Abstract
In recent years, the search for alternatives to the use of synthetic chemical additives in food has led to an increase in research focused on essential oils (EOs). Among the EOs with food applications already described in the literature, the essential oil extracted from basil (Ocimum basilicum L.) stands out, which presents several biological activities. This review was conducted to discuss the basil essential oil (BEO), presenting information on the different extraction methods and their influence on the compounds, in addition to presenting biological and toxicological aspects. The extraction method and the qualitative and quantitative variation of the compounds are highly dependent on factors such as type of cultivar; date, place, and conditions of cultivation; and seasonal variation and harvest. In addition, the main biological activities of basil essential oil are dependent of the chemical constituents, such as estragole, linalool, eugenol, eucalyptol, and bergamotene, and can act in isolation or in synergism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Essential oils (EOs) are products resulting from the secondary metabolism of plants, composed of complex mixtures of low molecular weight compounds and obtained from different methods of extraction (Chouhan et al., 2019). Currently, several applications of EOs have been described in the literature, including in the food and beverage industry, flavor and fragrance industry, agriculture field, alternative medicine, natural therapies, and, mainly, pharmaceutical industry. Especially in the food area, the application of EOs could be highly valuable due to the growing concerns on adverse health effects of synthetic preservatives and the consequent demand for natural preservatives. Essential oils could be applied to extend the shelf life of foods, owing to its antimicrobial and antioxidant properties, besides its use as a flavoring agent.
Among essential oils with food applications already described in the scientific literature is the oil from basil (Ocimum basilicum L.), an herb belonging to the Lamiaceae family, considered the most cultivated aromatic herb variety worldwide. They are mainly found in the tropical regions of Asia, Africa, and Central and South America. The basil leaves are widely used in culinary, in fresh or dry form, as food flavoring, and as an ornamental plant in house garden (Filip et al., 2016). There are several varieties of O. basilicum species, which differ mainly in their morphological structure. This consequently influences the content of essential oil, as well as its chemical composition. Overall, the major components of the basil essential oil (BEO) are the terpenes and phenylpropanoids, followed by alcohols and aldehydes (Milenković et al., 2019). The market value of Ocimum species is predominately based on their essential oil content and ornamental characters (Mahmoudi et al., 2020).
Several studies have reported biological activities of the essential oil from O. basilicum. These include antimicrobial, antifungal, insect repelling, antioxidant, anticancer, and anti-inflammatory activities. These biological activities are mainly attributed to their essential oils rich in linalool and phenolic compounds (Mahmoudi et al., 2020). However, despite being widely used worldwide and reported scientifically, review papers with a complete approach of the main properties of essential oil from basil (Ocimum basilicum L.) from extraction until its application were not found in the literature. This review summarizes the main methods used for BEO extraction, discussing their main chemical compounds, recent studies on its biological activities, toxicological effects, and some applications of BEO in the food area.
Extraction Methods
Basil essential oil content, as well as its yield and composition, is influenced by several factors, such as cultivars, chemotypes, growing conditions, and method of extraction and drying (Milenković et al., 2019). Several methods for the extraction of basil essential oil have been proposed in the literature. Table 1 shows the advantages and disadvantages of the main methods proposed for extraction of BEO, as well as for extraction of essential oils from other sources. Among these methods, steam distillation (SD) and hydrodistillation (HD) are the two most used methods for the extraction of essential oil from basil, due to their economic feasibility and simplicity (Shiwakoti et al., 2017).
Steam distillation involves the heating of the plant material by passing water steam, which promotes the opening of glands and essential oil evaporation, causing it to rise with the steam. Then, the essential oil is collected by condensation. On the other hand, hydrodistillation is based on immersion and boiling of material in water. In this method, essential oil is also collected in a condenser (Sefidkon et al., 2007).
Shiwakoti et al. (2017) evaluated the efficiency of the two BEO extraction methods (HD — hydrodistillation vs SD — steam distillation) and concluded that steam distillation showed better performance. The SD method presented a higher yield of essential oil (0.32–0.68 g of essential oil/100 g of dried leaves) than HD (0.08–0.32 g of essential oil/100 g of dried leaves) for both basil species tested.
Milenković et al. (2019) studied the effect of growing conditions and different times of harvest in the yield of basil essential oil. The basil was grown in the soil under two conditions: covered by color nets (50% shade index) or in unshaded condition (open field — control). The basil essential oil was obtained by Clevenger-type hydrodistillation with a ratio of 1:10 w/v (plant material:water) during 120 min. The second harvest from unshaded, control plants, showed the lowest accumulation of essential oils (1.02 mL/100 g), while the first harvest from shaded plants presented the highest oil accumulation (3.23 mL/100 g). The synthesis of essential oils is influenced by light quantity and quality. Shading affects solar radiation, regulating plant growth, development and biosynthesis of metabolites, such as essential oils (Carvalho et al., 2016; Ilić et al., 2017).
Alternative methods using microwave-assisted extraction have also been developed aiming to reduce the operational cost, shorten extraction time, and improve the extraction yield. Among microwave-assisted extraction techniques, these can also be mentioned: microwave-assisted hydrodistillation (MAHD), microwave-generated hydrodistillation (MGH) and microwave hydrodiffusion and gravity (MHG) (Asbahani et al., 2015).
In the MAHD process, the extraction occurs as the result of changes in the cell structure, which are caused by electromagnetic waves, and the heat and mass gradients occur in the same direction. On the other hand, in HD the mass transfer occurs from inside to the outside. This fact justifies the higher extraction yield in MAHD compared to traditional distillation methods (Kusuma & Mahfud, 2016). In MGH, the rapid heating is caused by molecular motion within polar or ionic components, which promotes a break of cell membrane and allows the release and transportation of essential oil by the water steam. MHG is a technique that associates microwave heating and gravity at atmospheric pressure (Binello et al., 2014).
Kusuma and Mahfud (2016) evaluated the kinetics of oil extraction from basil essential oil by microwave-assisted hydrodistillation (MAHD) and solvent-free microwave extraction (SFME). Although the extraction yield of basil oil obtained by MAHD and SFME is the same (0.78%), the results showed that this yield was reached in a shorter extraction time in SFME (60 min) when compared to MAHD (160 min). The basil oil recovery obtained by MAHD shows the following trend: under extraction time of 100 min (72.89%), 140 min (13.05%), and 160 min (5.57%). In comparison, the basil oil recovery obtained by the SFME method shows the following trend: under extraction time of 20 min (72.16%), 40 min (13.38%), and 60 min (6.69%).
Distillation methods have many disadvantages due to the high process temperature used, which promotes the thermal degradation, oxidation, and hydrolysis of some compounds of the essential oils (Elgndi et al., 2017; Filip et al., 2016). An alternative to traditional hydrodistillation is solvent-free microwave extraction (SFME), a green technology performed without any solvent or water and at atmospheric pressure. In addition to being environmentally friendly, this method has, as advantages, the effective heating and faster energy transfer (Chenni et al., 2020; Filly et al., 2014). The solvent-free microwave extraction has been patented in 2004 (Chemat et al., 2004).
Chenni et al. (2016) proposed a comparison between SFME and conventional hydrodistillation in relation to extraction time and yield of basil essential oil. SFME was conducted in a microwave laboratory oven of 2.45 MHz with a maximum power of 1000 W. One hundred fifty grams of O. basilicum was immersed in water (600 g) for 30 min at 600 W. The plant material was submitted to heating with a fixed power for 30 min. Outside the microwave, a cooling system was responsible for condensing the distillate with a Clevenger-type apparatus. The condensed water was continuously heated (100 °C) until no more essential oil was obtained. The essential oil was dried over anhydrous sodium sulfate and stored at 4 °C. The hydrodistillation method was performed in a Clevenger-type apparatus by immersion of 150 g of O. basilicum in 6 L water and heating for 1 h (until no more essential oil was obtained). The obtained essential oil was dried with anhydrous sodium sulfate, and stored at 4 °C.
During the extraction process, the system was heated at boiling temperature of water at atmospheric pressure (100 °C). In the SFME method, it was necessary to heat the sample for only 5 min to reach this temperature, while in HD 20 min was necessary. SFME enabled a considerable reduction in extraction time, 30 min against 60 min for HD, providing similar yields (around 0.48%) of essential oil. These results show that SFME promotes an energy saving due to its shorter extraction times with similar yields, lower cost, and solvent saving (Chenni et al., 2016).
Besides distillation methods, other modern extraction techniques include supercritical fluid extraction, fast controlled pressure drop process extraction, organic solvent extraction, and Soxhlet extraction. They have been proposed with the aim of making BEO extraction faster and more efficient.
Supercritical fluid extraction (SFE) is an innovative technique for extracting and isolating EOs from aromatic herbs. It avoids the use of harmful organic solvents, thus being a clean and environmentally friendly technology. This method also provides quick extraction and requires only moderate temperatures. The most commonly used extraction fluid in SFE is carbon dioxide (CO2), due to advantages such as non-toxicity, high availability, and ease of removal from extracted products. However, in practice this method is not commonly employed due to its high cost of operation and complex design (Yousefi et al., 2019).
Filip et al. (2016) proposed a comparison between three methods of essential oil extraction from basil leaves: hydrodistillation, Soxhlet extraction, and supercritical carbon dioxide extraction. The hydrodistillation has been performed according to the methods described above. Soxhlet extraction consisted in extraction of basil leaves by methylene chloride using Soxhlet apparatus during 6 h. For SFE extraction, 50 g of ground basil was placed in the extractor vessel and submitted to a first extraction (E1) using carbon dioxide at the pressure of 100 bar and temperature of 60 °C. The material obtained after E1 extraction was again submitted to extraction by carbon dioxide, but at the pressure of 150 bar and temperature of 60 °C, for isolation of extract E2. Further, materials obtained after E1 and E2 extraction underwent another extraction with carbon dioxide at 200 bar and 60 °C, for preparation of extract E3. The same procedure was carried out for E4 extraction, using carbon dioxide at 300 bar and 60 °C. In the whole process of extraction, the same time and flow rate were applied: 4 h and 3.225 g CO2/min, respectively.
Comparing the different essential oil extraction methods, the higher yield of basil essential oil (BEO) was obtained by supercritical extraction, 22.06 g/kg, while the BEO yield using the standard hydrodistillation method was 5.65 g/kg and the BEO yield by Soxhlet procedure was 19.50 g/kg. During supercritical carbon dioxide extraction, the highest extraction yield was achieved in extract E2, using carbon dioxide at 150 bar and 60 °C. The isothermal pressure increase from 100 to 150 bar promoted an increase in solvent density, from 0.294 to 0.563 g/cm3, respectively, which may have contributed to the higher yield of extraction.
In a study conducted by Elgndi et al. (2017), supercritical fluid extraction was performed at temperature of 40 °C and two different pressures (100 and 300 bar). The authors reported two standard extraction periods: in the first, there was rapid extraction, expressed during the first 2 h and characterized by dissolution of the supercritical CO2; in the second, a slow extraction, with controlled diffusion. In addition, it was possible to notice enhancement of the kinetics, reduction of the extraction time, and increase of extraction efficiency, consequences of the increase of pressure from 100 to 300 bar, at constant temperature (40 °C). The increase of pressure promotes a consequent increase of the dissolving ability of CO2, which influences the solubility of high-molecular weight compounds in plant matrix, co-extracted with essential oil compounds. This results in a higher amount of extract.
Solvent extraction has been recommended for delicate materials, which are sensible to the heat. Different solvents such as acetone, hexane, petroleum ether, methanol, or ethanol can be used for this purpose. In general, the solvent is mixed with the sample and then heated at 50 °C for extraction of the essential oil, followed by filtration. Afterwards, the obtained material is concentrated by solvent evaporation. The concentrate is mixed with pure alcohol to extract the oil and then distilled at low temperatures (Cook & Lanaras, 2016; Tongnuanchan & Benjakul, 2014).
However, this method is considered an onerous process, spending a lot of time, which makes the process more expensive, compared to other methods. It can lead to losses of volatile components of the essential oil, and the solvent residues could be remaining in the extract owing to incomplete removal, causing several problems such as allergies and toxicity. Another major disadvantage is the amount of waste generated by the use of solvents and their inappropriate disposal (Cook & Lanaras, 2016; Tongnuanchan & Benjakul, 2014).
