Abstract
The present study reports the properties and hydration of blended cements with ferronickel slag, produced during the pyrometallurgical treatment of laterites for the production of ferronickel. For that purpose, the slag was ground to a specific surface area of 4,000 cm2/g and added in ratios 5, 10, 15, and 20 wt%. The produced blended cements were tested by determining their initial and final setting times, standard consistency, flow of normal mortar, expansibility and compressive strength at 2, 7, 28 and 90 days. X-ray diffraction and TG/DTG analyses were used for the determination of the hydration products, whereas the microstructure of the hardened cement pastes and their morphological characteristics were examined by scanning electron microscopy. The blended cements leachability behavior was determined by the toxicity characteristic leaching procedure test and the tank diffusion test for monolithic samples (NEN 7375). The results revealed that the concentrations of leached heavy metals were substantially below the regulatory thresholds. According to the results, the ferronickel slag could be readily utilized as cement additive, presenting environmental benefits in waste management practice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ferronickel slag (FNS) is a byproduct obtained from smelting of laterite ore in an electric arc furnace at a high temperature with the presence of a reducing agent, for the production of ferronickel alloy and it is produced by cooling the molten slag with water or air [1, 2]. In Greece, the limonitic laterites have been exploited to produce ferronickel via a pyrometallurgical route, which involves drying, preheating and partial reduction of the oxidic laterite ore in rotary kilns (750–800 °C), followed by reductive smelting of the product of these kilns in open bath submerged arc electric furnaces at about 1,600 °C [3, 4]. The ferronickel from this process is then refined and enriched in an Oxygen-Bottom Maxhütte (OBM) converter to a ferronickel (nickel content: 15–25 wt%), which is used directly in steel production. The FNS from the electro-reduction furnace is produced at a rate of approximately 2 Mt/y and for every tone of FeNi alloy the amount of FNS produced is estimated at 4 tones [1, 5]. Currently, FNS is granulated through sudden cooling in sea water, leading to an amorphous material, which, due to its properties it has been classified as non-hazardous waste, according to the European Catalogue for Hazardous Wastes (EWC) [6].
Today a small part of the produced FNS is used as a sand-blasting material, as a raw material in cement clinker production, as an inert additive in high strength concrete, instead of limestone aggregates in the concrete for the construction of the base of roads, or in producing anti-slippery pavement tiles [7]. Efforts have been carried out in laboratory and pilot-scale trials for the utilization of FNS in the production of high-alumina cement [1], whereas recent researches have proven that FNS can be also used as raw material in synthesizing inorganic polymers (geopolymers), after alkali activation [8–10].
However, the largest amount of the produced slag is temporarily disposed in areas closed to the metallurgical plant. Although FNS has been considered as non-hazardous, alternative ways for its exploitation should be introduced in order to reduce or eliminate cost of disposal and avoid potential soil and water contamination. On the other hand, the natural resources preservation by using wastes or by-products that could be recycled as raw materials is one of the general environmental topics nowadays.
The produced FNS during cooling with sea water presents a size of a 0–5 mm fraction. Its chemical composition consists of Fe2O3: 36–43 wt%, SiO2: 34–36 wt%, CaO: 2–5 wt%, Al2O3: 7–12 wt%, MgO: 2–5 wt%, Cr2O3: 2–3 wt% and NiO: 0.10–0.15 wt%. Due to its high content in silicon oxide it could be consider as an additive in replacement of clinker in blended cements, because it presents pozzolanic characteristics, reacting with Portlandite in order to form calcium silicate hydrate [11]. On the other hand Portland cement is the one of the most widely used construction material, but it accounts for approximately 4–5 wt% of greenhouse gas emissions [12]. The demand for reduction of the atmospheric emissions and for energy cost savings has led to the introduction of alternative building materials. The traditional way to utilize metallurgical slags in cementing materials is to partially replace clinker in Portland cement, which usually results in a lower early strength and longer setting times. Slags with high amorphous silicon oxide content are today used as a substitute for clinker, acting as a pozzolanic material and presenting influence in enhancing mechanical properties and durability [12, 13].
