Abstract
Purpose
Epidemiological data are pivotal for the estimation of disease burden in populations.
Aim
Of the study was to estimate the incidence and prevalence of acromegaly in Italy along with the impact of comorbidities and hospitalization rates as compared to the general population.
Methods
Retrospective epidemiological study (from 2000 to 2014) and case control-study. Data were extracted from the Health Search Database (HSD). HSD contains patient records from about 1000 general practitioners (GPs) throughout Italy, covering a population of more than 1 million patients. It includes information about patient demographics and medical data including clinical diagnoses and diagnostic tests.
Results
At the end of the study period, 74 acromegaly patients (out of 1,066,871 people) were identified, resulting in a prevalence of 6.9 per 100,000 inhabitants [95% CI 5.4–8.5]. Prevalence was higher in females than men (p = 0.004), and showed a statistically significant trend of increase over time (p < 0.0001). Overall, incidence during the study period was 0.31 per 100,000 person-years. Hypertension and type II diabetes mellitus were the comorbidities more frequently associated with acromegaly (31.3 and 14.6%, respectively) and patients were more likely to undergo a high frequency of yearly hospitalization (≥3 accesses/year, p < 0.001) compared to sex-age matched controls.
Conclusions
This epidemiological study on acromegaly carried out using a large GP-based database, documented a disease prevalence of about 7 cases per 100,000 inhabitants. As expected, acromegaly was associated with a number of comorbidities (mainly hypertension and type II diabetes mellitus) and a high rate of patients’ hospitalization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acromegaly is a rare disease resulting in a complex systemic syndrome, caused by elevated circulating levels of growth hormone (GH) and insulin-like growth factor I (IGF-I), usually due to the presence of a GH-secreting pituitary adenoma (>95% cases) [1].
The first epidemiological studies of acromegaly, and more widely of pituitary adenomas, were from surgical series or cancer registries, which all represent very selected populations, thus hindering a reliable incidence or prevalence estimation in the general population [2, 3]. However, most of the studies carried out in Europe and US in the last decade [4,5,6,7,8,9,10,11,12,13] reported a 2 to 6-fold increase in acromegaly incidence and prevalence as compared to the previous ones (reviewed in ref. [3]). Moreover, results from autopsy and magnetic resonance imaging studies showed an overall estimated prevalence of pituitary adenomas around 17% in the general population, with a considerable presence of secreting tumors based on their immunohistochemical evaluation [14].
Tumor incidence and prevalence data are pivotal for the estimation of disease burden in populations and are often used to calculate health care resource distribution within and among clinical specialties [5]. Currently, there is a lack of well-designed epidemiological studies, carried out in representative samples of the Italian population, that are aimed at evaluating the actual incidence and prevalence of this disease in our country.
Noteworthy, acromegaly frequently results in a number of comorbidities, including cardiovascular diseases, metabolic complications, respiratory disorders and arthropathies, which may induce chronic disabilities and impair patients’ quality of life [1, 15,16,17,18]. In addition, a number of studies demonstrated that uncontrolled acromegaly is associated with a 1.5 to 3-fold increase in the standardized mortality ratio (SMR) compared to general population [19,20,21,22]. Data from National registries suggest that, using multimodal therapeutic strategies, about 70% of patients currently achieve disease control [23]. Particularly, a recent analysis of the French Acromegaly Registry showed that about 75% of acromegaly patients present normal age-adjusted IGF-I levels at the end of follow-up (median duration: 7 years) [23].
In this context, surgery still represents the first-line therapy in the majority of patients, despite additional medical (pharmacological) treatment is often required [16]. Chronic medical therapy for acromegaly is generally well tolerated, although a life-long treatment is expected for most patients [16, 24]. Most common adverse events related to first-generation somatostatin analogs (SSA) therapy mainly include disturbance of the gastrointestinal tract (abdominal cramps, flatulence, and diarrhea), which usually abate with continued treatment and, more rarely, an impairment of glucose control [16]. On the other hand, treatment with the GH-receptor antagonist (GHRA) pegvisomant may result in injection-site reactions, local discomfort, reversible lipohypertrophy/lipoatrophy and, in a very small percentage of patients, a significant increase of liver enzyme levels [16]. Disease control in acromegaly is crucial, since life expectancy in well-controlled patients approximates that of normal population. However, the cost-of-illness of acromegaly (both direct costs and production loss) has a huge burden on patients and health system. In a Swedish study evaluating the cost-of-illness of acromegaly, the authors computed a total annual cost per patient due to acromegaly and its comorbidities of about 12.000 € during 2013 [25]. More in detail, costs directly due to acromegaly seems to contribute per about 75% of total expenses and, particularly, medical treatments for acromegaly account for about 70% of the total direct medical costs (mainly due to SSA and GHRA usage) [25]. On the other hand, 25% of total cost of acromegaly seems to be related to comorbidities and patients’ hospitalizations. Interestingly, some authors demonstrated that the presence of selected comorbidities (e.g. cardiovascular complications) correlates with an increased odds of patients hospitalization and with a higher annual cost of disease management [25, 26].