Abed et al. (2018) compared two organic solvents (n-hexane and petroleum ether) in the process of extraction of essential oil from basil leaves. Dry leaves and solvents in a ratio of 1:40 w/v (g solids/mL solvent) were placed in an extraction reaction flask, immersed in water in order to control the temperature and continuously stirred to ensure homogenization of the mixture. The optimal experimental conditions were defined as follows: n-hexane at 60 °C and 300 rpm agitation speed.
Mustapha (2018) obtained basil essential oil by Soxhlet method, with an extraction yield of 1.89%. This is a method that also uses solvents: it is a solid–liquid extraction based on the transference of the target compounds from the solid to appropriate organic solvents. The contact between extraction solvent and sample must be maintained continuously during the extraction process. The procedure consists of adding around 500 g of the dried sample into the Soxhlet extractor and 1.5 L of n-hexane solvent in a distillation flask submitted to heating. The Soxhlet extractor is coupled on top of the flask and a reflux condenser is placed on top of the extractor. The sample is extracted by refluxing of the solvent during around 6 h, until the solvent is cleared.
Table 2 presents a summary of the main methods for basil essential oil extraction mentioned above, showing extraction method conditions and the essential oil yield obtained.
Chemical Variation of Different Extraction Methods
The chemical composition of the BEO is influenced by several factors, such as geographical variation, seasonality, genetics, and others already discussed. Additionally, another significant parameter is the extraction method used. The efficiency of the extraction is dependent on the technique and its operational conditions, such as the choice of the specific solvent, temperature, pressure, particle size, flow rate, water, and others (Yousefi et al., 2019).
Abbas et al. (2017) reported the variation in the yield and chemical composition of essential basil oils isolated by HD and supercritical fluid extraction (SFE). The most significant variances were observed in the following compounds: linalool (21.79% SFE and 36.06% HD), estragole (17.86% SFE and 12.79% HD), menthol (10.47% SFE and 1.19% HD), and carvone (5.70% SFE and 0.66% HD). The yields were 0.45% and 0.82% for SFE and HD, respectively. This chemical variation is the result of thermal and hydrolytic degradation of the compounds, by oxidation or transesterification, due to the temperature and extraction time applied to the method (Drinić et al., 2020). This degradation occurs in oxygenated monoterpenes and favors the formation of hydrocarbon monoterpenes (Jugreet & Mahomoodally, 2020). This is what occurred with α-pinene, β-pinene, and camphene, which increased with the HD method, possibly due to the detriment of oxygenated compounds. The higher yield observed in the HD method may be due to the effective volatilization of the terpenoid components and better recovery of BEO (Abbas et al., 2017).
The operational variation of the extraction method can also promote changes in the chemical composition of essential oils, as reported by Elgndi et al. (2017). The authors extracted EO from Ocimum basilicum L. using SFE under different pressures (100 and 300 bar). The results showed that the pressure of 300 bar promoted higher extraction yield (2.07%) compared to pressure of 100 bar (1.56%). There were also changes in the concentration of the monoterpene hydrocarbons (27.40 and 1.00 mg/g) and oxygenated (434.30 and 317.80 mg/g). This increase in extraction yield and compound concentration is due to the increase in pressure applied, which promotes a better mass transfer and increases the potential of the solvent, easing the release of the EO by the plant (Yousefi et al., 2019).
A comparison between the SFME technique and conventional HD was studied by Chenni et al. (2020), who reported the variation of linalool (43.5% and 48.4%), methyl chavicol (13.3% and 14.3%), and 1.8-cineol (6.8% and 7.3%) for SFME and HD methods, respectively. Chenni et al. (2016) did not observe a significant variation in yield (0.48% for SFME and 0.48% for HD); on the other hand, the authors reported a slightly lower amount of total oxygenated compounds in SFME (89.6%) when compared to HD (89.8%), and a slight increase of total non-oxygenated compounds of SFME (9.7%) compared to HD (9.2%). Linalool was reported as the main component of BEO; however, the amount differed in the extraction methods (43.5 for SFME and 48.4 for HD). These results contradict the behavior observed in other studies, which showed higher proportions of oxygenated compounds extracted by microwave compared to HD, due to the reduction of thermal and hydrolytic damage (Figueredo et al., 2012).
Higher amounts of oxygenated compounds and lower amounts of monoterpene hydrocarbons are frequently reported in EO extraction by SFME when compared to HD. The proportion of oxygenated compounds is higher due to the higher dipolar moments in these organic compounds. They facilitate extraction, in contrast to monoterpene hydrocarbons, which have lower dipolar moments, resulting in lower proportions (Filly et al., 2014). Another characteristic of the SFME extraction technique is the higher yield when compared to HD. This is due to the internal atmospheric pressure, which promotes the swelling of the oil glands and cell wall rupture, releasing EOs that are spontaneously evaporated by azeotropic distillation (Asbahani et al., 2015; Jugreet et al., 2020; Li et al., 2013). In addition, the extraction time is shorter in SFME than in HD; 5 min are needed to obtain 1 drop of the EO using the SFME method, while for the HD method, the process would take about 90 min (Lucchesi et al., 2004).
The use of alternative methods to replace HD was reported by Binello et al. (2014), who compared HD, MGH, and MHG in EO extraction from basil (Ocimum basilicum L.). The results showed no differences in BEO yields; however, the extraction time was shorter for the alternative methods (20 min) compared to HD (45 min). The amount of compounds was higher for the alternative methods, such as oxygenated compounds linalool (24.68% MGH and 22.96% MHG), eugenol (15.19% MGH and 15.93% MHG), and α-cadinol (9.88% MGH and 9.93% MHG) when compared to HD (18.72, 13.5, and 9.6%, respectively). The shortest extraction time and the highest content of compounds are two important effects of the microwave on the plant. The incidence of radiation tends to penetrate deeper into the plant matrix, affecting the glands containing oil and resulting in better extraction of the compounds (Binello et al., 2014; Jugreet et al., 2020). Moreover, the predominance of oxygenated compounds is due to the lower thermal and hydrolytic degradation effects when compared to HD, and the high dipolar moment of these compounds in comparison to monoterpene hydrocarbons (Jugreet et al., 2020).
The chemical composition of BEO obtained by HD and SD methods was compared by Shiwakoti et al. (2016), who reported an extraction yield of 0.32 (g/100 g of a dry leaf) for SD and 0.08 (g/100 g of a dry leaf) for HD. Regarding chemical compounds, the SD method indicated superior performance in the extraction of hydrocarbons over HD. This behavior is justified by the higher heat transfer in SD vapors than in HD solubilization. The direct contact of the plant with the water vapors carries the volatile materials and results in a rapid elution of the compounds, and consequently a higher accumulation of them (Asbahani et al., 2015; Shiwakoti et al., 2016).
Optimization of the extraction method was studied by Martins et al. (2012), who reported the better-operating conditions to extract a higher content of methyl chavicol from Ocimum basilicum. The authors observed that higher temperatures generate higher amounts of distillates (hydrocarbons). However, the control of the distillation time is very important, as excessive distillation can trigger thermal degradation, hydrolysis, and water solubilization of some compounds (Shiwakoti et al., 2016).
The application of electromagnetic waves in the presence of water is commonly used for EO extraction, as in microwave-assisted hydro-distillation (MAHD). This method of dielectric heating by microwave is applied to several plants for EO extraction. Studies comparing this method with other conventional methods, such as HD, are reported in the literature. Studies have found 67% reduction in extraction time using MAHD when compared to HD (Megawati et al., 2019) and extraction starting in 10 min in MAHD, compared to 30 min in HD (Moradi et al., 2018). This behavior is due to the most efficient heat flow in microwaves. Better yields have been in the extraction time of 10 to 40 min of the procedure, where there is an increase in the absorption of microwave energy with an increase in the MAHD time, which improves the dissolution of essential oil into the water. However, longer times (> 40 min) showed a reduction in EO compounds (Mollaei et al., 2019), and this behavior is due to the degradation that compounds can suffer from the increase in time of the MAHD extraction.
Microwave energy can also be varied to obtain a higher EO yield. The use of up to 500 w helps to increase yield, and consequently the content of compounds (Mollaei et al., 2019). However, the increase above 500 w tends to decrease the amount of compounds. This is motivated by molecular movements caused by electromagnetic waves, which heat the water inside the plant and break the cell walls of the plant. This breakage increases the EO extracted into the water, but the water at a high temperature facilitates the degradation of compounds, consequently reducing yield and quality (Mollaei et al., 2019). The chemical compounds suffer low qualitative variation; however, quantitative differences were observed. Moradi et al. (2018) noticed an increase in oxygenated compounds and reduction of monoterpene hydrocarbons in MAHD when compared to HD. This behavior is justified by the loosening of the plant matrix and increase of essential oil release, as previously mentioned in methods SFME, MGH and MHG.
All the methods presented for the extraction of basil essential oil have advantages and disadvantages in terms of extraction yield, quality of the final product, ease of processing, cost, and waste production. However, its choice is of increasing interest for the preservation of all biological properties of the extracted essential oil, with the aim of application in several areas of science.
Chemical Composition
Essential oils are mixtures of aromatic, volatile organic, and natural compounds produced by many plant species, as a secondary metabolite to play a defense role in the plant; they can be extracted from the various parts of the plant, including flowers, roots, barks, leaves, and seeds (Abdel-Kader et al., 2019; Binello et al., 2014). These complex mixtures of volatile secondary metabolites are present in the form of saturated and unsaturated hydrocarbons, terpenes, oxides, esters, alcohols, aldehydes, ethers, ketones, and terpenes (Abdel-Kader et al., 2019), and are directly linked to the plant’s defense and communication mechanism.
A plant of the same botanical family and the same genus may present qualitative similarity of components (Silvestre et al., 2019). Besides that, the chemical compounds of the EO of the same plant may suffer quantitative variations depending on their genetic traits, the stage of development, climate conditions, drying conditions, storage conditions, and soil properties (Ahmed et al., 2019; Silvestre et al., 2019).
Although there are several studies regarding the main chemical components of basil essential oil, the majority agrees that the main compounds are estragole, linalool, eugenol, eucalyptol, and bergamotene, in varying concentrations. Table 3 shows clearly that the chemical composition of the essential oil varies, being difficult to determine the percentage of each compound present in the essential oil of basil. These variations are attributed to the part of the plant used in oil extraction, the region of collection and the extraction method used. However, it is possible to observe the main components present in the samples.
The qualitative and quantitative differences within the same genus Ocimum basilicum L., in terms of climatic conditions, cultivation and soil, are described in the following studies. Falowo et al. (2019) reported that the chemotypes estragole (41.40%), 1,6-octadien-3-ol, 3.7-dimethyl (29.49%) [synonym: linalool], bergamotene (5.32%), and eucalyptol (3.51%) were the main components extracted by hydrodistillation from basil leaves in the South Africa region. In the study conducted by Stanojevic et al. (2017), linalool (31.6%), methyl chavicol (23.8%), β-elemene (6.9%), and α-bulnesene (4.5%) were the main components extracted by hydrodistillation from the plant (aerial parts), collected from the northwest of the Republic of Srpska, Bosnia and Herzegovina. Al Abbasy et al. (2015) found linalool (69.86%), geraniol (9.75%), p-allylanisole (6.01%), and 1.8-cineole (4.89%), among others, in aerial parts (stem and leaves) of the plant, in the Muscat Governorate region of the Sultanate of Oman. Brada et al. (2011) studied O. basilicum from the Ain-Defla region located in northern Algeria, and found the compounds linalool (44.70%), linalyl acetate (14.00%), 1.8-cineole (6.70%), myrcene (5.60%), and α-terpineol (5.10%). Khelifa et al. (2012) studied O. basilicum from the northern region of Algeria, and found linalool (32.83%), elemol (7.44%), geranyl acetate (6.18%), and myrcene (6.12%) as the main compounds.
According to Ahmed et al. (2019) and Ladwani et al. (2018), the classification of chemotypes varies depending on their geographical source. Plants grown in Europe, USA, and Africa have linalool and methylchavicol as their main compounds. In the Comoros, the Seychelles and Reunion Island, plants have a high concentration of methylchavicol. In India (Assam region, West Bengal, Bihar, Uttar Pradesh, Madhya Pradesh, Maharashtra, and Jammu), they present methylchavicol and linalool. In the original tropical part of India, Pakistan, Guatemala, Haiti, and Africa, they are rich in methyl cinnamate. In northern Africa, Russia, eastern Europe, and some parts of Asia, eugenol is found as a predominant (Ladwani et al., 2018; Padalia et al., 2017). Given the above, several factors may be related to this chemical differentiation by geographic regions. These can be solar radiation, shading, the nutritional quality of the soil, and temperature, among other factors, that can alter/affect genes or metabolic pathways involved in the synthesis of volatiles and terpenes (Carvalho et al., 2016).
