Abstract
The effects of CaO/Al2O3 ratio on viscosity and structure of the CaO-Al2O3-based fluoride-free mould fluxes were investigated with the CaO/Al2O3 ratio varied from 1 to 4 and the content of SiO2 fixed at 7 wt.%. The increase in the CaO/Al2O3 ratio from 1 to 2 lowered the viscosity of the flux melts. The viscosity increased slightly with the CaO/Al2O3 ratio from 2 to 3, and this increase became significant with further increasing CaO/Al2O3 ratio to 4. Both break temperature and apparent activation energy were found to be increased with the increase in CaO/Al2O3 ratio from 2 to 4. There was no break temperature available in the flux with CaO/Al2O3 ratio of 1. Changing the CaO/Al2O3 ratio from 1 to 2 decreased the apparent activation energy. Equilibrium phases of the fluxes were calculated using FactSage 7.1 and the major phases were found to be varied with the CaO/Al2O3 ratio. Structures of tested mould fluxes were analysed using Fourier transform infrared spectroscopy. The results showed that the increase in the CaO/Al2O3 ratio from 1 to 2 decreased the complexity of the structure, leading to a reduced viscosity. With the increase in the CaO/Al2O3 ratio from 2 to 4, both solid phase precipitation and melt structure contributed to the variation of viscosity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In steel production, continuous casting is a major operation. Mould fluxes play irreplaceable roles in the steel continuous casting, such as lubricating the stand, preventing steel from freezing, absorbing inclusions from molten steel, insulating steel from oxidation, and controlling heat transfer between the steel and mould [1,2,3,4]. Viscosity is one of the key properties of mould fluxes, which prominently affects the lubrication during casting, reaction kinetics, and diffusion in mould fluxes in high temperature region [5,6,7]. Temperature and composition are two major factors which affect the viscosity of mould fluxes.
High aluminium steel, such as transformation-induced plasticity (TRIP) steel, possesses outstanding mechanical properties and superior formability, making it attractive for application in the automotive industry [8]. However, in the process of continuous casting, [Al] in the steel reacts with (SiO2) in the conventional CaO–SiO2-based mould fluxes, resulting in the reduction of SiO2 and the oxidation of Al. Severe flux-metal reaction leads to changes of the flux chemical composition with a significant influence on the flux properties and causes reduction of the refining capacity of the flux and insufficient lubrication of flux with a detrimental effect on surface quality [9,10,11,12].
Studies on the modification of the CaO–SiO2-based mould fluxes by optimizing the ratios of CaO/SiO2 [13] as well as Al2O3/SiO2 [14,15,16], and the amount of Al2O3 [17] showed that heat flux between steel and mould is unstable at the initial stage, and the experimental mould fluxes present a very strong crystallization tendency. Therefore, optimizing the conventional mould fluxes to restrict the flux-steel reaction is extremely hard. The fundamental way of solving the reaction problem is to develop a CaO–Al2O3-based mould flux system which partially suppresses the reaction between [Al] in the steel and (SiO2) in the mould flux [18]. Wang et al. [19] also proved that the CaO–Al2O3-based mould flux can markedly depress the flux-metal reaction and obviously enhance the slab surface quality. There are several studies [20,21,22] focusing on the application of CaO–Al2O3-based mould flux. However, most of them focused on the effects of other components, such as BaO [23,24,25], Li2O [13, 26, 27], Na2O [13, 28], MgO [23], and B2O3 [9], on mould flux performance, but few paid attentions to the effect of CaO/Al2O3 ratio. Therefore, it is essential to investigate the effect of CaO/Al2O3 ratio in a broad range on the viscosity and structure of lime alumina-based mould flux systematically.
Furthermore, it is known that CaF2 is a volatile compound, causing health hazards, environmental pollution, and equipment corrosion [29, 30], which forces researchers using the substitute of fluoride, such as B2O3 [31,32,33,34,35] and Na2O [36, 37]. However, very few studies of fluoride-free CaO–Al2O3-based mould fluxes have been reported. Gao et al. [23] studied the effect of alkaline earth metal oxides on the viscosity and structure of CaO–Al2O3-based mould flux with a limited fluoride content (7 wt.%).
The aim of this study is to investigate the effect of CaO/Al2O3 ratio on the mould flux structure and viscosity and to provide further fundamental understanding of the effect of this parameter on flux properties which will be used for developing CaO–Al2O3 fluorine-free mould fluxes for high-Al steel casting.
