Abstract
Drought affects the normal growth and development of soybeans. Melatonin reportedly alleviates drought stress-induced growth inhibition and plant injury, thus, its foliar application presumably has considerable potential in agriculture. However, few studies have investigated the mechanism responsible for its effects on soybean nitrogen metabolism. In this study, pot culture and plant physiological detection, qPCR, and other methods were used for analysis. The purpose of this study was to explore the effects of melatonin and melanin on glutathione metabolism. The results showed that drought stress led to an increase in soluble protein and proline content, concomitantly with a decrease in the activity of nitrogen metabolism-related key enzymes, an increase in inorganic nitrogen content, and a reduction in nitrogen accumulation and transport. Exogenous melatonin application under drought stress significantly increased the expression of key genes involved in nitrogen metabolism and the activity of key enzymes including, GOGAT, NR, Gs and GDH. Enhanced enzyme activity promotes the conversion of nitrate nitrogen in plants, increases proline, soluble protein, and ureide contents, and, consequently, nitrogen accumulation. Altogether, these changes were conducive to greater nitrogen assimilation and transport. Therefore, under drought stress, melatonin application upregulated key genes involved in nitrogen metabolism, thereby enhancing the activity of related enzymes and restoring growth, stable biomass production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soybean (Glycine max L.) is an important global food and oil crop whose growth and yield can be severely inhibited by drought. Further, according to long-term projections of global rainfall and temperature changes, the frequency and intensity of drought events and the resulting dry regions are expected to increase annually (Harrison et al. 2014; Lobell et al. 2014). Grain filling is the most important period for the formation of soybean yield and quality. Additionally, it is the most vigorous and complex stage of nitrogen (N) metabolism. Drought stress limits the growth of soybeans during the filling period, affects N assimilation at the drumming stage, and alters normal N accumulation, distribution, and transport (Zou et al. 2019). Nitrogen metabolism involves almost all physiological processes in plants, thus playing a vital role in crop resistance to drought (Breda et al. 2019).
Nitrogen is an essential nutrient for plant growth. Furthermore, soil mineral N and nodular N fixation are equally important for yield formation (Robertson and Vitousek 2009). Inorganic nitrides, including NH4+ and NO3−, are the main sources of N for crop plant growth and development (Di Martino et al. 2019). As crops require less energy to absorb NH4+, they usually prefer to use it as an N source; however, high concentrations of NH4+ can inhibit the growth of substances and cause N metabolism disorders (Eva et al. 2011).
Drought stress significantly reduces soil water potential, thereby inhibiting N transport to the roots, retarding root growth, and significantly reducing root absorption surface area (Cen et al. 2020). Metabolic disorders due to a plant water deficit inevitably affect the synthesis, decomposition, and metabolism of nitrogenous compounds in the plant body (Zhong et al. 2017). Drought accelerates the death of roots, thereby inhibiting N uptake (Zhong et al. 2018). Drought stress has been shown to reduce N use efficiency of wheat and rice plants, and to significantly reduce N accumulation in wheat and rice grains (Liu et al. 2017; Cao et al. 2018). Specifically, in soybeans, drought not only affects root absorption of inorganic N but more importantly, it also inhibits N fixation by root nodules (Das et al. 2017).
GOGAT, NR, Gs, GDH are key enzymes involved in N metabolism in crop plants, and their activities fully reflect the level of crop N assimilation. In addition, they play an important role in helping crops respond to drought stress (Zhazira et al. 2020). Indeed, crop plants that experience drought stress during growth show inhibition of root NO3– uptake and ineffective transport of NO3– to the leaves, which in turn can cause a significant reduction in NR activity, eventually reducing NO3– uptake, and hindering NH4+ assimilation. Overall, this can result in an insufficient supply of GS substrate and, consequently, a reduction in GS activity (Lacuesta et al. 2006). Consistently, a previous study found that drought stress at the booting stage in rice reduced leaf GS activity (Zhao et al. 2017a, b). Similarly, PEG-simulated drought treatment showed that drought stress reduced NR and GS activities in soybean leaves, while GOGAT activity showed an initial upward trend followed by a decline (Xu et al. 2016).
Melatonin has been detected in the pineal gland of cattle, and its structure has been identified (Lerner et al. 1958). Preliminary research suggests that melatonin can regulate circadian rhythms in animals, improve sleep, and boost immunity in animals(Galano et al. 2011; Carmen et al. 2012; Calvo et al. 2013). In addition, the study also found that melatonin can alleviate important regulators of the plant stress response (Arnao and Hernández 2014; Zhang et al. 2015). Recently, melatonin has been effectively used to improve plant stress resistance by enhancing antioxidant enzyme activity and the regulatory capacity of the ASA-GSH system to control the level of H2O2 in plant tissues through the elimination of the stress-induced excess of reactive oxygen species (Wang et al. 2013). Consistently, the application of exogenous melatonin enhanced tomato drought tolerance and significantly increased net photosynthetic rate, stomatal conductance, transpiration rate, and PSII electron transfer rate (Liu et al. 2015). In addition, melatonin treatment promoted the increase of osmotic adjustment substances in soybean leaves under drought stress, and also had an osmoprotective effect on alfalfa (Cao et al. 2019; Antoniou et al. 2017). In plants growing under water deficit conditions, melatonin can regulate ABA synthesis, thereby changing ABA content and affecting kernel development (Fu et al. 2017; Zhao et al. 2017a, b). However, although numerous studies have confirmed that melatonin improves the growth and development of soybean under drought stress, most studies have focused on improving the ability of the crop to resist oxidative stress and improve photosynthetic capacity. Meanwhile, the mechanism whereby melatonin promotes soybean development and yield under drought stress as it might relate to the regulation of N metabolism remains unknown (Lerner et al. 1958).
