Abstract
A previous effort to characterize respirable coal mine dust in 16 US mines turned up a curious finding: particle-based analysis using scanning electron microscopy (SEM) tended to overpredict the abundance of dust sourced from rock strata, and underpredict the abundance of coal, when compared to mass-based thermogravimetric analysis (TGA). One possible explanation is the occurrence of coal-mineral microagglomerates (MAGs). Coal particles covered with fine mineral dust could be mostly coal by mass but classified as minerals by SEM due to their surface elemental content. In the current study, a subset of the previously analyzed mine dust samples was re-examined, and SEM images and elemental mapping showed that MAGs are indeed present. Furthermore, dust samples were created and sampled passively in the laboratory, demonstrating that MAG formation can occur due to dust generation processes and the sampling environment, rather than as a mere artifact of respirable dust sampling procedures. Finally, experiments were conducted to evaluate dispersibility of MAGs in liquid suspensions, which might shed some light on their possible fate upon inhalation. Results indicated that sonication in deionized water was effective for MAG dispersion, and a solution that mimics natural lung surfactant also appeared to enhance dispersibility. An understanding of MAG occurrence might be important in terms of exposure assessment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The specific activities in underground coal mines generate dust with diverse constituents and characteristics. However, the major components of respirable coal mine dust (RCMD) can generally be associated with three primary sources [1,2,3]. First, the coal being mined, crushed, and transported is expected to contribute mostly coal. Second, the rock strata surrounding the coal seam, which is often drilled for roof bolting and may be mined to some extent and handled along with the coal, are expected to contribute mostly mineral particles. In many mines, the rock strata are rich in silicates and silica (e.g., sandstone, shale, slate), though some mines encounter carbonate-rich strata (e.g., limestone). Third, the rock dust products applied to mine surfaces to mitigate explosion hazards can contribute to the RCMD. These products are often composed of high-purity limestone powder, and thus should contribute mostly carbonate minerals. Moreover, in mines operating diesel equipment, diesel particulate matter (DPM) can also contribute to the respirable particle fraction, mostly in the submicron range (i.e., less than about 0.3 µm) [1, 4].
While RCMD monitoring is frequently done on the basis of total mass concentration (mg/m3), specific constituents can have different effects in terms of occupational health. In fact, respirable crystalline silica has long been recognized as particularly hazardous [5,6,7], so exposures in coal mines are specifically regulated in many countries [8]. However, other RCMD constituents are not often identified or tracked.
The resurgence of lung disease among coal miners in the central Appalachian region of the USA has highlighted the need to understand more about the whole composition of RCMD [8]. Radiographic evidence indicates that crystalline silica exposures are a significant factor in resurgent disease pathology [9, 10]. Yet, mine monitoring data does not clearly explain when or where those exposures might have occurred [11]—and so there has been much speculation on these points, as well as other possible causal factors. Given the geographic clustering of disease, there has been a particular emphasis on the role of respirable dust sourced from rock strata. The rock encountered by many central and southern Appalachian mines is believed to be the primary source of respirable crystalline silica [12,13,14]. Mines in these regions also tend to exploit relatively thin coal seams, often cutting significant amounts of rock along with the coal [8].
Several studies have investigated RCMD constituents and their likely sources [15,16,17,18]. Recent work by Pokhrel et al. [19] specifically looked at non-carbonate mineral content as a proxy for rock-strata sourced dust in RCMD. The study used different techniques to analyze a total of 93 sample sets from 16 mines. These included thermogravimetric analysis (TGA), which was used to fractionate the total RCMD mass between non-carbonate minerals (e.g., silicates and silica), carbonates, and coal. Also, scanning electron microscopy with energy dispersive X-ray (SEM–EDX) was used to bin individual particles into pre-defined classes, and then the particle distributions were used to estimate the relative mass percentage of the RCMD in each class. It is noted that the SEM–EDX classes were such that they could be summed for comparison with TGA (i.e., SEM silica + silicates vs. TGA non-carbonates; SEM carbonates vs. TGA carbonates; SEM carbonaceous + mixed carbonaceous vs. TGA coal).
