Abstract
Mineral process wastes, such as metallurgical slags nowadays, are of significant metals and materials resources in the circular economy. The usability of iron-making slag is well established; however, steel slag still needs to be utilized due to various physicochemical constraints. In this context, we have reviewed the global steel slag generation, processing, characteristics, and potential application with suggestions for futuristic possibilities to address the technical, economic, and environmental aspects of steel slag recycling. The steel slags inherit characteristics from ore chemistry and their processing routes, which may be Blast Furnace-Basic Oxygen Furnace (BF-BOF) or Direct Reduced Iron-Electric Arc Furnace (DRI-EAF) steel-making routes with/without treatment in a Ladle Furnace (LF). The average generation of slags in BOF, EAF, and LF processes accounts for 110 kg, 70 kg, and 40 kg per tonne of steel, respectively. Considering global steel production of 1878 million tonnes in 2020, the associated slag by-products possess a significant resource of major components like FeO, CaO, SiO2, Al2O3, etc. Selecting a recycling technique depends on the slag's end use, logistics, and physicochemical characteristics. BOF and EAF slag are used ~ 48% in road construction applications and ~ 10% for industrial specific metallurgical use. EAF slag is preferred as cementitious material as compared to BOF. Hot-stage modification of BOF slag has emerged as a preferred method to achieve desired chemical and subsequent mechanical properties for end use. BOF slag has high carbon dioxide sequestration potential for in-plant remediation among steel slags.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
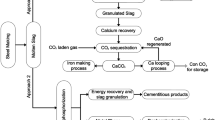
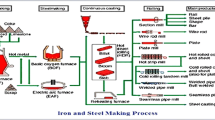
In the twenty-first century, steel has become one of the most utilized engineering materials, from house utensils to the automotive sector. According to the World Steel Organization, the world produced a total of 1951 million tons of crude steel in 2021, in which China is the top producer of crude steel with 1032.8 million tonnes, followed by India and Japan with 118.2 and 96.3 million tonnes of crude steel [1]. The enormous crude steel production generates massive solid waste, i.e., slag. The slags generated by steel making in the Basic Oxygen Furnace (BOF), Ladle Furnace (LF), and Electric Arc Furnace (EAF) are collectively called steel slag. In 2017, Japan claimed ~ 14 million tonnes of steel slag production, while China reported 100 million tonnes of steel slag in the year 2016 [2, 3], which is in line with the available data of 10 to 15% of waste by-product during steel production [4]. The percentage of slag production directly depends upon the steel-making processes. It also depends on the quality of the iron ore and other raw materials, namely, fluxes, ferroalloys, etc. Figure 1 shows the schematic diagram of steel production and associated chemical reactions for slag formation. Many studies have been published on recycling steel industry slags (as shown in Fig. 2) for various applications. The published data on the recycling of steel industry slag (from 2013 to 2022) is shown in Fig. 2, indicating that the researchers have paid more attention to recycling the steel slags irrespective of the process route.
Looking at the merits and associated benefits, some of the top steel-producing countries have adopted or upgraded with a recycling technique of steel slags from time to time for various applications (as shown in Fig. 3), such as for road construction, soil enrichment, cement production, etc. Notably, recycling steel slag for a particular application is associated with challenges the scientific community strives to overcome by developing and advancing the existing recycling technique. Therefore, a critical review has been conducted highlighting the different physicochemical properties of steel slag, the comparison of different recycling techniques developed worldwide, and various state-of-the-art applications.
2 Characteristics of Steel Slag
The chemistry of the slag depends on the raw materials, which are quite distinct for EAF and BOF processes. Approximately 70% of steel slag produces through the BOF process, whereas it is nearly 28.8% through the EAF process and around 0.4% from the secondary refining in LF. In addition, the rate of slag production by the BOF process is also very high compared to the EAF process. LF refining slag process has a different chemistry than that of BOF and LF due to the addition of desulfurizing (Ca, Mg, CaSi, CaC2) and oxidizing (Si, Al) agents in the refining process [6]. Table 1 compares various steel slags worldwide, confirming the difference in the physicochemical properties of BOF, EAF, and LF slag.
Figure 4 shows the total waste generation from the steel manufacturing process and how much industry and academic research got attention in various applications. Their chemical composition, along with BOF, EAF, and LF slag, is shown in Table 2. It is apparent that the BOF and EAF slags have almost identical chemical components, but a higher amount of CaO and MgO in EAF slag makes it more alkaline in nature. The LF slags still have higher alkalinity due to the presence of CaO and MgO compared to the BOF slags and EAF slags (shown in Table 2). Besides that, some other solid wastes like flue dust, sludge, fly ash, and mill dust get generated during the steel manufacturing process. This waste is rich in iron and can be recycled with steel slags [8]. Other than its chemical properties, the physical properties of slag are also examined. In past review studies it is seen the BOF slag has a bulk density 2.9–3.7 g/cm2, water absorption of 0.2–2.4%, and specific gravity between 3.1 to 3.7 g/cm3, a los angeles abrasion value of 12.5–22% [33]. In comparison to BOF slag, the EAF slag has higher water absorption in the range from 0.95—4.0% with a density value of 1.54–3.34 g/cm3[34].
Table 2 lists the main elements found in steel slags: calcium and silicon. Numerous studies have shown that these elements have a significant influence on the majority of observed phases, including portlandite (Ca(OH)2), calcite (CaCO3), dicalcium silicate (C2S), tricalcium silicate (C3S) and larnite (Ca2SiO4). In addition to calcium and silicon, the presence of aluminum and magnesium influence phases such as the Alkaminite-gehlenite series (Ca2(Al. Mg)(Si, Al)SiO7) and Diopside alumina (Ca(Mg)(Al, Si)SiO7) [33, 34]. The number of phases in this system is determined primarily by the mass percentages of Ca, Mg, Al, and Si. When the basicity of the slag is high, the formation of free-CaO and free-MgO was seen in their diffraction peaks, especially in BOF and LF slag. Furthermore, the BOF and EAF slags contain significant iron contents. X-ray diffraction (XRD) analysis also revealed the presence of iron in the form of fayalite (Fe2SiO4), wustite (FeO), hematite (Fe2O3), and a trace of magnetite (Fe3O4) [34, 37]. A few of the other minors phases of iron with calcium and/or magnesium were also reported in some studies [33, 34].
3 Environmental Concern About Steel Slag
The unscientific dumping of steel slag may lead to several environmental implications. Figure 5 shows a schematic representation of the interaction of steel slag with the environment. Steel slag from a specific iron and steel industry contains toxic elements like alkalis, heavy metals, and metalloids. These toxic elements get released into the environment and affect living organisms through water and air. Some part of the elements leached out from the landfilled slag and traveled up to groundwater and further mixed with a significant water source (river, oceans). Researchers have also claimed that the leaching tendency of the slags contaminated the surrounding soil and changed the pH value of agricultural land. The presence of toxic elements and heavy metals varies the pH value of water sources, which destroy aquatic life and affect the human body once it gets introduced to the food chain [38].
Steel industry slag consists of metals like Cr, Ni, V, Zn, and many more [39, 40]. The composition of slags depends upon the used raw material and various steel production processes. Some studies monitored the leaching potential of slag components to the environment. According to the U.S. Environment Protection Agency (EPA) standards, less than 1 mg/L of metal is under toxicity-leaching potential. The metals detected above 1 mg/L are Mn and Ba [40]. The leaching potential of V is higher than Cr, affecting soil quality in the vicinity [41]. Cr in the form of Cr6+ and V in soil reduces the organic matter available in soil [42]. Some standard leaching experiments and their parameters are shared in Fig. 6 to get an overview of the techniques.
Using steel slag for various landfilling applications enhances the soil pH. The steel slags are highly alkaline because CaO and MgO are the principal constituents. The slag leached out in the presence of groundwater increases the pH value of the groundwater up to 10.4–11.8 and increases the amount of calcium, chromium, and fluoride in the groundwater source [43]. When this alkaline groundwater meets with the primary water body, the aquatic life gets affected, as shown in Fig. 7. These water bodies, if used for plantation, drinking, and daily uses, harm the human body [44]. Hence, the leachability test of every steel slag should be done mandatorily to ensure a minimal environmental effect.
4 Industrial Approach to Slag Recycling
Many steel industries worldwide usually use steel slag for various construction and civil applications, avoiding landfill and dumping in the open environment. Some leading Indian steel industries, such as Jindal Steels and Tata Steels, first process the steel slag to recover valuable metals, then convert the remaining material into slag chips and slag powder for further resources [45]. Nippon Slag Association (NSA) of Japan Steel Industries adopted a similar approach [46]. However, most of the steel industries of Japan converted the steel slag into powder form to recover valuable metals. However, significant energy consumption is the primary constraint in this approach.