Therefore, due to the importance of epidemiological data, particularly in rare diseases, the main aim of this study was to evaluate the incidence and prevalence of acromegaly in the Italian population using the Italian primary care database (Health Search Database) and to characterize the clinical features of acromegaly patients.
Material and methods
Patient database
Data have been extracted from the Health Search Database (HSD). The HSD is a clinical and epidemiological research longitudinal observational database that is representative of the general Italian population. It was established in 1998 by the Italian College of General Practitioners and it contains data from computer-based patient records from about 1000 general practitioners (GPs) homogeneously distributed across Italy, covering a total population of more than one million patients (1,066,871 people at the end of 2014). Noteworthy, due to the current regulation of the National Health Care System, almost 100% of Italian people refer to a GP. Indeed, a 2013 analysis of the Italian National Institute of Statistics (ISTAT) reports the presence of 45,203 GPs in our country, each taking care a mean of 1160 inhabitants and thus covering a total of more than 52 million patients. This number nearly represents the entire Italian population aged ≥18 years old (total population in 2013: 60,782,668 inhabitants) (http://www.istat.it). Therefore, a carefully managed GP-based database can be representative of the Italian general population.
The database includes information about patient demographics and medical data such as clinical events and diagnoses (as free text notes or coded using the International Classification of Diseases, 9th Revision, Clinical Modification, ICD9-CM), hospital admissions, laboratory tests and radiological exams. To be considered for participation in epidemiological studies, GPs should meet “up-to-standard” quality criteria. These quality criteria pertain to the levels of coding, the prevalence of well-known diseases, the mortality rates, and years of recording. Briefly, GPs included in the HSD analyses are general practitioner who carefully register all the events related to patients’ clinical history, with a particular focus on clinical diagnoses. The population of the selected GPs is superimposable to that reported from ISTAT and therefore representative of Italian population. In order to collect standardized data and have complete information, all GPs are trained for data collection, data entry and software use. Every 6 months, GPs undergo a data check to verify the completeness of the information collected and entered into HSD (www.healthsearch.it). Noteworthy, GPs has to collect into their medical records all clinical diagnoses of patients, even those performed by other specialists (as for acromegaly in the majority of cases).
HSD complies with European Union guidelines on the use of medical data for research and has been previously demonstrated to be a valid data source for scientific research [27,28,29].
Source population
We selected from HSD all patients aged ≥18 years registered in the lists of participating GPs at the beginning of the study period (1st January 2000) and with at least 1 year of recorded history prior to the start of the study.
Case definition
Primary outcome of the study was the occurrence of acromegaly, which was identified using both the related ICD9-CM code (253.0: acromegaly and gigantism) coupled with the specific key word “acromegaly” in code description (thus excluding cases of gigantism).
To increase the specificity of the case definition, only patients identified with the above mentioned criteria and, during the same study year, at least one recorded event for GH evaluation (national code 90.35.1), IGF-I evaluation (code 90.40.6) or MRI of the sella turcica (code 88.97) were identified as reliable acromegaly cases and included in the data analysis.
Data analysis
The prevalence and incidence of acromegaly during the years 2000–2014 was calculated (study period: 1st January 2000 to 31st December 2014). Regarding the yearly prevalence, for each observation year the number of patients with old and new diagnoses of acromegaly, alive and present in the GPs’ lists up to 31st December of the year (numerator), was divided by the total number of patients alive and active in the same year (denominator) (Online Resource 1).
As for the yearly incidence (computed as incidence density), the number of new cases in each year of observation (numerator) was divided by the person-time at risk of developing acromegaly (denominator) at 1st January of each year of observation. For the calculation of the incidence rate, person-time of follow-up was censored upon the first occurrence of one of the following events: occurrence of acromegaly, transferring out of general practice or death (Online Resource 1). The prevalence and incidence were expressed as rates per 100,000 inhabitants and 100,000 person years (PYs), respectively.
To characterize the patients with a diagnosis of acromegaly, demographic data (age, sex) of cases were evaluated at the date of first code (index date) being registered in the database. Moreover, using a case-control study design, patients with acromegaly were compared to matched controls for the presence of selected comorbidities (ICD9-CM codes listed in Table 1), known to be associated with acromegaly (hypopituitarism, hyperglycemia, type II diabetes mellitus, hypertension, cardiac hypertrophy, carpal tunnel syndrome, infertility, arthropathy, sleep apnea, colorectal polyposis, goiter) and to the yearly frequency of patient hospitalization. Up to 10 controls were matched to each case on sex, age and duration of follow-up (namely, time of patient history recorded into the HSD).