Therefore, in this study, we simulated drought during grain filling in the soybean cultivar Suinong 26 and assessed the effects of melatonin application on plant N metabolism. Our work clearly showed that melatonin regulated N assimilation, distribution, and transport in soybean under drought stress, thereby maintaining C and N balance, and ultimately achieving the stability of yield and quality. These results provide a sound theoretical basis for breeding drought-resistant soybeans in the future.
Materials and methods
Experimental design
The experimental site is located at Bayi Agricultural University in Heilongjiang, China. Drought susceptible soybean variety Suinong 26 was used in experiments conducted in 2018 and 2019. The number of days from emergence to maturity for Suinong 26 is approximately 120, and the active accumulated temperature ≥ 10 °C is approximately 2400 °C. Seeds were sterilized three times with 5% sodium hypochlorite (w/v), washed with distilled water, and germinated in a germination box at 22 °C for 1 d in the dark. Three whole seedlings (~ 1 cm in length) were selected and transplanted onto experimental pots prepared as follows. Three 1-cm diameter holes were drilled in the bottom of white plastic pots (0.33 m high and 0.3 m in diameter), and a layer of gauze was placed to cover the holes. Thereafter, each pot was filled with 16 kg of culture soil prepared by combining perlite, vermiculite, and hematite in a 1:3:12 (V/V/V) ratio. Water content of the growth media was kept at 80% of the field capacity until the grain filling (R5) stage. Plants were divided into four groups and treated as follows. In the normal water supply (WW) treatment, the soil moisture content was maintained at 80% of the field moisture content throughout the experimental period. According to our previous results, such water content is the best growth environment for soybeans (Zou et al. 2021). In turn, in pots of the drought stress (D) group, watering was withheld, and the pots were weighed at 18:00 every day to monitor water content until it reached 50% of field capacity, which was observed on the 10th day from the initiation of the experiment. Thereafter, water was supplied to maintain soil water content at that level. Then, on the 28th day, soil water content was restored to 80% of field water capacity until harvest. Lastly, in the pots marked for foliar application of melatonin under normal water supply (WW + M) and in those marked for foliar application of melatonin under drought stress (D + M), plants were subjected to the corresponding water supply treatments, as described for the WW and D groups, respectively, and melatonin was applied (sprayed) at a concentration of 100 μmol⋅L−1 at 21:00 on the 11th, 12th, and 13th night of the experiment.
Plant samples were collected at 18, 23, and 28 days after the onset of water treatments.
Measurement of nitrogen metabolism
The concentration of proline was determined by the method of Bates et al. (1973),
Soluble protein concentration was estimated spectrophotometrically according to the method described by Smith et al. (1985). Briefly, freshly harvested leaf samples (1.0 g FW) were homogenized in 0.1 M phosphate buffer (pH 6.75). The homogenates were centrifuged at 15,000 × g for 15 min. Supernatant samples (5 μL) were transferred to tubes and mixed with 1.5 mL BCA reagent (bicinchoninic acid + FeCl3), incubated in boiling water for 5 min, and then cooled to room temperature. The absorbance at 562 nm was measured using a spectrophotometer (Jenway 6850 UV–Vis, Cole-Parmer Ltd., UK), and the concentration of soluble protein was expressed as mg g−1.
Nitrate reductase (NR) activity was determined using the method described by Jaworski (1971), in turn, GS and GOGAT activities were determined as described by González et al. (1998). Finally, GDH activity was determined following the method described by Miflin and Habash (2002). Ammonium (NH4+) and nitrate N (NO3−) contents were determined following the method described by Oliveira et al. (2013).
The ureide content was determined according to the method of Xu and Liu (1986).
Photosynthetic index determination
At 11:00 on the day of sampling, select the top 2 leaves of the plant (the second compound leaf from the top to the bottom, the new and fully functional leaves). The net photosynthetic rate (Pn), leaf stomatal conductance (Gs), transpiration rate (Tr), and intercellular CO2 concentration (Ci) were measured using a (Li-6400; LI-COR Inc., USA) photosynthesis instrument. Between 9:00 AM and 11:00 AM, use the LI-6400 Portable Photosynthesis System (LI-COR Inc., USA). Use a leaf chamber equipped with a red/blue LED light source. All measurements were performed at a constant flow rate of 500 mL min−1 and a CO2 concentration of approximately 400 μmol mol−1 at a PAR of 1000 μmol m−2 s−1.