As a protocol, the steel industries now follow a series of pretreatment processes of steel slag so that the recovery process of the metal will be effective. Pretreatment of steel slag (such as quenching, crushing, and screening), shown in Fig. 8, helps to adjust the requirement for effectively recycling steel slag in a specific application. Figure 8(a) and (b) show the pretreatment process flowchart of slag recycling in Japanese industries [47, 48] and India Industries [45].
The world steel organization report 2019 shows that steel slag production is nearly 190 to 280 × 106 kg/t worldwide. Europe recycled around ~ 43% of steel slag in road construction after extracting valuable metals. Turkey’s steel industry has adopted the same practice; however, it stores ~ 80–88% of steel slags for reusing and recycling in the plant itself, and 1% goes for cement production [49]. However, the overall rate of steel slag use by japan is almost ~ 98% in various sectors [46]. Some of the leading steel producing countries like China, India and Russia, still have a low rate of steel slag recycling. It is now a unanimous view that if slag management is not done correctly, it can affect the environment badly, irrespective of the economic status of a country.
5 Recycling of Steel Slags
Recycling of steel slag follows an extensive range of applications in several sectors. The processing of slags to recover the metal values or direct use in metallurgical processes is categorized as a metallurgical application. All other applications of the steel slag, irrespective of their type, are grouped as non-metallurgical applications. Some potential applications concerned with steel slag in terms of its chemical and physical properties are discussed in this section. Recycling studies have been focused on applications like construction, gas absorption, water filtration, and as an ingredient of fertilizer [22]. A schematic presentation of the use of steel slag in various applications is shown in Fig. 9. These applications are discussed in detail in the following sections.
5.1 Metallurgical Applications
5.1.1 Recovery of Metals From Slag
Steel slag from different steel-making processes has an extensive range of beneficial constituents, as shown in Table 1. Its components are mainly divided into ferrous (iron) and non-ferrous (calcium, silica, phosphorus, aluminum, manganese, and many more). Various studies have also suggested that the constituents present in steel slag can be recovered by three major approaches: (a) mechanical separation, (b) acid leaching, and (c) carbothermic reduction. The process flow chart for different metal recovery techniques from steel slag is schematically shown in Fig. 10. However, the selection of a metal recovery technique depends upon the nature and properties of components extracted from the steel slag.
Mechanical Separation Method
Mechanical separation is an effective technique to recover ferromagnetic metals like iron and nickel from steel slag. In this approach, the magnetic property separates the ferromagnetic metals from the rest of the slags [50]. However, the size reduction process is performed before the separation process for efficient liberation. Figure 11 shows different process flow charts (conventional, modified non-ferrous removal process, and optimized method) for extracting various ferrous constituents.
The iron recovery can rise to 45% for 10–20 mm particles and 75–80% for micron particle size of iron of steel slag [51]. The addition of screening equipment by Lan et al. [52] further improved the mechanical process by mapping sample size with the screening equipment's help. They have improved the efficiency of the magnetic separator by more than 8% compared to the original process (shown in Fig. 11a and b). A numerical study [53] concludes that the particles' size and shape are reasonably dependent upon magnetic roller speed and intensity. An experimental study revealed the effect of sample size and magnetic intensity relation during the magnetic separation [54]. Low magnetic power (hand-magnet) can achieve more than 95% recovery efficiency for sample sizes greater than 4 mm, which reduces to 50% for a sample size of less than 4 mm. Therefore, a high magnetic intensity with a current intensity of 1A was suggested to improve the separation efficiency, which shows about 95% efficiency for 0.3 mm sample size. Apart from separating the ferrous material, the magnetic separation technique has also been used to recover non-ferrous material like phosphorous using a magnetic separation tube [55]. The relevant process flow chart is shown in Fig. 11c. Using a magnetic separation tube helps recover up to 80% phosphorus. Other oxides of slag can also be retrieved, giving an extra advantage to the whole process. Figure 11d shows the optimized method for iron extraction by magnetic separation route.
Hydrometallurgical Method
The hydrometallurgical method involves leaching metallic values from the slag in an aqueous acidic or alkaline medium. The typical process steps are shown in Fig. 12. However, a prior slag grinding to a fine powder is necessary to effectively leach the metals. The finely grounded slag particles are mixed with the appropriate leaching agent in the required solid/liquid ratio and stirred for a fixed time at a specific temperature to obtain the metals-laden pregnant leach solution (PLS). The PLS is further processed by solvent extraction to separate the desired metal selectively. Based on the materials and leaching process, acid (HCL, NH4Cl, CH3COOH, and H2SO4), alkali (Na3C6H5O7 and C2H7NO2), both types of solvent can be used for the recovery of valuable metals. The acidic-based solution mainly extracts calcium, iron, and silicon metals [56, 57]. The alkaline leaching solution extracts chromium, vanadium, etc. [58]. However, a prior oxidation or reduction roasting of slag makes it more amenable to leaching, as shown in Fig. 12(a-c) [59]. Figure 12a illustrates an indirect reduction process for reducing iron and manganese using reduction gas agents like CO and H2. With the help of indirect reduction, 80% of magnesium and 50% of iron can be extracted from steel slag. Similarly, recovery of chromium by using alkali roasting is discussed in Fig. 12b. The alkaline salt like NaOH or NaOH + NaNO3 is used as an oxidizing agent combined with the water leaching process. The process operates at a higher temperature, and the maximum chromium recovery achieved was 46%. Figure 12c shows the recovery of zinc by using the acid-leaching method.
Pyrometallurgical Method
Pyrometallurgical methods essentially involve heating slag for the chemical conversion of one or more constituents in oxidizing or reducing conditions. The carbothermic reduction-based recovery process for steel slag is shown in Fig. 13(a,b), where size reduction followed by carbothermic reduction at higher temperatures (1000- 1800 °C) occurs in the presence of coal/coke. The flow chart for metal/non-metal and phosphorous separation via carbothermic reduction and microwave heating is shown in Fig. 13b [60]. Coal acts as a reducing agent under the heating by a microwave source [60, 61] as shown in Fig. 13a. The advantage of this process over other processes is that it can provide sensible and lateral heat for other operations [62]. The significant advantage is that the secondary slag, i.e., CaO-SiO2 slag is now free from phosphorus and can be subjected to an application similar to that for typical blast furnace slag.
As far as the extraction of valuable metallic elements from the steel slags is concerned, it can be concluded that the steel industries slag contains a wide range of valuable metals and non-metals (like Fe, Ni, Mg, Al, V, Si, P, and Ti) in which some materials like V and Ti are present in small amounts (Table 1). The researchers have claimed that the carbothermic reduction-based recycling process is suitable for Fe, P2/P4, and Mg elements. On the other hand, the mechanical separation techniques for iron and zinc elements have been found suitable. The elements like V, Si, Mg, and Cr are easily recycled using the selective leaching process in a suitable leaching solvent, as the acid leaching process involves an acidic solvent that has to be recycled effectively so that the environment remains unaffected.
5.1.2 Use of Slag as a Metallurgical Flux
A large amount of CaO presence in the slag can be used as a flux to iron making process after some treatment, as shown in Fig. 14. The use of steel slag as charging material (fluxing agent for iron making) in the iron-making process is an innovative way to recycle waste. Depending upon the pretreatment process, the size of the slag lies in the range of millimeters to centimeters (asymmetry in size). Agglomeration of fine ore and lime (obtained from slag) can be attained by sintering [63] or palletization [64] techniques. The pelletization technique is more effective than the sintering technique as it restricts the use of micron-size fine particles [65]. The product can be more economical if the steel slag is reused for iron making. However, the scientific community has not explored steel slag as a charge flux for iron making. The recycling technique of steel slag can be improved further in pellet preparation by adopting micro-pelletizing technology [65].
5.2 Non-Metallurgical Applications
Steel slag usage for non-metallurgical applications covers a wide range of recycling outside the iron-steelmaking industries. The various potential applications concerned with steel slag in terms of its chemical and physical properties are discussed in this section. The following applications have been established in the non-metallurgical area.
5.2.1 Rail Ballast
The steel slag has been used for railway track beds as ballast. The ballasted track has two individual layers of crushed stones: the ballast layer and the sub-ballast layer. Ballast use is essential for normalizing rail shaft load by spreading large ballast sites and preventing plant growth [66]. The researchers have compared the slag-based ballast with standard ballast (granite ballast, limestone ballast) and found that the slag-based ballast has higher shear strength [67], lower deformation [68], better track stiffness, good stability, greater strength, and excellent durability than standard/conventional ballast [69]. Since steel slags are abundant, using slag as rail ballast may help decrease the price, maintain cost, and reduce environmental impact, and it is undoubtedly an alternative to natural aggregate. Figure 15 illustrates a process flow chart for reusing steel slag as a rail ballast [70]. According to Indian Standard, the information about rail ballast quality and protocols is given in Table 3.