In Greece, the slag from the ferronickel production had been used for a short period in the past (in the late 90s) as an additive in the production of cement, but for the moment local legislation has limited its use in the building materials sector [7, 14]. The practice of using a waste as an additive material is acceptable when it does not pose any negative effect. One of the biggest concern in using FNS is the relative high chromium content that is found as a trivalent chromium, which is stable and not water soluble. In case of using FNS as raw material, the oxidizing conditions during sintering process, at certain stages of clinker manufacture, may transform trivalent chromium into hexavalent chromium, which is soluble in water. However, using the slag as an additive, replacing clinker in blended cements, the Cr(III) contained in FNS mainly in the spinel phase is not expected to be leached out during hydration process.
The so far published literature has given little attention to the use of FNS as an additive for producing composite Portland cement. Furthermore, although FNS is typically considered safe, according to the EWC [6], there are no studies investigating the heavy metals leaching behavior of composite cement with FNS.
In view of the growing environmental concern of slag disposal in the metallurgical plants area, the possibility of using the ferronickel slag as an additive for composite Portland cements was examined. One reference cement and four mixtures containing up to 20 wt% FNS were prepared. Water demand, setting times, soundness and compressive strength were measured. XRD, TG/DTG and SEM were applied in order to study the hydration products and the microstructure of the cement-slag system. Toxicity Characteristic Leaching Procedure (TCLP) tests were performed, using crushed hydrated samples after 28 days of curing to determine the concentration of heavy metals in the extract and subsequently to assess the potential toxicity of the produced composite cements [15]. Furthermore, the leachability characteristics of heavy metals from the produced FNS cements were also examined according to the Dutch standard NEN 7375 [16], which is designed for the determination of the extent of leaching of inorganic components from moulded or monolithic materials.
Experimental
Ferronickel Slag
FNS, supplied from LARCO smelting plant located at Larymna, was added to a CEM I 52.5 Portland cement produced by Heracles General Cement Company of Greece. FNS exhibits a relatively high particle size as determined by sieve analysis (presented in Fig. 1); this is mostly attributed to the cooling process using sea water as mentioned above. Chemical analyses carried out with X-ray Fluorescence (Spectro–Xepos), Atomic Absorption Spectrophotometry (Perkin Elmer 4100) and Inductively Coupled Plasma Mass Spectrometry (ICP-MS X Series II, Thermo Scientific) are shown in Table 1, along with the physical characteristics of FNS. The concentrations of trace metals are given in Table 2.
The crystalline phases of FNS and CEM I 52.5 used in this study were determined by XRD analysis, using a Bruker D8-Focus diffractometer with nickel-filtered CuKa radiation (λ = 1.5406 Å), at 40 kV and 40 mA. FNS morphology and microstructure was examined by Scanning Electron Microscopy with a Jeol 6380LV, equipped with an Oxford INCA Energy Dispersive Spectrometer.
The reactive SiO2 of FNS was determined using the standard procedures specified in EN 196-2 [17]; defined as the fraction of silica which, after the treatment with hydrochloric acid, is soluble in boiling potassium hydroxide solution, in a 4 h extraction. The determination of slag pozzolanicity was carried out according to the Hellenic National Standard 244/1980 [14], which measures the ability of slag to combine with lime due to pozzolanic reactions [18].
FNS Blended Cements
Prior to the preparation of the blends with cement, the FNS (size fraction: 0–5 mm) was ground, in a 1 kg laboratory ball mill using steel balls as grinding medium, to a specific surface area of 4,000 cm2/g, defined according to the Blaine air permeability method [19]. Following, the blended cements were produced by mixing the slag and the cement. The mixing ratios, as well as the physical characteristics of the cements are given in Table 3.