2 Experimental
2.1 Experimental material preparation
The chemical compositions of samples used in this study are listed in Table 1. All these fluxes were prepared using analytical reagent grade chemicals CaCO3, SiO2, Al2O3, B2O3, Na2CO3, MgO, and Li2CO3. The well-mixed components were fully ground in an agate mortar for 20 min and then premelted in a high-purity graphite crucible at 1400 °C for 20 min. At high temperature, CaCO3, Na2CO3, and Li2CO3 were decomposed, emitting CO2 gas and forming CaO, Na2O, and Li2O, respectively. After that, the melt was water quenched and dried at 120 °C for 4 h and then ground into fine powders with an average size of 75 μm for the following experimental use. The CaO/Al2O3 weight ratio was fixed at 1, 2, 3, and 4, which covers a broad range of the CaO/Al2O3 ratios, allowing to reliably establish the effect of this ratio on the viscosity and the structure of the fluxes. The concentrations of B2O3 and Li2O were determined by inductive couple plasma (ICP), and other components were analysed by X-ray fluoroscopy (XRF). Due to the evaporation during melting process, weight losses of B2O3 and Na2O were observed; the measured concentrations of B2O3 and Na2O were below the designated values of 11 and 6 wt.%, respectively. The concentrations of Na2O in sample 1 and B2O3 in sample 4 were very close to the designated values.
2.2 Viscosity measurement
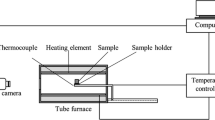
Mould flux viscosity was measured using a rotating cylindrical viscometer (model ZC-1600, China). The schematic layout of the experimental set-up is given in Fig. 1. During the experiment, the Mo spindle (diameter: 15 mm; height: 30 mm) was rotating at a fix speed of 12 r/min; a Pt/Rh thermocouple (B-type) was placed right beneath the bottom of a high-purity graphite crucible (inner diameter: 40 mm; inner height: 160 mm). Protective nitrogen gas was employed to prevent the oxidation of a graphite crucible and a molybdenum spindle. In every measurement, 140 g flux sample was placed in the crucible, heated to 1400 °C, and held for 20 min to obtain a homogeneous melt. The temperature of the furnace was controlled within ± 2 °C. It decreased with a rate of 5 °C/min, and the viscosity of experimental flux was continuously recorded. The experimental set-up and procedure were presented in detail elsewhere [38].
2.3 Fourier transform infrared spectroscopy
The effect of CaO/Al2O3 ratio on the structure of mould fluxes was studied using a Fourier transform infrared spectrometer (FTIR, Spotlight 400; PerkinElmer, USA). The as-quenched samples were ground into fine powders with the average size of 100 μm. For FTIR testing, 2.0 mg sample powder and 200 mg KBr were well mixed in an agate mortar and pressed into a cylindrical pellet. FTIR-transmitting spectra with a KBr detector were taken in the range of 4000 to 450 cm−1 with a resolution of 2 cm−1. The FTIR transmission spectra of studied samples were mainly focused in the range of 1600 to 400 cm−1 [39,40,41].
3 Results and discussion
3.1 Effect of CaO/Al2O3 ratio on flux viscosity
The results of viscosity measurements are plotted in Fig. 2 (where η is the viscosity of mould flux, Pa s). The starting measurement temperature for all fluxes was 1400 °C. During continuous cooling process, when the flux was in the liquid state, the viscosity was low. With the decrease in temperature, the viscosity increased gradually. For samples 1–3 with low CaO/Al2O3 ratios, the viscosity of liquid flux was in the range of 0.12 to 0.60 Pa s, while for sample 4, the viscosity range increased to 1.2–1.5 Pa s. When the temperature dropped to a certain value, the viscosity increased sharply, which corresponds to the break temperature (Tbr). At this temperature, a large number of bulk crystals appeared and formed a solid–liquid state [42,43,44].
For sample 1 with CaO/Al2O3 of 1, the viscosity increased gradually in the cooling process and no sharp change in viscosity was observed, and thus, no corresponding Tbr was found. Viscosity curves of mould fluxes with CaO/Al2O3 ratios of 2 and 3 had a similar profile (Fig. 2b, c) where viscosity increased sharply when the break temperature was reached. Viscosity of sample 4 with CaO/Al2O3 of 4 (Fig. 2d) increased with different rates in different temperature ranges. The break temperatures of fluxes Tbr are listed in Table 2.