A study characterizing seasonal effects was carried out by Carvalho et al. (2016), who used different wavelengths of light-emitting diodes (LED) to affect the yield and metabolic content of Ocimum basilicum L. cv “Caesar.” The study simulated what could occur in open fields throughout the year, and it resulted in higher subset levels of volatile monoterpenoids (blue/red/yellow or blue/red/green wavelengths) and volatile sesquiterpenoids (blue/red/red lengths) [% of chemical compounds not shown]. These wavelengths are detected by plants, in the photoreceptors, which absorb specific spectra. When these photosensors are stimulated, they activate light-sensing chromophores, which absorb this specific wavelength, and direct cytosolic changes in the phosphorylation of proteins in the cell nucleus, responsible for gene expression. This region is related to physiological regulation, which includes growth, plant development, and primary and secondary metabolic regulators (Carvalho et al., 2016; Folta & Carvalho, 2015).
Dörr et al. (2019) applied artificial sunlight (AS) and sulfur plasma light (SPL) (higher blue and green light content) as an alternative light source to the usual high-pressure-sodium (HPS), and evaluated the secondary metabolites and morphology of basil (O. basilicum L.). This simulation found the highest variations of eugenol of 474.0% and 490.6% (AS and SPL, respectively), linalool (110.2% and 107.7%) and eucalyptus (76.3% and 74%) when compared to the HPS (eugenol — 404.9%, linalool — 84.5%, and eucalyptus 51.6%). This light stimulation affects the morphology of the plant Ocimum basilicum in several aspects, such as leaf growth, alters the content of phenolic compounds and chlorophyll, and modulates a physiological response, with variation to the development of compounds. This is similar to what occurs in seasonality, which directly influences the plant's response to the external environment. The reduction of chlorophyll content and consequent photosynthesis causes the plant to increase secondary metabolite compounds with antioxidant action, in order to combat reactive oxygen species (ROS). This metabolic compensation meets the need of the plant to invest more resources in the synthesis of plant protectors instead of enzymes involved in primary metabolism (Dörr et al., 2019).
The different temperatures and the solar radiation that incides in the plant can negatively affect the growth of basil, as well as the occurrence of physiological disturbances, caused by heat in the leaves. Exposure or shading can alter plant development, leaf anatomy, and accumulation of secondary metabolites. They can also favor the development of other compounds that respond to external stimuli, e.g., the condition of 50% shading favored eugenol, whereas exposure favored linalool (Milenković et al., 2019).
The different fertilizers also significantly affect the chemical composition of the plant’s secondary metabolites. In the study conducted by Hanif et al. (2017), who used zinc (Zn) as fertilizer, the O. basilicum, from the Pakistan region, presented variation in the following compounds: linalool (90.84%), estragole (4.21%), α-bergamotene (1.27%), and tau-cadinol (0.87%). Zn acted on several functions: activation of vegetative enzymes, metabolism of carbohydrates, maintenance of cell membranes, synthesis of proteins, and regulation of hormones, as well as the regulation and maintenance of gene expression and other plant metabolites (Jeshni et al., 2017).
Nawaz et al. (2017) used copper (Cu) as a fertilizer in O. basilicum from the Pakistan region. The major BEO components obtained were linalool (70.44%), estragole (14.43%), tau-cadinol (4.13%), and α-bergamotene (3.71%). As previously reported, metallic compounds (Cu, Zn, Cd, Pb) are essential micronutrients of several enzymes in the plant: they act as a functional, structural, and regulatory cofactor for the metabolism of saccharides, photosynthesis, and protein synthesis. The photosynthetic action (CO2 and glucose) is a precursor of monoterpenes, as the synthesis of terpenoids is favored by saccharides (Ahl & Omer, 2009).
Metals have important activities on the metabolites of the plant: their absence or excess interferes in the regulation and maintenance of the plant, favoring, or not the accumulation of important chemical compounds. Similar behavior has been reported by Kunwar et al. (2015) and Singh et al. (2014), in research using O. basilicum L. and O. basilicum cv. CIM-Saumiya from India, respectively. The authors reported that the addition of metals (such as Cu, Zn, Cd, Pb, and others) enabled the production of O. basilicum with specific quality of compounds, favoring the linalool compound.
Fertilizers can lead to improvement and quality of basil harvest, meeting the plant’s need for macro- and microelements. According to the Dehsheikh et al. (2020), fertilizers were used to correct precarious soil in a region of Iran, in order to cultivate O. basilicum var. thyrsiflorum. The study used nitrogen-fixing bacterium, phosphate-solubilizing bacteria, and their combinations, in addition to considering the presence and absence of humic acid. Predominant constituents were methyl chavicol (52.02–62.29%), eucalyptol (5.75–10.94%), α-bergamotene (6.47–8.03%), linalool (2.92–5.42%), and α-cadinol (2.93–4.59%). The variation results from the use of biofertilizers and humic acid, which can interfere in the biosynthetic pathway of compounds. These changes may be caused by the activation or inactivation of enzymes present in the pathways of mevalonate, methylerythritol phosphate, and shikimic acid.
The use of fertilizers was also studied by Burducea et al. (2018). The authors used the cultivars of green-leafed ‘Aromat from Buzau’ and purple-leafed ‘Violet from Buzau’. These plants had the addition of commercial fertilizers (biosolids, Orgevit®, Micoseed®, and Nutrispore®), and there was a control group without their addition. The essential oil of flowers and leaves was extracted by HD, and variation was found for both cultivars. The green-leafed ‘Aromat de Buzau’ presented variations in β-linalol (33.73–39.70%), methyl chavicol (34.43–39.44%), taucinolin (4.70–5.75%), and eucalyptol (3.09–4.79%), among other compounds in a smaller proportion. For the purple-leafed cultivar ‘Violet de Buzau’, there were variations in β-linalool (39.16–54.49%), eucalyptol (8.61–12.85%), β-elemene (3.89–5.91%), and limonene (3.01–5.03%), among others. This study demonstrates that the organic fertilizers Orgevit® and Micoseed® increased β-linalool. These same fertilizers reduced methyl chavicol, whereas Nutrispore® favored this compound in the EO. The chemical variations of the fertilizers, such as different proportions of micronutrients, phosphorus, nitrogen, zinc, copper, and other elements, may have structural and regulatory action in the plant (Ahl & Omer, 2009). These variations will define structurally how the plant will respond to the presence of a fertilizer.
Another study on the use of fertilizers was conducted by Onofrei et al. (2018), who studied foliar fertilizers Fylo®, Geolino Plants and Flowers®, Crompax®, and Fitokondi®. The predominant compounds found were linalool (37.44–49.46%), α-muurolol (11.26–19.26%), and methyl chavicol (2.87–10.39%), among others. The use of Fylo® caused little variation; Geolino Plants and Flowers® favored eucalyptol and methyl chavicol and reduced α-bergamotene, germacrene D, and γ-cadinene; Crompax® favored linalool and eucalyptol; and Fitokondi® favored methyl chavicol. Besides the peculiarities of the chemical elements present in fertilizers from different manufacturers, there is also the ability of the plant to redirect nutrients to produce compounds of silent metabolism and/or produce new secondary metabolites. This is made in order to stimulate the production of plant hormones in response to the external environment, whether in defensive protection or pollination. This activity can favor or harm specific compounds.
Soil management, including macronutrients, significantly influences the quantity and quality of the plant chemotypes. Nurzyńska-Wierdak et al. (2013) demonstrated the correlation of different concentrations of nutrients nitrogen (N) (0.2–0.9 g dm−3) and potassium (K) (0.4–0.8 g dm−3), in response to chemical compounds of O. basilicum L. of Kasia and Wala cultivars. An increase in the concentration of linalool and germacrene D has been reported due to the addition of nitrogen and 1,8-cineol under the influence of potassium. According to the study, the increase in nitrogen and potassium and the interaction of the two doses of macronutrients are related to the increase in concentration of the compounds. The nitrogen fertilization increases the concentration of essential oil in leaves, indicating an increase in oil biosynthesis. This increase is possibly motivated by the increase in the number of leaves in the plant; this increases photosynthetic rates and also the biosynthesis of chemical compounds.
Sifola and Barbieri (2006) mention the behavior of basil leaf increase by nitrogen fertilization, improving photosynthetic and biosynthetic efficiency of terpenes. The nutritional variation of K also affects photosynthetic and biosynthetic rates. Nguyen et al. (2010) shows that the K present in the plant has an activating action of important enzymes of photosynthesis. It also acts on the biosynthesis of starch and proteins, among other metabolites, thus interfering in the concentrations of compounds.
The application of elicitors (such as phytohormone) can also influence the production of secondary metabolites. According to Złotek et al. (2016), the use of jasmonic acid in different concentrations, as an inducer in the plant O. basilicum L., promotes the variation of the following chemical compounds: methyl eugenol (10.84–40.69%), eugenol (15.29–24.88%), 1,8-cineole (9.05–20.34%), linalool (4.98–20.88%), and (Z)-caryophyllene (4.53–9.53%). The application of jasmonic acid, a phytohormone of the plant, caused abiotic stress in the plant. This, in a protective way, induced the quantitative production of compounds, and even qualitative ones, such as camphor, bornyl acetate, α-humulene, and trans-β-farnesene.
In agricultural systems, salinity causes stresses that reduce plant production. According to Tarchoune et al. (2013), salts, such as NaCl and Na2SO4, alter the chemical composition of the O. basilicum essential oil. In addition, the authors reported that methyl eugenol is favored when eugenol decreases. The bioconversion of these compounds suggests a change in the release of enzyme activities, since the conversion of eugenol to methyl eugenol, via O-methyltransferase, is catalyzed by S-adenosyl-L-methionine (SAM), which replaces eugenol in para-hydroxy position to give rise to methyl eugenol. Methyltransferases are enzymes that catalyze the transfer of a methyl group to a substrate, producing O-, N-, S-, and C-methyl derivatives and S-adenosyl-homocysteine. According to Heidari (2011), the salt stress of basil genotypes demonstrates the reduction in the plant’s osmotic action, affecting its ability to absorb water. In addition, specific salts can enter the transpiration stream, damaging leaf cells and directly interfering with photosynthetic capacity and cell growth. As a consequence, there is decrease in stomatal and mesophilic CO2 conductance. The reduction in photosynthesis can be attributed to a decrease in the content of chlorophyll present. However, this behavior can vary between plants, e.g., salt-tolerant plants have higher chlorophyll content than intolerant plants.
Studies conducted by Farsaraei et al. (2020) and Talebi et al. (2018) used elements to reduce the harmful effects of salt stress, such as superabsorbent polymers (SAPs) and methyl jasmonate, respectively. The results of Farsaraei et al. (2020) show that the severity of NaCl increased 1,8-cineol and tau-muurolol, and decreased linalool and α-cadinol. However, when the different SAPs were applied, the main components found were linalool, eugenol, tau-muurolol, α-cadinol, and α-eudesmol. This chemical differentiation of the main compounds is due to the relief of salinity stress that SAPs promote to the plant. SAPs increase the capacity of the soil to retain water and nutrients, improving plant growth and thereby reducing salt stress.
According to Talebi et al. (2018), the salt stress applied in O. basilicum increased the concentration of 1,8-cineol, linalool, β-maaliene, and α-cadinol and decreased α-bergamotene for the cultivar Genove. The cultivar Ruby increased 1,8-cineol and eugenol and decreased linalool, α-bergamotene, β-maaliene, and α-cadinol. After the application of methyl jasmonate (0.5 mM), there was an increase in the compounds linalool and 1,8-cineol (for cv. Genove) and 1,8-cineole, α-cadinol, and β-maaliene (for cv. Ruby). The application of methyl jasmonate increases the regulating function that is present in the synthesis of secondary metabolites. This function occurs in response to biotic and abiotic stresses, and it can also have an influence on gene regulation and enzyme activity in the metabolic pathways.