Total RNA extraction and gene expression analysis
The total RNA of leave samples was purified from five days post-treated plants. Axygen Plant RNA Extraction Kit (Axy Prep) was used to extract total RNA while the RNA quantity and integrity were assessed by NanoDrop 2000. Prime Script RT Enzyme Mix I with TB Green q PCR (Takara) was used to reverse-transcribe cDNA while the reversed transcription products were diluted tenfold, and then used for qRT-PCR in a 10 µL reaction volume via TB Green® Premix Ex Taq™ II (Takara), which the reaction solution had shown in The was made by mixing 5 µL TB Green Premix Ex Taq II, 3 µL distilled water, 0.5 µL PCR forward primer, 0.5 µL PCR reverse primer, and 1 µL diluted cDNA.
QRT-PCR analysis was performed by CFX-96 (Bio-rad), in which the reaction solution was held at 95 °C for 30 s, then performed for 40 cycles with the following cycle profile: 5 s denaturation step at 95 °C, 5 s annealing step at 60 °C. Primers were designed by Premier 5.0 and listed in Table 1 while the Gm-Actin was used as a reference gene. Relative gene expression levels between different treatments were calculated using the operational formula 2−△△Ct , with biological replicates and technical replicates.
Statistical analysis
Calculations were performed using SPSS (21.0) software, and all data were subjected to analysis of variance (ANOVA) and Duncan multiple range test and calculate the Pearson coefficient. Figures were drawn using the OriginPro 9.1 Software.
Results
Effects of exogenous melatonin on proline and soluble protein concentration in soybean leaves under drought stress
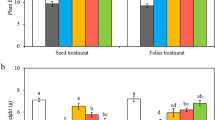
Drought stress increased proline and soluble protein concentrations (Fig. 1). Compared with well-watered plants, proline content increased by 30.30%, 37.88%, and 53.13% in drought-stressed plants, and by 14.99%, 18.78%, and 16.44% in drought-stressed plants pretreated with melatonin after 5, 10, and 15 d of drought stress, respectively (Fig. 1A). Similarly, compared with well-watered plants, soluble protein content increased by 14.64%, 65.82%, and 34.33% in drought-stressed plants and by 13.93%, 31.54%, and 16.44% in drought-stressed plants pretreated with melatonin after 5, 10, and 15 d of drought stress, respectively (Fig. 1B). These results suggest that the application of melatonin effectively contributed to maintaining a higher water potential and cell turgor in drought-stressed soybean leaves.
Effect of melatonin on proline (A) and soluble protein (B) concentrations in soybean leaves under well-watered and drought stress conditions. Data are means ± SE (n = 4). Different letters above horizontal lines indicate significant differences (P < 0.05) among treatments. WW, well-watered conditions; WW + M, well-watered conditions + melatonin; D, drought stress; D + M, drought stress + melatonin
Effects of exogenous melatonin on key enzyme activities of nitrogen metabolism in soybean leaves under drought stress
Drought stress significantly reduced NR, GS, GOGAT, and GDH activities in soybean leaves. NR, GS, and GOGAT activities gradually decreased, while GDH activity initially increased and then decreased with increasing drought duration. Throughout the entire sampling period, NR activity decreased by 13.20%, 17.19%, and 29.57% (Fig. 2A); GS decreased by 15.86%, 13.71%, and 24.65% (Fig. 2B); GOGAT decreased by 12.29%, 21.30%, and 23.21% (Fig. 2C), and GDH decreased by 23.20%, 16.97%, and 20.67% (Fig. 2D), at 5, 10, and 15 days under D treatment, respectively, compared with the WW treatment. As per ANOVA, compared with D, D + M treatment increased NR activity by 8.98%, 11.01%, and 15.59% (Fig. 2A); GS activity by 10.66%, 15.89%, and 18.69% (Fig. 2B); GOGAT activity by 3.90%, 10.21%, and 12.78% (Fig. 2C); and GDH activity by 12.78%, 8.95%, and 14.68% (Fig. 2D) at 5, 10, and 15 days after treatment, respectively. Further, compared to D + M, D and control treatment groups differed significantly. After 10 and 15 d of normal water supply, GDH activity in soybean leaves significantly increased with melatonin pretreatment, while GDH activity increased by 1.54% and 6.48% after 10 and 15 d of the WW + M treatment, respectively, compared to the WW treatment (Fig. 2D).
Effect of melatonin on NR (A), GS (B), GOGAT (C), and GDH (D) activity in soybean leaves under well-watered and drought stress conditions. Data are means ± SE (n = 4). Different letters above horizontal lines indicate significant differences (P < 0.05) among treatments. WW, well-watered conditions; WW + M, well-watered conditions + melatonin; D, drought stress; D + M, drought stress + melatonin
Effects of exogenous melatonin on ammonium nitrogen content of soybean under drought stress
Throughout the growth period, at 5, 10, and 15 days after D treatment initiation, NH4+ content in soybean organs decreased gradually under WW conditions, compared to which, drought stress resulted in a marked increase in NH4+ content in soybean organs, which increased gradually with the duration of the drought stress period from 5 to 10, and to 15 d. During the grain filling period, NH4+ content in the leaf, stem, root, pod wall, and grain increased by 13.35–49.65%, 11.07–39.42%, 10.18–45.17%, 16.08–73.75%, and 20.93–52.21%, respectively. At grain filling, leaf and stem NH4+ contents increased by 13.34%, 24.42%, and 40.39%, and by 11.07%, 12.10%, and 39.42%, respectively. Meanwhile, NH4+ increased by 10.18%, 26.59%, and 44.64% in the roots, and by 16.29%, 44.83%, and 73.75% in the pod skins, respectively, while in the grains, it increased by 20.93% ~ 52.21%. Further, application of exogenous melatonin under drought stress increased NH4+ content in soybean organs; however, compared with D, D + M treatment resulted in a decrease in NH4+, i.e., 2.09%, 6.66%, and 9.23% in the leaves; 7.54%, 7.86%, and 16.61% in the stems; 34.64%, 24.09%, and 22.06% in the roots; 10.87%, 15.85%, and 10.43% in the pods; and 14.22%, 16.87%, and 14.64% in the seeds, at 5, 10, and 15 d after treatment initiation, respectively. Soybean roots showed the greatest decrease in NH4+ content after exogenous application of melatonin under drought stress conditions, followed by the grains. ANOVA indicated that NH4+ content in soybean leaves on the fifth day of the grain filling stage was significantly different from that in the leaves of the D and D + M treatment groups (Table 2).
