Abstract
Waste paper, a major source of cellulosic biomass, could be utilized as a potential substrate for cellulase production. In this work, different pretreated waste papers were used as substrates for cellulase production. Among them, cardboard treated with 0.1% H2SO4 was found to be the best substrate for cellulase production by Bacillus subtilis S1 (Accession number MG457704). The optimization of the culture conditions for cellulase production was performed using the Plackett-Burman design (PB) and response surface methodology (RSM). The factors considered for PB design were cardboard concentration, yeast extract, inoculum concentration, cultivation temperature, and pH, with cellulase activity (FPase) as a response. PB design at 30 h was highly significant (F = 0.0018 and R2 = 0.99). Cardboard, yeast extract, and inoculum concentrations were the variables selected for optimization by RSM. The model with 15 runs was highly significant (F = 0.0004). The model that predicted a maximum FPase activity of 2.956 U/mL could be achieved with 7.78% inoculum concentration, 3.99 g/L yeast extract, and 25.89 g/L cardboard. The results showed that the predicted values agreed well with the experimental values. The validation experiment proved the adequacy and accuracy of model. This study demonstrates that cardboard could serve as a low-cost substrate for cellulase production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lignocellulosic biomass is widely considered as a promising feedstock for sustainable production of chemicals and fuels. A significant amount of plant-derived carbohydrates available in the municipal solid waste (MSW) could be used as a source of biomass. The organic fraction of MSW contains lignocellulose in the form of waste paper products which could be an adequate raw material to produce value-added products [1]. In MSW, the major cellulosic wastes are paper, cardboard, wood, and agricultural residues and discarding these wastes in the landfills pollute the environment and cause the emission of greenhouse gases [2]. Hence, utilizing these paper wastes as a biomass resource for microbial fermentations would be a better alternative way to solving this challenge. Waste paper could be utilized as a potential carbon source for cellulase enzyme production as it consists of 40–80% cellulose [3]. Considering its huge abundance in the environment, utilizing it to produce useful products has become a trend in the global community. Most of the previous studies have focused on utilizing office paper and newspaper as substrates [3,4,5,6,7] to produce commercially important products. However, very few studies have been carried out using cardboard as a resource [8]. Cardboard is widely used as a packing material and is found in large quantities all over the globe. Cardboard waste contains at least 50% of cellulose [9], and the physical properties of cardboard make it as a favorable candidate for biodegradation and bioproduction [10, 11].
The lignocellulosic biomass can be converted into fermentable sugars using cellulase. In most of the biorefinery research, commercially available cellulases have been used for this conversion which further increases the cost of the processes. Hence, in this study, we tried to produce cellulase by bioprocessing the cardboard waste. Cellulases are inducible enzymes produced by bacteria and fungi during their growth on cellulosic substrates [12, 13]. They are responsible for the hydrolysis of the β − 1, 4 glycosidic bonds in the cellulose polymer to produce glucose units [14]. To achieve complete hydrolysis, a cellulase catalytic system consisting of three classes of soluble extracelluar enzymes named cellobiohydrolase, endoglucanase or carboxymethylcellulase (CMCase), and beta-glucosidases [15] are required to act together and the challenge is to develop processes that are economically profitable.
Cellulases are widely used in many industrial applications such that, in the textile industry for cotton softening and denim finishing in laundry detergents for color care, cleaning [16, 17], in the food industry for mashing [18, 19], in the pulp and paper industries for drainage improvement and fiber modification [20], and they are also used in animal feed industry [21]. Therefore, the demand for more stable, highly active, and specific enzymes is growing rapidly [22]. Enzyme production is closely controlled in microorganisms, and for improving its productivity, these controls can be enhanced. Bacterial cellulases are known to be more effective catalysts, and they may also be less inhibited by the presence of material that has already been hydrolyzed [23]. In fact, the greatest possible advantage is the ease with which bacteria can be genetically engineered [24]. In addition, the high growth rate of bacteria as compared to fungi and its ability to utilize a wide range of substrates as a carbon source makes the bacteria as a potential microorganism to be used in cellulase production [25, 26]. Cellulase yields appear to depend upon a complex relationship involving a variety of factors like inoculum size, pH, temperature, presence of inducers, medium additives, aeration, and growth time [12].