Figure 3 shows the viscosity of the mould fluxes as a function of CaO/Al2O3 ratio in the temperature range from 1325 to 1400 °C. The viscosity of flux with CaO/Al2O3 ratio of 4 at 1325 °C is not shown as this temperature is below the break temperature. It can be seen from Fig. 3 that the viscosity of the melt decreased with the increasing CaO/Al2O3 ratio from 1 to 2. A slight rise in the viscosity was observed when the CaO/Al2O3 ratio increased from 2 to 3. A significant rise in viscosity occurred with a further increase in the CaO/Al2O3 ratio from 3 to 4. In general, reducing temperature increased the viscosity.
3.2 Activation energy and break temperature
Liquid flux can be assumed to be a Newtonian fluid when temperature is above the break temperature. The change in the viscosity with temperature follows the Arrhenius equation:
where T stands for the absolute temperature, K; A is a constant; Ea represents the apparent activation energy for viscous flow, kJ mol−1; and R is the ideal gas constant which equals to 8.314 J mol−1 K−1. The activation energy can be found using Eq. (2).
The plot of \({ \ln }\eta \;{\text{versus}}\;1/T\) is shown in Fig. 4 with an excellent linear fitting for all samples (coefficient of determination R2 ≥ 0.95 in Table 2), and the activation energy (with R2 values) is listed in Table 2. As mentioned before, Tbr was not obtained for flux with CaO/Al2O3 of 1. The apparent activation energy for this flux was found to be 175 kJ mol−1. The apparent activation energy increased from 168 to 180 kJ mol−1 with the increase in CaO/Al2O3 ratio from 2 to 4.
Table 2 also presents the calculated liquidus temperatures (Tliq) and calculated solidus temperatures (Tsol) of fluxes with different CaO/Al2O3 ratios obtained using thermochemical software FactSage 7.1.
The Tliq of sample 4 with CaO/Al2O3 of 4 was extremely high (above 1500 °C), compared with those of the other three samples. However, the Tsol of sample 4 showed the lowest value (793 °C). With increasing CaO/Al2O3 ratio from 1 to 2, the Tsol increased from 842 to 879 °C; with further increasing CaO/Al2O3 ratio to 3, the Tsol decreased from 879 to 793 °C; and sample 3 (CaO/Al2O3 = 3) and sample 4 (CaO/Al2O3 = 4) had the same Tsol value.
3.3 Calculated phase composition
The equilibrium phases of the experimental mould fluxes calculated using thermochemical software FactSage 7.1 are shown in Fig. 5. The phase equilibrium calculation revealed that, when CaO/Al2O3 ratio was 1 (Fig. 5a), the dominant phases were Na2CaAl4O8 and Mg3B2O6. With the increase in CaO/Al2O3 ratio from 1 to 2 (Fig. 5a, b), phase Ca11B2Si4O22 became significant and Mg3B2O6 disappeared; Na2CaAl4O8 still existed but tended to be less noticeable; Ca2SiO4, however, appeared and became the main crystal phase at the high temperature.
With further increasing CaO/Al2O3 ratio from 2 to 3 (Fig. 5b, c), the region of Ca2SiO4 became larger in the high temperature region. CaO also became an apparent high-temperature phase. The area of Ca11B2Si4O22 decreased a bit; the area of Na2CaAl4O8 trended to shrink and existed in the relatively low-temperature zone. With further increase in CaO/Al2O3 ratio from 3 to 4 (Fig. 5c, d), CaO phase became more pronounced, and Ca2SiO4 was partially substituted by CaO; the regions of both Ca11B2Si4O22 and Na2CaAl4O8 remained less prominent.
3.4 FTIR spectroscopy
Results of FTIR analysis of as-quenched mould fluxes with varying CaO/Al2O3 ratio are shown in Fig. 6. Three wave number regions can be distinguished in the FTIR spectra, 450–800, 800–1100, and 1100–1600 cm−1. Detailed assignment of FTIR band groups corresponding to different wave number regions is presented in Table 3. The reflection peak located at 500–600 cm−1 represents the asymmetric stretching vibration of [AlO6]-octahedron [27]; the transmittance spectrum around 650 cm−1 is assigned to the symmetric stretching vibration of [AlO4]-tetrahedral [9]; the transmittance curve placed at 800–1200 cm−1 corresponds to the [SiO4]-tetrahedral structural units region [45]; and bands at around 1250, 1350, and 1450 cm−1 were assigned to B–O stretching vibration of varied borate groups in [BO2O−], B–O stretching vibration of varied borate groups in [BO3] units, and B–O stretching vibration of [BO3] in boroxol rings [46], respectively.