Effects of exogenous melatonin on the nitrate nitrogen content of soybean under drought stress
Nitrate–N content in all soybean organs analyzed gradually decreased with growth under conditions of unlimited water supply, whereas it increased significantly under drought stress, and gradually increased with drought stress duration. Compared with the WW treatment group, NO3− content in soybean leaves, stems, roots, and seeds increased by 19.73–38.32%, 12.90–24.48%, 22.08–41.64%, and 12.82–30.30%, respectively, and by 11.08%, 37.51%, and 34.23% in the pod walls, at 5, 10, and 15 d after D treatment, respectively. Meanwhile, the exogenous application of melatonin under drought stress alleviated the increase in NO3− content in soybean organs. Thus, compared with D, D + M treatment reduced NO3− content by 11.19%, 16.42%, and 19.07% in the leaves; by 8.32%, 10.64%, and 17.15% in the stems; by 11.03%, 16.41%, and 12.24% in the roots; by 7.83%, 15.43%, and 10.07% in the pod walls; and by 7.91%, 10.95%, and 15.82% in the seeds, at 5, 10, and 15 d after treatment, respectively. Except for the stem on the fifth day of the grain filling period, NO3- contents in the other organs under study were significantly different from those in the D + M treatment group. Compared with the WW treatment group, NO3− content in the grains in the WW + M treatment group decreased significantly at 15 d after treatment initiation (Table 3).
Effects of exogenous melatonin on ureide content of soybean under drought stress
Compared with the WW treatment, drought stress significantly reduced the ureide content in all soybean organs under study. Thus, ureide content decreased by 38.99%, 46.95%, and 49.44% in the leaves; by 27.55%, 20.89%, and 26.98% in the stems; by 25.64%, 19.27%, and 33.90% in the roots; by 15.70%, 26.45%, and 39.87% in the pod walls; and by 25.26%, 10.83%, and 14.89% in the grains at 5, 10, and 15 d after D treatment, respectively. Conversely, the exogenous application of melatonin increased ureide content in different soybean organs under drought stress. Further, compared with D, D + M treatment increased the ureide content by 23.29%, 19.79%, and 25.90% in the leaves; by 17.75%, 12.96%, and 22.87% in the stem; by 6.00%, 13.97%, and 11.00% in the roots; and by 5.84%, 22.11%, and 20.13% in the pod walls after 5, 10, and 15 d of treatment, respectively. Meanwhile, the ureide content in the grain increased by 3.10%, 4.43%, and 14.08% after 5, 10, and 15 days of treatment, respectively (Table 4).
Effects of exogenous melatonin on nitrogen accumulation in soybean under drought stress
The accumulation of N in the leaves, stems, and roots first increased and then decreased under drought stress. Compared with the WW treatment, N accumulation in leaves, stems, and roots increased by 10.71%, 10.22%, and 2.07%, respectively, at 5 d after D treatment, with significant differences observed among values recorded for the stem. In contrast, compared with WW treatment, N accumulation in the leaves, stems, and roots decreased by 16.16–24.43%, 22.76–46.08%, and 32.24–38.68%, respectively, between the 10th and the 15th day after D treatment initiation. Drought stress reduced N accumulation in soybean pods, grains, and whole plants. Specifically, compared to the WW treatment, N accumulation in the pods decreased by 15.44%, 32.74%, and 25.65% at 5, 10, and 15 d after D treatment initiation, respectively. In turn, N accumulation in the grains decreased by 11.59%, 22.89%, and 25.06%, and by 1.91%, 24.14%, and 27.10% in the whole plant at 5, 10, and 15 days after D treatment initiation, respectively. Except for whole-plant N accumulation on day 5, differences between WW and D treatment groups were significant. Finally, N accumulation in soybean leaves, stems, roots, pods, seeds, and whole plants increased by 0.97–12.56%, 0.71–38.94%, 8.84–26.17%, 14.34–25.79%, 6.57–16.94%, and 2.61–17.10%, respectively, at 5, 10, and 15 d after D + M treatment initiation. Further, differences between the D and D + M treatment groups were significant, except for N accumulation in the leaves, stems, whole plants, and 10-day stems at 5 d after treatment initiation. These findings indicate that melatonin showed a larger promoting effect during the middle and late stages of the drought stress period. Compared with WW treatment, whole-plant N accumulation increased significantly 15 d after WW + M treatment initiation (Table 5).