Therefore, the present study has been conducted to utilize the cellulose available in the cardboard for bacterial cellulase production. The ability of soil Bacillus subtilis S1 to utilize cardboard as a carbon source was investigated, and the culture conditions for cellulase production were optimized using the Plackett-Burman design (PB) and response surface methodology (RSM). These statistical designs offer advantages over conventional one variable at a time approach as the latter disregards the complex interactions among the various factors in the experiment [26], while the statistical design techniques can effectively be used to study the effects of the factors and to search for optimum levels of parameters for a desired response [27].
2 Materials and methods
2.1 Isolation and screening for cellulolytic bacteria
Bacteria were isolated from soil samples collected from gardens and farms in different areas of Oman using the traditional serial dilution technique and plated on minimal salt medium (g/L: 0.5 yeast extract, 1 KH2PO4, 0.5 MgSO4, 10 carboxymethyl cellulose (CMC), 1 NaCl, 1 NaNO3, 1 KCl, and 17 agar, pH 7) [25]. The plates were incubated at 40 °C for 24–48 h, followed by flooding with 1% Congo red. After decolorization with 1 N NaCl, the ratio of the clear zone diameter to colony diameter indicating the hydrolysis capacity (HC) was measured [28]. Isolates with HC > 3 were selected, purified by streaking, and preserved at 4 °C for further studies. The selected isolates were screened for cellulase production in a CMC broth medium. In 250-mL conical flask, 100 mL of CMC broth was inoculated with 2 mL of the bacterial strain. Flasks were incubated in a shaker at 40 °C with an agitation speed of 180 rpm. Samples were withdrawn aseptically at every 24 h and were centrifuged at 10,000×g for 10 min at 4 °C. The clear supernatant obtained after centrifugation was considered as the crude enzyme and assayed for endoglucanase or carboxymethyl cellulase activity (CMCase) and filter paper assay (FPase activity).
2.2 Cellulase enzyme assay
The total cellulase activity was measured following the method of Miller and Ghose [29, 30], with slight modification. The endoglucanase activity was determined by measuring the reducing sugar released after incubating 0.5 mL of the supernatant with 0.5 mL of substrate solution containing 2% carboxymethyl cellulose in 0.05 M phosphate buffer saline (PBS pH 7) for 30 min at 50 °C. Bacterial cultures with high endoglucanase activity were further assayed for total cellulase activity (FPase activity) by incubating 0.5 mL of the supernatant diluted in 1 mL PBS with filter paper strip of 1.0 × 6.0 cm (≈ 50 mg) for 1 h at 50 °C. In both assays, reducing sugars were estimated spectrophotometrically with 3, 5-dinitrosalicylic acid (DNS) reagent using glucose as a standard. Cellulase activity and endoglucanase activity were presented as U/mL in which one unit (U) of activity is defined as the amount of enzyme that releases 1 μmole of reducing sugar under the assay conditions.
2.3 Identification of the best cellulase producer
The best cellulase producing bacterium was subjected to molecular identification using 16S rRNA sequencing. The identification was carried out at the central analytical and applied research unit (CAARU), College of Science, Sultan Qaboose University, Al Khod, Oman. Genomic DNA was extracted using Power soil DNA isolation kit (MO BIO laboratories. Inc.), and amplification was performed using the primers set: B27f (5′-AGAGTTTGATCCTGGCTCAG-3′) and U1492r (5′-GGTTACCTTGTTACGACTT-3′) followed by agarose gel electrophoresis. PCR purification was done using PCR purification kit (BioRad Cat# 28106). Cycle sequencing was performed and the amplicon was purified using big dye X terminator purification kit and sequenced using genetic analyzer (Applied Biosystem Hitachi, USA). Taxonomic affiliation of the S7/2 isolate was obtained from the blast algorithm (http://blast.ncbi.nlm.nih.gov/Blast) to determine the most related sequences in the NCBI nucleotide sequence database.