5.2.2 Road Construction
A large volume of industrial waste, primarily steel slag, is used in road construction without compromising the road's quality or performance/durability. The presence of steel slag in the raw mix helps to enhance hydraulically bound mixes with natural aggregates[72], which develops bonding between steel industries slag and natural aggregate and produces better working performance in terms of mechanical and physical properties like water absorption, apparent and bulk specific gravity and abrasion value(as shown in Fig. 16) [73].
Comparison of different mechanical properties studies between steel slag and natural stone aggregate used samples. A-G shows the various natural stones used during past studies. A- Granite; B- Basalt; C- Limestone; D- Natural Aggregate; E- Crushed Stone; F- Dolerite; G- Flint Gravel (a) Bulk specific gravity, b Apparent specific gravity, c Water Absorption and (d) Aggregate Soundness ([70] and reference therein)
Using 100% of the fine and coarse aggregate of steel industries wastes is not preferred for hot mixing asphalt, as this combination generates a large air voids structure which allows air and water to pass through it and weaken the overall system causing the bulking and flushing problem. Two commercial methods are used for highway research programs: Marshall mix design and Hveem mix design. The process flow charts are shown in Fig. 17. The Marshall mix design is the most common because of its simple test steps and equipment. However, the Marshall method has some limitations with significant parameters. Another way, i.e., the Superpave mix design, is usually followed during the Strategic Highway Research Program using mixed composition.
Different combinations of slag with natural aggregates such as slag + dolomite, water + crushed quartz + crushed dolomite [74], reinforced with synthetic fiber [75], and slag + bottom ash [76] have been used. The primary sample preparation, testing, and properties for road construction related to asphalt binder and a combination of asphalt binder and fiber are shown in Fig. 18 [75, 77]. The combination of waste slag and natural aggregates has helped to enhance fatigue performance. It was also observed that a higher slag content with a minimum mixture of dolomite, water, and sand decreases the sample's strength [74]. The free lime and magnesia in the steel slag lead to volume expansion when it comes in contact with water and leads to structural failure [75].
Some advantages and disadvantages are shown in Table 4 for different uses of steel slag and the challenges faced during road construction. Hence, using 100% slag for road construction is not considered since the free Ca2+ and Mg2+ ions have the property of expansion with time. Pure steel slag sample also shows swelling when it interacts with water, ultimately forming local cracks. The prolonged water treatment helps reduce the slag's free lime and magnesia content by up to 68%. Using steel industry slags with furnace bottom ash also avoids the volume expansion problem. However, the literature has reported that the furnace bottom ash has a lower strength value than steel, with more significant iron oxides (Fe2O3/FeO) enhancing the mechanical properties [76]. Hence, the bulk use of steel industry slag for road construction is impossible in the current scenario unless treated with water.
5.2.3 Landfilling Covering Material (Structural Fill)
Landfills are an alternative approach to reducing gas emissions and leaching processes. It minimizes the interaction of steel slag with the environment. Many researchers have scrutinized the stability of steel industry slag for a longer time and the effect of the aging process [82]. The old slag leached under the detection limit [83]. The schematic diagram for a basic understanding and its working principle of landfilling cover and processing steps are depicted in Fig. 19a, b. During landfilling, the samples are kept in a chamber so the slag cannot interact with the environment. However, the leakage of the chamber walls is continually monitored using a pH monitoring system. It is suggested that combining EAF slag with LF slag can replace a large amount of construction material for landfill cover material [84]. More research is necessary for this domain to explore the optimum factors further to enhance the utilization of steel slags.
5.2.4 Acid Mine Drainage (AMD) Neutralizer
Acid mine drainage (AMD) is the formation and movement of highly acidic (pH < 5) water rich in metals. It forms through the chemical reaction of water (rainwater) with rocks containing sulfur-bearing minerals. Untreated AMD significantly decreases the pH value of the soil and water resources. It destroys the surrounding plantation and aquatic life in the vicinity. The oxidation reaction during AMD formation is shown in Fig. 20a [85]. The technologies to use alkaline wastes to minimize the toxic nature of AMD are well-established and widely adopted in many countries [86].
A) Chemistry behind the neutralizing acidic effects, (b) mechanism of slag reacting with acid mining drainage, and (c) SEM image of steel industries slag before and after reacting with acid mining drainage [84]
The steel slag with pH > 9 acts as a leaching bed for AMD, in which the slag helps add alkalinity to the AMD system and neutralizes its acidic effect [87]. An experimental test concludes that steel industry slag can have the potential to leach for up to 9 months; after this period, the efficiency of the leaching process get decreases drastically [86]. Apart from its neutralizing property, steel slag has been extensively studied to remove hazardous material from the AMD [88]. Steel slag as an adsorbent efficiently removes the cations (Cu+2 and Cd+2) and anions (SO42−, PO42−) pollutants from the AMD (Fig. 20b). The mechanism of removal is based on the formation of Ca(OH)2 & CaSO4, which acts as adsorbent to these pollutants. The SEM characterization of the steel slag after the intersection with the AMD is shown in Fig. 20c [89, 90]. The CaO/Ca(OH)2 in steel slag reacts with sulfate forming a sheet or rod-type structure identified as gypsum (Fig. 20c). The resulting sludge is useful as a supplementary cementitious material. Nonetheless, CaO/Ca(OH)2 is a neutralizer agent while mixing AMD with steel slag [91].
5.2.5 Glass Ceramics
Ceramic materials have many applications in the chemical, electric space, and manufacturing industries [92]. The silica, aluminum, calcium, and magnesium in steel slag can add value to ceramic fabrication like porous ceramics [93], glass–ceramic [94], and foam glass–ceramics (Table 1) [95]. A different way to fabricate various ceramic materials from the steel slag is discussed in Fig. 21. A range of properties like porosity, compressive strength, thermal conductivity, and many more, with the help of other ceramic fabrication techniques.
For instance, glass ceramic is usually used for decorating materials of buildings like floor tiles, floor covering, and wall construction [96]. It is fabricated using two primary methods, sintering, and melting, as shown in Fig. 21a. The process requires a low temperature with sufficient applied pressure [97] often resulting in unavoidable breakage during fabrication. The steel slag generally has primary crystalline phases, which can be seen in the ternary phase diagram shown in Fig. 21b. These crystalline phases tend to enhance the property of the glass–ceramic [98]. Using steel slag improves physical properties like low water absorption, high corrosion resistance, and bending strength energy [99]. The literature has mentioned that more than 30% of CaO and 10% of MgO has provided a low bending strength to the final product [100]. The ceramic obtained from steel slag is denser and acquires better physical and mechanical properties up to crystalline temperature. However, the ceramic porosity gets increased, and the mechanical properties get reduced when the operation temperature crossed the crystalline temperature of the material (as shown in Fig. 21(c)) [97]
Therefore, using steel slag for fabricating porous ceramics is cost-effective and performs excellently since it contains various crystal phases suitable for different ceramics production [101].
5.2.6 Concrete (As an Aggregate)
Using steel slag as aggregate depends on the factors like aggregate sizes, aggregate properties, curing temperature, and bonding with the cement paste [66]. The process flow charts to use the natural aggregate and the slag aggregate in concrete are compared and shown in Figs. 22, 23a and b. The general approach for natural and steel industries slag has the same production steps. Interestingly, it is found that the steel industry slag gives rise to better properties than natural aggregate for the same cement content during concrete production. A comparison of natural aggregate and EAF/BOF slag is shown in Fig. 23c [102].
The majority of the research approaches involving BOF slag, EAF, LF, and AOD slag report an improvement in the concrete properties [103, 104] and EAF [105,106,107] Only a few studies saw a negative impact or no improvement in their experimental study [108]. The comparison of various mechanical properties between natural aggregate and steel industries slag as an aggregate is shown in Fig. 24(a–c).
Comparison of various mechanical properties of natural aggregate for concrete and use of steel industries slag for concrete (a) compressive strength, b flexural Strength, c split tensile strength, and (d) comparison of mechanical properties for natural aggregate and different steel industries slags [63]
Apart from the slag types, the mixing ratio, slag size, and curing temperature are essential parameters to improve the mechanical properties of the concrete sample. Compressive strength and bulk density have contrasting effects. The void volume in the concrete decreases with the increase in its bulk density, as shown in Fig. 25(b). Besides, the compressive strength gets reduced with the decrease in the size of the slag material. Nonetheless, the impact of size on the mechanical properties is prominent because of slag particle size ~ 100 mm (as shown in Fig. 25c). Thus, removing the finer fraction of the steel slag aggregate is advisable before subjecting it to concrete admixture to obtain better mechanical properties. The curing time and curing temperature show analogous impacts on the compressive strength, as shown in Fig. 25(d) [66]
5.2.7 Cement
Steel slags have been used as a cement ingredient in many countries over the last two decades. The studies have claimed that the raw materials’ hydraulic properties (ability to absorb water) are essential to use as a cementitious material. The raw material with higher alkalinity is assumed to be rich in hydraulic properties and considered a suitable cementitious material [108]. Here, it is noteworthy that the LF slag is the most alkaline in nature, followed by EAF and BOF slag (Table 1).