Yearly hospitalization rate was stratified into tertiles, based on the distribution of hospitalizations in the Italian general population. Namely, I tertile: 0 hospitalization/year; II tertile: 1–2 hospitalizations/year; III tertile: ≥3 hospitalizations/year
Statistical analysis
STATA software, version 11 (STATACorp, College Station, TX) was used to perform statistical analyses, while graphs and figures were drawn by use of GraphPad Prism software version 5.02 (GraphPad Software Inc., San Diego, CA). A descriptive statistical analysis for categorical variables (absolute frequency, relative frequency, 95% confidence intervals (CIs)) and continuous variables (mean ± standard deviation (SD)) was carried out. A multivariable conditional logistic regression was estimated to compute the odds ratio (OR) and the related 95% CI as a measure of association between each comorbidity and cases of acromegaly. A Poisson model was adopted to test the increasing trend for prevalence of acromegaly over the years under study.
Results
Prevalence and incidence of acromegaly in the Italian general population
At the end of the study period (31st December 2014), the HSD included a total population of 1,066,871 patients. Overall, 74 acromegaly patients were identified (48 F, mean age 49.9 ± 19.3 years), thus yielding a prevalence of 6.9 per 100,000 inhabitants [95% CI 5.4–8.5]. The total prevalence for women was significantly higher than for men (8.6 per 100,000 vs. 5.1 per 100,000, respectively; p = 0.004).
During the study period we observed a significant increase in the prevalence of acromegaly cases, rising from 4.7 per 100,000 in 2000 to 7.0 per 100,000 inhabitants in 2012 (p < 0.0001). The increase in the prevalence of acromegaly was mainly observed during the first part of the study period (from 2000 to 2004), while prevalence was rather stable in the following decade (from 6.5 per 100,000 in 2005 to 6.9 per 100,000 inhabitants in 2014; see Fig. 1 and Online Resource 1). As expected, the increasing trend was observed for both women (p = 0.002) and men (p = 0.026) (Fig. 1).
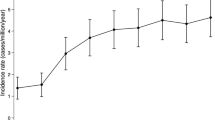
The incidence of acromegaly was variable in the study period, ranging from 0.0 per 100,000 PYs in 2014 to 0.74 per 100,000 PYs in 2004 (Fig. 2). As observed for the prevalence data, we found an increasing incidence of the disease in the first years of the study period (from 2001 to 2005), while it was still variable, although without showing remarkable peaks, during the remaining years analyzed (2006–2014). The incidence of acromegaly during the entire observation time was 0.31 per 100,000 PYs (0.22 per 100,000 PYs for males and 0.38 per 100,000 PYs for females).
Characterization of acromegaly patients
As previously described in the Methods section, patients with acromegaly were compared to matched controls to identify and analyze the frequency of the events (comorbidities and hospitalizations) in the two groups. In order to increase the statistical power of the analysis, we included in the case-control study also those cases not active at the end of the study period (e.g., death during the study). Therefore, 96 acromegaly patients (33 males, 63 females) were compared with 809 controls (Table 2).
For three out of eleven comorbidities selected for the study (hyperglycemia, arthropathy and sleep apnea), we did not find any event associated to the related ICD9-CM codes in acromegaly cases. However, all the other comorbidities evaluated (in which the number of reported events was enough to compute a statistical analysis), showed an OR > 1 (from 1.4 up to 20), as expected from the starting selection criteria, based on a known relation between the specific pathological condition and acromegaly [25] (Table 2, Fig. 3).
In this context, hypertension and type II diabetes mellitus emerged as the comorbidities more frequently associated with acromegaly (31.3 and 14.6% of cases), with an OR of 1.4 (95% CI 0.84–2.34) and 2.38 (95% CI 1.22–4.64) compared to the control group, respectively. The frequency of type II diabetes mellitus in acromegaly patients was significantly higher compared to controls (p = 0.01), as observed for the presence of hypopituitarism (OR 20, 95% CI 1.81–220.56; p = 0.01) and colorectal polyposis (OR 4.14, 95% CI 1.18–14.5; p = 0.03) (Table 2, Fig. 3). Moreover, carpal tunnel syndrome was present in 7.3% of cases, while goiter and colorectal polyposis in 5.2 and 4.2%, respectively.
Finally, we observed that acromegaly patients were more likely to undergo hospitalization, with a higher yearly frequency compared to the general population (Table 2, Fig. 3). Indeed, we found that a significantly higher percentage of acromegaly cases underwent ≥3 hospitalizations/year compared to controls (OR 2.8, 95% CI 1.51–5.19; p < 0.001).
Discussion
To our knowledge, this is the first epidemiological investigation of acromegaly carried out by use of a large longitudinal primary care database representative of the Italian general population.
Prevalence of acromegaly in Italy increased during the study years (2000–2014), reaching a value of 6.9 per 100,000 inhabitants at the end of the observation period (31st December 2014). Prevalence estimates of acromegaly, evaluated using different methods (e.g., multicenter studies and international registries, insurance databases, GP or referral center reports), vary in the different studies carried out across Europe and US and range from 3 to 10 cases per 100,000 people [4,5,6,7,8,9, 12, 14, 30,31,32]. In this context, our estimate is in line with a number of recent reports which documented an average prevalence of acromegaly around 6–8 cases per 100,000 inhabitants [8, 30,31,32].