Standard consistency and setting times of cement pastes were determined using a Vicat apparatus according to the European Standard EN 196-3 [20]. The determination of the normal mortar flow was carried out according to ASTM C1437 [21]. Expansions of the cement pastes were determined by the Le Chatelier method [20]. Compressive strength measurements were conducted at the ages of 2, 7, 28 and 90 days on mortar specimens (dimensions 40 mm × 40 mm × 160 mm), prepared and tested in accordance with European Standard EN 196-1 [22].
To examine the hydration products, cement pastes were prepared by mixing 300 g of cement-FNS mixtures with 75 ml of water. Following, the pastes were left to cure in temperature controlled sealed vessels containing tap water (20 ± 2 °C). At the ages of 2, 7, 28 and 90 days, the hydration was stopped by means of acetone and ether extraction. The hydration products were mineralogically determined by X-ray diffraction, using a Bruker D8-Focus diffractometer. Thermal Gravimetric Analysis (TGA) was carried out to evaluate hydration rate using a Mettler-Toledo TGA/SDTA 851 instrument. The exact boundaries for the temperature intervals were defined from the derivative curve (DTG). Type R thermocouple (Pt-13 % Rh/Pt) was used for temperature measurements. Specimens were put in ceramic crucible and heated from room temperature to 900 °C, at a constant rate of 10 °C/min, using nitrogen as medium under static condition. Finally, morphological analysis and observation of hydration products were performed by SEM.
Leachability Tests
The FNS and the C20 blended cement mortar (with 20 wt% cement substitution) were subjected to TCLP leachability test [15]. Following compressive strength determination at 28 days, the fractured mortar sample was prepared by grinding it to 0–9.5 mm, while the FNS was tested as received. The extraction solution was prepared using glacial acetic acid to obtain a pH value of 2.88. The crushed samples and acetic acid solution were placed into beakers with liquid/solid ratio of 20:1 and extracted for 18 h.
The leachability of the C20 blended cements was also examined by the NEN 7375 monolithic tank test [16], which is suitable for monolithic structures, such as concretes or mortars. Freshly prepared mortar slurry was transferred to plastic molds blocks (10 cm3); after 2 days the mortars were removed from their molds and left to cure for 28 days applying the same conditions as mentioned in 2.2. After the initial 28-day curing, specimens were immersed in distilled water (pH = 7) which was used as a leaching agent for a period of 64 days; the leachant was renewed at a fixed schedule at 0.25, 1, 2.25, 4, 9, 16, 36 and 64 days. The volume ratio of leachant to mortar (VLeachant/Vsample) was kept at 5, constantly ensuring that all sides of the mortar specimens were sufficiently covered by the leachant (2 cm on each side). In both cases, ICP-MS was used to determine the leachable heavy metals.
Furthermore, all samples were analyzed for water soluble Cr(VI), in accordance with European Standard EN 196-10 [23]. The method consists mainly of the hexavalent chromium dissolution by treating the initial solid cement with water, at ambient temperature for a predetermined period of time (15 ± 1 min). The extracts were subsequently reacted with diphenylcarbizide in acid solution and the Cr(VI) determination was based on the formation of pink organometallic complex and on its measurement by a Bausch & Lomb Spectronic 20 spectrophotometer at a wavelength of 540 nm.
Results and Discussion
Ferronickel Slag
The ferronickel high rate cooled slag appeared black–grey, opaque and glassy. Its particle size distribution is presented in Fig. 1. 90 wt% of it exhibits a grain size smaller than 2 mm, whereas the 50 wt% was below 0.9 mm. Chemical analysis is given in Tables 1 and 2. Iron and silicon were the main constituents and accounted for 81 wt% of the slag mass. Other constituents such as Al2O3, CaO, MgO were found in lower quantities. Cr2O3 determined at about 3 wt% and NiO constituted approximately 0.13 wt% of slag mass; minor presence of MnO (0.52 wt%) and TiO2 (0.12 wt%) was also observed. Regarding FNS pozzolanic activity, the percentage of reactive silica was determined at 40.71 wt%, whereas its pozzolanicity was determined at 5.9 MPa (mortars of slag with Ca(OH)2 and standard sand).