Mandoulakani et al. (2017) studied the abiotic effect of drought stress on the expression of the genes involved in the biosynthesis of phenylpropanoid. In O. basilicum cv. Keshkeni Luvelou, the authors observed the genes chavicol O-methyltransferase (CVOMT), eugenol O-methyl transferase (EOMT), cinnamate-4-hydroxylase (C4H), 4-coumarate coA ligase (4CL), and cinnamyl alcohol dehydrogenase (CAD). These genes are involved in the pathway of biosynthesis of phenylpropanoids (methylchavicol and methyleugenol) and the expression of these genes is related to the accumulation of these compounds. It was observed that water stress increased the amount of methylchavicol, methyleugenol, β-mirceno, and a-bergamotene; there was an increase in CVOMT and EOMT expression, a decrease in the 4CL and C4H gene, and the continuity of CAD. Genetic manipulation in order to increase chemical compounds is possible: enzymatic reactions through metabolic channels are catalyzed by the enzymes CVOMT and EOMT, involved in biosynthesis. They catalyze the conversion step of eugenol and chavicol to methyleugenol and methylchavicol using S-adenosyl methionine (SAM) as the methyl donor.
The water stress on basil essential oil was studied by Ekren et al. (2012), who cultivated O. basilicum L. in 2007 and 2008, and used 4 different levels in the irrigation water. It was necessary to raise root zone soil water to field capacity (I50 50%, I75 75%, I100 100%, and I125 125% of field capacity), and the main compounds found were linalool, eugenol, and methyl chavicol. The greatest variation was of linalool, 69.10–73.18%, in the highest water level (I125) in 2007, and in the year 2008 the range found was 61.02–68.88%, in the water level I100. The chemical variations arising from water stress are due to the negative effects on carbon gain/flow, which is caused by the closure of stomata, reducing the capacity for CO2 assimilation, and the biosynthesis of the phenylpropanoid pathway. However, there is an increase in the biosynthesis of terpenoids as a defense response to the external environment.
The water stress of Ocimum basilicum in response to the composition of the essential oil has been reported by Mota et al. (2020). They described the water stress applied to Ocimum basilicum L. and analyzed the chemical profile in two stages of growth: vegetative (PV) and flowering (FP). Variations were found in the following chemical compounds: linalool — 17.0% (PV) and 46.0% (PF) in the control treatment, and linalool — 25.1% (PV) and 52.8% (FP) in water stress. For the eugenol compound, the control treatment was 16.2% (PV and FP), and in stress, the values found were 50.8% (PV) and 10.9% (FP). The water stress behavior was reported. However, another behavior was observed: chemical differentiation in the vegetative and flowering phases. This is justified, as the vegetative phase has a higher level of transcripts of eugenol-o-methyltransferase (EOMT); in contrast, in the flowering phase occurs the reduction of EOMT transcripts, demonstrating the variety of chemotypes. This strengthens the theory that the metabolic pathways have a transcriptional reprogramming associated with a decrease in the accumulation of metabolites.
Basil varieties have different chemical profiles. According to Koroch et al. (2017), the 10 varieties of O. basilicum showed different chemical profiles, but linalool and methyl chavicol were the components with the greatest variations (0.2 to 59.4% and 0 to 59.5%, respectively). In the study reported by Baldim et al. (2018), the varieties of O. basilicum named Green Basil and purpurascens presented linalool (24.7–60.2%), eucalyptol (0.95–30.04%), and α-trans-bergamotene (0–20, 33%), among others. Couto et al. (2019) studied 20 commercial cultivars and 4 experimental hybrids, and detected linalool, methyl chavicol, neral, geranial, eugenol, and (E)-methyl cinnamate at highest amounts. Other examples of studies describe the chemical diversity of basil varieties, as studied by Padalia et al. (2017), who found methyl chavicol (56.1–89.7%) and linalool (1.0–33.7%) as the main compounds of three O. basilicum var. OB-1G, OB-5P, and CIM-Soumya. These results prove that the chemical differentiation is related to the biosynthetic pathways of the secondary metabolites of each cultivar; for example, the compounds methylchavicol and eugenol have common biosynthesis of the same precursors (L-phenylalanine and cinnamic acid). However, the compound linalool presents another biogenetic pathway, that of mevalonic acid via geranyl pyrophosphate (Marotti et al., 1996). In this way, different cultivars of basil have the genetic capacity to generate and maintain different sets of chemical compounds within the same species.
The wide qualitative and quantitative variation in the compounds of basil essential oil is a common phenomenon. It is known that each plant with the same genetic background (Ocimum) will present a profile of distinct compounds, which are determined to be biotic or abiotic in nature. However, it is difficult to determine clearly what are the genetic and environmental contributions to the chemical compounds, since the process of compound biosynthesis is complex. Therefore, the exploration of several specific metabolic pathways for each compound is necessary. There may even be an interrelation between primary and secondary metabolism, making it difficult for the entire process of identification (Dehsheikh et al., 2020).
Therefore, this review allows understanding that it is possible to adapt the planting and cultivation of basil and obtain high yield and good quality of chemical compounds of interest and that they can present biological activities so important for new applications and industrial products. In addition, it has been shown that basil essential oil can exhibit various chemical compounds and that they can suffer the most diverse influences. The literature has so far provided the identification of about 100 molecules, which can be expanded due to improved methods of extraction and identification. However, some of them are more often reported, as mentioned in Table 3.
In this review, several studies of O. basilicum and its essential oil were addressed, emphasizing chemotypes and evaluating biotic and abiotic factors in response to their quantity and quality of compounds. In addition, there is a considerable amount of data that investigates the relationship between extraction methods and the chemical profiles of basil essential oil, which will be discussed in the next section.
Biological Activities
Over the years, the application and use of EOs have received attention due to their possible biological activities (Avetisyan et al., 2017; Baldim et al., 2018; Złotek et al., 2016). Several biological activities have been reported in the literature, such as antiseptic, analgesic, antidiabetic, wound healing, radiation protection, immunomodulatory, antifertility, anti-inflammatory, antistress, anticarcinogenic, antiviral, antimicrobial, antifungal, and antioxidant activities (Avetisyan et al., 2017; Baldim et al., 2018; Dehsheikh et al., 2020; Gaio et al., 2015; Koroch et al., 2017; Singh & Chaudhuri, 2018).
The antibacterial, antifungal, antioxidant, anti-inflammatory, and insecticidal activities, summarized in Table 3, will be addressed in more detail below.
Antibacterial Activity
Essential oils are widely known by their antimicrobial activity. Contrary to what is expected for healthy cells, the application of essential oils on deteriorating or pathogenic microorganisms aims at their inhibition or elimination. According to Baldim et al. (2018), basil essential oil has effective antimicrobial action against Gram-positive bacterial strains such as Bacillus cereus, Staphylococcus aureus, and Listeria monocytogenes, and against Gram-negative bacteria such as Salmonella sp. and Pseudomonas aeruginosa. However, it demonstrates that the main component (linalool) is not the only one responsible for this biological activity, depending also on other components in a smaller proportion (synergistic effect). This synergistic effect is possible, as linalool has higher cell membrane fluidity and changes cell permeability. This facilitates the entry of the components in smaller proportions.
In another study with different cultivars of O. basilicum, Avetisyan et al. (2017) observed that cultivars O. basilicum var. thyrsiflora and O. basilicum var. purpureum have proven effective against S. aureus and B. subtilis, respectively. They showed differences in the main chemical composition, such as 57.3% methyl-chavicol (estragole) for var. purpureum and 68% linalool for var. thyrsiflora. This difference helps in understanding that different portions of chemical components may favor synergisms among compounds with lower portions, and can define broader antimicrobial actions (different bacterial strains).
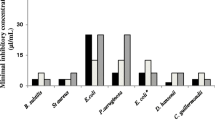
Due to its antibacterial effect, the basil essential oil can be compared with commercial antibiotics, as reported by Stanojevic et al. (2017), who compared BEO with ciprofloxacin and gentamicin, against 10 bacterial strains. The BEO showed a high inhibitory effect, with larger inhibition zone diameters (mm) for strains of Salmonella enterica (28.33 mm), Providencia stuartii (35 mm), Coagulase-positive Staphylococcus (40 mm), and Streptococcus group D (30 mm), when compared to ciprofloxacin (25.00, 0.00, 31.33, and 26.00 mm, respectively) and gentamicin (25.00, 5.00, 26.66, and 27.33 mm, respectively). The antimicrobial activity of BEO is a consequence of all the compounds present, as a synergistic action (Jugreet & Mahomoodally, 2020). This variation in the BEO, as well as in other EOs, can prevent the emergence of resistance, since several bacterial targets need to adapt to hamper the effects of each essential oil compound (Yap et al., 2014).
The indiscriminate use of antibiotics led to the development of bacterial resistance. The mechanism of action of antibiotics tends to interfere with cell wall biosynthesis, inhibition of protein synthesis, interference with nucleic acid synthesis, and inhibition of metabolic pathways, among others (Yap et al., 2014). Once resistance to these multiple antibiotics is acquired, it results in the selection of stronger drugs (Freitas et al., 2020), causing greater health problems. The favoring of bacterial resistance intensified the search for studies aimed at identifying new natural antimicrobial compounds.
This growing interest of the scientific community for new alternatives to complement antimicrobial therapies motivates the application of basil essential oil in several bacterial strains, in order to evaluate the concentration capable of inhibiting their proliferation and/or proportioning their death. In this context, this review summarizes the action of basil essential oil against several bacterial strains, such as Acinetobacter sp., Aeromonas sp., Citrobacter freundii, Enterococcus faecalis, Klebsiella pneumoniae, Micrococcus luteus, Salmonella choleraesuis, Sarcina sp., Serratia sp., Shigella flexneri, Streptococcus mutans, and Yersinia enterocolitica (Gaio et al., 2015); Bacillus megaterium, Listeria monocytogenes, Shigella boydii, Shigella dysenteriae, Vibrio mimicus, and Vibrio parahaemolyticus (Hossain et al., 2010); Bacillus cereus (Al Abbasy et al., 2015; Baldim et al., 2018; Elansary et al., 2016; Hossain et al., 2010; Maggio et al., 2016; Tavallali et al., 2019); Bacillus subtilis (Carović-Stanko et al., 2010; Hossain et al., 2010; Joshi, 2014; Maggio et al., 2016; Shirazi et al., 2014; Tavallali et al., 2019); Escherichia coli (Al Abbasy et al., 2015; Carović-Stanko et al., 2010; Elansary et al., 2016; Hossain et al., 2010; Koroch et al., 2017; Maggio et al., 2016; Predoi et al., 2018; Shirazi et al., 2014; Sienkiewicz et al., 2013; Tavallali et al., 2019); Proteus mirabilis, Pseudomonas aeruginosa (Gaio et al., 2015; Maggio et al., 2016); Proteus vulgaris (Gaio et al., 2015; Maggio et al., 2016; Shirazi et al., 2014); Salmonella enteritidis, Salmonella paratyphi (Shirazi et al., 2014); Salmonella typhimurium (Al Abbasy et al., 2015; Hossain et al., 2010; Maggio et al., 2016; Tavallali et al., 2019); Staphylococcus aureus (Al Abbasy et al., 2015; Chenni et al., 2016; Elansary et al., 2016; Gaio et al., 2015; Hossain et al., 2010; Joshi, 2014; Koroch et al., 2017; Maggio et al., 2016; Predoi et al., 2018; Shirazi et al., 2014); Staphylococcus epidermidis (Gaio et al., 2015; Joshi, 2014; Maggio et al., 2016); and Streptococcus faecalis (Joshi, 2014; Maggio et al., 2016).
Previous reports showed higher antibacterial effects on Gram-positive rather than Gram-negative bacteria. This is because the external membrane of Gram-negatives is composed of lipopolysaccharides (hydrophilic), which restricts the diffusion of hydrophobic compounds of essential oils (Gaio et al., 2015; Koroch et al., 2017; Maggio et al., 2016; Tariq et al., 2019). EO compounds have important hydrophobic characteristics, which favors the association with membrane lipids and mitochondria of bacterial cells. This association disturbs the cell structure by facilitating wall permeability, leading to bacterial death due to the leak of molecules and cell ions (Tariq et al., 2019).
Antifungal Activity
Many in vitro studies have evaluated the antifungal activity of basil essential oils. The antifungal bioactive components in BEO may target various chemical pathways or cell structures, such as cell wall degradation, membrane damage, and interruption of proton motive force (Bhavaniramya et al., 2019).