A first epidemiological study conducted in Italy reported a prevalence of acromegaly of 9.7 per 100,000 inhabitants [33]. However, this report referred to a specific area of Italy (province of Messina, Sicily) and almost all patients were registered by a single tertiary center for pituitary diseases. The study mainly focused on the influence of environmental factors on acromegaly, showing a great variability of the estimated prevalence in four different sub-areas analyzed (from 2.6 to 21.0 per 100,000 inhabitants) [33].
On the other hand, our data are in line with the estimated prevalence of acromegaly (about 60 cases per million) assumed in a following retrospective multicenter study endorsed by the Italian Society of Endocrinology and performed involving 24 Italian tertiary referral centers across the Country [22].
Moreover, our prevalence data are supported by the incidence estimates of acromegaly reported in the present study (incidence of period 2001–2014: 0.31 cases per 100,000 PYs). Incidence rates published across Europe are remarkably similar, ranging around 0.2–0.4 cases per 100,000 PYs.
Noteworthy, a population-based study conducted in Finland reported a standardized incidence rate (SIR) for acromegaly of 0.34 cases per 100,000 PYs [6], a cross-sectional analysis conducted on Maltese population a SIR of 0.31 cases per 100,000 PYs [34], and the Spanish study by Etxabe and colleagues reported an average incidence of 0.31 cases per 100,000 PYs [30]. Therefore, our incidence estimate, although computed using a completely different method compared to the above mentioned studies, is in line with previous reports, thus supporting the reliability of our study design and the use of the HSD also for epidemiological studies evaluating rare diseases such as acromegaly.
In this light, the mean age at diagnosis observed for our cases (49.9 ± 19.3 years) fits with the results of the great majority of published reports. Indeed, the fifth decade of life is often reported as the period in which most acromegaly patients are diagnosed [3, 22, 25, 35].
Looking more in detail to our data, we observed that the increasing prevalence found during the study period is mainly due to a significant trend present in the first years of observation time (particularly from 2000 to 2004), which is mirrored by the increasing incidence observed in the same period (peak of incidence in 2004). In this context, other epidemiological studies, investigating the trend of acromegaly incidence and/or prevalence during a time frame >10 years, report different courses for the incidence of the disease (e.g., stable over time, constantly increasing, up and down in different periods) [6, 11, 13, 30], while prevalence data almost unanimously show an increase over time (reviewed in ref. [3]). Therefore, the increasing incidence observed in our study from 2001 to 2005 could represent a “physiological” peak of incidence in the context of a variable trend over time, as observed during other long-term longitudinal evaluations [13]. However, another reason could be represented by the gradual inclusion into the database of cases diagnosed before HSD start (1998).
As for the female predominance observed in our study, this finding has been already described in two epidemiological studies conducted in Spain (61% females in the study by Mestron and 64% in the work from Extabe) [12, 30], one survey conducted on the Maltese population (58%) [34], some National registries, including the German (54%), the French (55%) and the Mexican one (59%) [36,37,38], a very recent large multicenter study (54%) [39] and, in particular, the previously mentioned Italian multicenter study (59% females) [22]. Although most of these studies report a higher frequency/percentage of females among acromegaly patients, instead of the true prevalence estimation, these measures usually show a strong direct correlation. However, the finding of gender predominance in acromegaly is still debated. Indeed a number of other studies report a similar prevalence and/or incidence among the two sexes [4, 6, 8, 10, 33, 40], and two reports (carried out in Belgium and Iceland) even lay for a male predominance [5, 7].
Briefly, after performing an accurate case definition, the HSD comes out as a reliable tool for the evaluation of the incidence and prevalence of acromegaly. The strict inclusion criteria strongly reduce the presence of false positive cases, while a (slight) underestimation of the disease represent a possible limitation of the study design that is counterbalanced by the fact that our data are in line with the majority of previous reports in literature.
Another important objective of our study was to use the HSD as a tool to perform a clinical characterization of acromegaly patients, based on the evaluation of the presence of a number of selected comorbidities, and to evaluate the impact of acromegaly on patients’ hospitalization, compared to the general population.
Despite the intrinsic rarity of the disease and the relative low number of cases identified, it was possible to compute the odds ratio (OR), as well as the related p values, for the majority of the selected comorbidities, comparing acromegaly cases to controls in a (nearly) 1:10 ratio.
As already mentioned, hypertension and type II diabetes mellitus emerged as the comorbidities most commonly associated to acromegaly (31.3 and 14.6%, respectively). Noteworthy, these data are almost superimposable with those presented in the above mentioned Italian multicenter survey, including a large number of acromegaly patients (n = 1512). Indeed, in the study by Arosio et al. hypertension and type II diabetes mellitus were reported in 33 and 16% of cases, respectively [22]. Furthermore, our data on the impact of these two comorbidities on acromegaly are also in line with the results recently reported in a nationwide population-based study conducted in Sweden [25], as well as in a retrospective analysis of the French Registry [23]. In this context, a meta-analysis including a total of 2562 patients from 18 series reported a mean prevalence of hypertension in about 35% of acromegaly patients [41].