Aluminium ion in the flux structure occupied both tetrahedral and octahedral positions, which reflects an amphoteric nature of aluminium oxide. With increasing CaO/Al2O3 ratio, the transmittance trough of [AlO6]-octahedron at 500–600 cm−1 was first gradually deepened (CaO/Al2O3 ratio from 1 to 2), then remained constant (CaO/Al2O3 ratio from 2 to 3), and finally became shallower (CaO/Al2O3 ratio from 3 to 4), indicating the complex structure of mould flux [27]. With the increase in CaO/Al2O3 ratio, the transmittance trough of symmetric stretching vibration of [AlO4]-tetrahedral at about 650 cm−1 shifted towards lower wave number region and the width of the band tended to become narrower, which suggested a decrease in the degree of polymerization (DOP) as Al2O3 exhibited property of a network-forming oxide. Similar results were obtained by Yan et al. [47] in their study of the effect of CaO/Al2O3 ratio on structure of CaO–Al2O3–20% CaF2–10% B2O3–10% Na2O–5% SiO2 system.
In the silicate structures, increasing CaO/Al2O3 ratio from 1 to 2 moved the band to the left towards lower wave number and the band width of the silicate region became narrower. Further increasing CaO/Al2O3 ratio from 2 to 4 made no noticeable band shift and width change. The increase in CaO/Al2O3 ratio also had a minor effect on the borate structure in the region of 1100–1600 cm−1.
For quantitative analyses of the structural units, absorbance–wave number curves of FTIR spectra should be employed, as the area of the structural unit in the absorbance–wave number curve is in proportion to its concentration in the sample, which is invalid in the corresponding transmittance–wave number curve [48]. FTIR spectra of mould fluxes can be converted into absorbance–wave number curves from the original transmittance–wave number curves by using the Beer law [49].
where \(T^{\prime}\) and \(A^{\prime}\) are light transmittance and absorbance; I is the transmitted light intensity; and I0 is the initial light intensity. The absorbance–wave number profiles can be obtained from the original transmittance–wave number ones using Eq. (5):
The absorbance curves for fluxes with varied CaO/Al2O3 ratios are presented in Fig. 7 for the wave number between 450 and 1100 cm−1. IR spectra (infrared spectra) were fitted using the Gaussian deconvolution method as shown also in Fig. 7. The band around 400–500 cm−1 is related to the deformation of tetrahedron and octahedron units [50]. The band around 550 cm−1 is assigned to the Al–O–Al bond bending vibrations [51]. According to Kamitsos et al. [46], characteristic band at around 760 cm−1 reflects the B–O–B bond bending vibrations in pentaborate and band at around 900 cm−1 represents the B–O asymmetric stretching vibrations of [BO4]-tetrahedral. The band in the range of 780–800 cm−1 was assigned to the Si–O-Si bending vibration [52]. The characteristic peaks located at 840–870, 920–930, 970–1000, 1050–1075, and 1100–1150 cm−1 were assigned to the Si–O− symmetric stretching of various structural units, containing SiO44− monomers, Si2O76− dimers, SiO32− chains or rings, Si2O52− sheets, and SiO2 three-dimensional units [52, 53]. The IR spectra are used to estimate fractions of bridging and non-bridging oxygen in different structural units, which are characterized by Qn, where n is a number of bridging oxygens per silicon ion: Q0 (NBO/Si = 4), Q1 (NBO/Si = 3), Q2 (NBO/Si = 2), Q3 (NBO/Si = 1), and Q4 (NBO/Si = 0).
4 Discussion
As shown above, CaO/Al2O3 ratio affects viscosity, equilibrium phases, and structures of mould fluxes. Above the break temperature, the viscosity of the melt decreased with increasing the CaO/Al2O3 ratio from 1 to 2. With increasing the CaO/Al2O3 ratio from 2 to 3, there was a slight rise of the viscosity. The viscosity increased significantly when the CaO/Al2O3 ratio increased from 3 to 4. The break temperature and activation energy were found to increase with the increase in CaO/Al2O3 ratio from 2 to 4, while for sample 2, the activation energy was lower than that for sample 1 (Table 2). This observation is generally considered to be related to varied phase formation and flux structure at different temperatures during cooling process as discussed below.
4.1 Effect of CaO/Al2O3 ratio on flux structure
According to the deconvolution of IR spectra for quenched fluxes with different CaO/Al2O3 ratios (Fig. 7), area fractions (f) of different characteristic peaks referred to total area in the whole measured wave number range are listed in Table 4. The relative area percentages of various silicate structural units in reference to total silicates are shown in Fig. 8, and area fractions of aluminate unit structures are shown in Fig. 9.