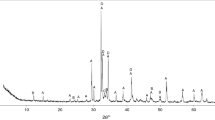
X-ray diffraction pattern of FNS (presented in Fig. 2) reveals its amorphous nature, as reflected by the presence of a diffuse wide band from the glassy phase, located approximately at 2θ 30° and is extended in the range of 20°–40°. The spinel phase [(Fe2+,Mg)(Fe3+,Al,Cr)2O4] was the only crystalline mineralogical phase that was detected in the slag. The formation of crystalline phases is a function of both the chemical composition of the melt and its cooling rate. In case of silica high content, the slag vitrifies (forms a glassy phase), when it is rapidly cooled.
The above observations were also confirmed by SEM analysis (Fig. 3). The glass phase appears colourless and is chemically heterogeneous, as it is formed mostly by silicon, iron, aluminium and magnesium. It also exhibits elevated concentrations of Ca. The metal alloy is easy to distinguish as it exhibits higher reflectance when compared to other minerals. Although the coarse alloy has been recovered, the ferronickel slag still contains small amounts of metallic particles with various complicated forms (drop-like, vermicular and oval). EDS analysis of metallic parts revealed the presence mainly of Fe and Ni with smaller amount of Cr.
Backscattered electron micrographs of the as received FeNi slag polished sections. Fine to coarse-sized euhedral crystals of spinel (Sp) and pure magnetite (Mgn) embedded in glassy matrix (Gl). Parallel growths of columnar elongated olivine-group crystals (Ol) in the amorphous glass (Gl) phase. Drop-like and vermicular metallic particles composed of FeNi (Me)
Within the isotropic glass, complex spinel phases [(Fe2+,Mg)(Fe3+,Al,Cr)2O4] are the most abundant and some magnesium silicate crystalline phases are less common. Spinels appeared most frequently as cubic to octahedral euhedral crystals. Occasionally they exhibit skeletal form mostly in cases where the cores are hollow. The detection of spinel phases, except Fe3O4, indicates that the trivalent iron was able to be combined with chromium, aluminum and magnesium to produce the spinel phase upon cooling from the melt. As the spinel-group phase is amongst the first to crystallize, chromium ferrite and magnetite developed through crystallization from the silicate melt into idiomorphic individuals, but sometimes exits also in eutectic growth.
The formation of rich iron magnesium silicate phases is due to the presence of magnesium oxide in the slag. They were detected with the form of elongated prismatic crystals. Olivine-group phases, which represent an isomorphic mixture of forsterite Mg2SiO4 and fayalite Fe2SiO4, probably are the last silicates crystallizing from the liquid. It should be noticed that in slags with lower Mg content only pyroxenes would be crystallized instead of olivine.
Physical and Mechanical Properties of Blended Cements
Table 4 presents the cement water demand, setting times and normal mortar flow of the tested cements. The “water demand” is generally considered to be the quantity of water required for the preparation of a cement paste with standard consistency, as specified in EN 196-3. The FNS containing cements demanded less water than the reference, a fact that was attributed to the delayed hydration of slag. The flow tests confirmed the above results and showed that the FNS addition improves the mortar workability. The replacement of cement with the less reactive slag, lead to reduced amounts of hydration products formed during the early hydration stages, thus resulting to higher workability of the mortar.
Furthermore, the blended cements showed longer setting times than the reference. In case of C20, the initial and final setting times were reached at 175 and 220 min respectively, 40 min longer than those of reference sample. Cement replacement by FNS is expected to result in delaying the hydration process thus increasing setting times. On the contrary, in case of reference sample as the hydration products increased, the slurry from the suspending state started to be agglomerated faster and the setting times was shortened. The expansion, measured according to the Le Chatelier process, was well below the maximum accepted value of 10 mm [20].