Effects of exogenous melatonin on soybean photosynthesis under drought stress
It can be seen from Table 6 that drought stress inhibited the photosynthesis of soybean. Compared with the WW treatment, the Pn, Ci, Gs, and Tr were significantly reduced by 13.85%, 15.54%, 10.48%, and 29.86% at 5 d after the D treatment. (P < 0.05);On the 10th day, Pn, Ci, Gs, Tr decreased by 12.87%, 20.39%, 17.95%, 34.41% (P < 0.05); on the 15th day, Pn, Ci, Gs, Tr decreased by 19.56%, 22.54%, 13.68%, 32.33% (P < 0.05). Compared with the treatment D, the Pn, Ci, Gs, and Tr of the D + M treatment were significantly increased on the 5th day after the drought, and the increases were 9.57%, 6.79%, 6.58%, 17.00% (P < 0.05);On the 10th day, Pn, Ci, Gs, Tr increased by 10.28%, 4.79%, 11.89%, 21.18% (P < 0.05);On the 15th day, Pn, Ci, Gs, Tr increased by 16.25%, 13.52%, 9.44%, 22.81% (P < 0.05);
Relative expression levels of nitrogen metabolism genes
The transcriptional regulation of LOC100813471, LOC100818103, LOC100818642, INR2, LOC100775519, NIR, LOC100782969, LOC100787888, LOC100803196, LOC100807710, NRT2, LOC100809032, LOC100788009 were studied via qRT-PCR analysis to assess the nitrogen metabolism progression of melatonin treated soybean under drought stress. Compared with the control, drought stress significantly down-regulated the expression of LOC100818103, LOC100787888, LOC100803196, LOC100807710, NRT2, LOC100775519, NIR, LOC100788009. Moreover, the expression level of these genes in D + M treatment was higher than that in WW. Melatonin up-regulated the expression of LOC100813471, LOC100818642, INR2, LOC100782969, NRT2 and LOC100809032 (Fig. 3).
Effect of melatonin on nitrogen metabolism related genes and pathways (A), Assimilatory nitrate reduction (B), Nitrogen metabolism (C), Relative expression levels of nitrogen metabolism regulation genes in soybean treated with melatonin under rought stress. Gene expression levels of WW under normal conditions were set to 1 as the normalization for qRT-PCR analysis using the operational formula 2−△△Ct. Data are expressed as means ± SD of three independent experiments (each with three technical repeats). For the same cultivar, different letters indicate statistically significant differences (P < 0.05) among treatments
Discussion
The accumulation of proline and soluble proteins under drought stress reportedly contributes to the maintenance of high water potential and osmotic pressure in the cells, thereby, effectively protecting the stability of the cell membrane and improving crop drought resistance (Jinyou et al. 2004; Małgorzata et al. 2017). In this study, we found that drought stress led to a considerable increase in proline and soluble protein contents in soybean plants, and that exogenous application of melatonin during drought stress further promoted the increase in proline and soluble protein contents. These results suggest that melatonin helped to maintain normal cell osmotic potential, which in turn reduced water loss and protected the normal functioning of all cellular organelles.
The activity of key enzymes involved in N metabolism in plants reflects N assimilation, protein synthesis, and the overall level of N metabolism. Therefore, the reactions catalyzed by these key enzymes play a crucial role in crop plant growth and development (Sil et al. 2020; Gangwar and Singh 2011). In this study, soybean leaf NR, GS, GOGAT, and GDH activities significantly decreased under drought stress, likely because hydrolase activity was enhanced, and protein synthesis was reduced by drought. Therefore, the reduction of NR activity and the significant reduction of GS, GOGAT, and GDH activities resulted from a chain reaction of substrate deficiency. Under adverse conditions, plant growth regulators have been shown to enhance leaf N metabolism and the activity of key enzymes involved in N metabolism, while reducing the rate of leaf aging by regulating N metabolism to improve soybean stress resistance (Zhang et al. 2004). In this study, NR, GS, GOGAT, and GDH activities significantly increased when exogenous melatonin was applied to soybeans plants under drought stress. These findings are consistent with those of previous studies, indicating that exogenous melatonin can promote the maintenance of a relatively stable level of N assimilation by upregulating key enzyme activities, and effectively reducing the adverse effects of drought stress on these key enzyme activities involved in N metabolism. Presumably, exogenous melatonin can increase the activity of key enzymes of N metabolism to a certain extent, and reduce NO3− to NH4+, thereby increasing the rate of the GS/ GOGAT cycle and improving the overall level of soybean N metabolism.
Inorganic N forms, including NH4+ and NO3−, are the main N source for crop plants. As crops require less energy to absorb NH4+, they usually prefer to use it as an N source; however, high concentrations of NH4+ can inhibit the growth of substances and cause N metabolism disorders (Eva et al. 2011). A previous study demonstrated that drought stress increased NH4+ content and decreased NO3− content in plants (Jinghong et al. 2012). In this study, drought stress caused a significant increase in both NH4+ and NO3− N contents in various soybean organs. Further, exogenous application of melatonin can reduce NH4+ and NO3− N in plants; therefore, exogenous melatonin can accelerate N assimilation and increase N metabolism. Differences in inorganic N absorption capacity by crop plants growing under drought stress may be the result of differences in cultivation conditions. Thus, for example, the absorption of inorganic N may be seriously affected under certain experimental conditions, causing a reduction in plant inorganic-N accumulation. In this study, drought stress increased inorganic N content hindered metabolism, and reduced N assimilation ability; however, exogenous application of melatonin restored normal N metabolism.