Interestingly, when Pokhrel et al. [19] compared the particle- and mass-based results, the SEM–EDX tended to overpredict the mass percentage of non-carbonate minerals (i.e., rock-strata sourced dust, especially aluminosilicates), but underpredict the mass percentage of coal, relative to the TGA. This suggests a significant number of particles that are predominantly coal by mass were instead classified as minerals by the SEM–EDX. Moreover, this trend was particularly evident for samples collected just downwind of production activities (i.e., where coal and rock strata are being cut and dust is being generated) and in the return airways (i.e., which are highly influenced by production activities). Since these samples also tended to have higher loading than samples from other locations, one obvious explanation could be that many coal particles were simply misclassified by the SEM–EDX due to mineral particles depositing in close proximity on the sample filter (Fig. 1b). However, misclassification of coal particles in the Pokhrel et al. [19] mine dust samples could have happened for other reasons too, e.g., due to impurities ingrained within the coal itself (Fig. 1c) or the occurrence of micro-agglomerates (MAGs)Footnote 1 (Fig. 1d). The former seems unlikely as a primary explanation in that study because many of the samples actually had a significant number of particles classified as carbonaceous (i.e., coal, Fig. 1a)—including samples collected in the same mines as those with apparently high coal particle misclassification in production or return samples. On the other hand, the occurrence of MAGs cannot be easily ruled out.
The presence of MAGs in RCMD samples has been indicated in SEM–EDX images captured by Zazouli et al. [20] and Pandey et al. [21]. Su et al. [22] looked at passively deposited dust (i.e., total dust rather than only the respirable fraction), which was collected from various surfaces in underground coal mines, and some of their images also indicate MAGs in the respirable size range. Moreover, MAGs including coal particles were also reported by Cvetković et al. [23]. They looked at passively deposited dust particles in the vicinity of an opencast coal mine and power plant and emphasized that misclassification by SEM–EDX may be inevitable for mixed coal-mineral particles (i.e., since classification as coal relies on the absence of substantial EDX response from elements indicative of minerals such as Al and Si).
While evidence of MAGs in RCMD samples can be found (e.g., [20, 21]), the formation and relative adhesion or persistence of these particles have not been widely addressed. The presence of MAGs in passively collected total airborne dust samples (e.g., [21]) suggests that formation could be related to the dust generation processes or conditions in the sampling environment (e.g., particle concentration, humidity). However, because the collection of RCMD, specifically, is typically done using a size selector (e.g., cyclone), the presence of MAGs in such samples is potentially also an artifact of the sampling procedure itself [24].
From the perspective of RCMD exposure assessment, it would be valuable to elucidate if MAGs are likely formed in the mine atmosphere or merely due to sampling effects. Additionally, some indication of the relative persistence of MAGs could shed light on possible health implications. For example, upon respiration, are MAGs likely to remain intact or disperse easily? Nichols [25] describes agglomerates as being either “hard” or “soft,” with the distinction being that hard agglomerates require a substantial external force to disperse whereas soft agglomerates may disperse easily. Dispersibility may influence lung penetration, clearance, or toxicity of MAGs, which can be dependent on particle size or surface characteristics.
Based on the above, the objectives of the current work were threefold: (1) re-examine the RCMD samples studied by Pokhrel et al. [19] for evidence of MAGs; (2) demonstrate the occurrence of respirable-sized MAGs in passively collected dust samples generated from real coal and rock strata materials; and (3) explore the persistence of MAGs when subjected to mechanical forces or surfactants that mimic lung fluid.