Antifungal activity was studied by Vieira et al. (2014), who examined the action of different Ocimum species with anticandidal properties (Candida strains). The species O. micranthum (main compounds: eugenol — 64.8% and E-caryophyllene — 14.3%), showed inhibition of all Candida species. The species O. selloi (main compounds: trans-anethole — 52.2% and linalool — 16.8%) showed antifungal activity against all Candida species. O. americanum (main compounds: (Z)-methyl cinnamate — 29.4%, 1.8-cineole — 25.9%, linalool — 9.6%, and α-terpineol — 9.3%) presented antifungal activity only for C. tropicalis, C. parapsilosis, and C. albicans. The species O. basilicum var. purpurascens (main compounds: linalool — 41.5% and α-muurulol — 11.8%) showed antifungal activity only for C. parapsilosis and C. albicans. The species O. basilicum var. minimum (main compounds: linalool — 44% and 1,8-cineole — 15.5%) did not inhibit any Candida, which is probably due to the presence of the monoterpene alcohols as main constituents.
Nawaz et al. (2017) studied the effect of different concentrations of Cu as a fertilizer on the chemical composition of basil essential oil and its biological activity. They observed that different concentrations of Cu increased the concentration of the main components linalool, estragole, and gamma-terpinene, significantly increasing the antifungal activity of basil essential oil. The authors reported that the biological action is due to the presence of linalool and other oxygenated monoterpenes. This bioactivity may occur in several ways, by the adherence of the compounds to the fungal cell wall. This causes an anomaly in membrane fluidity, which leads to cytoplasmic loss and consequent interruption of ATP assembly. These affect the Ca2+ and H+ homeostasis, causing loss of ions, and consequently, loss of cell viability. Besides that, the permeability of mitochondria may be altered by the compounds, causing an alteration in the flow of electrons and leading to apoptosis and cell necrosis (Gundewadi et al., 2018; Raut & Karuppayil, 2014; Tariq et al., 2019).
Studies used different strains of fungi and showed promising results for antifungal properties in vitro of BEO, e.g., the following strains: Alternaria alternata (Ahmad et al., 2016; Marco et al., 2020); Alternaria tenuissima (Marco et al., 2020); Aspergillus flavus (Ahmad et al., 2016; El-soud et al., 2015; Gundewadi et al., 2018); Aspergillus fumigatus (Ahmad et al., 2016; Beatović et al., 2015); Aspergillus ochraceus, Aspergillus versicolor, Penicillium funiculosum, Penicillium ochrochloron, and Trichoderma viride (Beatović et al., 2015); Aspergillus niger (Ahmad et al., 2016; Beatović et al., 2015; Hanif et al., 2017; Tavallali et al., 2019); Candida albicans (Tavallali et al., 2019); Penicillium (Ahmad et al., 2016); Penicillium notatum (Hanif et al., 2017); Penicillium chrysogenum (Gundewadi et al., 2018); Penicillium nalgiovense, Penicillium sp. (Saggiorato et al., 2012); and Rhizopus solanai (Ahmad et al., 2016).
The effectiveness of basil essential oil is proven by the studies presented; the identification of isolated or combined phytochemicals helps to identify the compounds responsible for antifungal activities, the mechanism of action and which cells are least resistant.
Antioxidant Activities
Over the last few years, some research has reported the antioxidant activity of essential oils, in response to growing consumer demand for the replacement of synthetic antioxidants by natural products (Sharma et al., 2019). The antioxidant activities of basil essential oil have been addressed using various free radical scavenging methods, such as ABTS, DPPH, and the ability to reduce iron (FRAP).
The improvement of techniques for identification of the essential oil compounds, as well as improvements in the analytical methods of antioxidant action, showed that this action is the result of a synergistic effect among the compounds, not referring to only one isolated compound (Tavallali et al., 2019). The antioxidant action can come through different mechanisms, such as free radical sequestration, hydrogen donation, and metal ion chelation.
The methylation behavior was reported by Koroch et al. (2017), who mentioned that the highest antioxidant activity is associated with linalool-eugenol, with a predominance of the highest eugenol content; on the other hand, essential oils with the highest concentrations of linalool and methyl chavicol presented the lowest reducing activity. This behavior was observed and correlated to the hydroxyl group (OH) present in some compounds, such as methyl eugenol, which is blocked by methylation, thus decreasing the antioxidant activity. The authors also reported that additional methoxy groups considerably increase antioxidant activity, which explains the high antioxidant activity of eugenol (presence of the methoxy group in the molecule).
The higher antioxidant potential of the BEO is correlated with the highest proportion of compounds containing a phenolic ring with an OH group, not excluding minor components. Chenni et al. (2016) reported that the main component does not always determine the antioxidant activity of an EO, being necessary to identify compounds in lower concentrations due to their synergistic effects when combined with other compounds. Furthermore, Couto et al. (2019) reported that the mixture of compounds can overcome this bioactivity when compared with isolated compounds. According to Złotek et al. (2016), the application of jasmonic acid considerably increased the antioxidant activities when compared to the control, and this biological activity is attributed to the higher levels of linalool and eugenol. In addition, the authors reported a possible correlation between the proportion of eugenol and the antioxidant capacity, where the increase in the eugenol concentration influences the increase in bioactivity. This behavior was also mentioned by Chenni et al. (2016).
Hydroxyl groups have been reported as primarily responsible for the high antioxidant activity of basil essential oil. They are characterized by the ability to donate hydrogen atoms to free radicals, sequestering and inactivating them (Rodklongtan & Chitprasert, 2017; Tavallali et al., 2019). However, the absence of an aromatic ring in the molecule containing an OH group (i.e., 1,8-cineole and linalool) and some other monoterpenes results in low antioxidant properties (Koroch et al., 2017).
Overall, the bioactivity depends on the structure and variation of the chemical compounds present in basil essential oil.
Anti-inflammatory Activity
Inflammation is a response of the body, whose function is to protect the organism against attacks by an endogenous or exogenous etiological agent. In response, the body alters vascular and cellular functions, sending defense chemicals to inflammation. When the inflammatory process persists, it can cause tissue damage caused by offensive tissue cells (such as macrophages and lymphocytes) and present symptoms potentially harmful to the body, such as the development of lethal chemicals, both for the inflammatory aggressor and the human body itself (Rodrigues et al., 2016).
The study conducted by Złotek et al. (2016) evaluated the anti-inflammatory action of BEO, reporting that an increase in chemical compounds of basil essential oils, such as eugenol, linalool, and limonene, showed a high anti-inflammatory activity. The authors also reported that these compounds may be responsible for inhibiting cyclooxygenase 2 (COX-2), an enzyme that acts in the inflammatory process.
A study comparing steroidal and non-steroidal anti-inflammatory drugs was reported by Rodrigues et al. (2016). The estragol in Ocimum basilicum caused reduction in acute and chronic systemic inflammation in paws of rats, when compared to the steroidal anti-inflammatories (dexamethasone and indomethacin). This action occurs through the inhibition of receptor mechanisms and chemical mediators from the inflammatory process.
Studies also report the correlation between inflammatory response and antioxidant activities. According to Miguel (2010), the inflammatory process of the body favors the release of oxidative compounds and reactive oxygen species (ROS), and the free radical scavenging efficacy is considered important to reduce inflammation (Raut & Karuppayil, 2014). In addition, other activities are linked to the mechanism of inhibition or reduction of inflammation, such as inhibition of lipoxygenase, prevention of leukotriene synthesis, inhibition of the COX-2 enzyme, inhibition of pro-inflammatory cytokines, interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α), and suppression of pro-inflammatory genes (Miguel, 2010; Raut & Karuppayil, 2014).
Insecticidal Action
Insecticides, repellents, and synthetic pesticides are considered toxic to the human body (Pandey et al., 2014), and alternative control measures are needed. Basil essential oil (O. basilicum L.) has presented insecticidal activity, being able to replace synthetic chemicals. This replacement aims at human and environmental safety (Benelli et al., 2019; Wang et al., 2019).
According to the studies reported by Beier et al. (2014), linalool is the compound responsible for insecticidal action against fruit and house flies. This action can be explained by the effects that this compound promotes on the central nervous system. Wang et al. (2019) reported that monoterpenoids have powerful actions on this system, with the inhibition of acetylcholinesterase (AChE) and the modification of γ-aminobutyric acid (GABA) receptors and octopamine receptors.
Chenni et al. (2020) reported that the main essential oil compounds of O. basilicum L. — linalool, methyl chavicol and methyl eugenol — had a toxic effect, by contact and ingestion, against cereal insects S. oryzae, R. dominica, and T. castaneum, being indicated as a potential environmentally friendly insecticide. Ottai et al. (2012) presented the insecticidal activity of BEO from two cultivars (Egyptian and French) against the insects Rhyzopertha dominica. The results showed that BEO had an insecticide effect of 100%, attributed to the presence of linalool.
The fumigation of basil essential oil was reported by Hossain et al. (2014), who studied the mortality of adult S. oryzae insects and found that BEO was highly toxic to the insect, confirming the results of insecticidal and repellent properties. Follett et al. (2014) report that fumigation caused high mortality in rice insects (Sitophilus oryzae L.) and can be used to control pests.
The secondary metabolites of plants serve ecological functions, such as defensive or protective, mainly against insect attacks (Oliveira et al., 2014). These metabolites, such as EOs, have potent insecticidal activity against a wide spectrum of insects and other pests, and therefore could be used as an alternative to synthetic insecticides.
Anticancer Activity
Anticancer activity is intended to prevent the proliferation and growth of some cancer cells (Sharma & Jana, 2020). Several studies report the important influence of the essential oil compounds on biological pathways and regulation of cell cycles, such as angiogenesis and metastasis (Mahmoud & Abdelrazek, 2019). The compounds can induce preventive mechanisms, act in the tumor cell itself, and interact with environmental factors that promote the development of cancer (Bouyahya et al., 2020).
Numerous published reports have suggested the anticancer potential of monoterpenes against various cell lines by apoptosis; however, few studies report the anticancer activity attributed to the basil essential oil. Taie et al. (2010) used the line Ehrlich ascites carcinoma cells (EACC) and observed a decrease in cell viability with the increase in BEO concentration. This bioactivity may be due to the monoterpenes present in BEO: camphor, limonene, thymol, citral, geraniol, and linalool.
Singh and Chaudhuri (2018) reported that the essential oil extracted from the leaves of plants of the genus Ocimum showed antiproliferative activity against cells of a mammary lineage that present tumor phenotype at MCF-7. The authors mentioned terpenoids and flavonoids as the main compounds responsible for this behavior.
Few studies reported the anticancer activity of BEOs, and as a consequence, there is a lack of information related to their mechanism of action. Despite this, linalool, a main compound of BEO, has been widely reported in the literature: several studies described its anticancer action. According to Pereira et al. (2018), linalool induces the stopping of the cycle of human prostate cancer cells (DU145). Chang et al. (2015) reported that this compound induced apoptosis in the line of myeloid leukemia cells (U937). Cerchiara et al. (2015) demonstrated that linalool has pro-apoptotic effects on a human melanoma cell line (RPMI 7932). Besides these, other studies describe the anticancer activity of linalool in different cell types, such as hepatocellular carcinoma cells (HepG2) (Rodenak-Kladniew et al., 2018), human-alveolar-adenocarcinoma cells (A549) (Rodenak-Kladniew et al., 2014), among others.
Toxicological Effects
The chemical constituents of EOs are studied for their potential in many functions: anticancer, antioxidant, anti-inflammatory, antimicrobial, antistress, analgesic, diuretic, and insecticide, among others (Gündel et al., 2018; Shirazi et al., 2014).
The application of EOs directly to food or packaging to control microorganisms is also a growing trend as an alternative source to the chemicals used. Chenni et al. (2020) evaluated the toxicity of O. basilicum essential oil extracted by HD and SFME methods, encapsulated with maltodextrins and gum arabic, in insects collected from stored cereals. The insects examined were Sitophilus oryzae (rice weevil), Rhyzopertha dominica (smaller borer), and Tribolium castaneum (red flour beetle), by the technique of direct contact and ingestion. The maximum tested concentration (1 g/kg) of essential oil obtained by encapsulated SFME was the most efficient, causing the mortality of 93.68% by contact and 85.26% by ingestion for R. dominica. No concentrations of essential oil tested, obtained by different extraction techniques, had potential to combat T. castaneum through direct contact or ingestion.