The bacterial isolate showing highest FPase activity was further screened for its ability to utilize waste paper as a carbon source for cellulase production.
2.4 Cellulase production using waste paper
2.4.1 Preparation of waste paper as a substrate
Office paper (OP), magazine (MAG), newspaper (NP), and cardboards (CB) were used as substrates for cellulase production. Following the method described by Guerfali et al. [31], the materials were shredded into 2 by 6 mm pieces using a paper shredder (Atlas, CC0660, China) and soaked in deionized water at 60 °C and washed by continuous stirring. Water was removed by squeezing and the washing procedure was repeated for 3–4 times followed by overnight drying in oven at 50 °C. The dry materials were then grinded using a mixer grinder. The grinded material was subjected to either 0.1% sulfuric acid (H2SO4) or 0.1% dilute hydrogen peroxide (H2O2) treatments at 60 °C. After 2 h of treatment, the treated paper was washed to neutralize the pH and dried again overnight. The ground paper, treated and untreated, was used as substrate.
2.4.2 Cellulase production
Production of cellulase was carried out following same conditions and medium applied in the initial screening experiments replacing CMC with 1% of different waste papers as carbon source. The flasks were inoculated with the bacterial culture, incubated at 40 °C at 180 rpm, and the endoglucanase and FPase activities were measured at 24, 48, and 72 h of fermentation. The waste paper substrate influencing better cellulase activity was chosen for further optimization studies.
2.5 Statistical optimization of cellulase production
The PB experimental design was used to study the significant factors followed by RSM to optimize the fermentation conditions using Design expert trial version 7 software.
2.5.1 Analysis of significant parameters by PB design
Factors selected for screening by PB design were yeast extract, amount of cardboard substrate, temperature, pH of the medium, and inoculum concentration. The selected factors and their levels are shown in Table 1. The inoculum was developed in LB medium for 18 h at 37 °C and 180 rpm, standardized at absorbance (OD600nm = 1.0 ± 0.2). Bacterial strain was cultured in 100-mL medium containing the following (g/L): KH2PO4, 1; MgSO4, 0.5; NaCl, 1; NaNO3, 1; and KCl, 1 in 250-mL flasks with different concentrations of cardboard and yeast extract at different initial pH of the medium and incubated at different temperatures in a shaker at 180 rpm as per the PB experimental design in Table 1. Samples were collected at 6, 24, 30, and 48 h to measure the cellulase activity.
2.5.2 Response surface methodology
Response surface methodology with central composite design was used to optimize the contributing and significant factors. The factors chosen from PB design were inoculum concentration, yeast extract, and substrate concentration, and the ranges of levels for the chosen factors are presented in Table 2. Fifteen experiments were run with five replicates at the center points to evaluate the error. All the runs were performed in triplicate. Finally, the predicted model and experimental conditions obtained by RSM were selected for the validation experiment. Data obtained from the RSM for cellulase production were subjected to analysis of variance (ANOVA). The RSM experimental results were fitted with the response surface regression using the following second order polynomial equation:
where Y is the predicted response, Xi and Xj are the independent factors, β0 is the intercept, βi is the linear coefficient, βii is the quadratic coefficient, and βij is the interaction coefficient. The fitted polynomial equation was expressed as three-dimensional surface plots to demonstrate the relationship between interaction effects of the variables and the responses.