2 Experimental Details
2.1 Existing RCMD Samples and SEM–EDX Data
As mentioned earlier, the previous study by Pokhrel et al. [19] used SEM–EDX to analyze a total of 93 RCMD samples representing 16 underground coal mines. The samples were collected in five standard locations: in the intake airway(s), just downwind of the production face, adjacent to the feeder breaker, just downwind of an active roof bolter, and in the return airway. The sample collection, preparation, and SEM–EDX procedures were detailed by Pokhrel et al., and are briefly summarized as follows: samples were collected using standard equipment for RCMD (i.e., personal air sampling pumps operated at 2.0 L/min and 10-mm nylon cyclones), and deposited on polycarbonate (PC) filters (37-mm, 0.4-µm pore size) housed inside 2-piece styrene cassettes. For each sample collected for SEM–EDX analysis, replicates were collected on additional PC and polyvinyl chloride (PVC) filters for other analysis (i.e., TGA and gravimetric). A 9-mm subsection was cut near the edge of each PC filter sample routed for SEM–EDX analysis, and the subsection was sputter-coated (Au/Pd).
The SEM–EDX analysis was done using two computer-controlled routines to identify, size, and collect elemental data on a total of about 800 particles per sample: the first routine targeted supramicron particles (~ 1–10 µm) and the second routine targeted submicron particles (~ 0.1–1 µm). The EDX data (i.e., normalized atomic percentage of values for eight elements: carbon, oxygen, aluminum, silicon, calcium, magnesium, iron, and titanium) was used to bin each particle into one of the following mineralogy classes: carbonaceous (C), mixed carbonaceous (MC), aluminosilicates (AS, which could be further subclassified as kaolinite or other), silica (S), other silicates (SLO), carbonates (CB), heavy minerals (HM), or other (O). The specific classification criteria were given by Pokhrel et al. [19].
2.1.1 Analysis of EDX Metadata
While Pokhrel et al. [19] only focused on the relative abundance of particles in each mineralogy class per sample, the metadata on individual particles (i.e., the elemental percentage of values) was archived (for 91 of the 93 samples). Considering that Pokhrel et al. [19] found an inordinate abundance of AS that was countered by relatively low abundance of C, especially in production and return samples, the aim here was to explore the possibility that aluminosilicates interfered with the classification of coal dust particles.
For the current study, the elemental data associated with all particles (1–10 µm) classified as either C or AS were analyzed. (this included a total of 12,282 C and 18,002 AS particles across the 91 samples with available data). Specifically, the sum of aluminum and silicon content (Al + Si%) in each C or AS particle was determined. In essence, the difference between C and AS particles—per the SEM–EDX classification criteria—can be thought of as a continuum along a scale of Al + Si%. If the content of these and other elements (except for carbon and oxygen) is sufficiently low, a particle will be classified as C. However, if the Al and Si contents are sufficiently high, the particle will be classified as AS. Thus, in samples with significant interference from aluminosilicates, some coal dust particles might be misclassified as AS, but, even coal particles classified as C might be expected to have elevated Al + Si% (i.e., nearing the upper limits of the C class).
The Pokhrel et al. [19] SEM–EDX metadata was also used to estimate particle loading density (particles/µm2 of the analyzed filter area) on the 91 samples with available data. As mentioned earlier, it is acknowledged that high sample loading could be one explanation for significant EDX interference between rock-strata sourced dust (e.g., aluminosilicates) and coal dust particles.
2.1.2 Follow-up SEM–EDX Analysis
From the large set of RCMD samples analyzed by Pokhrel et al. [19], a subset of eight samples was also selected for follow-up SEM–EDX analysis here. Included were the production location samples from four different mines, plus the return location samples collected in the same mines. This work aimed to explore for MAGs and document specific instances using SEM images with EDX elemental mapping. The SEM–EDX analysis was done on the same 9-mm filter subsections referenced above using the same instrumentation—i.e., a FEI Quanta 600 FEG environmental SEM (FEI, Hillsboro, OR) equipped with a Bruker Quantax 400 EDX spectroscope (Bruker, Ewing, NJ). The following instrument parameters were used for analysis: 10,000 × magnification, 12.5-mm working distance, 5.5-μm spot size, and accelerating voltage of 5 kV to allow surface features to be resolved.