These evidences support the reliability of the HSD for the clinical characterization of acromegaly patients, particularly regarding the presence of hypertension and type II diabetes mellitus, two pathological conditions with a peculiar impact on acromegaly. Interestingly, a previous study already validated the HSD as a reliable tool for the identification of hypertension and type II diabetes mellitus in the general population [42]. Furthermore, as expected, the presence of hypopituitarism (OR 20, 95% CI 1.81–220.56) and colorectal polyposis (OR 4.14, 95% CI 1.18–14.5) was significantly higher in acromegaly cases compared to controls.
On the other hand, our results show that the frequency of other acromegaly associated conditions (carpal tunnel syndrome, goiter, cardiac hypertrophy and infertility) is lower compared to that reported in the majority of previous studies [12, 23, 25, 26, 43], while for hyperglycemia, arthropathy and sleep apnea we did not find any event associated to the related codes. A possible explanation could reside in the choice of more strict and specific ICD9-CM codes compared to those used in other reports [25, 26, 44], thus leading to the underestimation of the associated comorbidities. This could be the case for arthropathy and sleep apnea. However, we can also assume that the acromegaly cases identified from the HSD electronic records are representative of the Italian scenario, where about 50% of patients refers to tertiary referral centers [22]. The other half is likely diagnosed and/or followed up by specialists working in public/private practices, first level regional and district hospitals and, in some cases, by GPs themselves. Therefore, the underestimation of specific comorbidities could reflect a real lack of diagnoses, more likely occurred outside the referral centers, where procedures like polysomnography are rarely performed. In this light, the impact of the different comorbidities is largely variable in the different studies and data from multicenter surveys (mainly including tertiary centers) usually show higher percentages compared to population-based studies (e.g., presence of carpal tunnel syndrome ranging from 1.4 to 30% of patients) [23, 25].
Finally, we showed that acromegaly patients are more likely than the general population to undergo high rate of yearly hospitalizations (≥3 accesses/year). Since acromegaly related comorbidities have been demonstrated to increase the odds of hospitalization, this finding is somehow expected [26]. However, our study represents the first report on this specific feature in the Italian population, furthermore performed comparing patients with “non-acromegaly” controls and using the modern approach of the automatic identification of clinical events from GPs’ electronic medical records. This observation emphasizes the need for an improvement of disease control in acromegaly. Indeed, the increased number of cured or controlled patients would allow us to significantly decrease the number of disease related comorbidities and patient hospitalization, thus improving both patient life expectancy and quality of life and reducing the disease burden on health care resources [25, 26].
In this context, a limitation of our study is represented by the lack of information about the disease status of the patients included in the case-control study. This is mainly due to the relatively low number of specific biochemical parameters (namely, GH and IGF-I values) currently reported in the database and to the lack of a specific claim (code) associated to the disease control. Likely, future developments of the HSD will allow us to overcome most of the current limitations and to shore up the present strengths, particularly in the setting of rare diseases.
In conclusion, by use of strict inclusion criteria in order to minimize the presence of false positive cases, the present study documented a prevalence of acromegaly of about 7 cases per 100,000 inhabitants [95% CI 5.4–8.5]. Acromegaly was associated with a number of comorbidities (mainly hypertension and type II diabetes mellitus) and with a higher rate of yearly hospitalizations compared to controls. Noteworthy, these data have been extracted from a representative sample of the Italian general population by use of a large longitudinal and observational GP-based database.