The slag from various steel industries contains the dicalcium silicate (C2S) and tricalcium silicate (C3S) phases. The presence of di and tricalcium silicate reduces the activation factor of steel slag compared to available cement clinker; the reason is ascribed to the crystal size and less porous structure formed due to high-temperature operation [109]. Apart from the alkalinity, the slag's cooling technique also contributes to the reactivity of the available phases of slags, as shown in Fig. 26 [110]. Free-CaO and -MgO in slag show hydraulic nature when used with cement, improving the cement products' mechanical behaviors [111]. Some mineral phases are shown in Fig. 27. The presence of the C2S γ − phase in slag makes it non-hydraulic in nature [114], which restricts the binding properties of materials. The presence of the RO phase (solid solution of CaO-FeO-MnO-MgO) limits the hydration process and performs unfavorable mechanical properties. However, the RO phase helps to create an interface bridge between available phase particles and the availability of C-S–H gel in hydrated cement. The presence of free Ca2+ and Mg2+ ions or CaCO3 also negatively affects soundness, swelling, expansion, and shrinkage. Steel slag can be used as an alkaline binder instend of cement supplementary material every time. Due to the above reason, direct uses of steel slag in the blended cement have not been recommended. Among the BOF slag, EAF slag and LF slags, the LF slag and BOF slag were found better than EAF slag in terms of the resulting mechanical properties [111].
The effect of the mineral phase when the different cooling technique is used to cool molten steel industries slag (a) Original, b water granulation, c Water splashing, d Air cooling, e controlled furnace cooling [106]
5.2.8 CO 2 Sequestration
Carbon dioxide (CO2) emission is one of the biggest environmental problems the world is facing. The primary CO2 emission from the steel industries is about 3–4% of worldwide emissions [115]. Lately, steel slag is a good CO2 absorption material, rich in calcium and magnesium, with basicity always > 1 [116]. So far, two different direct carbonization processes, (i) solid–gas carbonization and (ii) liquid–gas carbonization, have been studied. Working flow charts of those methods are depicted in Fig. 28a. Dry carbonation (also known as solid–gas carbonation) is one of the direct types of dry carbonation techniques available for CO2 absorption. In this process, CO2 gas flows through alkaline porous slag at optimized temperature and pressure [117]. The schematic diagram of the chemical interaction of carbon dioxide and alkaline slag is shown in Fig. 28b. The exothermic heat of the process can be further used for electricity generation. Parameters involved during the solid–gas carbonation experimental study are mentioned in Table 5 with the carbonization efficiency.
On the other hand, the aqueous carbonation (also known as liquid–gas carbonation) method is preferably a direct wet carbonation technique in which carbon dioxide gets absorbed with the help of alkaline waste minerals in the presence of an aqueous medium. The carbonate precipitation starts when CO2 dissolves to yield carbonic acid (H2CO3). The dissociation of H2CO3, H+ ions, and bicarbonate (HCO−3) initiate the series of reactions where H+ ions help to emancipate free metal ions (Mg2+ or Ca2+) from available minerals. Finally, bicarbonate ions and metal ions react to form metal carbonates [121], as shown in Fig. 28b. Alternatively, the aqueous process can be carried out in two steps: the Ca/Mg ions leached out in the first step, then carbon dioxide dissolved in the leached solution, leading to carbonate precipitation [122]. Table 6 summarizes the studies on the use of steel slag for CO2 sequestration by the aqueous method. Process effluent has a pH ̴ 8, which is almost near neutral. A schematic illustration of the indirect aqueous CO2 sequestration in Fig. 29 shows essential steps in the indirect process [123, 124].
It is apparent from Tables 5 and 6 that the aqueous carbonation can absorb more CO2 per kg slag than the dry carbonation process. The following equation calculates the steel slag's carbonization degree \(\xi_{Ca} (\% )\) [130].
where Catotal is the total calcium content in slag, MWCa and MWCO2 are the molar mass of calcium, and the molar mass of CO2. However, this equation only works for carbonization by calcium, negating the effect of magnesium and other components. Yadav and Mehra [130] used the thermogravimetric analysis (TGA) to measure the carbonization degree on the slag sample using Eq. (2) follows between the carbonated sample (\(\Delta m_{{CaCO_{3} }}\)) and the non-carbonation sample (\(m_{{at27^{o} C}}\)). The CO2 sequestration efficiency was monitored by the weight of the slag sample after and before the carbonization process.
Equations (1) and (2) signify that solid carbonation has a low degree of carbonization compared to aqueous carbonation due to carbon dioxide's slow carbonization chemical reaction with calcium or magnesium oxide. It is reported that the response gets faster if the carbon dioxide is converted to bicarbonate. Environmental parameters like pressure and temperature significantly affect the carbonation process. A scanning electron microscopy study showed a significant impact of working temperatures and pressure on forming a carbonate layer on slag samples [120]. Table 7 summarizes the effect of direct and indirect carbonation on the various physicochemical properties of slag. Direct carbonation forms calcite and silico-carbonate of calcium, whereas indirect carbonate captures the CO2 as calcite and aragonite. The porosity and density are also affected by carbonation. The leaching of heavy metals like chromium decreases after the direct carbonation of slag.
Table 7 summarizes the direct and indirect carbonation study for carbon dioxide sequestration using steel slag.
6 Future Aspects
Furthur developments and improvements in old process for holistic utilization of slag requires innovative approaches. Besides, a viable process for economically extracting metals from slag is much required to save natural resources. However, the scalability and fit ability of the type of steel slag in a particular application is a matter of further research. An unexplored area is entails the recent trends for decarbonizing the steel making process. The modified steel making processes would have slag byproducts with different physico-chemical properties. It is advisable to be ready beforehand with the new compositions of steel slag for conventional and newer applications. The suggested techniques need to be more innovative and economical so that the utilization rate of steel slag can increase and quickly be adopted by industries. Another metallurgical applications, for instance, the lime content in steel slag can be exploited in various metallurgical processes such as binder in the sintering process, as a slag conditioner for high alumina slags. Another approach could be based on generation of calcium by metallothermic reduction of steel slag under vacuum. However in-depth theoretical and experimental investigation are necessary to understand the fundamentals to establish the processes. The basic understanding will help to up scale the process. It is also recommended for utilizing the iron recovered from steel slag back into the conventional steelmaking process. It can be done by subsequent refining by EAF route to make alloy steel.
In order to ensure maximum utilization, newer non-metallurgical niche area applications like flame retardants, screed uses, and carbon-slag composites must be tried. Process innovation for bulk utilization is critical for futuristic technology development. Most studies focus on making construction materials such as concrete, cementitious, and road construction. So far, steel slag could replace about 15% of construction material due to the deleterious effect of iron on strength and durability when it comes in contact with water and oxygen. Recently, Tata Steel, India has launched a product called “Tata Agreto,” a BOF slag-based material to replace aggregates for road making. It is believed to conserve about 16,500 tonnes of natural aggregates for every kilometer of road. Such an innovative approach is the need of the hour to put the so-called waste slag back into the circular economy.
The process developed must have the potential to integrate with existing plants. The resulting slag may find application in a variety of sectors. For instance, a study for the carbothermic reduction of BOF slag can produce pig iron and modified slag for subsequent steel and cement-making applications. However, a thorough thermodynamic investigation and tests on a pilot scale are necessary for acceptance by the industry. A hot stage slag modification on a tonnage scale needs to be tested in industrial EAF for subsequent in-plant integration where the slag from the BOF can be directly received in an EAF for necessary modification like removal of iron and phosphorus. Besides, other applications like refractory materials, radiation shield materials, 3D printing materials, and heat transfer applications, which have not been much explored till now, must be attempted to conserve the natural resource and minimize environmental alteration.
Hence, further research focusing on a sustainable solution to the holistic utilization of steel slag is essential in the circular economy. Extensive upscale tests, particularly field trials, must be attempted to achieve a higher Technology Readiness Level. Moreover, the recommended solution arising from this research should be scalable and integrated with the current industrial setup. In addition, the environmental impact and ecological benefits of recycling steel slag must be continually discussed. If the direct use of steel slag has a low-economical effect, its long-term environmental and ecological impact should also be considered.
7 Conclusions
Steel slag is a by-product of various steel manufacturing steps. The authors in this study have reviewed the generation volume, chemical/physical characteristics of the steel slag, and all the recycling processes. The environmental concerns in the unscientific dumping of slags have also been reviewed. It was revealed that the average generation of slags in BOF, EAF, and LF processes was 110 kg, 70 kg, and 40 kg per tonne of steel, respectively. Approximately 70% of steel slag produces through the BOF process, whereas it is nearly 28.8% through the EAF process and around 0.4% from the secondary refining in LF. The actual use of these slags is meager, in the range of 10–15%, in a helpful manner. The steel slags contained large amounts of valuable metals, such as Fe, Si, etc., and toxic elements, such as Cr, Ni, V, and Zn.