3 Results and discussion
3.1 Isolation, screening, and identification of cellulolytic bacteria
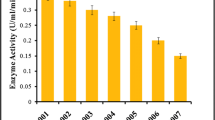
Soil samples collected from different gardens and farms were suspended in CMC medium to isolate cellulose-degrading bacteria. The cellulose hydrolysis was observed by the clear zone around the colonies. Among the 100 bacterial isolates, only 32 isolates with HC > 3 were further screened for endoglucanase or CMCase activity using the CMC broth medium. The isolate S2/7 showed the maximum activity (4.41 ± 0.002 U/mL) followed by S6/2 (4.26 ± 0.004 U/mL), S2/10 (4.05 ± 0.004 U/mL), and S2/15 (3.85 ± 0.006 U/mL). These isolates were tested for their FPase activity and results are demonstrated in Fig. 1. Bacterial isolate S7/2 had the highest FPase activity (2.80 ± 0.050 U/mL) among the four isolates. Maximum FPase activity of the isolated S7/2 bacteria was observed at 48 h, and it declines after that which may be due to an enzyme inhibitory effect of reducing sugar accumulation in the medium.
The isolated bacterium S7/2 was identified by the 16S rRNA sequencing and the sequence was compared using BLAST (NCBI). The isolate S/7 showed 100% homology with Bacillus subtilis strain IAM 12118 (NR_112116). The evolutionary history was inferred using the UPGMA method. The optimal tree with the sum of branch length = 0.21449571 is shown in (Fig. 2). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the maximum composite likelihood method and are in the units of the number of base substitutions per site. The analysis involved 15 nucleotide sequences. All ambiguous positions were removed for each sequence pair. There were a total of 1569 positions in the final dataset. Evolutionary analyses were conducted in MEGA7 [32]. Sequence data of the strain B. subtilis S1 was submitted to the gene bank database (Accession number MG457704).
3.2 Cellulase production using different waste paper substrates
The isolated B. subtilis S1 was tested for its ability to utilize different treated and untreated waste paper as substrates to produce cellulase enzymes. Figures 3 and 4 represented the ability of the B. subtilis S1 to utilize different waste paper substrates for cellulase production. Bacteria grown in media with untreated waste paper were showing less cellulase activity. Maximum FPase activity without treatment was achieved from NP and CB (1.78 ± 0.104 and 1.77 ± 0.149 U/mL) at 72-h incubation. However, the FPase activity for treated waste paper ranged from 1.70 ± 0.159 to 2.12 ± 0.009 U/mL and the highest activities were reached at 48 h. Pretreatment, mainly with dilute acid, is a good choice as they solubilize the hemicellulose components, increase cellulose accessibility [33], and make cellulose more vulnerable to the enzyme, without hydrolysis [34].
The endoglucanase activity with acid-treated CB was highest among all (2.90 ± 0.042 U/mL). Although the FPase activity using acid-treated OP was highest (2.12 ± 0.009 U/mL) followed by acid-treated CB (2.07 ± 0.077 U/mL), the latter was considered as the best choice for proceeding this study due to the following considerations. In addition to its ability to serve as cellulase inducer, CB is also having the advantage of being less in ink content compared to other waste papers, and therefore, less de-inking process is involved. Printing dyes are known to be the most common type of pollutants of waste paper; thus, having negative effect on cellulase activity [35] and the de-inking process may require higher acid concentrations which can affect the cellulose content of the substrate by reducing it and can produce other unfavorable by-products which may require additional steps to overcome that may increase the cost of the final bioproducts [36]. Also, it was observed that during media preparation, it is much easier to adjust and control the pH in media with CB compared to other papers. pH is an important factor in bacteria growth, enzyme production, and activity [25, 37]. Therefore, CB is a substrate that has less effect on pH fluctuations during the medium preparation and even cultivation.