2.2 Laboratory-Generated Dust Samples
To replicate dust generation at the production face of a coal mine, bulk samples of run-of-mine coal and/or rock strata materials were pulverized in the laboratory. The materials were obtained from an industry partner, pulled directly from the production belt. To carry out the dust generation and sample collection, the pulverizer was placed in a small enclosure and operated in two iterations. First, only coal was pulverized and during the pulverization (i.e., active dust generation in the enclosure), several dust samples were collected onto 37-mm PC filters (0.4-µm pore size; Zefon International, Ocala, FL) in open-face cassettes. To collect the samples, the cassette was simply held inside the enclosure for a relatively short period of time (about 10 s) with the filter surface oriented upward to collect the settling dust. While this setup did not allow sampling of only respirable-sized particles, it minimized the possibility MAGs present in the dust are due to the sample collection procedure, rather MAGs are likely due to the dust generation mechanisms or environmental conditions (e.g., high particle concentration). In the second iteration, both coal and rock strata (in roughly equal amounts) were pulverized simultaneously in the enclosure. Again, samples were collected in open-face cassettes during the dust generation; this time, samples were collected over relatively short and longer durations (about 120 s).
2.2.1 SEM–EDX Analysis
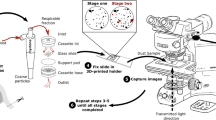
From one of the coal-only and four of the coal + rock samples collected over short durations, a 10-mm subsection was cut from the filter center and sputter-coated with Pt/Pd. These were used to collect baseline data with the SEM–EDX regarding the relative abundance of MAGs versus individual particles contained in the laboratory-generated dust samples (Table 1). For this work, the same instrumentation referenced above was used with the following procedure: a systematic approach was used for frame selection, where the first five frames from the center of the filter subsection were employed to acquire images with the secondary electron (SE) detector, targeting approximately 50 particles per sample (samples with a relatively low loading density required additional frames to reach this target). Next, elemental maps were obtained using the mapping tool in Bruker’s Espirit 1.9 software, with aluminum and silicon highlighted in the SE images using green and red, respectively. Magnification was kept at 2500 × to provide a constant frame area for the particle counts. The working distance, spot size, and accelerating voltage were kept the same as in the “Follow-up SEM–EDX Analysis” section.
Following image collection, a single analyst reviewed all images to identify and bin viewable particles into one of five categories: (individual) coal, (individual) mineral, coal MAG, mineral MAG, or coal-mineral MAG. The analysis was limited to particles within the respirable size range, (about 0.5–10 µm). Figure 2 illustrates particle identification and categorization for one particular SEM–EDX frame imaged on one of the baseline coal + rock samples (pre-dispersion). For this frame, a total of five particles were identified: one individual coal, one coal MAG, one mineral MAG, and two coal-mineral MAGs.
Example of particle classification into individual or MAG categories. The left side shows an SEM image from one of the coal + rock lab-generated dust samples (pre-dispersion), and the yellow squares (5 × 5 µm) identify particles. The right image shows the same image area with overlaid elemental map (Si and Al). Four out of the five particle categories are viewable in this area; individual mineral particles were not observed
2.2.2 Dispersion of MAGs
To explore the persistence of MAGs in the laboratory-generated dust samples, two experiments were conducted. In the first experiment (refer to Fig. 3), the remnants from the five baseline filter samples referenced above were used (i.e., those collected for a short duration). Each remnant was carefully cut in half. The first halves were placed into clean glass test tubes and 10 mL of deionized water (DI) was added to each tube. The tubes were placed in an ultrasonic bath for 5 min at 30 °C, then the filter was carefully removed and the remaining dust suspension was sonicated for another 5 min. The second halves of the filter remnants (from three coal + rock samples) were also placed in clean test tubes and submerged in 10 mL of DI. Instead of being subjected to sonication, however, these tubes were gently swirled, again, for an initial 5 min before the filter was removed, and then for an additional 5 min. Following the sonication or swirling sequence, the suspension in each tube was redeposited for post-dispersion SEM–EDX analysis. Redeposition was done by pouring the contents of a tube over a clean 47-mm PC filter (0.4-µm pore size; Zefon International, Ocala, FL) mounted on a clean vacuum apparatus. The sample was vacuumed for approximately 12 h to complete dryness, before a 10-mm subsection was cut and sputter-coated (Pt/Pd). Finally, the same SEM–EDX analysis procedure described above for the baseline samples was used to count and categorize particles in all eight of the post-dispersion samples.