Basil essential oil also had its toxicity tested against human cells, which showed that it is not toxic to healthy cells. Stanojevic et al. (2019) revealed the absence of skin irritation after topical application of BEOs under occlusion. This oil could be used in skin hyperpigmentation treatment, addressing problems such as the appearance of dark spots and freckles caused by aging. However, it can also decrease skin moisture in all sites, and its frequent use could cause increased dryness in skin.
Hemolytic activity is an important test for evaluating essential oils that have potent biological activity: if they have hemolytic effects, they are not useful for applications in food, packaging, and pharmacological preparations. Basil essential oil isolated by SFE method was tested in vitro in human erythrocytes (O blood groups) and presented minimum activity (18.59%), while the hydro-distilled essential oil exhibited higher value (35.83%). Therefore, it is possible to infer that SFE is a better method for extraction of basil essential oil with lower hemolytic activity, when compared to the HD method (Abbas et al., 2017).
Shirazi et al. (2014) applied O. basilicum essential oil in nasopharyngeal cancer (KB) and liver hepatocellular carcinoma (HepG2) cell lines, using a modified MTT assay. They concluded that low concentrations (1–10 µg/mL) had no effect on KB and HepG2 viability. However, at higher concentrations (10–100 µg/mL), cell viability was significantly reduced in a concentration-related manner, with the maximum effect at 200 µg/mL. IC50 was 45 ± 4 µg/mL for KB and 40 ± 3 µg/mL for HepG2. Among the several possible applications of essential oils, one could study their cytotoxicity against human tumor cells and see if they are promising for use in the treatment of diseases.
Onyebuchi and Kavaz (2019) developed chitosan (OGEO-CSNPs) and N,N,N-trimethyl chitosan (OGEO-TMCNPs) nanoparticles loaded with O. gratissimum essential oil (OGEO). The authors evaluated cell viability using different concentrations (5 to 100 µL/mL) of these nanoparticles and pure essential oil in breast cancer cells MDA-MB-231. It was found that the viability of the cells decreased with a corresponding increase in concentration in all treatments. In addition, OGEO-TMCNPs nanoparticles significantly inhibited the viability of the cancer cell line at much lower concentrations compared to other treatments. The high antiproliferation activity of cells with the application of pure essential oil or in nanoparticles is due to the generation of oxidative stress with the cell membrane.
Cytotoxicity studies contribute in proving that BEO is safe for application in the fields of medicine and food. Furthermore, cytoxicity approaches show that this EO can be added directly to food, in nanoemulsions, capsules, or packaging, due to its antioxidant, antimicrobial, and antifungal effects, among other bioactivities. In addition, different parameters can be used, such as dosages, contact time, and form of application (direct, by spray, encapsulated). This allows verifying which concentration and application condition is safe and does not promote undesirable toxicological effects. Table 4 summarizes some cytotoxic studies of BEO, showing the influence of the extraction method, its form of application, and the main results in different cells tested.
In general, EOs do not produce a cytotoxic effect when used in low concentrations. However, further research is needed on the isolated interference of each chemical compound present in EOs, in order to assess their interference with the toxicity characteristics in cells and therefore predict also its possible negative effect in healthy cells. In addition, normalization of the dose and incubation time in cell and animal models will allow a better understanding of the biological activities and mechanisms of EOs.
Applications of Basil Essential Oil in Food
Basil essential oil has been widely used in food production, flavoring, preservation, and safety. Due to its pleasant fragrance, basil oil is used as an additive in foodstuffs such as sausages, cheeses, and alcoholic and nonalcoholic beverages. In addition, BEOs are widely used in combination with other spices and herbs in a variety of foods: confectionary and bakery products, sweets, puddings, condiments, vinegars, ice creams, mustard, and pickled vegetables (Li & Chang, 2016).
Besides that, the use of BEO as a natural food preservative has steadily gained recognition in the literature. There are several studies on the application of BEO with this purpose, reporting the following uses: natural antioxidant additive in meat, improving color and lipid oxidative stability (Falowo et al., 2019); antibacterial agent in Italian-type sausage, reducing the count of Staphylococcus aureus until the 14th day of storage (Gaio et al., 2015); antifungal compound in Italian-type sausage (Saggiorato et al., 2012) and fermented sausages (Kocić-Tanackov et al., 2020), being a possible substitute for chemical additives; and incorporated in fish protein isolate/fish skin gelatin-ZnO nanocomposite film to increase the shelf life of refrigerated sea bass slices (Arfat et al., 2015). In addition, the BEO can be added to dairy products, being an antioxidant and antimicrobial agent and enhancing the flavor of these products (Licon et al., 2020). Some of these applications are described in Table 5.
The chemical composition of BEO is directly influenced by the place of harvest, cultivars (green and purple), climatic conditions, collection, and stages of harvesting (Singh & Chaudhuri, 2018). Moreover, the composition interferes in the aroma, personality, and application of EOs (Li & Chang, 2016).
These oils can be incorporated or coated onto synthetic packaging films, due to their antimicrobial effect on food products and consequent increase of shelf life (Adelakun et al., 2016). The choices of EO and its concentration are important when adding it to a given food: this addition can cause sensory changes, even in small quantities. The intense aroma of the oil can affect the flavor of food, but synergistic combinations of EOs with each other or with other barrier techniques can reduce this effect (Mariod, 2016).
The methods and temperatures used in drying basil directly interfere with the sensory characteristics of the product. Higher temperatures usually imply decrease in attributes of odor such as fresh, floral, and herbaceous, and increase in others: spicy, hay-like, sweet, earthy, woody, and infused (Calín-Sánchez et al., 2012).
In addition, the volatile compounds present in different species of essential oils are responsible for the characteristic aroma notes. Jirovetz et al. (2003) reported that in O. americanum, the herbal-fruity and sweet-balsamic aspects of odor are observed by the presence of methyl cinnamates, camphor due to some monoterpenes, pine of pinane structures, and green notes of hexane derivatives. In O. basilicum, linalool and its oxides would generate notes of linalool and eugenol, as well as spicy notes of methyl eugenol with a direction of clover. In O. gratissimum, pepper notes can be attributed to (Z)-β-ocimene, germacrene D, and β-caryophyllene. In O. sanctum, spicy green notes are due to methyl eugenol and spicy pepper notes which are derived from β-caryophyllene and its oxide and germacrene.
The antifungal, antibacterial, and antioxidant properties of essential oils can benefit foods without interfering negatively in their sensory aspects. Possible alternatives are to encapsulate these before application in foods or add them to packaging, making them active (Arfat et al., 2015). The use of techniques that do not use high temperatures is promising for the encapsulation of BEO and isolated EO compounds, allowing their slow and gradual release into food. Such techniques can be coacervation (Ngamakeue & Chitprasert, 2016), emulsion freeze-drying (Chenni et al., 2020), and inclusion complex by ultrasound method (Siva et al., 2020). Another option is the development of nanocomposite film that can be used as an edible active coating or primary packaging in foods with the incorporation of BEOs (Arfat et al., 2015; Bonilla et al., 2014) or emulsions with micro- and nanometric size particles (Shokri et al., 2020; Xiong et al., 2020). Although the studies carried out by Shokri et al. (2020) and Xiong et al. (2020) used EOs of Ferulago angulata and oregano, the development principle can be extrapolated to incorporate BEO, due to the similarity of the hydrophobic characteristic of the compounds.
The encapsulation of EO and the use of nanotechnologies are fields that have great potential to be explored. They can allow the use of lower concentrations that are still capable of maintaining antimicrobial efficacy. This is key for the implementation of EOs in the preservation of food, without undesirable organoleptic effects.
Concluding Remarks and Future Perspectives
There is a growing market demand for natural products such as essential oils, since their bioactive material can be applied in food systems. However, this area presents some challenges. A fundamental point regards the variations in the chemical profile of BEO, conditioned by factors as geographical region of production, climatic conditions, and genetics. They need to be considered, besides the method or technology used for oil extraction. Consequently, the different chemical characteristics of BEO influence its potential and effectiveness of application in different areas.
Moreover, despite the wide range of studies investigating the bioactive properties of BEO, its effective application in the food area is very limited and there seems to be a lack of more detailed in situ and in vivo experiments. The possibility of direct application of EO seems to be limited, due to causing undesired sensory effects. The encapsulation of this bioactive compound to create delivery systems is a promising technique in the areas of food and packaging. It allows slow and gradual release of compounds; they present their bioactive effects, but are not able to interfere negatively in sensory aspects.
To conclude, this review demonstrated the main advantages and challenges of extraction and identification of compounds present in basil essential oils of different species, from different geographic regions and obtained by various extraction methods. In addition, antibacterial, antifungal, and antioxidant activities were presented. Thus, the studies reported in this review suggest great perspectives for the use of BEO, but further investigation is needed to clarify some points: how the compounds act, whether they act alone or synergistically in their bioactive actions, and their mechanisms of toxicity in relation to the most diverse cells.
The use of EOs in the food, cosmetic, and pharmaceutical markets has been growing considerably in recent years, due to the market appeal of natural products. However, aspects still to be ensured are quality and safety in their use. BEO presents a wide variation in chemical profile; thus, this oil needs further exploration. Only by guaranteeing biosafety can BEO have potential application in the food, cosmetic, and pharmaceutical areas.
This overview of BEO addressed the following points: extraction methods, variations of chemical compounds, biological activity, and toxicology. The chemical constituents undergo variations involving extraction techniques (conventional and alternative), geographical variation, seasonality, genetics, and other factors. The chemical structure of the compounds can favor biological activities: antibacterial, antifungal, antioxidant, anti-inflammatory, insecticide, and anticancer. Although the results presented in this review constitute a rich database about the essential oil of basil (Ocimum basilicum L.), future research on its use is of extreme relevance. Therefore, more studies are needed on the following topics: the improvement of techniques for the identification of volatiles; the exploration of pharmacological activities, and therapeutic potential of other compounds present in BEO; and the expansion of in vivo studies and clinical trials of BEO, in order to identify the effects on consumers and expand the application of this oil to different food systems.
References
Abbas, A., Anwar, F., & Ahmad, N. (2017). Variation in physico-chemical composition and biological attributes of common basil essential oils produced by hydro-distillation and super critical fluid extraction. Journal of Essential Oil-Bearing Plants, 20, 95–109. https://doi.org/10.1080/0972060x.2017.1280418.
Abdel-Kader, M. S., Soliman, G. A., Alqarni, M. H., Hamad, A. M., Foudah, A. I., & Alqasoumi, S. I. (2019). Chemical composition and protective effect of Juniperus sabina L. essential oil against CCl4 induced hepatotoxicity. Saudi Pharmaceutical, 27, 945–951. https://doi.org/10.1016/j.jsps.2019.07.003.
Abed, K. M., Kurji, B. M., Abdulmajeed, B. A. (2018). Extraction of Ocimum basillicum oil by solvents methods. Asian Journal of Chemistry 30, 958–960. https://doi.org/10.14233/ajchem.2018.21032
Adelakun, O. E., Oyelade, O. J., & Olanipekun, B. F. (2016). Use of essential oils in food preservation. In V. J. Preedy (Ed.), Essential oils in food preservation, flavor and safety (pp. 71–84). Elsevier Inc.
Ahl, H. A. H., & Omer, E. A. (2009). Effect of spraying with zinc and/or iron on growth and chemical composition of coriander (Coriandrum sativum L.) harvested at three stages of development. Journal of Medicinal Food Plants, 1, 30–46.
Ahmad, K., Khalil, A., & Somayya, R. (2016). Antifungal, phytotoxic and hemagglutination activity of methanolic extracts of Ocimum basilicum. Journal of Traditional Chinese Medicine, 36, 794–798. https://doi.org/10.1016/S0254-6272(17)30017-1.
Ahmed, A. F., Attia, F. A. K., Liu, Z., Li, C., Wei, J., & Kang, W. (2019). Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Science and Human Wellness, 8, 299–305. https://doi.org/10.1016/j.fshw.2019.07.004.
Al Abbasy, D. W., Pathare, N., Al-Sabahi, J. N., & Khan, S. A. (2015). Chemical composition and antibacterial activity of essential oil isolated from Omani basil (Ocimum basilicum Linn.). Asian Pacific Journal of Tropical Disease, 5, 645–649. https://doi.org/10.1016/S2222-1808(15)60905-7.