Thus, the slag must be further recycled and refined towards environmentally friendly resource recovery. Suggested methods for metallurgical recycling comprised recovery of Fe values by mechanical, hydrometallurgical, or pyrometallurgical methods. The mechanical methods are based on crushing-grinding-magnetic separation unit operations. A low magnetic intensity (hand-magnet) could achieve more than 95% recovery for sample sizes greater than 4 mm, whereas a high magnet with 1 T intensity was sufficient to recover 95% metal values from a size less than 0.3 mm. Acid or alkali leaching followed by solvent extraction works well to recover elements like V and Cr. However, the recovery of these elements remains very low and consumes a large quantity of reagents. Pyrometallurgical steel slag processing by roasting/smelting effectively recovers the iron value. BOF slag was successfully used in iron ore pellet and sinter, supplying necessary CaO for binding. Direct use of slag in non-metallurgical applications such as rail ballast, aggregate in road construction and concrete, landfill covering material, neutralizing agent for acid mine drainage, and raw material for glass ceramics and cement manufacturing has been demonstrated. The presence of iron in the steel slag is a limiting factor to achieving desirable properties in many applications. For instance, it affects the strength of the structure when slag is used as construction material. The modified slag obtained after the recovery of iron finds subsequent use in other non-metallurgical applications. Being rich in CaO and MgO, the steel slags have been a successful candidate for CO2 sequestration, preferably by a wet method where an acidic solution leaches Ca2+ and Mg2+, and CO2 is captured as corresponding carbonates/bicarbonates. Despite evident progress in this field, some technical and environmental challenges/problems still exist. Therefore, improving the recycling efficiency and removal/immobilization of harmful elements to address environmental concerns is necessary. Thus, improved processes for the metallurgical and non-metallurgical recycling and modification of steel slags for high recycling and energy efficiency and minimum secondary environmental pollution should be the focus of the research and development trend in this area.
Data Availability
Data and results presented in this manuscript are available with the journal’s permission.
References
World Steel (2021) https://www.worldsteel.org/media-centre/press-releases/2021/world-steel-in-figures-2021.html. Accessed Sep. 19, 2021
Das P, Mondal GC, Singh S, Singh AK, Prasad B, Singh KK (2018) Effluent treatment Technologies in the Iron and Steel Industry - A state of the art review. Water Environ Res 90(5):395–408. https://doi.org/10.2175/106143017x15131012152951
Guo J, Bao Y, Wang M (2018) Steel slag in China: Treatment, recycling, and management. Waste Manag 78:318–330. https://doi.org/10.1016/J.WASMAN.2018.04.045
Proctor DM et al (2000) Physical and chemical characteristics of blast furnace, basic oxygen furnace, and electric arc furnace steel industry slags. Environ Sci Technol 34(8):1576–1582. https://doi.org/10.1021/es9906002
Slag recycling - recovery (2021) https://www.recovery-worldwide.com/en/artikel/slag-recycling_3528047.html. Accessed 22 Oct 2021
Schoenberger H (2001) Final draft: best available techniques reference document on the production of iron and steel. Publ. EC Eur. Comm. Jt. Res. Centre, IPTS, Eur. IPPC Bur
Xue Y, Wu S, Hou H, Zha J (2006) Experimental investigation of basic oxygen furnace slag used as aggregate in asphalt mixture. J Hazard Mater 138(2):261–268. https://doi.org/10.1016/J.JHAZMAT.2006.02.073
Zhang N, Wu L, Liu X, Zhang Y (2019) Structural characteristics and cementitious behavior of basic oxygen furnace slag mud and electric arc furnace slag. Constr Build Mater 219:11–18. https://doi.org/10.1016/J.CONBUILDMAT.2019.05.156
Fang K, Wang D, Zhao J, Zhang M (2021) Utilization of ladle furnace slag as cement partial replacement: Influences on the hydration and hardening properties of cement. Constr Build Mater 299:124265. https://doi.org/10.1016/J.CONBUILDMAT.2021.124265
Reddy AS, Pradhan RK, Chandra S (2006) Utilization of basic oxygen furnace (BOF) slag in the production of a hydraulic cement binder. Int J Miner Process 79(2):98–105. https://doi.org/10.1016/J.MINPRO.2006.01.001
Sabapathy YK, Balasubramanian VB, Shankari N Shiva, Kumar A Yeshwant, Ravichandar D (2017) Experimental investigation of surface modified EOF steel slag as coarse aggregate in concrete. J King Saud Univ - Eng Sci 29(4):388–393. https://doi.org/10.1016/J.JKSUES.2016.07.002
Gollapalli V, Tadivaka SR, Borra CR, Varanasi SS, Karamched PS, Rao MB Venkata (2020) Investigation on stabilization of ladle furnace slag with different additives. J Sustain Metall 2020 61 6(1):121–131. https://doi.org/10.1007/S40831-020-00263-W
Doucet FJ (2010) Effective CO2-specific sequestration capacity of steel slags and variability in their leaching behaviour in view of industrial mineral carbonation. Miner Eng 23(3):262–269. https://doi.org/10.1016/J.MINENG.2009.09.006
Gao X, Okubo M, Maruoka N, Shibata H, Ito T, Kitamura SY (2015) Production and utilisation of iron and steelmaking slag in Japan and the application of steelmaking slag for the recovery of paddy fields damaged by Tsunami. Trans Inst Min Metall Sect C Miner Process Extr Metall 124(2):116–124. https://doi.org/10.1179/1743285514Y.0000000068
Zhao J, Wang Y, Fang K, Zheng Y, Wang D (2020) The Characteristics of the Phase Transition of Air-Quenched Ladle Furnace Slag. JOM 2020 734 73(4):1071–1079. https://doi.org/10.1007/S11837-020-04464-2
Bodor M et al (2016) Laboratory investigation of carbonated BOF slag used as partial replacement of natural aggregate in cement mortars. Cem Concr Compos 65:55–66. https://doi.org/10.1016/J.CEMCONCOMP.2015.10.002
Durinck D et al (2007) EAF stainless steel refining - Part II: Microstructural slag evolution and its implications for slag foaming and chromium recovery. Steel Res Int 78(2):125–135.https://doi.org/10.1002/SRIN.200705869
Mombelli D, Mapelli C, Barella S, Di Cecca C, Le Saout G, Garcia-Diaz E (2016) The effect of chemical composition on the leaching behaviour of electric arc furnace (EAF) carbon steel slag during a standard leaching test. J Environ Chem Eng 4(1):1050–1060. https://doi.org/10.1016/J.JECE.2015.09.018
Pan S-Y, Chung T-C, Ho C-C, Hou C-J, Chen Y-H, Chiang P-C (2017) CO2 mineralization and utilization using steel slag for establishing a waste-to-resource supply chain. Sci Rep 2017 71 7(1):1–11. https://doi.org/10.1038/s41598-017-17648-9
Wang WC, Wang HY, Tsai HC (2016) Study on engineering properties of alkali-activated ladle furnace slag geopolymer. Constr Build Mater 123:800–805. https://doi.org/10.1016/J.CONBUILDMAT.2016.07.068
Belhadj E, Diliberto C, Lecomte A (2012) Characterization and activation of basic oxygen furnace slag. Cem Concr Compos 34(1):34–40. https://doi.org/10.1016/J.CEMCONCOMP.2011.08.012
Adegoloye G, Beaucour AL, Ortola S, Noumowe A (2016) Mineralogical composition of EAF slag and stabilised AOD slag aggregates and dimensional stability of slag aggregate concretes. Constr Build Mater 115:171–178. https://doi.org/10.1016/J.CONBUILDMAT.2016.04.036
Polettini A, Pomi R, Stramazzo A (2016) Carbon sequestration through accelerated carbonation of BOF slag: Influence of particle size characteristics. Chem Eng J 298:26–35. https://doi.org/10.1016/J.CEJ.2016.04.015
Rondi L, Bregoli G, Sorlini S, Cominoli L, Collivignarelli C, Plizzari G (2016) Concrete with EAF steel slag as aggregate: A comprehensive technical and environmental characterisation. Compos Part B Eng 90:195–202. https://doi.org/10.1016/J.COMPOSITESB.2015.12.022