In the second experiment (refer to Fig. 4), one of the laboratory-generated coal + rock samples collected for a longer duration was used to explore whether surfactant similar to that contained in the lung could be observed to enhance dispersion of MAGs relative to a DI control. For this, a small volume of the lung surfactant drug Survanta® was obtained; it is a natural bovine lung extract that contains phospholipids expected to mimic human pulmonary fluid. The methodological approach loosely followed that elicited by Wallace et al. [26] and Steuckle et al. [27]. First, a solution of the surfactant was prepared by adding 60 µg of Survanta® to 10 mL of DI and incubated for 3 h at 37 °C, with subsequent sonication for 10 min. Immediately after the solution was prepared, a small mass of the dust was scraped from the sample filter (using a stainless steel spatula) into each of the four test tubes. To two tubes, 5 mL of the prepared surfactant solution was added; 5 mL of DI was added to the other two tubes. All four tubes were capped and centrifuged at 2500 RPM for 10 min. Next, half of the volume of each tube was decanted and replaced with fresh DI to rinse the dust (i.e., and remove residual surfactant), and then centrifuged for another 10 min. The rinsing procedure was repeated twice more, and finally, the dust was redeposited for post-dispersion SEM–EDX analysis after sonicating the test tubes for 5 min at 30 °C, following the same procedures outlined above (i.e., vacuum filtration on a clean PC filter, 10-mm subsection cut, and sputter coated).
3 Results and Discussion
3.1 Re-Examination of Existing RCMD Samples
Figure 5 shows the mean Al + Si% for C and AS particles in all 91 RCMD samples with available SEM–EDX metadata included in the Pokhrel et al. [19] study. The box and whisker plots for C particles clearly indicate a higher Al + Si% in the samples from production (P) and return (R) sampling locations. The Al + Si% also tends to be higher in the AS particles from these locations. As noted earlier, one obvious explanation for these findings could be that the affected samples are just highly loaded, which challenges the SEM–EDX classification. Figure 6, however, shows the mean Al + Si% as a function of loading density for each sample. Notably, C particles in the production and return samples exhibit a relatively high Al + Si% even in samples with a low loading density.
Box and whisker plots of Al + Si% for individual C (left) and AS particles (right) shown by sampling location: Bolter (B), Feeder (F), Intake (I), Production (P), and Return (R). For C particles (B, n = 1865; F, n = 2951; I, n = 4007; P, n = 928; R, n = 2531; total particles = 12,282). For AS particles (B, n = 3110; F, n = 2302; I, n = 1074; P, n = 7387; R, n = 4129; total particles = 18,002)
Moreover, during the follow-up SEM–EDX work, MAGs were indeed observed in all eight of the samples examined. Illustrative images are provided in Table 2 (loading density was clearly high in the production location samples selected for this work, but MAGs could be identified nonetheless). It is noted that most of the MAGs observed in these samples fit the description of coal-mineral MAGs, with a primary coal particle serving as a platform for finer aluminosilicate and/or silica particles. This might be related to the nature of dust generation at the mine face, where particle concentration is locally very high and the particles—especially those sourced from the rock strata—tend to be finer than in other locations [3, 28]. Under such conditions, it is possible that MAGs form due to electrostatic forces, impaction or diffusion, and humidity or particle wetness could also play a role due to capillary action [22].