For silicate units, in fluxes with CaO/Al2O3 ratio from 1 to 3, Q0 was the dominant unit, while among samples with CaO/Al2O3 ratio of 2, 3, and 4, Q3 was not distinguishable in the IR spectra. No Q4 was detected for all fluxes. Area fractions of Q0, Q2, and Q3 decreased, while that of Q1 increased significantly from 12% to 37% with increasing CaO/Al2O3 ratio from 1 to 2. With increasing CaO/Al2O3 ratio from 2 to 3, the relative area fractions of silicate groups varied in an opposite direction; the relative fraction of Q1 sharply decreased from 37% to 3%, while that of both Q0 and Q2 increased, especially for Q2 raising from 11% to 34%. With further increase in CaO/Al2O3 ratio from 3 to 4, Q2 became the dominant unit, while Q1 disappeared. The degree of depolymerization of silicate group can be evaluated by non-bridging oxygen per silicon (NBO/Si) of the silicate group which can be estimated from the following equation [53] by using relative silicate unit fractions in Fig. 8:
where \(F_{{{\text{Q}}^{4 - n} }}\) is the peak area fraction of \({\text{Q}}^{4 - n}\) structure unit in total area of silicate units.
The values of NBO/Si are shown in Fig. 8 (also listed in Table 4) which indicate that with increasing the CaO/Al2O3 ratio from 1 to 4, NBO/Si decreased slightly for CaO/Al2O3 ratio from 1 to 3 but significantly for CaO/Al2O3 ratio from 3 to 4. The decrease in NBO/Si indicates an increase in the degree of polymerization of silicate group.
The effect of aluminate group becomes important because of high alumina concentration compared with the conventional silica-based flux. In the aluminate structure, the area fraction of [AlO6]-octahedron increased, while that of [AlO4]-tetrahedron decreased with the increase in CaO/Al2O3 ratio from 1 to 3, and the tendency of fraction variation for both aluminate structures was stable with the further increase in CaO/Al2O3 ratio to 4.
McMillan and Piriou [54] suggested that continually adding CaO into the aluminates instigated the weakening of the network of tetrahedral aluminate units and the formation of octahedral aluminate units with higher average coordination linkage for accommodating the redundant oxygen.
The area fraction of [AlO6]-octahedron increased, while that of [AlO4]-tetrahedron decreased with the increase in CaO/Al2O3 ratio from 1 to 3, indicating the reforming of the network structure. After CaO/Al2O3 ratio exceeding 3, the area fractions of both [AlO6]-octahedra and [AlO4]-tetrahedra tend to be flat because no more [AlO4]-tetrahedra left.
For borate units, only [BO4]-tetrahedral and B–O–B pentaborate were quantitatively analysed. Therefore, no relative area fractions of these units referred to total borate units were given, as for silicate and aluminate cases in Figs. 8 and 9. According to Table 4, the area fraction of [BO4]-tetrahedral structural units first descended with increasing CaO/Al2O3 ratio from 1 to 2 and then continuously increased with further increase in CaO/Al2O3 ratio to 4. For B–O–B bending vibrations from pentaborate, the area fraction of boroxol ring structure [35] first increased with increasing the CaO/Al2O3 ratio from 1 to 2 and then decreased with increasing CaO/Al2O3 ratio to 3, but finally increased with further increasing CaO/Al2O3 ratio to 4. The variation of B–O–B bending vibrations with CaO/Al2O3 ratio is in an opposite direction with that of [BO4]-tetrahedral structural units in the same CaO/Al2O3 ratio range of 1 to 3. The reason for the variation in both areas of B–O–B bending vibrations and [BO4]-tetrahedral structural units could be explained by the transformation between varied borate units, which is affected by the contents of other components, e.g. CaO and Al2O3. According to Klyuev and Pevzner [55], the transformation between [BO3]-triangular structural units and [BO4]-tetrahedral structural units depends on CaO content. When CaO content is higher than 40 mol% which is the case for all fluxes investigated, [BO3]-triangular structural units are produced at the expense of [BO4]-tetrahedral structural units, thus reforming the structure [55]. With the increase in CaO/Al2O3 ratio, the relative content of Al2O3 decreased, and as a result, [BO4]-tetrahedral structural units were formed which increased the degree of flux polymerization [9]. Therefore, there is a balance between the decrease in [BO4]-tetrahedral structural units caused by CaO content increase and the increase in [BO4]-tetrahedral structural units caused by Al2O3 decrease.