Arfat, Y. A., Benjakul, S., Vongkamjan, K., Sumpavapol, P., & Yarnpakdee, S. (2015). Shelf-life extension of refrigerated sea bass slices wrapped with fish protein isolate/fish skin gelatin-ZnO nanocomposite film incorporated with basil leaf essential oil. Journal of Food Science and Technology, 52(10), 6182–6193. https://doi.org/10.1007/s13197-014-1706-y
Asbahani, A. E., Miladi, K., Badri, W., Sala, M., Addi, E. H. A., Casabianca, H., et al. (2015). Essential oils: From extraction to encapsulation. International Journal of Pharmaceutics, 483, 220–243. https://doi.org/10.1016/j.ijpharm.2014.12.069.
Avetisyan, A., Markosian, A., Petrosyan, M., Sahakyan, N., Babayan, A., & Aloyan, S. (2017). Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complementary and Alternative Medicine, 17, 60–67. https://doi.org/10.1186/s12906-017-1587-5
Baldim, J. L., Silveira, J. G., Fernandes Almeida, A. P., Carvalho, P. L. N., Rosa, W., Schripsema, J., Chagas-Paula, D. A., Soares, M. G., & Luiz, J. H. H. (2018). The synergistic effects of volatile constituents of Ocimum basilicum against foodborne pathogens. Industrial Crops and Products, 112, 821–829. https://doi.org/10.1016/j.indcrop.2017.12.016
Beatović, D., Krstić-Milošević, D. T., & S., Šiljegović, J., Glamočlija, J., Ristić, M., Jelačić, S., . (2015). Chemical composition, antioxidant and antimicrobial activities of the essential oils of twelve Ocimum basilicum L. cultivars grown in Serbia. Rec. Nat. Prod., 1, 62–75.
Beier, R. C., Ii, J. A. B., Kubena, L. F., Hume, M. E., Mcreynolds, J. L., Anderson, R. C., & Nisbet, D. J. (2014). Evaluation of linalool, a natural antimicrobial and insecticidal essential oil from basil: Effects on poultry. Poultry Science, 93, 267–272. https://doi.org/10.3382/ps.2013-03254
Benelli, G., Pavela, R., Maggi, F., Wandjou, J. G. N., Fofie, N. B. Y., Koné-bamba, D., Sagratini, G., Vittori, S., & Caprioli, G. (2019). Insecticidal activity of the essential oil and polar extracts from Ocimum gratissimum grown in Ivory Coast: Efficacy on insect pests and vectors and impact on non-target species. Industrial Crops and Products, 132, 377–385. https://doi.org/10.1016/j.indcrop.2019.02.047
Bhavaniramya, S., Vishnupriya, S., & Al-aboody, M. S. (2019). Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Science Technology, 2, 49–55. https://doi.org/10.1016/j.gaost.2019.03.001.
Binello, A., Orio, L., Pignata, G., Nicola, S., Chemat, F., & Cravotto, G. (2014). Effect of microwaves on the in situ hydrodistillation of four different Lamiaceae. Comptes Rendus Chimie, 17, 181–186. https://doi.org/10.1016/j.crci.2013.11.007.
Bonilla, J., Vargas, M., Atarés, L., & Chiralt, A. (2014). Effect of chitosan essential oil films on the storage-keeping quality of pork meat products. Food and Bioprocess Technology, 7, 2443–2450. https://doi.org/10.1007/s11947-014-1329-3
Bouyahya, A., Belmehdi, O., Benjouad, A., Ameziane El Hassani, R., Amzazi, S., Dakka, N., & Bakri, Y. (2020). Pharmacological properties and mechanism insights of Moroccan anticancer medicinal plants: What are the next steps? Industrial Crops and Products, 147, 112198. https://doi.org/10.1016/j.indcrop.2020.112198
Brada, M., Khelifa, L. H., Achour, D., Wathelet, J. P., & Lognay, G. (2011). Essential oil composition of Ocimum basilicum L. and Ocimum gratissimum L. from Algeria. Journal of Essential Oil-Bearing Plants, 14, 810–814. https://doi.org/10.1080/0972060X.2011.10644009.
Burducea, M., Zheljazkov, V. D., Dincheva, I., Lobiuc, A., Teliban, G., Stoleru, V., & Zam, M. (2018). Fertilization modifies the essential oil and physiology of basil varieties. Industrial Crops and Products, 121, 282–293. https://doi.org/10.1016/j.indcrop.2018.05.021
Calín-Sánchez, Á., Lech, K., Szumny, A., Figiel, A., & Carbonell-Barrachina, Á. A. (2012). Volatile composition of sweet basil essential oil (Ocimum basilicum L.) as affected by drying method. Food Research International, 48, 217–225. https://doi.org/10.1016/j.foodres.2012.03.015.
Carovic´-Stanko, K., Orlic´, S., Politeo, O., Strikic, F., Kolak, I., Milos, M., Satovic, Z., . (2010). Composition and antibacterial activities of essential oils of seven Ocimum taxa. Food Chemistry, 119, 196–201. https://doi.org/10.1016/j.foodchem.2009.06.010
Carvalho, S. D., Schwieterman, M. L., Abrahan, C. E., Colquhoun, T. A., & Folta, K. M. (2016). Light quality dependent changes in morphology, antioxidant capacity, and volatile production in sweet basil (Ocimum basilicum). Frontiers in Plant Science, 7, 1–14. https://doi.org/10.3389/fpls.2016.01328
Cerchiara, T., Straface, S. V., Brunelli, E., Tripepi, S., Gallucci, M. C., Chidichimo, G. (2015). Antiproliferative effect of linalool on RPMI 7932 human melanoma cell line: Ultrastructural studies. Natural Product Communications, 10, 547–549. https://doi.org/10.1177/1934578X1501000401
Chang, M. Y., Shieh, D. E., Chen, C. C., Yeh, C. S., & Dong, H. P. (2015). Linalool induces cell cycle arrest and apoptosis in leukemia cells and cervical cancer cells through CDKIs. International Journal of Molecular Sciences, 16, 28169–28179. https://doi.org/10.3390/ijms161226089
Chemat, F.; Smadja, J.; Lucchesie, M.E. Solvent free microwave extraction of volatile natural compound. EP Patent 1,439,218 B1, 28 July 2004.
Chenni, M., El Abed, D., Neggaz, S., Rakotomanomana, N., Fernandez, X., Chemat, F. (2020). Solvent free microwave extraction followed by encapsulation of O. basilicum L. essential oil for insecticide purpose. Journal of Stored Products Research, 86, 101575. https://doi.org/10.1016/j.jspr.2020.101575
Chenni, M., El Abed, D., Rakotomanomana, N., Fernandez, X., Chemat, F. (2016). Comparative study of essential oils extracted from Egyptian basil leaves (Ocimum basilicum L.) using hydro-distillation and solvent-free microwave extraction. Molecules, 21, 113, 21010113. https://doi.org/10.3390/molecules21010113.
Chouhan, K. B. S., Tandey, R., Sen, K. K., Mehta, R., & Mandal, V. (2019). Critical analysis of microwave hydrodiffusion and gravity as a green tool for extraction of essential oils: Time to replace traditional distillation. Trends in Food Science & Technology, 92, 12–21. https://doi.org/10.1016/j.tifs.2019.08.006
Cook, C. M., Lanaras, T. (2016). Essential oils: Isolation, production and uses. Encyclopedia of food and health, 552–557.https://doi.org/10.1016/b978-0-12-384947-2.00261-0
Couto, H. G. A., Blank, A. F., Silva, A. M. O., Nogueira, P. C. L., Arrigoni-Blank, M. F., Nizio, D.A.C., Pinto, J. A. O. (2019). Essential oils of basil chemotypes: Major compounds, binary mixtures, and antioxidant activity. Food Chemistry, 293, 446–454. https://doi.org/10.1016/j.foodchem.2019.04.078
Dehsheikh, A. B., Sourestani, M. M., Zolfaghari, M., & Enayatizamir, N. (2020). Changes in soil microbial activity, essential oil quantity, and quality of Thai basil as response to biofertilizers and humic acid. Journal of Cleaner Production, 256, 120439. https://doi.org/10.1016/j.jclepro.2020.120439
Dörr, O. S., Brezina, S., Rauhut, D., Mibus, H. (2019). Plant architecture and phytochemical composition of basil (Ocimum basilicum L.) under the influence of light from microwave plasma and high-pressure sodium lamps. Journal of Photochemistry and Photobiology B: Biology, 19, 111678. https://doi.org/10.1016/j.jphotobiol.2019.111678
Drinić, Z., Pljevljakušić, D., Živković, J., Bigović, D., & Šavikin, K. (2020). Microwave-assisted extraction of O. vulgare L. spp. hirtum essential oil: Comparison with conventional hydro-distillation. Food and Bioproducts Processing, 120, 158–165. https://doi.org/10.1016/j.fbp.2020.01.011
Ekren, S., Sönmez, Ç., Özçakal, E., Kurttaş, Y. S. K., Bayram, E., & Gürgülü, H. (2012). The effect of different irrigation water levels on yield and quality characteristics of purple basil (Ocimum basilicum L.). Agricultural Water Management, 109, 155–161. https://doi.org/10.1016/j.agwat.2012.03.004
Elansary, H. O., Yessoufou, K., Shokralla, S., Mahmoud, E. A., & Skalicka-wo, K. (2016). Enhancing mint and basil oil composition and antibacterial activity using seaweed extracts. Industrial Crops and Products, 92, 50–56. https://doi.org/10.1016/j.indcrop.2016.07.048
Elgndi, M. A., Filip, S., Pavlić, B., & J., Pavlić, Stanojković, T., Žižak, Z., Zeković, Z., . (2017). Antioxidative and cytotoxic activity of essential oils and extracts of Satureja montana L., Coriandrum sativum L. and Ocimum basilicum L. obtained by supercritical fluid extraction. J. of Supercritical Fluids, 128, 128–137. https://doi.org/10.1016/j.supflu.2017.05.025
El-soud, N. H. A., Deabes, M., El-kassem, L. A., & Khalil, M. (2015). Chemical composition and antifungal activity of Ocimum basilicum L. essential oil. Open Access Maced J Med Sci., 3, 374–379. https://doi.org/10.3889/oamjms.2015.082
Falowo, A. B., Mukumbo, F. E., Idamokoro, E. M., Afolayan, A. J., & Muchenje, V. (2019). Phytochemical constituents and antioxidant activity of sweet basil (Ocimum basilicum L.) essential oil on ground beef from Boran and Nguni Cattle. International Journal of Food Science, 2019, 1–8. https://doi.org/10.1155/2019/2628747
Farsaraei, S., Moghaddam, M., & Pirbalouti, A. G. (2020). Changes in growth and essential oil composition of sweet basil in response of salinity stress and superabsorbents application. Scientia Horticulturae, 271, 109465. https://doi.org/10.1016/j.scienta.2020.109465
Figueredo, G., Ünver, A., Chalchat, J. C., Arslan, D., & Özcan, M. M. (2012). A research on the composition of essential oil isolated from some aromatic plants by microwave and hydrodistillation. Journal of Food Biochemistry, 36, 334–343. https://doi.org/10.1111/j.1745-4514.2011.00542.x
Filip, S., Vidović, S., Vladić, J., Pavlić, B., Adamović, D., & Zeković, Z. (2016). Chemical composition and antioxidant properties of Ocimum basilicum L. extracts obtained by supercritical carbon dioxide extraction: Drug exhausting method. J. of Supercritical Fluids, 109, 20–25. https://doi.org/10.1016/j.supflu.2015.11.006
Filly, A., Fernandez, X., Minuti, M., Visinoni, F., Cravotto, G., & Chemat, F. (2014). Solvent free microwave extraction of essential oil from aromatic herbs: From laboratory to pilot and industrial scale. Food Chemistry, 150, 193–198. https://doi.org/10.1016/j.foodchem.2013.10.139
Follett, P. A., Rivera-leong, K., & Myers, R. (2014). Rice weevil response to basil oil fumigation. J. Asia. Pac. Entomol., 17, 119–121. https://doi.org/10.1016/j.aspen.2013.11.008
Folta, K. M., Carvalho, S. D. (2015). Photoreceptors and control of horticultural plant traits. HortScience, 50, 1274–1280. https://doi.org/10.21273/hortsci.50.9.1274
Freitas, P. R., Araújo, A. C. J., dos Barbosa, C. R., & S., Muniz, D.F., Rocha, J.E., Neto, J.B. de A., Silva, M.M.C., Pereira, R.L.S., Silva, L.E., Amaral, W., Deschamps, C., Tintino, S.R., Ribeiro-Filho, J., Coutinho, H.D.M., . (2020). Characterization and antibacterial activity of the essential oil obtained from the leaves of Baccharis coridifolia DC against multiresistant strains. Microbial Pathogenesis, 145, 104223. https://doi.org/10.1016/j.micpath.2020.104223
Gaio, I., Saggiorato, A. G., Treichel, H., Cichoski, A. J., Astolfi, V., Cardoso, R. I., Toniazzo, G., Valduga, E., Paroul, N., & Cansian, R. L. (2015). Antibacterial activity of basil essential oil (Ocimum basilicum L.) in Italian-type sausage. J. Für Verbraucherschutz Und Lebensmittelsicherheit, 10, 323–329. https://doi.org/10.1007/s00003-015-0936-x
Gündel, S. S., Velho, M. C., Diefenthaler, M. K., Favarin, F. R., Copetti, P. M., De Oliveira Fogaça, A., et al. (2018). Basil oil-nanoemulsions: Development, cytotoxicity and evaluation of antioxidant and antimicrobial potential. Journal of Drug Delivery Science and Technology, 46, 378–383. https://doi.org/10.1016/j.jddst.2018.05.038.