The mortars of the blended cements under investigation were tested for compressive strengths after 2, 7, 28 and 90 days of curing and the obtained results are shown in Fig. 4. It is observed that slag cements developed lower strength, at all ages, compared to reference cement. After 2 days of hydration, the compressive strength of the reference sample is approximately 28 % higher (24.1 MPa) than that of the C20 sample (17.2 MPa), whereas the decrease in case of C5 (23.3 MPa) is about 3 %. The rate of strength development of reference cement depends mainly on its hydration rate, while in cement–slag systems it also depends on the hydration and the latent hydraulic reactions of slag. The same observation was made at 7 days of curing, where the presence of FNS resulted in decrease of mortar compressive strength, in inverse proportion to FNS replacement ratio. At 28 days, slag containing specimens presented a decrease in compressive strength relative to the reference specimen; this was calculated at 2.2, 6.8, 10 and 13 % for specimens with 5, 10, 15 and 20 wt% slag content respectively. At early ages, strength development is governed mostly by the hydration of cement while FNS does not appear to contribute in any way to hydration reactions. At later ages the pozzolanic reaction of slag contributes to strength development, since it is known to occur at a lower rate than standard cement hydration. Thus, the “gap” in strength between reference and slag containing specimens is gradually reducing with hydration age. At 90 days the compressive strength of the reference sample is 8 % higher (63.5 MPa) than that of the C20 sample (58.2 MPa) and approximately 1 % of C5 (62.8 MPa). It should be noted that all specimens studied in this work satisfy the requirements for the strength class 42.5 as per standard EN 197-1.
Cement Hydration
XRD was used to identify the phases formed during hydration. Figure 5 shows the X-ray diffraction patterns of the blended cement with 20 wt% FNS hydrated for 2, 7, 28 and 90 days. It can be seen that the main hydration products were C–S–H, Ca(OH)2, ettringite (Ca6Al2(SO4)3·32H2O) and carbonate-AFm phases, as well as unhydrated C3S and C2S. The amount of portlandite increases continuously at the first days mainly due to the hydration of calcium silicate phases. However, after 28 days of curing, the production of crystalline Ca(ΟH)2 seems to diminish. It is known that the slag derived amorphous silica reacts with lime produced during the early hydration of alite, to form C–S–H gel, which contributes to the strength of the cement paste. As a result, the decrease on the evolution of portlandite is directly related to the pozzolanic reactions. The peaks of the calcium silicates phases diminished, especially at the age of 90 days.
X-ray diffraction patterns (Fig. 6) of all blended cements hydrated for 28 days showed that the main hydration products include C–S–H, portlandite (CH) and aluminate phases such as ettringite and carbonate-AFm. Ettringite was formed within the first hours of mixing, while after 7 days of hydration C3S is the main hydration component. The initiation of the pozzolanic reaction due to amorphous silica of FNS is characterized by the relative decrease of the Portlandite peaks intensity after 28 days of hydration. The glassy particles of the slag were dissolved in the alkali environment of the hydrated cement and were further react with Ca(OH)2 to produce more C–S–H gel and AFm, from the reaction of soluble alumina and calcium hydroxide phases. The peaks associated with C–S–H seem to be intensified with the increase of slag content and C2S is the only unhydrated clinker phase still detected after 28 days of hydration.
Figures 7 presents the results of TG/DTG of the blended cement with 20 wt% FNS hydrated for 2, 7, 28 and 90 days. The TG curves showed mass loss at about 120, 470 and 720 °C, representing dehydration of C–S–H and ettringite, dehydroxylation of Ca(OH)2 and decomposition of calcium carbonate, respectively. The broad peak in the range 100–150 °C was evidence of the existence of colloidal C–S–H gel and ettringite. It can be observed as a wide band that shifts to higher temperatures when the hydration age increases. The second main mass loss can be observed at around 470 °C, which represents the decomposition of crystalline Ca(OH)2 produced by the hydration of calcium silicate phases of the blended cements. The endothermic peak of Ca(OH)2 at 90 days is relatively lower than that of 28 days, a fact that confirmed the increased pozzolanic reactivity of slag with lime. The comparatively smaller peaks of Portlandite, with the wider C–S–H endothermic peak at 120 °C and with almost disappearing belite phase, as it was shown from the XRD data, indicated an increased pozzolanic reactivity of the FNS slag at this period of hydration.