References
S. Melmed, Medical progress: acromegaly. N. Engl. J. Med. 355(24), 2558–2573 (2006). https://doi.org/10.1056/NEJMra062453
F.G. Davis, V. Kupelian, S. Freels, B. McCarthy, T. Surawicz, Prevalence estimates for primary brain tumors in the United States by behavior and major histology groups. Neuro. Oncol. 3(3), 152–158 (2001)
A. Lavrentaki, A. Paluzzi, J.A. Wass, N. Karavitaki, Epidemiology of acromegaly: review of population studies. Pituitary 20(1), 4–9 (2017). https://doi.org/10.1007/s11102-016-0754-x
A. Fernandez, N. Karavitaki, J.A. Wass, Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin. Endocrinol. 72(3), 377–382 (2010). https://doi.org/10.1111/j.1365-2265.2009.03667.x
A.F. Daly, M. Rixhon, C. Adam, A. Dempegioti, M.A. Tichomirowa, A. Beckers, High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J. Clin. Endocrinol. Metab. 91(12), 4769–4775 (2006). https://doi.org/10.1210/jc.2006-1668
A. Raappana, J. Koivukangas, T. Ebeling, T. Pirila, Incidence of pituitary adenomas in Northern Finland in 1992-2007. J. Clin. Endocrinol. Metab. 95(9), 4268–4275 (2010). https://doi.org/10.1210/jc.2010-0537
T.T. Agustsson, T. Baldvinsdottir, J.G. Jonasson, E. Olafsdottir, V. Steinthorsdottir, G. Sigurdsson, A.V. Thorsson, P.V. Carroll, M. Korbonits, R. Benediktsson, The epidemiology of pituitary adenomas in Iceland, 1955-2012: a nationwide population-based study. Eur. J. Endocrinol. 173(5), 655–664 (2015). https://doi.org/10.1530/EJE-15-0189
T. Burton, E. Le Nestour, M. Neary, W.H. Ludlam, Incidence and prevalence of acromegaly in a large US health plan database. Pituitary 19(3), 262–267 (2016). https://doi.org/10.1007/s11102-015-0701-2
M. Bex, R. Abs, G. T’Sjoen, J. Mockel, B. Velkeniers, K. Muermans, D. Maiter, AcroBel--the Belgian registry on acromegaly: a survey of the ‘real-life’ outcome in 418 acromegalic subjects. Eur. J. Endocrinol. 157(4), 399–409 (2007). https://doi.org/10.1530/EJE-07-0358
A. Tjornstrand, K. Gunnarsson, M. Evert, E. Holmberg, O. Ragnarsson, T. Rosen, H. Filipsson Nystrom, The incidence rate of pituitary adenomas in western Sweden for the period 2001-2011. Eur. J. Endocrinol. 171(4), 519–526 (2014). https://doi.org/10.1530/EJE-14-0144
J. Dal, U. Feldt-Rasmussen, M. Andersen, L.O. Kristensen, P. Laurberg, L. Pedersen, O.M. Dekkers, H.T. Sorensen, J.O. Jorgensen, Acromegaly incidence, prevalence, complications and long-term prognosis: a nationwide cohort study. Eur. J. Endocrinol. 175(3), 181–190 (2016). https://doi.org/10.1530/EJE-16-0117
A. Mestron, S.M. Webb, R. Astorga, P. Benito, M. Catala, S. Gaztambide, J.M. Gomez, I. Halperin, T. Lucas-Morante, B. Moreno, G. Obiols, P. de Pablos, C. Paramo, A. Pico, E. Torres, C. Varela, J.A. Vazquez, J. Zamora, M. Albareda, M. Gilabert, Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Espanol de Acromegalia, REA). Eur. J. Endocrinol. 151(4), 439–446 (2004)
G.T. Hoskuldsdottir, S.B. Fjalldal, H.A. Sigurjonsdottir, The incidence and prevalence of acromegaly, a nationwide study from 1955 through 2013. Pituitary 18(6), 803–807 (2015). https://doi.org/10.1007/s11102-015-0655-4
S. Ezzat, S.L. Asa, W.T. Couldwell, C.E. Barr, W.E. Dodge, M.L. Vance, I.E. McCutcheon, The prevalence of pituitary adenomas: a systematic review. Cancer 101(3), 613–619 (2004). https://doi.org/10.1002/cncr.20412
A. Colao, D. Ferone, P. Marzullo, G. Lombardi, Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr. Rev. 25(1), 102–152 (2004). https://doi.org/10.1210/er.2002-0022
L. Katznelson, E.R. Laws Jr., S. Melmed, M.E. Molitch, M.H. Murad, A. Utz, J.A. Wass, S. Endocrine, Acromegaly: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 99(11), 3933–3951 (2014). https://doi.org/10.1210/jc.2014-2700
S.M. Webb, X. Badia, Quality of Life in Acromegaly. Neuroendocrinology 103(1), 106–111 (2016). https://doi.org/10.1159/000375451
T. Brue, F. Castinetti, The risks of overlooking the diagnosis of secreting pituitary adenomas. Orphanet. J. Rare. Dis. 11(1), 135 (2016). https://doi.org/10.1186/s13023-016-0516-x
I.M. Holdaway, R.C. Rajasoorya, G.D. Gamble, Factors influencing mortality in acromegaly. J. Clin. Endocrinol. Metab. 89(2), 667–674 (2004). https://doi.org/10.1210/jc.2003-031199