3.2 Laboratory-Generated Samples
Table 3 summarizes the results of the two experiments conducted on the lab-generated samples. For each sample, the observed relative abundance (number %) of respirable-sized particles between individual or MAG categories is shown for the baseline and dispersion case(s). Baseline results for all samples in the first experiment clearly demonstrate that MAG formation is possible due to only the dust generation processes and environmental conditions; these samples were collected passively without the use of an air pump, tubing, or size separator.
Figure 7 presents the relative change in the abundance of particles in each category attributed to either sonication or gentle swirling (in DI water) of the dust recovered from the lab-generated samples used in Experiment 1. The sonication produced a consistent increase in the abundance of individual coal particles, which was countered by a consistent decrease in the abundance of coal and/or coal-mineral MAGs. In the two samples with very high abundance of mineral-containing MAGs pre-dispersion (C + R_1 and C + R_4), the sonication appears to have effectively dispersed mineral particles too (i.e., as indicated by the increase individual mineral particles). On the other hand, mixed results were observed when the dust suspension was only gently swirled. This suggests that at least some of the MAGs were persistent (i.e., not dispersed) when subjected to relatively weak mechanical agitation versus the stronger sonication.
Figure 8 compares the results of the suspension of the lab-generated dust in the surfactant solution versus DI water. The surfactant appears to have effectively dispersed both coal and mineral particles from MAGs. In the DI water, the results were somewhat different. While the relative abundance of coal-mineral MAGs did decrease, only the individual mineral particles increased; the abundance of individual coal particles actually decreased. This might mean that coal particles included in MAGs pre-dispersion were not effectively dispersed by the DI water, or that they re-agglomerated during the sample preparation procedure (similar explanations might also apply to the C + R_3 sample results shown in Fig. 7).
Relative change (number %) in particle distributions following dispersion of lab-generated dust in either DI water or prepared surfactant solution. The y-axis values show the difference between the dispersed sample and the mean results for baseline coal + rock samples analyzed in Experiment 1 (i.e., C + R_1, C + R_2, C + R_3, C + R_4)
Though MAG occurrence in coal mine environments seems quite likely, such particles have not been widely considered in RCMD monitoring, characterization studies, or exposure assessments. There may be a range of implications, however, regarding the conceptual understanding of lung response: Due to their size, MAGs that do not disperse easily should penetrate less deeply into the lung than the primary particles they include [29] and the larger MAGs might be cleared more efficiently as well [30, 31]. That said, the MAG constituents, especially those that sit on the agglomerate’s surface, should also be important to clearance since the function of lung macrophages can be influenced by particle surface chemistry [30]. Likewise, the MAG constituents and dispersibility might impact toxicity. For example, the toxicity of mixed-type MAGs might be dependent on agglomerate and primary particle sizes, structure (i.e., the relative position of the constituent primary particles within the agglomerate or on the surface), and adherence (i.e., whether the primary particles disperse efficiently in the lung).
Knowledge of MAG formation could also have implications for the design of better dust controls: By enhancing the conditions that promote MAG formation, it may be possible to improve the efficiency of controls that work well on relatively larger particles (e.g., physical filtration systems). Thus, further research on the underlying mechanisms of MAG formation is recommended.
4 Conclusions
This study evidenced the presence of MAGs in respirable coal mine dust samples and demonstrated that MAGs can indeed occur in the mine environment rather than as a mere artifact of sample collection. Additionally, some understanding was gained regarding the relative dispersibility of MAGs under different conditions. Ultrasonic vibration in DI water suspension was clearly effective for dispersion of the types of MAGs produced during lab pulverization of real coal and rock materials—and even gentle swirling of the suspension was able to disperse some MAGs. Moreover, a surfactant solution that mimics natural lung fluid was shown to enhance dispersion over DI water alone. While these results are not directly translatable to health outcomes, they offer important insights for those considering how to characterize respirable coal mine dust or conduct realistic exposure assessments. A better understanding of the mechanisms responsible for MAG formation could also prove valuable in efforts to improve dust controls in mines.