3.3 Analysis of significant parameters by PB design
The PB design was used as a screening method to investigate the contributing factors in the cellulase production by B. subtilis S1 using the acid-treated CB as cellulase inducer. Totally, 13 runs were performed with one center point. PB runs were conducted by varying five factors: CB concentration, yeast extract concentration, inoculum concentration, cultivation temperature, and pH, with enzymatic activity (FPase) as a response being measured at 6, 24, 30, and 48 h. Table 3 represents the analysis of the significance of the PB models showing that models of 24, 30, and 48 h are statistically significant as they have F value > 1 but the highest R2 was achieved by the experimental model of 30 h, hence was chosen for further optimization by RSM. The respective FPase values and the cultivation conditions of the 30-h model are presented in Table 4. ANOVA was used to define the significant factors in FPase activity in the model (Table 5). Values of “Prob˃ F” less than 0.0500 indicate that model terms are significant. In this case, inoculum, yeast extract, pH, and temperature are significant model terms. Inoculum concentration had the greatest influence on the FPase activity of the isolated B. subtilis S1, strain, followed by yeast extract concentration, temperature, and pH.
In a study by Dias et al. [38], the effect of yeast extract, inoculum concentration, and temperature, on cellulase production by Bacillus spp. isolated from sugarcane cultivated soil was investigated. Yeast extract concentration proved to be important in the production and activity, but increasing it to more than 2 g/L had negative effect where bacteria showed lower activity which could be due to enzyme production inhibition. Although this may appear contradicting with our findings which reported a positive influence of yeast extract, it is important to note that the maximum value in our experimental design is the minimum value used in the experiments by Dias et al. Unlike our study which shows a significant positive effect of inoculum size, they reported that the inoculum size showed no significant activity variations suggesting a better evaluation of this factor in the process. It was also reported that the temperature had the greatest influence on the cellulase production by the isolated strain [38] where maximum activity was achieved at 32 °C which was the minimum temperature used for cultivation in the experiment. In our study, similar finding was observed, where the design reported that the temperature was significant and increasing it has a negative effect on cellulase production; therefore, the temperature 32 °C was chosen and fixed for further optimization experiments. This finding contradicts with other studies on different Bacillus spp. including B. subtilis having the optimum temperature for cellulases that ranged from 37 to 40 °C [39,40,41].
The pH of growth medium is known to influence many enzymatic reactions by affecting the transport of chemical products and enzyme across the cell membrane [42]. The optimum pH for cellulase production reported by other studies was 7.2 [40, 41, 43]. The contribution of the pH effect in the current study, although was significant, but had a negative influence on cellulase production, so the minimum pH of 4 was chosen for further work.
Cardboard concentration had a positive effect on cellulase production but not significant. Studies on cellulase production using other types of substrate as carbon source have reported substrate concentration to be influential [26, 41, 44]. Most of previous studies have used either pure cellulose in the form of CMC or Avicel as an example or lignocellulose from agricultural waste as carbon source for cellulase production. A study by Szijarto et al. has reported cellulase production on CB as a novel substrate, but the effect of substrate concentration was not investigated [8]. Therefore, it is wise to further optimize CB concentration, though not been reported significant in the current study.
3.4 Optimization of cellulase production using RSM
CB, yeast extract, and inoculum concentrations were the variables selected for optimization by RSM while fixing the temperature and pH at minimal values. The experimental design with corresponding cellulase activity is shown in Table 6. ANOVA of the quadratic regression model (Table 7) implies that it was a significant model as the Fisher’s F test with a low probability value. The model F value of 38.68 implies that the model is significant. There is only a 0.04% chance that a “model F value” this large could occur due to noise. Values of “Prob > F” less than 0.0500 indicate that model terms are significant. The coefficients and P values of all the variables of linear (A, B, C), quadratic (A2, B2, C2), and interaction (AB, AC, BC) terms were determined and presented in Table 7. Among the linear coeffcients, B and C (yeast extract and cardboard concentration) had a significant effect on cellulase production. While all the quadratic coefficients were significant, only the interaction AC (inoculum and cardboard concentration) had a significant effect on the cellulase production. The 3D plots are used to study the interactions between the response (FPase activity in this case) and the experimental level of the combined effects of the analyzed variables (Fig. 5).
