of all countries, and subsequently be reported to the WHO. Unfortunately, the current systems are ineffective[37] because underreporting of adverse reactions in relation to the use of herbal medicines remains a fact in many countries.
Pharmacovigilance requirements for herbal medicines are generally the same as for licensed medicines. Patient information on labels of herbal medicines is similar to that for any over-the-counter medicine, with an additional requirement for a statement on labels identifying at least the part of the herb used, type of extraction, dose and, in advertisements, that the indication is based on traditional use. This is related to herbal medicines used as raw material. Scientific recommendations on the use of herbal medicines and their coadministration with conventional drugs should be based on robust scientific data rather than on the inadequate data of case reports only. A thorough evaluation of the interaction of herbal medicines with other medicines should be carried out. Little attention has been paid to this issue to date. Current literature data are unsubstantiated, with conclusions based on single or limited reports. These data are generally insufficient to predict the incidence of HDIs. Thus, HDIs need to be investigated through greater research, particularly by meta-analysis of prospective and clinical studies using large population samples in order to avoid the problems with individual susceptibility.[38] Prospective randomized clinical trials that include assessment of HDIs are urgently needed.
4. Pharmacovigilance Inspections
Public pressure on industry and regulatory bodies to improve the monitoring of health product safety has led to expansion of national pharmacovigilance departments in many countries. The pharmaceutical industry has an ethical and legal responsibility to ensure that the products they sell will not harm the patients they are intended for.[39] Indeed, the current postmarketing safeguards include good manufacturing practices, batch safety testing, inspections and pharmacovigilance. But when herbal medicines are used conjointly with conventional drugs, with limited information about the pharmacokinetics, pharmacodynamics and manufacturing properties of these products, or when they are not regulated, pharmacovigilance inspection is confronted with several challenges.
5. The Need for Educational Sessions in Pharmacovigilance
It is important for health professionals, consumers and other interested stakeholder groups, including regulatory authorities and suppliers of herbal medicines, to be aware of the possible adverse effects and drug interactions caused when herbal medicines are co-administered with conventional drugs. Consumers frequently self-select herbal medicines, without the advice of a qualified health provider.[40] They should be encouraged to disclose their use of herbal medicines to their physicians and pharmacists, who will then be aware of potential HDIs and should report them to national pharmacovigilance centres. More effective communication between all these partners is needed, and information must be accessible to all[41] so that responsibility of safety information is shared.
National pharmacovigilance centres continue to play a significant role in increasing awareness of drug safety. Various methods can be considered for all relevant target audiences, such as involvement of the mass media and patient/consumer associations, including translation into local languages for the public in general. For health professionals, information may be disseminated via the delivery of adverse reaction bulletins or articles and scientific meetings. This education should concern all other partners in pharmacovigilance: herbal medicines providers, academics, researchers/scientists, and the pharmaceutical and herbal medicines industries. The clinical importance of HDIs depends on many factors associated with the particular herb, drug and patient. Herbal medicines should be appropriately labelled to alert consumers to potential interactions when concomitantly used with conventional drugs.
Reporting of adverse reactions is an important aspect of postmarketing surveillance.[42,43] Pharmacovigilance educational and feedback sessions should integrate the importance of reporting of adverse reactions resulting from HDIs. It is imperative that physicians are aware of all medications, both conventional medicines and herbal medicines, that their patients are taking, in order to provide the best care. This should be possible by direct patient questioning. Physicians must regularly ask their patients about their use of herbal medicines, particularly elderly patients,[44] and those whose disease is not responding to treatment as expected. This is important because adverse reactions observed following use of conventional drugs might, in reality, be due to herbal medicines or HDIs. This assessment cannot be made when physicians and other healthcare providers are unaware of the extent of a patient’s self-medication with herbal medicines.[45] The reporting of all adverse reactions, including HDIs, should be encouraged by all stakeholders.