Previous studies showed that ureide is an important N metabolite in symbiotic N fixation by Rhizobium and is the main form of N storage and transport. Ureide content has been shown to significantly and positively correlate with soybean nitrogenase activity (Vadez 2000; Purcell et al. 2004). This study showed that ureide content in soybean organs was significantly reduced under drought stress, indicating that drought inhibited N storage and transport. Conversely, exogenous application of melatonin under drought stress promoted an increase in ureide content in various soybean organs, presumably because melatonin alleviated the inhibitory effect of drought on symbiotic N fixation by nodules and helped to improve the activity of N fixation enzymes in Rhizobium nodules, which in turn likely promoted soybean growth during grain filling.
Photosynthesis occurs in the structure of plant chloroplasts and participates in plant material metabolism and energy conversion (Xu et al. 2013). A study (Bohnert and Jensen 1996) found that drought stress can accelerate leaf senescence and gradually decrease photosynthetic capacity. Past research (Su et al. 2019; Xu et al. 2010) revealed that exogenous melatonin could effectively alleviate the adverse effects of water stress on the chlorophyll content of cucumber and corn, and improved the photosynthetic rate, thereby increasing light absorption and transformation efficiency of plants. According to this study, when soybean leaves are stressed by drought, they conserve water by closing their stomata. The stomatal conductance and transpiration rate of leaves decrease, and the stomata closely cause insufficient CO2 absorption. Under this compound factor, the phenomenon of inhibiting photosynthesis occurs. A study (Cao et al. 2019) revealed that long-term drought could cause damage to the photosynthetic system and leaf tissues. However, drought stress can also reduce starch accumulation in plant leaves. In this study, Drought stress reduces the photosynthetic capacity of soybean This demonstrates that prolonged drought stress, inhibits photosynthetic transport, and ultimately results in a photosynthetic system breakdown. Melatonin can maintain normal net photosynthetic rate and transpiration rate, and stabilize leaf stomatal conductance and intercellular CO2 concentration, These findings are consistent with those previously reported for corn (Wang et al. 2021) and tomato (Liu et al. 2015). This demonstrates that melatonin treatment alleviated drought stress-induced photosynthesis inhibition, and was beneficial to the normal growth of soybeans in the later period. At the same time, the Pearson coefficient results showed that under drought stress, nitrogen metabolism-related enzymes were negatively correlated with both photosynthesis and Pro (Supplementary Table 3), and photosynthetic indexes were positively correlated with nitrate nitrogen after melatonin treatment. This indicated that the enhancement of plant photosynthetic capacity also increased the accumulation of nitrate nitrogen in soybean. (Supplementary Table 4).
Crop N accumulation is closely related to dry matter quality, which determines the final yield (Xu et al. 2005). The results of this study indicated that under drought stress, N accumulation in leaves, stems, and roots first increased and then decreased, whereas, in the pods, grains, and the whole plant, N accumulation decreased significantly. Exogenous melatonin was able to reduce the adverse effects of drought stress by significantly increasing N accumulation in soybean organs. Therefore, we showed that exogenous melatonin can promote N accumulation in soybean plants under normal water supply and under drought stress conditions, and can improve the N utilization rate of soybean plants. Finally, our results showed that melatonin significantly increased the expression of NR (loc100813471, loc100818103, loc100818642, inr2), NIRA (loc100775519, NIR), NRT (loc100782969, loc100787888, loc100803196, loc100807710, nrt2) and gdhA (loc100809032, loc100788009). Therefore, overall, the transformation efficiency of NO3−-N and total N content were effectively improved.
Conclusions
Drought stress led to an increase in soluble protein and proline contents, together with a decrease in the activities of key enzymes in N metabolism (NR, GS, GOGAT, GDH), and an increase in inorganic N content. Furthermore, drought hindered N accumulation and transport and Inhibit photosynthetic capacity. In contrast, exogenous melatonin significantly increased the activities of key enzymes of N metabolism under drought stress; further, N assimilation pathway-related genes were upregulated, and the transformation of nitrate N was increased, thereby increasing proline, soluble protein, and ureide contents, and promoting an overall increase in N accumulation in the plant and increased photosynthetic capacity. These changes were conducive to N assimilation and transport (Fig. 4).