Notes
Agglomerates are generally defined as a cluster of particles in which one relatively larger particle serves as a platform for other finer particles that adhere to it. In some cases, the cluster may be formed of similarly sized particles. In the current work, no distinction is made between these cases. Rather, the emphasis is on the relative size of the cluster itself. If the entire cluster is in the respirable range (i.e., less than about 10 µm), herein it is termed a micro-agglomerate (MAG).
References
Sarver E, Keles C, Rezaee M (2019) Beyond conventional metrics: Comprehensive characterization of respirable. Int J Coal Geol 207:84–95
Agioutanti E, Keles C, Sarver E (2020) A thermogravimetric analysis application to determine coal, carbonate, and non-carbonate minerals mass fractions in respirable mine dust. J Occup Environ Hyg 17:1–12
Sarver E, Keleş Ç, Afrouz SG (2021) Particle size and mineralogy distributions in respirable dust samples from 25 US underground coal mines. Int J Coal Geol 247:14
Kittelson DB (1998) Engines and nanoparticles: a review. J Aerosol Sci 29(5–6):575–588
Peters JM (1986) Silicosis. Occupational Respiratory Diseases 86–102:219–237
Castranova V, Vallyathan V (2000) Silicosis and coal workers’ pneumoconiosis. Environ Health Perspect 108(4):675–684
Leung CC, Yu ITS, Chen W (2012) Silicosis. Lancet 379(9830):2008–2018
National Academies of Sciences, Engineering, and Medicine (2018) Monitoring and sampling approaches to assess underground coal mine dust exposures. The National Academies Press, Washington, DC. https://doi.org/10.17226/25111
Blackley DJ, Crum JB, Halldin CN, Storey E, Laney AS (2016) Resurgence of progressive massive fibrosis in coal miners — Eastern Kentucky. Morb Mortal Wkly Rep 65(49):1385–1389
Blackley D, Reynolds L, Short C, Carson R, Storey E, Halldin C, Laney A (2018) Progressive massive fibrosis in coal miners from 3 clinics in Virginia. JAMA 319(5):500–501
Doney BC, Blackley D, Hale JM, Halldin C, Kurth L, Syamlal G, Laney AS (2019) Respirable coal mine dust in underground mines. Am J Ind Med 62(6):478–485
Schatzel SJ (2009) Identifying sources of respirable quartz and silica dust in underground coal mines in southern West Virginia, western Virginia, and eastern Kentucky. Int J Coal Geol 78(2):110–118
Pollock DE, Potts JD, Joy GJ (2010) Investigation into dust exposures and mining practices in mines in the southern Appalachian Region. Min Eng 62(2):44–49
Abbasi B, Wang X, Chow JC, Watson JG, Peik B, Nasiri V, Riemenschnitter KB, Elahifard M (2021) Review of respirable coal mine dust characterization for mass concentration, size distribution and chemical composition. Minerals 11(4):1–36
LaBranche N, Keles C, Sarver E, Johnstone K, Cliff D (2021) Characterization of particulates from Australian underground coal mines. Minerals 11(5):447
Pan L, Golden S, Assemi S, Sime MF, Wang X, Gao Y, Miller J (2021) Characterization of particle size and composition of respirable coal mine dust. Minerals 11(3):1–12
Trechera P, Moreno T, Córdoba P, Moreno N, Amato F, Cortés J, Zhuang X, Li B, Li J, Shangguan Y, Dominguez AO, Kelly F, Mhadhbi T, Jaffrezo JL, Uzu G, Querol X (2021) Geochemistry and oxidative potential of the respirable fraction of powdered mined Chinese coals. Sci Total Environ 800:149486
Shekarian Y, Rahimi E, Rezaee M, Su WC, Roghanchi P (2021) Respirable coal mine dust: A review of respiratory deposition, regulations and characterization. Minerals 11(7):1–25