6. Conclusions
Interactions between herbal medicines and conventional drugs are a major patient safety concern. The concurrent use of herbal medicines with conventional drugs must take into account their safety, efficacy, consistency and quality. The safety of herbal medicines requires strict control of the presence of adulterants, the dosage labelling, contraindications, manufacturing techniques and a list of all ingredients. There is often no requirement to list each ingredient of every herbal preparation on the label; more significantly, only some of the ingredients are listed, but not others that may be harmful. Unfortunately, there is also no requirement to precisely state the dose of active ingredients contained in herbal preparations in some countries. Under these conditions, HDIs are possible and are difficult to monitor. This situation can be better controlled through pharmacovigilance processes and strict regulatory controls.
The current model of pharmacovigilance, and its associated tools, has been developed in relation to conventional drugs. Applying these methods to monitor the safety of herbal medicines and HDIs presents challenges that the pharmacovigilance system of the countries must manage. Increased reporting of HDIs to pharmacovigilance centres and the WHO will enable better assessment of this source of adverse reactions.
References
Butterwek V, Derendorf H. Potential of pharmacokinetic profiling for detecting herbal interactions with drugs. Clin Pharmacokinet 2008; 47(6): 383–97
Lin JH, Lu AY. Inhibition and induction of cytochrome P450 and the clinical implications. Clin Pharmacokinet 1998; 35: 361–90
Ioannides C. Pharmacokinetic interactions between herbal remedies and medicinal drugs. Xenobiotica 2002; 32: 451–78
Vasiliadis HM, Tempier R. Reporting on the prevalence of drug and alternative health product use for mental health reasons: results from a national population survey. J Popul Ther Clin Pharmacol 2011; 18(1): e33–43
Delgoda R, Younger N, Barrett C, et al. The prevalence of herbs use in conjunction with conventional medicines in Jamaica. Complement Ther Med 2010 Feb; 18(1): 13–20
Elmer GW, Lafferty WE, Tyree PT, et al. Potential interactions between complementary/alternative products and conventional medicines in a medicare population. Ann Pharmacother 2007 Oct; 41(10): 1617–24
Müller AC, Kanfer I. Potential pharmacokinetic interactions between antiretrovirals and medicinal plants used as complementary and African traditional medicines. Biopharm Dispos 2011 Nov; 32(8): 458–70
Rispler DT, Sara J. The impact of complementary and alternative treatment modalities on the care of orthopaedic patients. J Am Acad Orthop Surg 2011 Oct; 19(10): 634–43
Skalli S, Zaid A, Soulaymani R. Drug interactions with herbal medicines. Ther Drug Monit 2007 Dec; 29(6): 679–86
Haller CA, Benowitz NL. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. N Engl J Med 2000; 343: 1833–8
Samenuk D, Link MS, Homoud MK, et al. Adverse cardiovascular events temporally associated with ma huang, an herbal source of ephedrine. Mayo Clin Proc 2002; 77: 12–6
Skalli S, Soulaymani R. A propos des produits Herbalife. L’Officinal 2002; 28: 4
Chung MK. Vitamins, supplements, herbal medicines, and arrhythmias. Cardiol Rev 2004; 12: 73–84
Johns Cupp M. Herbal remedies: adverse effects and drug interactions. Am Fam Phys 1999; 59: 1239–47
Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 2004; 329(7456): 15–9
McCabe BJ. Prevention of food-drug interactions with special emphasis on older adults. Curr Opin Clin Nutr Metab Care 2004; 7: 21–6
Jordan SA, Cunningham DG, Marles RJ. Assessment of herbal medicinal products: challenges, and opportunities to increase the knowledge base for safety assessment. Toxicol Appl Pharmacol 2010 Mar 1; 243(2): 198–216
Yue QY, Bergquist C, Gerden B. Safety of St John’s wort (Hypericum perforatum). Lancet 2000; 355: 576–7
Jiang M, Park M, Lee HC, et al. Antidiabetic agents from medicinal plants. Curr Med Chem 2006; 13: 1203–18