Branca TA, Colla V, Valentini R (2013) A way to reduce environmental impact of ladle furnace slag. 36(8):597–602. https://doi.org/10.1179/030192309X12492910937970
Fernández-González D, Prazuch J, Ruiz-Bustinza I, González-Gasca C, Piñuela-Noval J, Verdeja LF (2019) The treatment of Basic Oxygen Furnace (BOF) slag with concentrated solar energy. Sol Energy 180:372–382. https://doi.org/10.1016/J.SOLENER.2019.01.055
Badiee H, Maghsoudipour A, Dehkordi BR (2013) Use of Iranian steel slag for production of ceramic floor tiles. 107(2):111–115. https://doi.org/10.1179/174367608X263377
Manso JM, Ortega-López V, Polanco JA, Setién J (2013) The use of ladle furnace slag in soil stabilization. Constr Build Mater 40:126–134. https://doi.org/10.1016/J.CONBUILDMAT.2012.09.079
Calmon JL, Tristão FA, Giacometti M, Meneguelli M, Moratti M, Teixeira JESL (2013) Effects of BOF steel slag and other cementitious materials on the rheological properties of self-compacting cement pastes. Constr Build Mater 40:1046–1053. https://doi.org/10.1016/J.CONBUILDMAT.2012.11.039
Penteado CSG, Evangelista BL, dos S. Ferreira GC, Borges PHA, Lintz RCC (2019) Use of electric arc furnace slag for producing concrete paving blocks. Ambient. Construído 19(2):21–32. https://doi.org/10.1590/S1678-86212019000200305
Lateef KB, Rezan SA, Nurulakmal MS (2014) Assessment of EAF steel slag solubility by statistical design. Adv Mater Res 858:228–235. https://doi.org/10.4028/WWW.SCIENTIFIC.NET/AMR.858.228
Hui-Teng N et al (2021) Formulation, mechanical properties and phase analysis of fly ash geopolymer with ladle furnace slag replacement. J Mater Res Technol 12:1212–1226. https://doi.org/10.1016/J.JMRT.2021.03.065
Zago SC, Vernilli F, Cascudo O (2023) The reuse of basic oxygen furnace slag as concrete aggregate to achieve sustainable development: characteristics and limitations. Build 13(1193), 13(5):1193. https://doi.org/10.3390/BUILDINGS13051193
Teo P Ter et al (2020) Assessment of Electric Arc Furnace (EAF) steel slag waste’s recycling options into value added green products: A review. Met 10(10):1347. https://doi.org/10.3390/MET10101347
Bhatt A, Priyadarshini S, Mohanakrishnan A Acharath, Abri A, Sattler M, Techapaphawit S (2019) Physical, chemical, and geotechnical properties of coal fly ash: a global review. Case Stud Constr Mater 11:e00263. https://doi.org/10.1016/J.CSCM.2019.E00263
Wang Y, Liu Z, Zhang J, Mao R, Zhang Y (2020) Advanced converter sludge utilization technologies for the recovery of valuable elements: a review. J Hazard Mater 381:120902. https://doi.org/10.1016/J.JHAZMAT.2019.120902
Chandel S Singh, Randhawa N Singh, Singh P Kumar (2023) Thermodynamic and kinetic aspect of solid state reduction of Electric Arc Furnace slag through coke: An experimental study. Mater Today Proc. https://doi.org/10.1016/J.MATPR.2023.07.209
Rehman K, Fatima F, Waheed I, Akash MSH (2018) Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem 119(1):157–184. https://doi.org/10.1002/jcb.26234
Yang C-Y, Reijonen I, Yu H, Dharmarajan R, Seshadri B, Bolan NS (2018) Back to basic slags as a phosphorus source and liming material. Soil Amend Sustain. https://doi.org/10.1201/9781351027021-18
HS G, MI K, MA A, S D, PJ K (2018) Environmental risk assessment of steel-making slags and the potential use of LD slag in mitigating methane emissions and the grain arsenic level in rice (Oryza sativa L.). J Hazard Mater 353:236–243. https://doi.org/10.1016/J.JHAZMAT.2018.04.023
Chaurand P et al (2007) Environmental impacts of steel slag reused in road construction: A crystallographic and molecular (XANES) approach. J Hazard Mater 139(3):537–542. https://doi.org/10.1016/j.jhazmat.2006.02.060
Rakshit A, Sarkar B, Abhilash PC (2018) Soil amendments for sustainability: challenges and perspectives. p. 403
Hull SL, Oty UV, Mayes WM (2014) Rapid recovery of benthic invertebrates downstream of hyperalkaline steel slag discharges. Hydrobiologia 736(1):83–97. https://doi.org/10.1007/S10750-014-1894-5
Wang X, Li X, Yan X, Tu C, Yu Z (2021) Environmental risks for application of iron and steel slags in soils in China: A review. Pedosphere 31(1):28–42. https://doi.org/10.1016/S1002-0160(20)60058-3
Ministry of Steel (2016) https://steel.gov.in/. Accessed Oct. 25, 2021
Nippon Slag Association (2017) https://www.slg.jp/e/slag/product/kotuzai.html. Accessed Oct. 25, 2021
JFE Mineral Co., LTD. (2017) Slag, Iron and Steel JFE Mineral Co., LTD. https://www.jfe-mineral.co.jp/e_mineral/business/iron_and_steel/index.html. Accessed Sep. 19, 2021
Nippon Slag Association (2014) Available: https://www.slg.jp/e/statistics/ Accessed: Sep. 19, 2021. [Online]
Yüksel İ (2016) A review of steel slag usage in construction industry for sustainable development. Environ Dev Sustain 19(2):369–384. https://doi.org/10.1007/S10668-016-9759-X
Morgan D (2000) Separation, magnetic separation. Kirk-Othmer Encycl Chem Technol. https://doi.org/10.1002/0471238961.1301071413151807.A01
Menad N, Kanari N, Save M (2014) Recovery of high grade iron compounds from LD slag by enhanced magnetic separation techniques. Int J Miner Process 126:1–9. https://doi.org/10.1016/J.MINPRO.2013.11.001
Lan Y, Liu Q, Meng F, Niu D, Zhao H (2017) Optimization of magnetic separation process for iron recovery from steel slag. J Iron Steel Res Int 24(2):165–170. https://doi.org/10.1016/S1006-706X(17)30023-7
Tripathy SK, Singh V, Suresh N (2015) Prediction of separation performance of dry high intensity magnetic separator for processing of para-magnetic minerals. J Inst Eng Ser D 96(2):131–142. https://doi.org/10.1007/S40033-015-0064-X
Sakaroglou M, Anastassakis GN (2017) Nickel recovery from electric arc furnace slag by magnetic separation. J Min Metall A Min 53(1):3–15. https://doi.org/10.5937/JMMA1701003S
Lin L, Bao Y, Wang M, Jiang W, Zhou H (2014) Separation and recovery of phosphorus from P-bearing steelmaking slag. J Iron Steel Res Int 21(5):496–502. https://doi.org/10.1016/S1006-706X(14)60077-7
Yang L et al (2019) The stability of the compounds formed in the process of removal PB(II), CU(II) and CD(II) by steelmaking slag in an acidic aqueous solution. J Environ Manage 231:41–48. https://doi.org/10.1016/J.JENVMAN.2018.10.028
Ragipani R, Bhattacharya S, Akkihebbal SK (2020) Understanding dissolution characteristics of steel slag for resource recovery. Waste Manag 117:179–187. https://doi.org/10.1016/J.WASMAN.2020.08.008
Wan J et al (2020) Direct leaching of vanadium from vanadium-bearing steel slag using NaOH solutions: A case study. 42(4):257–267. https://doi.org/10.1080/08827508.2020.1762182
Binnemans K, Jones PT, Fernández Á Manjón, Torres V Masaguer (2020) Hydrometallurgical processes for the recovery of metals from steel industry by-products: A critical review. J Sustain Metall 6(4):505–540, Springer. https://doi.org/10.1007/s40831-020-00306-2
Agrawal S, Dhawan N (2020) Microwave carbothermic reduction of low-grade iron ore. Metall Mater Trans B 51(4):1576–1586. https://doi.org/10.1007/S11663-020-01883-Z
Liu Z, Bi X, Gao Z, Liu W (2018) Carbothermal reduction of iron ore in its concentrate-agricultural waste pellets. Adv Mater Sci Eng 2018. https://doi.org/10.1155/2018/2138268
Scheller PR, Lee J, Yoshikwa T, Tanaka T (2014) Applications of interfacial phenomena in process metallurgy. Treatise Process Metall 2:119–139. https://doi.org/10.1016/B978-0-08-096984-8.00022-7
Singh PK, Lava K Avala, Katiyar PK, Maurya R (2017) Agglomeration behaviour of steel plants solid waste and its effect on sintering performance. J Mater Res Technol 6(3):289–296. https://doi.org/10.1016/J.JMRT.2016.11.005