References
Antoniou C, Chatzimichail G, Xenofontos R, Pavlou JJ, Panagiotou E, Christou A (2017) Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J Pineal Res 62:12401
Arnao MB, Hernández RJ (2014) Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci 19:789–797
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bohnert HJ, Jensen RG (1996) Strategies for engineering water-stress tolerance in plants. Trends Biotechnol 14:89–97. https://doi.org/10.1016/0167-7799(96)80929-2
Breda FADF, Silv TFRD, Santos SGD, Alves GC, Reis VM (2019) Modulation of nitrogen metabolism of maize plants inoculated with Azospirillum brasilense and Herbaspirillum seropedicae. Arch Microbiol 201:547–558
Calvo JR, González YC, Maldonado MD (2013) The role of melatonin in the cells of the innate immunity: a review. J Pineal Res 55:103–120
Carmen V, José AG, Germaine E, Francisco O, López A, Carolina D, Laura GC, Luis C, Russel JR, Darío A (2012) Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res 52:217–227
Cao X, Zhu C, Zhong C, Hussain S, Zhu L, Wu L, Jin Q (2018) Mixed-nitrogen nutrition-mediated enhancement of drought tolerance of rice seedlings associated with photosynthesis, hormone balance and carbohydrate partitioning. Plant Growth Regul 84:451–465
Cao L, Jin XJ, Zhang YX (2019) Melatonin confers drought stress tolerance in soybean (Glycine max L.) by modulating photosynthesis, osmolytes, and reactive oxygen metabolism. Photosynthetica 57:812–829
Cen HF, Wang TT, Liu HY, Tian DY, Zhang YW (2020) Melatonin application improves salt tolerance of Alfalfa (Medicago sativa L.) by enhancing antioxidant capacity. Plants 9:220
Das A, Rushton PJ, Rohila JS (2017) Metabolomic profiling of soybeans (Glycine max L.) reveals the importance of sugar and nitrogen metabolism under drought and heat stress. Plants 6:21
Di Martino C, Fioretto A, Palmieri D, Torino V, Palumbo G (2019) Influence of tomato plant mycorrhization on nitrogen metabolism, growth and fructification on P-limited soil. J Plant Growth Regul 38:1183–1195
Eva SR, María MRW, Juan JR, Blasco B, Miguel R, Rubén M (2011) Ammonia production and assimilation: Its importance as a tolerance mechanism during moderate water deficit in tomato plants. J Plant Physiol 168:816–823
Fu J, Wu Y, Miao Y, Xu Y, Zhao E, Wang J (2017) Improved cold tolerance in Elymus nutans by exogenous application of melatonin may involve ABA-dependent and ABA-independent pathways. Sci Rep 7:39865
Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res 51:1–16
Gangwar S, Singh VP (2011) Indole acetic acid differently changes growth and nitrogen metabolism in Pisum sativum L. seedlings under chromium (VI) phytotoxicity: Implication of oxidative stress. Sci Hortic 129:321–328
González EM, Aparicio-Tejo PM, Gordon AJ, Minchin FR, Royuela M, Arrese-Igor C (1998) Water-deficit effects on carbon and nitrogen metabolism of pea nodules. J Exp Bot 770:1705–1714
Harrison MT, Tardieu F, Dong Z, Messina CD, Hammer GL (2014) Characterizing drought stress and trait influence on maize yield under current and future conditions. Glob Change Biol 20:867–878
Jaworski EG (1971) Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun 43:1274–1279
Jinghong H, Yang YI, Qingmao S, Chunjuan D, Zhigang AZ (2012) Effect of exogenous salicylic acid on nitrogen assimilation of cucumber seedling under drought stress. Acta Horticulturae Sinica 39:81–90
Jinyou D, Xiaoyang C, Wei L, Qiong G (2004) Osmoregulation mechanism of drought stress and genetic engineering strategies for improving drought resistance in plants. Forestry Studies in China 006:56–62
Lacuesta M, González B, González C, Muñoz-Ruedu A (2006) Time ourse of the phosphinothricin effect on gas exchange and nitrate reduction in Medicago sativa. Physiol Plant 89:847–853
Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W (1958) Isolation of melatonin, the pineal gland factor that lightens melanocytes\r1. J Am Chem Soc 80:2587–2587
Liu J, Wang W, Wang L, Sun Y (2015) Exogenous melatonin improves seedling health index and drought tolerance in tomato. Plant Growth Regul 77:317–326
Liu S, Li X, Larsen DH, Zhu X, Song F, Liu F (2017) Drought priming at vegetative growth stage enhances nitrogen-use efficiency under post-anthesis drought and heat stress in wheat. J Agron Crop Sci 203:29–40
Lobell DB, Roberts MJ, Schlenker W, Braun N, Little BB, Rejesus RM (2014) Greater sensitivity to drought accompanies maize yield increase in the U.S. Midwest. Science 344:516–519
Małgorzata K, InformationOlga FS, Katarzyna G, Agnieszka W, Jan S (2017) Cacl2 treatment improves drought stress tolerance in barley (Hordeum vulgarel.). Acta Physiol Plant 39:41
Miflin BJ, Habash DZ (2002) The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J Exp Bot 53:979–987
Oliveira HC, Freschi L, Sodek L (2013) Nitrogen metabolism and translocation in soybean plants subjected to root oxygen deficiency. Plant Physiol Biochem 66:141–149