The response measured for FPase activity (FPA) at 30 h for cellulase production by B. subtilis S1 using cardboard as substrate was correlated with the values of the factor, and the following regression equation was established:
The predicated R2 (0.7910) and adjusted R2 (0.9604) values for the cellulase production were in reasonable agreement with the value of R2 (0.9858), which is closer to 1.0, indicating the better fitness of the model in the experimental data. Figure 6 shows the observed cellulase activity in comparison with those from the empirical model. It is evident that the predicted values were in close agreement with the observed ones in the experimental region investigated. The model predicted a maximum FPase activity 2.956 U/mL achieved with 7.78% inoculum concentration, 3.99 g/L yeast extract, and 25.89 g/L CB. Predicted model was validated and experiments were conducted in triplicate under the optimal conditions suggested. The experimental value achieved (2.963 U/mL) was in high agreement with the predicted value indicating the validity and adequacy of the predicted model to optimize cellulase production. Previous studies have also reported the significance of the RSM on enhancing the production of metabolites using microorganisms [26, 45, 46]. The cellulase activity under optimum condition using cardboard waste was 1.43-fold higher than the cellulase activity obtained using CMC medium. Further, the reducing sugars released during the cellulase fermentation could be used a carbon source to produce various value-added products. Hence, this study suggests that the cardboard could be used as a low-cost substrate for cellulase production.
4 Conclusions
This study has revealed the ability of bacteria to utilize waste paper as carbon source. Although different waste paper substrates were tested, CB treated with 0.1% H2SO4 was found to be the best inducer of cellulase enzyme production by the isolated B. subtilis S1. The cellulase activity of 2.963 U/mL was achieved using the predicated model with the optimized conditions. Optimization process for the production of cellulases is important for increasing productivity and reducing the cost; therefore, it is a useful tool in production economics. In addition, the utilization of cardboard waste as a substrate would further reduce the production cost. Further studies with expanded scale are required as this is very promising for industrial applications.
References
Schmitt E, Bura R, Gustafson R, Cooper J, Vajzovic A (2012) Converting lignocellulosic solid waste into ethanol for the state of Washington: an investigation of treatment technologies and environmental impacts. Bioresour Technol 104:400–409
Sivakumar N, Al Zadjali A, Al Bahry S, Elshafie A, Eltayeb EA (2016) Isolation and characterization of cellulolytic Bacillus licheniformis from compost. Afr J Biotechnol 15:2434–2446
Ioelovich M (2014) Waste paper as promising feedstock for production of biofuel. J Sci Res Rep 3:905–916
Elliston A, Samuel RAC, Wilson DR, Roberts IN, Waldron KW (2013) High concentrations of cellulosic ethanol achieved by fed batch semi simultaneous saccharification and fermentation of waste-paper. Bioresour Technol 134:117–126
Pooja H, Rashmi A, Sabeena K, Bhatkal A, Sastry DN (2016) Production of ethanol from waste newspaper. Int J Eng Trends Technol 5:589–593
Annamalai N, Al-Battashi H, Al-Bahry S, Sivakumar N (2018) Biorefinery production of poly-3-hydroxybutyrate using waste office paper hydrolysate as feedstock for microbial fermentation. J Biotech 265:25–30
Annamalai N, Sivakumar N, Oleskowicz-Popiel P (2018) Enhanced production of microbial lipids from waste office paper by the oleaginous yeast Cryptococcus curvatus. Fuel 217:420–426