Singh PK, Katiyar PK, Kumar AL, Mishra DK, Behera A (2016) Agglomeration behavior of solid waste materials in steel plants. Emerg Mater Res 5(1):171–176. https://doi.org/10.1680/JEMMR.15.00014
Pal J (2018) Innovative development on agglomeration of iron ore fines and iron oxide wastes. 40(4):248–264. https://doi.org/10.1080/08827508.2018.1518222
Gencel O, Karadag O, Oren OH, Bilir T (2021) Steel slag and its applications in cement and concrete technology: A review. Constr Build Mater 283:122783. https://doi.org/10.1016/J.CONBUILDMAT.2021.122783
Delgado BG, Viana da Fonseca A, Fortunato E, Maia P (2019) Mechanical behavior of inert steel slag ballast for heavy haul rail track: Laboratory evaluation. Transp Geotech 20:100243. https://doi.org/10.1016/J.TRGEO.2019.100243
Esmaeili M, Nouri R, Yousefian K (2017) Experimental comparison of the lateral resistance of tracks with steel slag ballast and limestone ballast materials. Proc Inst Mech Eng Part F J Rail Rapid Transit 231(2):175–184. https://doi.org/10.1177/0954409715623577
Ministry of Railway (2017) https://books.google.co.in/books?hl=en&lr=&id=-yMbDQAAQBAJ&oi=fnd&pg=PA283&ots=OkaK4At5HD&sig=9ND06eh23umv3PsM6B1O8ipGXiE&redir_esc=y#v=onepage&q&f=false. Accessed Oct. 05, 2021
Chamling PK, Haldar S, Patra S (2020) Behaviour of steel slag ballast for railway under cyclic loading. Lect Notes Civ Eng 85:709–722. https://doi.org/10.1007/978-981-15-6086-6_57
Ministry of Railways (Railway Board) (2020) https://indianrailways.gov.in/railwayboard/view_section.jsp?lang=0&id=0,1. Accessed Sep. 22, 2021
Xiao Z et al (2019) Moisture susceptibility evaluation of asphalt mixtures containing steel slag powder as filler. Materials (Basel) 12(19). https://doi.org/10.3390/MA12193211
Kambole C, Paige-Green P, Kupolati WK, Ndambuki JM, Adeboje AO (2017) Basic oxygen furnace slag for road pavements: A review of material characteristics and performance for effective utilisation in southern Africa. Constr Build Mater 148:618–631. https://doi.org/10.1016/J.CONBUILDMAT.2017.05.036
Haritonovs V, Tihonovs J (2014) Use of unconventional aggregates in hot mix asphalt concrete. Balt J Road Bridg Eng 9(4):276–282. https://doi.org/10.3846/BJRBE.2014.34
Alnadish AM, Aman MY, Katman HYB, Ibrahim MR (2020) Laboratory assessment of the performance and elastic behavior of asphalt mixtures containing steel slag aggregate and synthetic fibers. Int J Pavement Res Technol 14 (4):473–481. https://doi.org/10.1007/S42947-020-1149-Y
Jattak Z Ali et al (2019) Characterization of industrial by-products as asphalt paving material. IOP Conf Ser Earth Environ Sci 220(1). https://doi.org/10.1088/1755-1315/220/1/012012
Skaf M, Pasquini E, Revilla-Cuesta V, Ortega-López V (2019) Performance and durability of porous asphalt mixtures manufactured exclusively with electric steel slags. Materials (Basel) 12(20). https://doi.org/10.3390/MA12203306
Kavussi A, Qazizadeh MJ (2014) Fatigue characterization of asphalt mixes containing electric arc furnace (EAF) steel slag subjected to long term aging. Constr Build Mater Complete(72):158–166. https://doi.org/10.1016/J.CONBUILDMAT.2014.08.052
Wen H, Wu S, Bhusal S (2016) Performance evaluation of asphalt mixes containing steel slag aggregate as a measure to resist studded Tire Wear. J Mater Civ Eng 28(5):04015191. https://doi.org/10.1061/(ASCE)MT.1943-5533.0001475
Chen Z, Jiao Y, Wu S, Tu F (2018) Moisture-induced damage resistance of asphalt mixture entirely composed of gneiss and steel slag. Constr Build Mater 177:332–341. https://doi.org/10.1016/J.CONBUILDMAT.2018.05.097
Hainin MR, Aziz MA, Ali Z, Jaya RP, El-Sergany MM, Yaacoba H (2015) Steel slag as a road construction material. J Teknol 73(4):33–38. https://doi.org/10.11113/JT.V73.4282
Dhoble YN, Ahmed S (2018) Review on the innovative uses of steel slag for waste minimization. J Mater Cycles Waste Manag 20(3):1373–1382. Springer. https://doi.org/10.1007/s10163-018-0711-z
Diener S, Andreas L, Herrmann I, Ecke H, Lagerkvist A (2010) Accelerated carbonation of steel slags in a landfill cover construction. Waste Manag 30(1):132–139. https://doi.org/10.1016/J.WASMAN.2009.08.007
Andreas L, Diener S, Lagerkvist A (2014) Steel slags in a landfill top cover – experiences from a full-scale experiment. Waste Manag 34(3):692–701. https://doi.org/10.1016/J.WASMAN.2013.12.003
Chandra AP, Gerson AR (2010) The mechanisms of pyrite oxidation and leaching: A fundamental perspective. Surf Sci Rep 65(9):293–315. https://doi.org/10.1016/J.SURFREP.2010.08.003
Goetz ER, Riefler RG (2014) Performance of steel slag leach beds in acid mine drainage treatment. Chem Eng J 240:579–588. https://doi.org/10.1016/J.CEJ.2013.10.080
Simmons J, Ziemkiewicz P, Black D Courtney (2014) Mine Water Environ 21(2):91–99. https://doi.org/10.1007/S102300200024
Masindi V, Ramakokovhu MM, Osman MS, Tekere M (2021) Advanced application of BOF and SAF slags for the treatment of acid mine drainage (AMD): A comparative study. Mater Today Proc 38:934–941. https://doi.org/10.1016/J.MATPR.2020.05.422
Masindi V, Osman MS, Mbhele RN, Rikhotso R (2018) Fate of pollutants post treatment of acid mine drainage with basic oxygen furnace slag: Validation of experimental results with a geochemical model. J Clean Prod 172:2899–2909. https://doi.org/10.1016/J.JCLEPRO.2017.11.124
Zheng Q, Zhang Y, Zhang Z, Li H, Wu A, Shi H (2020) Experimental research on various slags as a potential adsorbent for the removal of sulfate from acid mine drainage. J Environ Manage 270:110880. https://doi.org/10.1016/J.JENVMAN.2020.110880
Du Y, Lu Q, Chen H, Du Y, Du D (2016) A novel strategy for arsenic removal from dirty acid wastewater via CaCO3-Ca(OH)2-FE(III) processing. J Water Process Eng 12:41–46. https://doi.org/10.1016/J.JWPE.2016.06.003
Gao R et al (2017) The forming region and mechanical properties of SiO2-Al2O3-MgO glasses. J Non Cryst Solids 470:132–137. https://doi.org/10.1016/J.JNONCRYSOL.2017.05.004
Iacobescu RI, Koumpouri D, Pontikes Y, Saban R, Angelopoulos GN (2011) Valorisation of electric arc furnace steel slag as raw material for low energy belite cements. J Hazard Mater 196:287–294. https://doi.org/10.1016/J.JHAZMAT.2011.09.024
Tong Z, Sun J, Wang J, Tan Z, Liu S (2020) Iron reduction and diopside-based glass ceramic preparation based on mineral carbonation of steel slag. Environ Sci Pollut Res 28(1):796–804. https://doi.org/10.1007/S11356-020-10358-2
Tang Z et al (2020) Preparation of high strength foam ceramics from sand shale and steel slag. Ceram Int 46(7):9256–9262. https://doi.org/10.1016/J.CERAMINT.2019.12.179
Pei F, Zhu G, Li P, Guo H, Yang P (2020) Effects of CaF2 on the sintering and crystallisation of CaO–MgO–Al2O3–SiO2 glass-ceramics. Ceram Int 46(11):17825–17835. https://doi.org/10.1016/J.CERAMINT.2020.04.089
Shang W et al (2021) Production of glass-ceramics from metallurgical slags. J Clean Prod 317:128220. https://doi.org/10.1016/J.JCLEPRO.2021.128220
Deng L et al (2020) Influence of Cr2O3 on the viscosity and crystallization behavior of glass ceramics based on blast furnace slag. Mater Chem Phys 240. https://doi.org/10.1016/J.MATCHEMPHYS.2019.122212
Deng L et al (2020) Effect of SiO2/MgO ratio on the crystallization behavior, structure, and properties of wollastonite-augite glass-ceramics derived from stainless steel slag. Mater Chem Phys 239. https://doi.org/10.1016/J.MATCHEMPHYS.2019.122039
Lu J, Cong X, Lu Z (2016) Influence of magnesia on sinter-crystallization, phase composition and flexural strength of sintered glass-ceramics from waste materials. Mater Chem Phys 174:143–149. https://doi.org/10.1016/J.MATCHEMPHYS.2016.02.061