Figures 8 and 9 show backscattered electron micrographs images of pastes polished sections, after 2 and 90 days of hydration, for the CRef and C20 blended cements, respectively. It is possible to distinguish the following phases: (a) Unreacted anhydrous cement grains, (b) Ca(OH)2, which is slightly darker than anhydrous grains, (c) Inner C–S–H, which are observed as rim around the anhydrous grains, (d) Outer C–S–H, the hydration products which fills the cementitious matrix, (e) slag particles which are surrounded by secondary C–S–H gel and porosity.
As the hydration kinetics of slag is lower than that of pure cement, up to 2 days the clinker hydration process advanced more rapidly. Ca3SiO5 was detected mainly at 2 days samples, whereas after 90 days of curing only small amount of Ca2SiO4 was observed. The dark gray regions forming the matrix, in which the unreacted clinker phases were embedded, mainly consisted of hydration products. The C–S–H gel formed a fibrous dense network structure after 2 days. This showed that the hydration reaction of the examined mixtures was adequately fast and a number of hydration products were formed. C–S–H near the cement grains has been developed uniformly and it is much denser and stronger. At this age slag particles have not yet reacted with cement hydration products.
As the hydration proceeded, hydration products gradually filled the network structure and the cement paste structure became tight at the latter stage of hydration. At 90 days, a reduction in the amount of anhydrous cement grains and the pores content was observed. In case of C20 sample, partially hydrated slag particles were detected, showing a microstructure with a dense rim of hydration products. Secondary hydration products were produced due to the pozzolanic reaction of portlandite, which reacted with the ferronickel slag active silica to produce mainly secondary C–S–H. Also the presence of active SiO2 modified the microstructure of the cement paste and led to the filling of the pores with low density C–S–H gel and to the consuming of Ca(OH)2, thus improving the mechanical properties of the mortars.
Leachability Tests
The as-received ferronickel slag and the C20 mortar sample at 28 days of curing were subjected to TCLP leaching test and the results are presented in Table 5. It is evident that concentrations of heavy metals in leachates are below the limits defined by US EPA for non-hazardous waste, although regarding nickel leachability from FNS, the concentration in the extract is relatively higher (in relation with the other heavy metals). On the other hand, TCLP tests also revealed that the release of metals from the crushed C20 mortar sample was far below regulatory limits. The cement mortar provided suitable solid matrix for further immobilization of heavy metals and this was confirmed in the case of Ni, where its concentration in the leach liquor is lower. The immobilization in the cement mortar may proceed by chemical retention or physical encapsulation. Chemical retention involves the incorporation of metals in the hydrated cement phases, while physical encapsulation involves the physical incorporation of toxic metals in the cement matrix. Regardless of the degree of metals leaching from the FNS, their immobilization in cement matrix is higher and the level of release is lower. The above observations are in agreement with the results of EDS analyses, where the trace metals were found both entrapped inside the slag in the cement matrix and as a part of cement crystalline or gel phases. According to the results and the regulatory limits, it can be concluded that the ferronickel slag is not hazardous when used as a cementitious material.
Regarding NEN 7375 monolithic leaching test, the determination of elemental concentration in each elution stage (μg/L) enables further calculations of the cumulative concentrations per unit area (mg/m2) for each stage and for the entire test. The released concentrations of the heavy metals from CRef and C20 mortar blocks (μg/L), compared with the parametric value of European Directive 98/83/EC (μg/L) [24] for drinking water, as well as the cumulative leachable content of elements, compared with the corresponding UK regulatory limits for disposal in landfill [25], are presented in Table 6. In all cases, the results at 64 days release indicated that the amount of heavy metals leached from the cement mortar blocks were below the lowest limits, suggesting the environmental compatibility of ferronickel slag, when it is used as an additive in the cement production.