4.2 Effect of CaO/Al2O3 ratio on viscosity
It is well known that flux viscosity is related to the melt structure. For CaO–Al2O3-based fluxes, aluminate structure becomes dominant. The FTIR structural analysis revealed that increasing CaO/Al2O3 ratio from 1 to 3 leads to an increase in the fraction of [AlO6]-octahedron but an decrease in the fraction of [AlO4]-tetrahedron. This structural change can be correlated to the decrease in the structural complexity, and therefore, from this aspect, the viscosity should be decreased. This is in fact true for the CaO/Al2O3 ratio from 1 to 2 as shown in Fig. 3. Furthermore, the decrease in the fraction of [BO4]-tetrahedral structural unit with the increasing CaO/Al2O3 ratio from 1 to 2 could also lead to the reduction in viscosity due to the reduced amount of polymerized borate structure. For the silicate units, the NBO/Si determined in Table 4 as well as Fig. 8 showed no significant change when CaO/Al2O3 ratio increased from 1 to 2, suggesting the minor effect of silicate structural units on the DOP of these mould fluxes. Therefore, the decrease in viscosity for the CaO/Al2O3 ratio from 1 to 2 is attributed to the decrease in structural complexity of aluminates and borates.
With further increasing CaO/Al2O3 ratio from 2 to 3, the fractions of aluminate structures changed in a similar manner as those for 1 to 2, but the fraction of [BO4]-tetrahedral structure showed an opposite trend. For silicate group, with further increasing CaO/Al2O3 ratio to 3, the NBO/Si had a slight decrease. It is known that viscosity was affected by both the flux structure and the precipitation of the solid phase. Based on Sect. 3.3, the liquidus temperature of mould flux increased with the increasing CaO/Al2O3 ratio; especially for the range of 2 to 4, high-temperature phases such as Ca2SiO4 and CaO (Fig. 5) appeared. The comprehensive effect of melt structure and precipitation of the solid phase could result in no obvious change in viscosity for mould flux with CaO/Al2O3 ratio increasing from 2 to 3.
With further increasing CaO/Al2O3 ratio from 3 to 4, both the area fraction of [BO4]-tetrahedral structure and aluminate structures exhibited no significant change, while a noticeable decrease was observed in NBO/Si of silicate group. The calculated liquidus temperature of mould flux for Flux 4 is above 1500 °C, making the flux heterogeneous in the temperature range of viscosity measurements. By this consideration, a sharp rise in the flux viscosity when the CaO/Al2O3 ratio increases from 3 to 4 should be attributed to the increase in liquidus temperature of sample 4.
4.3 Effect of CaO/Al2O3 ratio on precipitation of crystal phases and its correlation with break temperature
The value of Tbr increased from 1289 to 1338 °C with increased CaO/Al2O3 ratio from 2 to 4. Tbr is attributed to the formation of solid substances in the melts, and therefore, it can correlate with the liquidus temperature of the flux. Equilibrium phase calculations showed that this temperature increased with the increase in CaO/Al2O3 ratio which is, in principle, consistent with the trend in the change of Tbr. For the flux with CaO/Al2O3 ratio of 2, Tbr was slightly higher than Tliq. Such imbalance between Tbr and Tliq can be related to the experimental error in the measurement of Tbr (± 10 °C), and some change in the flux composition due to vaporization of Na2O and B2O3 [38], which could lead to the increase in Tliq, and these factors were not considered in the calculation of Tliq. The calculated Tliq increased significantly when CaO/Al2O3 ratio increases to 3 and 4, while Tbr increased only by 18 °C. It follows from the phase composition in Fig. 5 that the increase in the liquidus temperature was caused by CaO precipitation, which started at CaO/Al2O3 ratio of 3 (Fig. 5c) and became significant at CaO/Al2O3 ratio of 4 (Fig. 5d). Precipitation of CaO could increase viscosity although the increase was not sharp. It should also be mentioned that the system was not in the equilibrium state in the process of cooling.
5 Conclusions
-
1.
The viscosity of the flux melts decreased with an increase in the CaO/Al2O3 ratio from 1 to 2. From CaO/Al2O3 ratio = 2 to CaO/Al2O3 ratio = 3, there was a slight rise of the viscosity. The viscosity increased significantly when the CaO/Al2O3 ratio increased from 3 to 4.
-
2.