Chen L, Ge X, Long Y, Zhou M, Wang H, Chen X (2020) Crystallization and properties of high calcium glass-ceramics synthesized from ferromanganese slag. J Non Cryst Solids 532. https://doi.org/10.1016/J.JNONCRYSOL.2019.119864
Andrade HD, de Carvalho JMF, Costa LCB, da F. Elói FP, do C. e Silva KD, Peixoto RAF (2021) Mechanical performance and resistance to carbonation of steel slag reinforced concrete. Constr Build Mater 298:123910. https://doi.org/10.1016/J.CONBUILDMAT.2021.123910
Lu TH, Chen YL, Shih PH, Chang JE (2018) Use of basic oxygen furnace slag fines in the production of cementitious mortars and the effects on mortar expansion. Constr Build Mater 167:768–774. https://doi.org/10.1016/J.CONBUILDMAT.2018.02.102
Lin W-T, Tsai C-J, Chen J, Liu W (2018) Feasibility and characterization mortar blended with high-amount basic oxygen furnace slag. Mater 12(1):6. https://doi.org/10.3390/MA12010006
De Domenico D, Faleschini F, Pellegrino C, Ricciardi G (2019) Corrigendum to ‘Structural behavior of RC beams containing EAF slag as recycled aggregate: Numerical versus experimental results. Construct Build Mater 201:879. Elsevier Ltd. https://doi.org/10.1016/j.conbuildmat.2019.01.061
Anastasiou EK, Papayianni I, Papachristoforou M (2014) Behavior of self compacting concrete containing ladle furnace slag and steel fiber reinforcement. Mater Des Complete(59):454–460. https://doi.org/10.1016/J.MATDES.2014.03.030
Moon EJ, Choi YC (2018) Development of carbon-capture binder using stainless steel argon oxygen decarburization slag activated by carbonation. J Clean Prod 180:642–654. https://doi.org/10.1016/J.JCLEPRO.2018.01.189
Brand AS, Fanijo EO (2020) A review of the influence of steel furnace slag type on the properties of cementitious composites. Appl Sci 10(22):8210. https://doi.org/10.3390/APP10228210
Jiang Y, Ling TC, Shi C, Pan SY (2018) Characteristics of steel slags and their use in cement and concrete—A review. Resour Conserv Recycl 136:187–197. https://doi.org/10.1016/J.RESCONREC.2018.04.023
Wang D et al (2012) Enrichment of Fe-Containing Phases and Recovery of Iron and Its Oxides by Magnetic Separation from BOF Slags. Steel Res Int 83(2):189–196.https://doi.org/10.1002/SRIN.201100216
Rashad AM (2019) A synopsis manual about recycling steel slag as a cementitious material. J Mater Res Technol 8(5):4940–4955. https://doi.org/10.1016/J.JMRT.2019.06.038
Palod R, Deo SV, Ramtekkar GD (2020) Sustainable approach for linz-donawitz slag waste as a replacement of cement in concrete: mechanical, microstructural, and durability properties. Adv Civ Eng 2020. https://doi.org/10.1155/2020/5691261
Lee NK, Jang JG, Lee HK (2014) Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem Concr Compos 53:239–248. https://doi.org/10.1016/J.CEMCONCOMP.2014.07.007
Jost KH, Ziemer B (1984) Relations between the crystal structures of calcium silicates and their reactivity against water. Cem Concr Res 14(2):177–184. https://doi.org/10.1016/0008-8846(84)90102-9
Yu J, Wang K (2011) Study on characteristics of steel slag for CO2 capture. Energy Fuels 25(11):5483–5492. https://doi.org/10.1021/EF2004255
Huijgen WJJ, Witkamp GJ, Comans RNJ (2005) Mineral CO2 sequestration by steel slag carbonation. Environ Sci Technol 39(24):9676–9682. https://doi.org/10.1021/es050795f
Tian S, Jiang Jianguo, Chen Xuejing, Yan Feng, Li K (2013) Direct gas-solid carbonation kinetics of steel slag and the contribution to in situ sequestration of flue gas CO(2) in steel-making plants. ChemSusChem 6(12):2348–2355. https://doi.org/10.1002/CSSC.201300436
Dananjayan RRT, Kandasamy P, Andimuthu R (2016) Direct mineral carbonation of coal fly ash for CO2 sequestration. J Clean Prod P5(112):4173–4182. https://doi.org/10.1016/J.JCLEPRO.2015.05.145
Mazzella A, Errico M, Spiga D (2016) CO2uptake capacity of coal fly ash: Influence of pressure and temperature on direct gas-solid carbonation. J Environ Chem Eng 4(4):4120–4128. https://doi.org/10.1016/J.JECE.2016.09.020
Ćwik A, Casanova I, Rausis K, Koukouzas N, Zarębska K (2018) Carbonation of high-calcium fly ashes and its potential for carbon dioxide removal in coal fired power plants. J Clean Prod 202:1026–1034. https://doi.org/10.1016/J.JCLEPRO.2018.08.234
O’Connor WK, Dahlin DC, Nilsen DN, Rush GE, Walters RP, Turner PC (2000) CO2 storage in solid form: a study of direct mineral carbonation. CSIRO, Collinwood, Victoria, Australia
Zhao Q et al (2020) Co-treatment of waste from steelmaking processes: Steel slag-based carbon capture and storage by mineralization. Front Chem 856. https://doi.org/10.3389/FCHEM.2020.571504
Bilen M, Altiner M, Yildirim M (2017) Evaluation of steelmaking slag for CO2 fixation by leaching-carbonation process. 36(3):368–377. https://doi.org/10.1080/02726351.2016.1267285
Wang C-Y, Bao W-J, Guo Z-C, Li H-Q (2018) Carbon dioxide sequestration via steelmaking slag carbonation in alkali solutions: Experimental investigation and process evaluation. Acta Metall Sin (English Lett) 31(7):771–784. https://doi.org/10.1007/S40195-017-0694-0
Bonenfant D et al (2008) CO2 sequestration potential of steel slags at ambient pressure and temperature. Ind Eng Chem Res 47(20):7610–7616. https://doi.org/10.1021/IE701721J
Baciocchi R, Costa G, Di Bartolomeo E, Polettini A, Pomi R (2010) Carbonation of stainless steel slag as a process for CO2 storage and slag valorization. Waste Biomass Valoriz 1(4):467–477. https://doi.org/10.1007/S12649-010-9047-1
Chang EE, Chen CH, Chen YH, Pan SY, Chiang PC (2011) Performance evaluation for carbonation of steel-making slags in a slurry reactor. J Hazard Mater 186(1):558–564. https://doi.org/10.1016/j.jhazmat.2010.11.038
Chang EE, Pan SY, Chen YH, Chu HW, Wang CF, Chiang PC (2011) CO2 sequestration by carbonation of steelmaking slags in an autoclave reactor. J Hazard Mater 195:107–114. https://doi.org/10.1016/j.jhazmat.2011.08.006
Baciocchi R, Costa G, Di Gianfilippo M, Polettini A, Pomi R, Stramazzo A (2015) Thin-film versus slurry-phase carbonation of steel slag: CO2 uptake and effects on mineralogy. J Hazard Mater C(283):302–313. https://doi.org/10.1016/J.JHAZMAT.2014.09.016
Yadav S, Mehra A (2017) Experimental study of dissolution of minerals and CO2 sequestration in steel slag. Waste Manag 64:348–357. https://doi.org/10.1016/J.WASMAN.2017.03.032
Li Y, Pei S, Pan SY, Chiang PC, Lu C, Ouyang T (2018) Carbonation and utilization of basic oxygen furnace slag coupled with concentrated water from electrodeionization. J CO2 Util 25:46–55. https://doi.org/10.1016/J.JCOU.2018.03.003
Librandi P, Nielsen P, Costa G, Snellings R, Quaghebeur M, Baciocchi R (2019) Mechanical and environmental properties of carbonated steel slag compacts as a function of mineralogy and CO2 uptake. J CO2 Util 33:201–214. https://doi.org/10.1016/J.JCOU.2019.05.028
Pan SY, Adhikari R, Chen YH, Li P, Chiang PC (2016) Integrated and innovative steel slag utilization for iron reclamation, green material production and CO2 fixation via accelerated carbonation. J Clean Prod 137:617–631. https://doi.org/10.1016/J.JCLEPRO.2016.07.112
Acknowledgements
The authors sincerely thank the affiliated institutes for providing facilities for the literature review. Mr. Sheshang Singh Chandel is grateful to IIT Ropar for his research fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chandel, S.S., Singh, P.K., Katiyar, P.K. et al. A Review on Environmental Concerns and Technological Innovations for the Valorization of Steel Industry Slag. Mining, Metallurgy & Exploration 40, 2059–2086 (2023). https://doi.org/10.1007/s42461-023-00886-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-023-00886-z