Regarding water-soluble chromium, the results showed that the 20 wt% substitution with FNS did not affect the final content in Cr(VI) and the values determined were 1.23 ppm for the reference sample and 1.18 ppm for the C20 blended cement. According to the Directive 2003/53/EC published by the Council of the European Union [26], a strict limitation was set regarding the use of cements containing more than 2 mg/kg of water-soluble Cr(VI) of the total weight of the cement. However, an excess of this limit can be affronted with measures already existing in the industrial practice with the use of a reducing agent such as ferrous (II) sulphate [27].
Conclusions
Ferronickel slag (FNS), a non hazardous waste obtained from reductive smelting of laterite ore in electric arc furnace, is a glassy material mainly consisting of amorphous silicate matrix, spinel phases, and partly of iron magnesium silicate phases of the olivine group. Owing to its properties, FNS was used as an additive up to 20 wt% for the production of blended cements. The slag containing cements exhibited lower water demand and longer setting times than the reference specimen produced with cement only. Furthermore, slag addition improved mortar workability.
FNS containing blended cements developed strengths at a lower rate compared to the reference specimen. The addition of FNS appeared to delay the hydration rate of the produced mixtures at early ages. At longer ages the pozzolanic reaction of the slag appeared to contribute to strength development. The amorphous silicate matrix was dissolved in the alkali environment of the hydrated cement and then reacted with Portlandite to form secondary C–S–H gel. This led to a modified microstructure of the final hydrated cement matrix. Higher slag replacement resulted in denser structure accompanied by reduction in pore size. It must be noted that all specimens satisfied the requirements for strength class 42.5 as per EN 197-1.
Leachability tests conducted with two standard methodologies (TCLP and NEN 7375) indicated that the concentrations of leachable heavy metals were substantially below the corresponding regulatory thresholds. As a result it can be concluded that ferronickel slag—a readily available byproduct—could be used as a cement constituent, thus contributing to the reduction or elimination of the disposal cost, and to the decrease of cement production operating cost, mainly due to clinker substitution in the final cement product.
References
Dourdounis, E., Stivanakis, V., Angelopoulos, G.N., Chaniotakis, E., Frogoudakis, E., Papanastasiou, D., Papamantellos, D.C.: High-alumina cement production from FeNi-ERF slag, limestone and diasporic bauxite. Cem. Concr. Res. 34(6), 941–947 (2004)
Diamantopoulos, A.S., Angelopoulos, G.N., Papamantellos, D.C.: Investigation of the sedimentation of FeNi fine grains entrained in ERF slag. Steel Res. 67, 179–187 (1996)
Agatzini-Leonardou, S., Zafiratos, I.G., Spathis, D.: Beneficiation of a Greek serpentinic nickeliferous ore: part I. Miner. Process. Hydrometall. 74(3–4), 259–265 (2004)
Kim, K.D., Huh, W.W., Min, D.J.: Effect of FeO and CaO on the sulfide capacity of the ferronickel smelting slag. Metall. Mater. Trans. B 45(3), 889–896 (2014)
Balomenos, E., Panias, D.: Iron recovery and production of high added value products from the metallurgical by-products of primary aluminum and ferro-nickel industries. In: Proceedings of the 3rd International Slag Valorisation Symposium, Leuven, Belgium, pp. 161–172 (2013)
European Waste Catalogue and Hazardous Waste List, Environmental Protection Agency, Ireland, Valid from 1 January 2002
Kirillidi, Y., Frogoudakis, E., Electricarc furnace slag utilization. In: Proceedings of the 9th International Conference on Environmental Science and Technology, Rhodes, Greece, pp. 768–772 (2005)