Purcell LC, Serraj R, Sinclair TR, De A (2004) Soybean N fixation estimates, ureide concentration, and yield responses to drought. Crop Sci 44:484–492
Robertson GP, Vitousek PM (2009) Nitrogen in agriculture: balancing the cost of an essential resource. Annu Rev Environ Resour 34:97–125
Sil P, Das P, Biswas AK (2020) Impact of exogenous silicate amendments on nitrogen metabolism in wheat seedlings subjected to arsenate stress. SILICON 12:535–545
Su X, Fan X, Shao R, Guo J, Guo L (2019) Physiological and iTRAQ-based proteomic analyses reveal that melatonin alleviates oxidative damage in maize leaves exposed to drought stress. Plant Physiol Biochem. https://doi.org/10.1016/j.plaphy.2019.07.012
Smith PK, Krohn RI, Hermanson GT (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 163:76–85
Vadez V (2000) Ureide degradation pathways in intact soybean leaves. J Exp Bot 51:1459–1465
Wang P, Sun X, Li C, Wei ZW, Liang D, Ma FW (2013) Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J Pineal Res 54:292–302
Wang YF, Guo YY, Zhao CF, Li HJ, Zhang RH (2021) Exogenous melatonin achieves drought tolerance by improving photosynthesis in maize seedlings leaves. Russ J Plant Physiol 68(4):718–727. https://doi.org/10.1134/S102144372104021X
Xu X, Liu C (1986) Determination of ureide content in legumes. Plant Physiol Commun 04:60–62
Xu ZZ, Yu ZW, Wang D, Zhang YL (2005) Nitrogen accumulation and translocation for winter wheat under different irrigation regimes. J Agron Crop Sci 191:439–449
Xu XD, Yan S, Bo S, Jian Z, Guo XQ (2010) Effects of exogenous melatonin on active oxygen metabolism of cucumber seedlings under high temperature stress. Chin J Appl Ecol 21(5):1295. https://doi.org/10.3724/SP.J.1142.2010.40521
Xu LX, Yu JJ, Han LB, Huang B (2013) Photosynthetic enzyme activities and gene expression associated with drought tolerance and post-drought recovery in Kentucky bluegrass. Environ Exp Bot 89:28–35. https://doi.org/10.1016/j.envexpbot.2012.12.001
Xu YH, Dong SK, Li XN, Gao YX, Wang LB, Liu LJ (2016) Effect of progressive drought stress on the key enzyme activities of nitrogen metabolism of spring soybean. J Nuclear Agricult Sci 30:164–170
Zhang MC, Li ZH, Tian XL, Duan LS, Wang BM, Zhai ZX, He ZP (2004) Effect of plant growth regulator SHK-6 on nitrogen metabolism of soybean leaf. Soybean Sci 01:15–20
Zhang N, Sun Q, Zhang H, Cao Y, Weeda S, Ren S (2015) Roles of melatonin in abiotic stress resistance in plants. J Exp Bot 66:647–656
Zhao H, Zhang K, Zhou X, Xi L, Wang Y, Xu H (2017a) Melatonin alleviates chilling stress in cucumber seedlings by up-regulation of CsZat12 and modulation of polyamine and abscisic acid metabolism. Sci Rep 7:4998
Zhao HW, Zhang B, Jia Y, Wang ZQ, Sun B, Gu HT (2017b) Effect of drought stress at booting stage on grain nitrogen formation and yield of rice in cold region. J Northeast Agric Univ 48:1–10
Zhazira YC, Léo B, Rouster J, Lenaïg B, Francoise G, Isabelle Q, Christophe S (2020) Bertrand H. NADH-GOGAT overexpression does not improve maize (Zea mays L.) performance even when pyramiding with NAD-IDH, GDH and GS. Plants 9:130
Zhong C, Cao XC, Bai ZG, Zhang JH, Zhu LF, Huang JL, Jin QY (2018) Nitrogen metabolism correlates with the acclimation of photosynthesis to short-term water stress in rice (Oryza sativa, L.). Plant Physiol Biochem 125:52–62
Zhong C, Cao XC, Hu JJ, Zhu LF, Huang JL, Jin QY (2017) Nitrogen metabolism in adaptation of photosynthesis to water stress in rice grown under different nitrogen levels. Front Plant Sci 8:1079
Zou JN, Jin XJ, Zhang YX (2019) Effects of melatonin on photosynthesis and soybean seed growth during grain filling under drought stress. Photosynthetica 57:512–520
Zou JN, Yu H, Yu Q (2021) Physiological and UPLC-MS/MS widely targeted metabolites mechanisms of alleviation of drought stress-induced soybean growth inhibition by melatonin. Ind Crops Prod 163:113323
Acknowledgements
Thanks to Heilongjiang Academy of Agricultural Sciences for providing seeds.
Funding
This study was funded by the Initiation Foundation for Heilongjiang Bayi Agricultural University Support Program for San Heng San Zong (ZRCQC202101), China Agriculture Research System (CARS-04-PS18), Natural Science Foundation of Heilongjiang Province of China (C2017049), Research Initiation Plan for Talent Introduction (XYB202011).
Author information
Authors and Affiliations
Contributions
Yuxian Zhang conceived and designed the study. Liang Cao and Bin Qin performed the experiments, collected the plant materials, analyzed the data. Liang Cao wrote the manuscript with contributions from all the authors. Zhenping Gong critically revised the manuscript. All of the authors read and approved the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, L., Qin, B., Gong, Z. et al. Melatonin improves nitrogen metabolism during grain filling under drought stress. Physiol Mol Biol Plants 28, 1477–1488 (2022). https://doi.org/10.1007/s12298-022-01219-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-022-01219-y