FTIR results showed that the flux structure changed with changing CaO/Al2O3 ratio. With the increase in CaO/Al2O3 ratio from 1 to 2, the melt structure was controlled by the aluminate and borate group units; as a result, the structure complexity was decreased, leading to a reduced viscosity. With the increase in CaO/Al2O3 ratio from 2 to 4, both solid phase precipitation and melt structure contributed to the variation of viscosity.
-
3.
With the increasing CaO/Al2O3 ratio, the apparent activation energy first decreased from 175 to 168 kJ mol−1 and then increased from 168 to 180 kJ mol−1, which is in the same trend as viscosity. The value of Tbr increased from 1289 to 1338 °C with the increase in CaO/Al2O3 ratio from 2 to 4.
References
L.J. Zhou, W.L. Wang, F.J. Ma, J. Li, J. Wei, H. Matsuura, F. Tsukihashi, Metall. Mater. Trans. B 43 (2012) 354–362.
K.C. Mills, A.B. Fox, Z. Li, R.P. Thackray, Ironmak. Steelmak. 32 (2005) 26–34.
W.L. Wang, A.W. Cramb, ISIJ Int. 45 (2005) 1864–1870.
A. Yamauchi, K. Sorimachi, T. Sakuraya, T. Fujii, ISIJ Int. 33 (1993) 140–147.
J. Jia, C. Bai, G. Qiu, D. Chen, Y. Xu, VII International Conference on Molten Slags, Fluxes and Salts, Johannesburg, South Africa, 2004, pp. 137–139.
I. Sohn, D.J. Min, Steel Res. Int. 83 (2012) 611–630.
S.F. Zhang, X. Zhang, W. Liu, X.W. Lv, C.G. Bai, L. Wang, J. Non-Cryst. Solids 402 (2014) 214–222.
O. Grassel, L. Kruger, G. Frommeyer, L.W. Meyer, Int. J. Plast. 16 (2000) 1391–1409.
G.H. Kim, I. Sohn, Metall. Mater. Trans. B 45 (2014) 86–95.
W.L. Wang, Z.C. Luo, H.H. Zhang, Metall. Mater. Trans. B 49 (2018) 1034–1045.
M. Lenoir, A. Grandjean, Y. Linard, B. Cochain, D.R. Neuville, Chem. Geol. 256 (2008) 316–325.
G.H. Kim, I. Sohn, J. Non-Cryst. Solids 358 (2012) 1530–1537.
B. Lu, K. Chen, W. Wang, B. Jiang, Metall. Mater. Trans. B 45 (2014) 1496–1509.
J. Liao, Y. Zhang, S. Sridhar, X. Wang, Z. Zhang, ISIJ Int. 52 (2012) 753–758.
Z. Zhang, G.H. Wen, P. Tang, S. Sridhar, ISIJ Int. 48 (2008) 739–746.
Z. Zhang, G. Wen, J. Liao, S. Sridhar, Steel Res. Int. 81 (2010) 516–528.
H.G. Ryu, Z.T. Zhang, J.W. Cho, G.H. Wen, S. Sridhar, ISIJ Int. 50 (2010) 1142–1150.
D.L. Zheng, C.B. Shi, Z.J. Li, J. Li, J.W. Cho, J. Iron Steel Res. Int. (Revised version appears online on 2019-01-04). https://doi.org/10.1007/s42243-018-0218-9.
W. Wang, B. Lu, D. Xiao, Metall. Mater. Trans. B. 47 (2016) 384–389.
K. Blazek, H.B. Yin, G. Skoczylas, M. McClymonds, M. Frazee, AIST Trans. 8 (2011) 232–240.
J.W. Cho, K. Blazek, M. Frazee, H.B. Yin, J.H. Park, S.W. Moon, ISIJ Int. 53 (2013) 62–70.
C.B. Shi, M.D. Seo, J.W. Cho, S.H. Kim, Metall. Mater. Trans. B 45 (2014) 1081–1097.
E.Z. Gao, W.L. Wang, L. Zhang, J. Non-Cryst. Solids 473 (2017) 79–86.
B. Lu, W. Wang, Metall. Mater. Trans. B 46 (2015) 852–862.
D. Xiao, W. Wang, B. Lu, Metall. Mater. Trans. B 46 (2015) 873–881.
L.J. Zhou, H. Li, W.L. Wang, D. Xiao, L. Zhang, J. Yu, Metall. Mater. Trans. B 49 (2018) 2232–2240.