R. Kauppinen-Makelin, T. Sane, A. Reunanen, M.J. Valimaki, L. Niskanen, H. Markkanen, E. Loyttyniemi, T. Ebeling, P. Jaatinen, H. Laine, P. Nuutila, P. Salmela, J. Salmi, U.H. Stenman, J. Viikari, E. Voutilainen, A nationwide survey of mortality in acromegaly. J. Clin. Endocrinol. Metab. 90(7), 4081–4086 (2005). https://doi.org/10.1210/jc.2004-1381
I.M. Holdaway, M.J. Bolland, G.D. Gamble, A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur. J. Endocrinol. 159(2), 89–95 (2008). https://doi.org/10.1530/EJE-08-0267
M. Arosio, G. Reimondo, E. Malchiodi, P. Berchialla, A. Borraccino, L. De Marinis, R. Pivonello, S. Grottoli, M. Losa, S. Cannavo, F. Minuto, M. Montini, M. Bondanelli, E. De Menis, C. Martini, G. Angeletti, A. Velardo, A. Peri, M. Faustini-Fustini, P. Tita, F. Pigliaru, G. Borretta, C. Scaroni, N. Bazzoni, A. Bianchi, M. Appetecchia, F. Cavagnini, G. Lombardi, E. Ghigo, P. Beck-Peccoz, A. Colao, M. Terzolo; Italian Study Group of, A., Predictors of morbidity and mortality in acromegaly: an Italian survey. Eur. J. Endocrinol. 167(2), 189–198 (2012). https://doi.org/10.1530/EJE-12-0084
L. Maione, T. Brue, A. Beckers, B. Delemer, P. Petrossians, F. Borson-Chazot, O. Chabre, P. Francois, J. Bertherat, C. Cortet-Rudelli, P. Chanson; French Acromegaly Registry, G., Changes in the management and comorbidities of acromegaly over three decades: the French Acromegaly Registry. Eur. J. Endocrinol. 176(5), 645–655 (2017). https://doi.org/10.1530/EJE-16-1064
A. Giustina, P. Chanson, D. Kleinberg, M.D. Bronstein, D.R. Clemmons, A. Klibanski, A.J. van der Lely, C.J. Strasburger, S.W. Lamberts, K.K. Ho, F.F. Casanueva, S. Melmed; Acromegaly Consensus, G., Expert consensus document: a consensus on the medical treatment of acromegaly. Nature reviews. Endocrinology 10(4), 243–248 (2014). https://doi.org/10.1038/nrendo.2014.21
E. Lesen, D. Granfeldt, A. Houchard, J. Dinet, A. Berthon, D.S. Olsson, I. Bjorholt, G. Johannsson, Comorbidities, treatment patterns and cost-of-illness of acromegaly in Sweden: a register-linkage population-based study. Eur. J. Endocrinol. 176(2), 203–212 (2017). https://doi.org/10.1530/EJE-16-0623
M.S. Broder, M.P. Neary, E. Chang, D. Cherepanov, L. Katznelson, Treatments, complications, and healthcare utilization associated with acromegaly: a study in two large United States databases. Pituitary 17(4), 333–341 (2014). https://doi.org/10.1007/s11102-013-0506-0
C. Cricelli, G. Mazzaglia, F. Samani, M. Marchi, A. Sabatini, R. Nardi, G. Ventriglia, A.P. Caputi, Prevalence estimates for chronic diseases in Italy: exploring the differences between self-report and primary care databases. J. Public Health Med. 25(3), 254–257 (2003)
G. Trifiro, P. Morabito, L. Cavagna, C. Ferrajolo, S. Pecchioli, M. Simonetti, E. Bianchini, G. Medea, C. Cricelli, A.P. Caputi, G. Mazzaglia, Epidemiology of gout and hyperuricaemia in Italy during the years 2005-2009: a nationwide population-based study. Ann. Rheum. Dis. 72(5), 694–700 (2013). https://doi.org/10.1136/annrheumdis-2011-201254
G. Mazzaglia, E. Ambrosioni, M. Alacqua, A. Filippi, E. Sessa, V. Immordino, C. Borghi, O. Brignoli, A.P. Caputi, C. Cricelli, L.G. Mantovani, Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation 120(16), 1598–1605 (2009). https://doi.org/10.1161/CIRCULATIONAHA.108.830299
J. Etxabe, S. Gaztambide, P. Latorre, J.A. Vazquez, Acromegaly: an epidemiological study. J. Endocrinol. Invest. 16(3), 181–187 (1993). https://doi.org/10.1007/BF03344942
I.M. Holdaway, C. Rajasoorya, Epidemiology of acromegaly. Pituitary 2(1), 29–41 (1999)
L.A. Naves, L.B. Porto, J.W. Rosa, L.A. Casulari, J.W. Rosa, Geographical information system (GIS) as a new tool to evaluate epidemiology based on spatial analysis and clinical outcomes in acromegaly. Pituitary 18(1), 8–15 (2015). https://doi.org/10.1007/s11102-013-0548-3
S. Cannavo, F. Ferrau, M. Ragonese, L. Curto, M.L. Torre, M. Magistri, A. Marchese, A. Alibrandi, F. Trimarchi, Increased prevalence of acromegaly in a highly polluted area. Eur. J. Endocrinol. 163(4), 509–513 (2010). https://doi.org/10.1530/EJE-10-0465