Pokhrel N, Agioutanti E, Keles C, Afrouz S, Sarver E (2021) Comparison of mineral content in respirable coal mine dust samples estimated using FTIR, TGA, and SEM-EDX. In Proceedings of the 18th North American Mine Ventilation Symposium, pp. 255–267
Zazouli MA, Dehbandi R, Mohammadyan M, Aarabi M, Dominguez AO, Kelly FJ, Khodabakhshloo N, Rahman MM, Naidu R (2021) Physico-chemical properties and reactive oxygen species generation by respirable coal dust: Implication for human health risk assessment. J Hazard Mater 405:124185
Pandey JK, Agarwal D, Gorain S, Dubey RK, Vishwakarma MK, Mishra KK, Pal AK (2017) Characterisation of respirable dust exposure of different category of workers in Jharia Coalfields. Arab J Geosci 10(7):1–10
Su X, Ding R, Zhuang X (2020) Characteristics of dust in coal mines in central North China and its research significance. ACS Omega 5(16):9233–9250
Cvetković Ž, Logar M, Rosić A, Ćirić A (2012) Mineral composition of the airborne particles in the coal dust and fly ash of the Kolubara basin (Serbia). Period di Mineral 81(2):205–223
Armbruster L (1988) Agglomeration of Coal Mine Dust and its Effect on Respirable Dust Sampling. Ann Occup Hyg 32(inhaled particles VI):393–401
Nichols G, Byard S, Bloxham MJ, Botteril J, Dawson NJ, Dennis A, Diart V, North NC, Sherwood JD (2002) Review of the terms agglomerate and aggregate with a recommendation for nomenclature used in powder and particle characterization. J Pharm Sci 91(10):2103–2109
Wallace WE, Harrison J, Keane MJ, Bolsaitis P, Eppelsheimer D, Poston J, Page SJ (1990) Clay occlusion of respirable quartz particles detected by low voltage scanning electron microscopy - X-ray analysis. Ann Occup Hyg 34(2):195–204
Stueckle TA, Davidson DC, Derk R, Wang P, Friend S, Schwegler-Berry D, Zheng P, Wu N, Castranova V, Rojanasakul Y, Wang L (2017) Effect of surface functionalizations of multi-walled carbon nanotubes on neoplastic transformation potential in primary human lung epithelial cells. Nanotoxicology 11(5):613–624
Gonzalez J, Pokhrel N, Jaramillo L, Keles C, Sarver E (2021) Respirable dust characterization using SEM-EDX and FT-IR: A case study in an Appalachian coal mine. In Proceedings of the 18th North American Mine Ventilation Symposium, 189–197
Thomas RJ (2013) Particle size and pathogenicity in the respiratory tract. Virulence 4(8):847–858
Oberdörster G (1993) Lung dosimetry: pulmonary clearance of inhaled particles. Aerosol Sci Technol 18(3):279–289
Porter DW, Hubbs AF, Chen BT, McKinney W, Mercer RR, Wolfarth MG, Battelli L, Wu N, Sriram K, Leonard S, Andrew M, Willard P, Tsuruoka S, Endo M, Tsukada T, Munekane F, Frazer DG, Castranova V (2012) Acute pulmonary dose–responses to inhaled multi-walled carbon nanotubes. Nanotoxicology 7(7):1179–1194
Funding
The authors would like to express our sincere appreciation to The National Institute for Occupational Safety and Health (NIOSH) for funding this study (under contract 75D30119C05529). The views and opinions expressed herein are solely those of the authors and do not imply any endorsement by research partners or funding source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gonzalez, J., Keles, C. & Sarver, E. On the Occurrence and Persistence of Coal-Mineral Microagglomerates in Respirable Coal Mine Dust. Mining, Metallurgy & Exploration 39, 271–282 (2022). https://doi.org/10.1007/s42461-022-00555-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-022-00555-7