J. Qi, C.J. Liu, M.F. Jiang, J. Non-Cryst. Solids 475 (2017) 101–107.
H. Wang, P. Tang, G.H. Wen, X. Yu, Ironmak. Steelmak. 38 (2011) 369–373.
L.J. Zhou, W.L. Wang, J. Wei, B.X. Lu, ISIJ Int. 53 (2013) 665–672.
Z.T. Zhang, G.H. Wen, Y.Y. Zhang, Int. J. Min. Met. Mater. 18 (2011) 150–158.
J. Wei, W. Wang, L. Zhou, D. Huang, H. Zhao, F. Ma, Metall. Mater. Trans. B 45 (2014) 643–652.
L. Zhou, W. Wang, J. Wei, K. Zhou, ISIJ Int. 55 (2015) 821–829.
J. Yang, J. Zhang, Y. Sasaki, O. Ostrovski, C. Zhang, D. Cai, Y. Kashiwaya, Metall. Mater. Trans. B 47 (2016) 2447–2458.
J. Yang, J. Zhang, Y. Sasaki, O. Ostrovski, C. Zhang, D. Cai, Y. Kashiwaya, ISIJ Int. 56 (2016) 574–583.
L. Zhang, W.L. Wang, S.L. Xie, K.X. Zhang, I. Sohn, J. Non-Cryst. Solids 460 (2017) 113–118.
N. Takahira, M. Hanao, Y. Tsukaguchi, ISIJ Int. 53 (2013) 818–822.
S.P. He, Q. Wang, D. Xie, Int. J. Min. Met. Mater. 16 (2009) 261–264.
L. Wang, C. Zhang, D.X. Cai, J.Q. Zhang, Y. Sasaki, O. Ostrovski, Metall. Mater. Trans. B 48 (2017) 516–526.
J.H. Park, D.J. Min, H.S. Song, ISIJ Int. 42 (2002) 344–351.
H. Kim, H. Matsuura, F. Tsukihashi, W.L. Wang, D.J. Min, I. Sohn, Metall. Mater. Trans. B 44 (2013) 5–12.
H. Kim, W.H. Kim, I. Sohn, D.J. Min, Steel Res. Int. 81 (2010) 261–264.
A.Y. Ilyushechkin, S.S. Hla, D.G. Roberts, N.N. Kinaev, J. Non-Cryst. Solids 357 (2011) 893–902.
Y. Kim, M.S. Oh, Fuel Process. Technol. 91 (2010) 853–858.
Z. Wang, I. Sohn, J. Am. Ceram. Soc. 101 (2018) 4285–4296.
Y.Q. Sun, J.L. Liao, K. Zheng, X.D. Wang, Z.T. Zhang, JOM 66 (2014) 2168–2175.
E.I. Kamitsos, M.A. Karakassides, G.D. Chryssikos, J. Phys. Chem. 91 (1987) 1073–1079.
W. Yan, W. Chen, Y. Yang, C. Lippold, A. McLean, Ironmak. Steelmak. 42 (2015) 698–704.
S.F. Weng, Fourier transform infrared spectrometer, 3rd ed., Chemistry Induction Press, Beijing, 2010.
R.C. Pinkerton, J. Chem. Educ. 41 (1964) 366–367.
J.H. Park, D.J. Min, H.S. Song, ISIJ Int. 42 (2002) 38–43.
T.S. Kim, J.H. Park, ISIJ Int. 54 (2014) 2031–2038.
S. Ueda, H. Koyo, T. Ikeda, Y. Kariya, M. Maeda, ISIJ Int. 40 (2000) 739–743.
G. Socrates, in: Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed., Wiley, New York, 2004.
P. McMillan, B. Piriou, J. Non-Cryst. Solids 55 (1983) 221–242.
V.P. Klyuev, B.Z. Pevzner, J. Non-Cryst. Solids 353 (2007) 2008–2013.
Acknowledgements
Financial supports from Baosteel-Australia Joint Research and Development Centre (BAJC) and Australian Research Council (ARC) Industrial Transformation Hub are greatly acknowledged. The first author also acknowledges the scholarship support from UNSW Australia (Tuition Fee Scholarship) and the China Scholarship Council (CSC Award).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Q., Yang, Ja., Zhang, C. et al. Effect of CaO/Al2O3 ratio on viscosity and structure of CaO–Al2O3-based fluoride-free mould fluxes. J. Iron Steel Res. Int. 26, 374–384 (2019). https://doi.org/10.1007/s42243-019-00248-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-019-00248-4