M. Gruppetta, C. Mercieca, J. Vassallo, Prevalence and incidence of pituitary adenomas: a population based study in Malta. Pituitary 16(4), 545–553 (2013). https://doi.org/10.1007/s11102-012-0454-0
J. Dal, N. Skou, E.H. Nielsen, J.O. Jorgensen, L. Pedersen, Acromegaly according to the Danish National Registry of Patients: how valid are ICD diagnoses and how do patterns of registration affect the accuracy of registry data? Clin. Epidemiol. 6, 295–299 (2014). https://doi.org/10.2147/CLEP.S63758
S. Fieffe, I. Morange, P. Petrossians, P. Chanson, V. Rohmer, C. Cortet, F. Borson-Chazot, T. Brue, B. Delemer; French Acromegaly, R., Diabetes in acromegaly, prevalence, risk factors, and evolution: data from the French Acromegaly Registry. Eur. J. Endocrinol. 164(6), 877–884 (2011). https://doi.org/10.1530/EJE-10-1050
S. Petersenn, M. Buchfelder, M. Reincke, C.M. Strasburger, H. Franz, R. Lohmann, H.J. Quabbe, U. Plockinger, Participants of the German Acromegaly, R.: Results of surgical and somatostatin analog therapies and their combination in acromegaly: a retrospective analysis of the German Acromegaly Register. Eur. J. Endocrinol. 159(5), 525–532 (2008). https://doi.org/10.1530/EJE-08-0498
L.A. Portocarrero-Ortiz, A. Vergara-Lopez, M. Vidrio-Velazquez, A.M. Uribe-Diaz, A. Garcia-Dominguez, A.A. Reza-Albarran, D. Cuevas-Ramos, V. Melgar, J. Talavera, A.J. Rivera-Hernandez, C.V. Valencia-Mendez, M. Mercado, Mexican Acromegaly Registry, G.: The Mexican Acromegaly Registry: Clinical and Biochemical Characteristics at Diagnosis and Therapeutic Outcomes. J. Clin. Endocrinol. Metab. 101(11), 3997–4004 (2016). https://doi.org/10.1210/jc.2016-1937
P. Petrossians, A.F. Daly, E. Natchev, L. Maione, K. Blijdorp, M. Sahnoun-Fathallah, R. Auriemma, A.M. Diallo, A.L. Hulting, D. Ferone, V. Hana Jr., S. Filipponi, C. Sievers, C. Nogueira, C. Fajardo-Montanana, D. Carvalho, V. Hana, G.K. Stalla, M.L. Jaffrain-Rea, B. Delemer, A. Colao, T. Brue, S. Neggers, S. Zacharieva, P. Chanson, A. Beckers, Acromegaly at diagnosis in 3173 patients from the Liege Acromegaly Survey (LAS) Database. Endocr.-Relat. Cancer 24(10), 505–518 (2017). https://doi.org/10.1530/ERC-17-0253
O. Kwon, Y.D. Song, S.Y. Kim, E.J. Lee; Rare Disease Study Group, S., Research Committee, K.E.S., Nationwide survey of acromegaly in South Korea. Clin. Endocrinol. 78(4), 577–585 (2013). https://doi.org/10.1111/cen.12020
M. Bondanelli, M.R. Ambrosio, E.C. degli Uberti, Pathogenesis and prevalence of hypertension in acromegaly. Pituitary 4(4), 239–249 (2001)
R. Gini, M.J. Schuemie, G. Mazzaglia, F. Lapi, P. Francesconi, A. Pasqua, E. Bianchini, C. Montalbano, G. Roberto, V. Barletta, I. Cricelli, C. Cricelli, G. Dal Co, M. Bellentani, M. Sturkenboom, N. Klazinga, Automatic identification of type 2 diabetes, hypertension, ischaemic heart disease, heart failure and their levels of severity from Italian General Practitioners’ electronic medical records: a validation study. BMJ Open 6(12), e012413 (2016). https://doi.org/10.1136/bmjopen-2016-012413
T.J. Reid, K.D. Post, J.N. Bruce, M. Nabi Kanibir, C.M. Reyes-Vidal, P.U. Freda, Features at diagnosis of 324 patients with acromegaly did not change from 1981 to 2006: acromegaly remains under-recognized and under-diagnosed. Clin. Endocrinol. 72(2), 203–208 (2010). https://doi.org/10.1111/j.1365-2265.2009.03626.x
T. Burton, E. Le Nestour, T. Bancroft, M. Neary, Real-world comorbidities and treatment patterns of patients with acromegaly in two large US health plan databases. Pituitary 16(3), 354–362 (2013). https://doi.org/10.1007/s11102-012-0432-6
Funding:
This research received the unconditional support of Pfizer srl.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
F.G. received fees for lectures and/or participation to advisory boards for Novartis, AMCo, and IONIS Pharmaceuticals. D.F. received grants and fees for lectures and participation to advisory boards for Novartis, Ipsen, and Pfizer. The remaining authors have declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Gatto, F., Trifirò, G., Lapi, F. et al. Epidemiology of acromegaly in Italy: analysis from a large longitudinal primary care database. Endocrine 61, 533–541 (2018). https://doi.org/10.1007/s12020-018-1630-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1630-4