Szijártó N, Réczey ZFK, Mézes M, Bersényi A (2004) Cellulase fermentation on a novel substrate (waste cardboard) and subsequent utilization of home-produced cellulase and commercial amylase in a rabbit feeding trial. Indus Crops Prod 20:49–57
Kinnarinen T, Shakhanova M, Hietanen E, Salmimies R, Häkkinen A, Louhi-Kultanen M (2012) Effect of mixing on enzymatic hydrolysis of cardboard waste: saccharification yield and subsequent separation of the solid residue using a pressure filter. Bioresour Technol 110:405–411
Kadar ZS, Szengyel ZS, Reczey K (2004) Simultaneous saccharification and fermentation (SSF) of industrial wastes for the production of ethanol. Ind Crop Prod 20:103–110
Yañez R, Alonso JL, Parajó JC (2004) Production of hemicellulosic sugars and glucose from residual corrugated cardboard. Process Biochem 39:1543–1551
Immanuel G, Dhanusha R, Prema P, Palavesam A (2006) Effect of different growth parameters on endoglucanase enzyme activity by bacteria isolated from coir retting effluents of estuarine environment. Int J Enviro Sci Technol 3:25–34
Lee SM, Koo YM (2009) Isolation, purification, characterization of cellulolytic enzymes produced by the isolate Streptomyces omiyaensis. J Microbiol Biotechnol 1:229–233
Nishida Y, Suzuki KI, Kumagai Y, Tanaka H, Inoue A, Ojima T (2007) Isolation and primary structure of a cellulase from the Japanese sea urchin Strongylocentrotus nudus. Biochimie 89:1002–1011
Bhat MK (2000) Cellulases and related enzymes in biotechnology. Biotechnol Adv 18:355–383
Hebeish A, Ibrahim NA (2007) The impact of fronteir sciences on textile industry. Colourage 54:41–55
Karmakar M, Ray RR (2011) Current trends in research and application of microbial cellulases. Research J Micro 6:41–53
Minussi RC, Pastore GM, Durán N (2002) Potential applications of laccase in the food industry. Trends in Food Sci Techol 13:205–216
de Carvalho LMJ, de Castro IM, da Silva CAB (2008) A study of retention of sugars in the process of clarification of pineapple juice (Ananas comosus, L. Merril) by micro- and ultra-filtration. J Food Eng 87:447–454
Mai C, Kües U, Militz H (2004) Biotechnology in the wood industry. Appl Micro Biotech 63:477–494
Dhiman TR, Zaman MS, Gimenez RR, Walters JL, Treacher R (2002) Performance of dairy cows fed forage treated with fibrolytic enzymes prior to feeding. Animal Feed Sci Techno 101:115–125
Cherry JR, Fidants AL (2003) Directed evolution of industrial enzymes: an update. Current Opinion in Biotechnol 14:438–443
Nakamura K, Kappamura K (1982) Isolation and identification of crystalline cellulose hydrolyzing bacterium and its enzymatic properties. J Ferment Technol 60:343–348
Ariffin H, Abdullah N, Umi K, Shirai Y, Hassan MA (2006) Production and characterization by Bacillus pumilus EB3. Int J Eng Technol 3:47–53
Sethi S, Datta A, Lal Gupta B, Gupta S (2013) Optimization of cellulase production from bacteria isolated from soil. ISRN Biotechnology 2013:1–7
Selvam K, Govarthanan M, Kamala-kannan S, Govindharaju M, Senthiillkimar B, Selvankumar T, Sengottaiyan A (2014) Process optimization of cellulase production from alkali-treated coffee pulp and pineapple waste using Acinetobacter sp. TSK-MASC. RSC Adv 4:13045–13051
Latifian M, Hamidi-Esfahani Z, Brzegar M (2007) Evaluation of culture conditions for cellulase production by two Trichoderma reesei mutants under solid-state fermination conditions. Bioresour Technol 98:3634–3637
Gupta P, Samant K, Sahu A (2011) Isolation of cellulose-degrading bacteria and determination of their celluloytic potential. Int J Micro 2012:1–5