Izzo AA, Di Carlo G, Borrelli F, et al. Cardiovascular pharmacotherapy and herbal medicines: the risk of drug interaction. Int J Cardiol 2005; 98: 1–14
Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs 2001; 61: 2163–75
Henderson L, Yue QY, Bergquist C, et al. St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol 2002; 54: 349–56
Kawaguchi A, Ohmori M, Tsuruoka S, et al. Drug interaction between St John’s and quazepam. Br J Clin Pharmacol 2004; 58: 403–10
Mills E, Wu P, Johnston BC, et al. Natural health product-drug interactions: a systematic review of clinical trials. Ther Drug Monit 2005; 27: 549–57
Wang EJ, Barecki-Roach M, Johnson WW. Quantitative characterization of direct P-glycoprotein inhibition by St John’s wort. J Pharm Pharmacol 2004; 56: 123–8
Ruschitzka F, Meier PJ, Turina M, et al. Acute heart transplant rejection due to Saint John’s wort. Lancet 2000; 355: 548–9
Hennessy M, Kelleher D, Spiers JP, et al. St. John’s wort increases expression of P-glycoprotein: implications for drug interactions. Br J Clin Pharmacol 2002; 53: 75–82
Zhou W, Chai H, Lin HL, et al. Molecular mechanisms and clinical applications of ginseng root for cardiovascular disease. Med Sci Monit 2004; 8: RA187–92
De Smet PA. Health risks of herbal remedies. Drug Saf 1995; 13: 81–93
Bartels CL, Miller SJ. Herbal and related remedies. Nutr Clin Pract 1998; 12: 5–9
Poppenga RH. Herbal medicines: potential for intoxication and interactions with conventional drugs. Clin Tech Small Anim Pract 2002; 17: 6–18
Singh SR, Levine M. Natural health product use in Canada:patterns of use and the risk of interactions with pharmaceuticals. Clin Pharmacol Ther 2004; 75: 28
Kaufman DW, Kelly JP, Rosenberg L, et al. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA 2002; 287: 337–44
Makino T, Inagaki T, Komatsu KI, et al. Pharmacokinetic interactions between Japanese traditional medicine (Kampo) and modern medicine (III): effect of Sho-seiryu-to on the pharmacokinetics of azelastine hydrochloride in rats. Biol Pharm Bull 2004; 27: 670–3
Alvarez-Requejo A, Carvajal A, Begaud B, et al. Underreporting of adverse drug reactions: estimate based on a spontaneous reporting scheme and sentinel system. Eur J Clin Pharmacol 1998; 54(6): 483–8
Hazell L, Shakir SAW. Under-reporting of adverse drug reactions: a systematic review. Drug Saf 2006; 29(5): 385–96
Walji R, Boon H, Barnes J, et al. Consumers of natural health products: natural-born pharmacovigilantes? BMC Complement Altern Med 2010 Feb 25; 10: 8
Aronson JK. Classifying drug interactions. Br J Clin Pharmacol 2004; 58: 343–4
Fitzgerald P. Pharmacovigilance inspections. Indian J Pharmacol 2008; 40 Suppl. 1: S21–3
Murray E, Pollack L, White M, et al. Clinical decision-making: patient’ preferences and experiences. Patient Educ Couns 2007; 65: 189–96
Lexchin J. Is there still a role for spontaneous reporting of adverse drug reactions? CMAJ 2006; 174: 191–2
Wysowski DK, Swartz L. Adverse drug event surveillance and drug withdrawals in the United States, 1969–2002: the importance of reporting suspected reactions. Arch Intern Med 2005; 165: 1363–9
Berry DC, Knapp PR, Raynor DK. Is 15% very common: informing people about the risks of medication side effects. Int J Pharm Prac 2002; 10: 145–51
Kales HC, Blow FC, Welsh DE, et al. Herbal products and other supplements: use by elderly veterans with depression and dementia and their caregivers. J Geriatr Psychiatry Neurol 2004; 17: 25–31
Woodward KN. The potential impact of the use of homeopathic and herbal remedies on monitoring the safety of prescription products. Hum Exp Toxicol 2005; 24: 219–33
Acknowledgements
No sources of funding were used to prepare this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Skalli, S., Bencheikh, R.S. Safety Monitoring of Herb-Drug Interactions. Drug Saf 35, 785–791 (2012). https://doi.org/10.1007/BF03261975
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03261975