Maragkos, I., Giannopoulou, I.P., Panias, D.: Synthesis of ferronickel slag-based geopolymers. Miner. Eng. 22(2), 196–203 (2009)
Zaharaki, D., Komnitsas, K.: Long term behaviour of ferronickel slag inorganic polymers in various environments. Fresenius Environ. Bull. 21(8C), 2436–2440 (2012)
Komnitsas, K., Zaharaki, D., Perdikatsis, V.: Effect of synthesis parameters on the compressive strength of low-calcium ferronickel slag inorganic polymers. J. Hazard. Mater. 161(2–3), 760–768 (2009)
Barnett, S.J., Soutsos, M.N., Millard, S.G., Bungey, J.H.: Strength development of mortars containing ground granulated blast-furnace slag: effect of curing temperature and determination of apparent activation energies. Cem. Concr. Res. 36(3), 434–440 (2006)
Mehta, P.K., Monteiro, P.J.M.: Concrete microstructure, properties, and materials, 3rd edn. McGraw-Hill, New York (2006)
Bohac, M., Palou, M., Novotny, R., Masilko, J., Vsiansky, D., Stanek, T.: Investigation on early hydration of ternary Portland cement-blast-furnace slag-metakaolin blends. Constr. Build. Mater. 64, 333–341 (2014)
Hellenic National Standard 244/80, Cement regulation for concrete construction works, Article 8, Greece (1980)
Us, E.P.A.: Toxicity characteristics leaching procedure (TCLP), method 1311. U.S. Environmental Protection Agency, USA (1992)
NEN 7375, Leaching characteristics determination of the leaching of inorganic components from moulded or monolithic materials with the diffusion test-solid earthy and stony materials, Netherlands Normalisation Institute Standard, Delft (2004)
British Standards Institution. EN 196-2: methods of testing cement. part 2: chemical analysis of cement (2005)
Aggelakopoulou, E., Bakolas, A., Moropoulou, A.: Properties of lime–metakolin mortars for the restoration of historic masonries. Appl. Clay Sci. 53(1), 15–19 (2011)
American Society for Testing and Materials-ASTM. ASTM C204: Standard test method for fineness of hydraulic cement by air permeability apparatus. ASTM International; 2000. Document Number: ASTM C204-00
British Standards Institution. EN 196-3: Methods of testing cement: part 3: determination of setting time and soundness (2005)
American Society for Testing and Materials-ASTM. ASTM C1437. Standard test method for flow of hydraulic cement mortar, ASTM International. Document number: ASTM C1437-01 (1999)
British Standards Institution. EN 196-1: methods of testing cement: part 1: determination of compressive strength. (2005)
British Standards Institution. EN 196-10. Methods of testing cement: part 10: determination of the water-soluble chromium(VI) content of cement (2006)
European Commission, Council Directive 98/83/EC on the quality of water intended for human consumption, The Council of the European Union, Official Journal of the European Community, L 330/33, Brussels (1998)
Environment Agency, Criteria for waste acceptable at landfills for hazardous waste, The Landfill (England and Wales) (Amendment), Regulations, UK (2005)
European Commission, Council Directive 2003/53/EC, 26th amendment of the marketing and use of certain dangerous substances and preparations (nonylphenol, nonylphenol ethoxylate and cement), The Council of the European Union, Official Journal of the European Community, OJ L 178/24, Brussels (2003)
Vangelatos, I., Angelopoulos, G.N., Boufounos, D.: Utilization of ferroalumina as raw material in the production of ordinary Portland cement. J. Hazard. Mater. 168(1), 473–478 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katsiotis, N.S., Tsakiridis, P.E., Velissariou, D. et al. Utilization of Ferronickel Slag as Additive in Portland Cement: A Hydration Leaching Study. Waste Biomass Valor 6, 177–189 (2015). https://doi.org/10.1007/s12649-015-9346-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-015-9346-7