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Ghose TK (1987) Measurment of cellulase activities. Pure Appl Chem 59:2257–2268
Guerfali M, Saidi A, Gargouri A, Belghith H (2015) Enhanced enzymatic hydrolysis of waste paper for ethanol production using separate saccharification and fermentation. Applied Biochem Biotechol 175:25–42
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
El-Zawawi WK, Ibrahim MM, Abdel-Fattah YR, Soliman NA, Mahmoud MM (2011) Acid and enzyme hydrolysis to convert pretreated lignocellulosic materials into glucose for ethanol production. Carbohydr Polym 84:865–871
Gamez S, Gonzalez-Cabriales JJ, Ramırez JA, Garrote G, Vazque M (2006) Study of the hydrolysis of sugar cane bagasse using phosphoric acid. J Food Eng 74:78–88
Brummer V, Jurena T, Hlavacek V, Omelkova J, Bebar L, Gabriel P, Stehlik P (2014) Enzymatic hydrolysis of pretreated waste paper- source of raw material for production of liquid biofuels. Bioresour Technol 152:543–547
Ioelovich M (2013) Plant biomass as a renewable source of biofuels and biochemicals. Lambert Academic Publishing, Germany
Patagundi BI, Shivasharan CT, Kaliwal BB (2004) Isolation and characterization of cellulase producing bacteria from soil. Int J Curr Microbiol App Sci 3:59–69
Dias P, Ramos K, Padilha I, Araujo D, Santos SFM, Silva FLH (2014) Optimization of cellulase production by Bacillus Sp. isolated from sugarcane cultivated soil. Chem Eng Trans 38:277–281
Ray AK, Bairagi KS, Ghosh A, Sen SK (2007) Optimization of fermentation conditions for cellulase production by Bacillus subtilis CY5 and Bacillus circulans TP3 isolated from fish gut. Acat Icht Et Pist 37:47–53
Ariffin H, Hassan MA, Shah UK, Abdullah N, Ghazali FM, Shirai Y (2008) Production of bacterial endoglucanase from pretreated oil palm empty fruit bunch by Bacillus pumilus EB3. J Biosci Bioeng 106:231–236
Deka D, Das SP, Sahoo N, Das D, Jawed M, Goyal D, Goyal A (2013) Enhanced cellulase production from bacillus subtilis by optimizing physical parameters for bioethanol production. ISRN Biotechnol 2013:1–11
Liang Y, Feng Z, Yesuf J, Blackburn JW (2010) Optimization of growth medium and enzyme assay conditions for crude cellulases produced by a novel thermophilic and cellulolytic bacterium, Anoxybacillus sp. 527. Appl Biochem Biotechnol 160(6):1841–1852
Rastogi G, Muppidi GL, Gurram RN, Adhikari A, Bischoff KM, Hughes SR, Apel WA, Bang SS, Dixon DJ, Sani RK (2009) Isolation and characterization of cellulose-degrading bacteria from the deep subsurface of the Homestake gold mine, lead, South Dakota, USA. J Ind Microbiol Biotechnol 36:585–598
Varotkar P, Tumane PM, Wasnik DD (2016) Bioconversion of waste paper into bioethanol by co- culture of fungi isolated from lignocellulosic waste. Int J Pure Appl Biosci 4:206–215
Chuprom J, Bovornreungroj P, Ahmad M, Kantachote D, Dueramae S (2016) Approach toward enhancement of halophilic protease production by Halobacterium sp. strain LBU50301 using statistical design response surface methodology. Biotechnol Rep 10:17–28
Patil MD, Shinde KD, Patel G, Chisti Y, Banerjee UC (2006) Use of response surface method for maximizing the production of arginine deiminase by Pseudomonas putida. Biotechnol Rep 10:29–37
Acknowledgements
The authors are greatly thankful to The Research Council (TRC), Oman for the financial support (Grant no: ORG/EBR/14/003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al Azkawi, A.S., Sivakumar, N. & Al Bahry, S. Bioprocessing of cardboard waste for cellulase production. Biomass Conv. Bioref. 8, 597–606 (2018). https://doi.org/10.1007/s13399-018-0309-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-018-0309-7