Gundewadi, G., Jyoti, D., Gaur, S., & Singh, D. (2018). Preparation of basil oil nanoemulsion using Sapindus mukorossi pericarp extract: Physico-chemical properties and antifungal activity against food spoilage pathogens. Industrial Crops and Products, 125, 95–104. https://doi.org/10.1016/j.indcrop.2018.08.076
Hanif, M. A., Nawaz, H., Ayub, M. A., Tabassum, N., Kanwal, N., Rashid, N., Saleem, M., & Ahmad, M. (2017). Evaluation of the effects of Zinc on the chemical composition and biological activity of basil essential oil by using Raman spectroscopy. Industrial Crops and Products, 96, 91–101. https://doi.org/10.1016/j.indcrop.2016.10.058
Heidari, M. (2011). Effects of salinity stress on growth, chlorophyll content and osmotic components of two basil (Ocimum basilicum L.) genotypes. African Journal of Biochemistry Research, 11, 379–384. https://doi.org/10.5897/ajb11.2572.
Hossain, F., Lacroix, M., Salmieri, S., Vu, K., & Follett, P. A. (2014). Basil oil fumigation increases radiation sensitivity in adult Sitophilus oryzae (Coleoptera: Curculionidae). Journal of Stored Products Research, 59, 108–112. https://doi.org/10.1016/j.jspr.2014.06.003
Hossain, M. A., Kabir, M. J., Salehuddin, S. M., Rahman, S. M. M., Das, A. K., Singha, S. K., & Rahman, A. (2010). Antibacterial properties of essential oils and methanol extracts of sweet basil Ocimum basilicum occurring in Bangladesh. Pharmaceutical Biology, 48, 504–511. https://doi.org/10.3109/13880200903190977
Ilić, S. Z., Milenković, L., Dimitrijević, A., Stanojević, L., Cvetković, D., Kevrešan, Ž, Fallik, E., & Mastilović, J. (2017). Light quality manipulation by color nets improve quality of lettuce from summer production. Scientia Horticulturae, 226, 389–397. https://doi.org/10.1016/j.scienta.2017.09.009
Jeshni, M. G., Mousavinik, M., Khammari, I., & Rahimi, M. (2017). The changes of yield and essential oil components of German Chamomile (Matricaria recutita L.) under application of phosphorus and zinc fertilizers and drought stress conditions. Journal of the Saudi Society of Agricultural Sciences, 16, 60–65. https://doi.org/10.1016/j.jssas.2015.02.003
Jirovetz, L., Buchbauer, G., Shafi, M. P., & Kaniampady, M. M. (2003). Chemotaxonomical analysis of the essential oil aroma compounds of four different Ocimum species from southern India. European Food Research Technology, 217, 120–124. https://doi.org/10.1007/s00217-003-0708-1.
Joshi, R. K. (2014). Chemical composition and antimicrobial activity of the essential oil of Ocimum basilicum L. (sweet basil) from Western Ghats of North West Karnataka, India. Ancient Science of Life, 33, 151. https://doi.org/10.4103/0257-7941.144618
Jugreet, B. S., & Mahomoodally, M. F. (2020). Essential oils from 9 exotic and endemic medicinal plants from Mauritius shows in vitro antibacterial and antibiotic potentiating activities. South African Journal of Botanists, 132, 355–362. https://doi.org/10.1016/j.sajb.2020.05.001.
Jugreet, B. S., Suroowan, S., Rengasamy, R. R. K., & Mahomoodally, M. F. (2020). Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends in Food Science & Technology, 101, 89–105. https://doi.org/10.1016/j.tifs.2020.04.025
Khelifa, L. H., Brada, M., Brahmi, F., Achour, D., Fauconnier, M. L., & Lognay, G. (2012). Chemical composition and antioxidant activity of essential oil of Ocimum basilicum leaves from the northern region of Algeria. Topclass J. Herb. Med., 1, 53–58.
Kladniew, B. R., Polo, M., Montero Villegas, S., Galle, M., Crespo, R., & García De Bravo, M. (2014). Synergistic antiproliferative and anticholesterogenic effects of linalool, 1,8-cineole, and simvastatin on human cell lines. Chemico-Biological Interactions, 214, 57–68. https://doi.org/10.1016/j.cbi.2014.02.013
Kocić-Tanackov, S., Dimić, G., Đerić, N., Mojović, L., Tomović, V., Šojić, B., Đukić-Vuković, A., Pejin, J., 2020. Growth control of molds isolated from smoked fermented sausages using basil and caraway essential oils, in vitro and in vivo. LWT, 109095.https://doi.org/10.1016/j.lwt.2020.109095
Koroch, R., Simon, J. E., & Juliani, H. R. (2017). Essential oil composition of purple basils, their reverted green varieties (Ocimum basilicum) and their associated biological activity. Industrial Crops and Products, 107, 526–530. https://doi.org/10.1016/j.indcrop.2017.04.066
Kunwar, G., Pande, C., Tewari, G., Singh, C., & Kharkwal, G. C. (2015). Effect of heavy metals on terpenoid composition of Ocimum basilicum L. and Mentha spicata L. J. Essent. Oil Bear. Plants, 5026, 818–825. https://doi.org/10.1080/0972060X.2014.935091
Kusuma, H. S., & Mahfud, M. (2016). Preliminary study: Kinetics of oil extraction from basil (Ocimum basilicum) by microwave-assisted hydrodistillation and solvent-free microwave extraction. South Africa Journal of Chemical Engineering, 21, 49–53. https://doi.org/10.1016/j.sajce.2016.06.001.
Ladwani, A. M. A., Salman, M., & Es, A. H. (2018). Chemical composition of Ocimum basilicum L. essential oil from different regions in the Kingdom of Saudi Arabia by using gas chromatography mass spectrometer. Journal of Medical Plants Study, 6, 14–19.
Li, Q. X., Chang, C. L., (2016). Basil (Ocimum basilicum L.) Oils, in: Preedy, V.J., (Eds), Essential oils in food preservation, flavor and safety. Elsevier Inc., London, UK, pp. 231–238. https://doi.org/10.1016/B978-0-12-416641-7.00025-0
Li, Y., Fabiano-Tixier, A. S., Vian, M. A., & Chemat, F. (2013). Solvent-free microwave extraction of bioactive compounds provides a tool for green analytical chemistry. Trends in Analytical Chemistry, 47, 1–11. https://doi.org/10.1016/j.trac.2013.02.007
Licon, C. C., Moro, A., Librán, C. M., Molina, A. M., Zalacain, A., Berruga, M. I., & Carmona, M. (2020). Volatile transference and antimicrobial activity of cheeses made with ewes’ milk fortified with essential oils. Foods, 9(1), 35. https://doi.org/10.3390/foods9010035
Lucchesi, M. E., Chemat, F., & Smadja, J. (2004). Solvent-free microwave extraction of essential oil from aromatic herbs: Comparison with conventional hydro-distillation. Journal of Chromatography A, 1043, 323–327. https://doi.org/10.1016/j.chroma.2004.05.083
Maggio, A., Roscigno, G., Bruno, M., Falco, E. D., & Senatore, F. (2016). Essential-oil variability in a collection of Ocimum basilicum L. (Basil) cultivars. Chemistry & Biodiversity, 13, 1357–1368. https://doi.org/10.1002/cbdv.201600069
Mahmoud, Y. K., & Abdelrazek, H. M. A. (2019). Cancer: Thymoquinone antioxidant/pro-oxidant effect as potential anticancer remedy. Biomedicine & Pharmacotherapy, 115, 108783. https://doi.org/10.1016/j.biopha.2019.108783
Mahmoudi, H., Marzouki, M., M’Rabet, Y., Mezni, M., Ouazzou, A.A., Hosni, K. (2020). Enzyme pretreatment improves the recovery of bioactive phytochemicals from sweet basil (Ocimum basilicum L.) leaves and their hydrodistilled residue by-products, and potentiates their biological activities. Arabian Journal of Chemistry. https://doi.org/10.1016/j.arabjc.2020.06.003.
Mandoulakani, B. A., Eyvazpour, E., & Ghadimzadeh, M. (2017). The effect of drought stress on the expression of key genes involved in the biosynthesis of phenylpropanoids and essential oil components in basil (Ocimum basilicum L.). Phytochemistry, 139, 1–7. https://doi.org/10.1016/j.phytochem.2017.03.006
Marco, A., Santos, S., Caetano, J., Pintado, M., Vieira, E., & Moreira, P. R. (2020). Basil essential oil as an alternative to commercial biocides against fungi associated with black stains in mural painting. Building and Environment, 167, 106459. https://doi.org/10.1016/j.buildenv.2019.106459
Mariod, A. A. (2016). Effect of essential oils on organoleptic (smell, taste, and texture) properties of food, in: Preedy, V.J., (Eds), Essential oils in food preservation, flavor and safety. Elsevier Inc., London, UK, pp. 131–137. https://doi.org/10.1016/B978-0-12-416641-7.00013-4
Marotti, M., Piccaglia, R., & Giovanelli, E. (1996). Differences in essential oil composition of basil (Ocimum basilicum L.) Italian cultivars related to morphological characteristics. Journal of Agriculture and Food Chemistry, 44, 3926–3929. https://doi.org/10.1021/jf9601067
Martins, P. F., Carmona, C., Martinez, E. L., Sbaite, P., Filho, R. M., Maciel, M. R. W., & Maciel, W. (2012). Short path evaporation for methyl chavicol enrichment from basil essential oil. Separation and Purification Technology, 87, 71–78. https://doi.org/10.1016/j.seppur.2011.11.024
Megawati, F., & D.S., Sediawan, W.B., Hisyam, A., . (2019). Kinetics of mace (Myristicae arillus) essential oil extraction using microwave assisted hydrodistillation: Effect of microwave power. Industrial Crops and Products, 131, 315–322. https://doi.org/10.1016/j.indcrop.2019.01.067
Miguel, M. G. (2010). Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules, 15, 9252–9287. https://doi.org/10.3390/molecules15129252
Milenković, L., Stanojević, J., Cvetković, D., Stanojević, L., Lalević, D., Šunić, L., Fallik, E., & Ilić, Z. S. (2019). New technology in basil production with high essential oil yield and quality. Industrial Crops and Products, 140, 111718. https://doi.org/10.1016/j.indcrop.2019.111718
Mollaei, S., Sedighi, F., Habibi, B., Hazrati, S., & Asgharian, P. (2019). Extraction of essential oils of Ferulago angulata with microwave-assisted hydrodistillation. Industrial Crops and Products, 137, 43–51. https://doi.org/10.1016/j.indcrop.2019.05.015
Moradi, S., Fazlali, A., & Hamedi, H. (2018). Microwave-assisted hydro-distillation of essential oil from Rosemary: Comparison with traditional distillation. Avicenna Journal of Medical Biotechnology, 10, 22–28.
Mota, I., Sánchez-Sánchez, J., Pedro, L. G., Sousa, M. J., (2020). Composition variation of the essential oil from Ocimum basilicum L. cv. Genovese Gigante in response to Glomus intraradices and mild water stress at different stages of growth. Biochemical Systematics and Ecology, 90, 104021. https://doi.org/10.1016/j.bse.2020.104021
Mustapha, A. (2018). Comparative analysis on the extraction of essential oil from lemongrass and basil leaves. International Journal of Innovative Research in Science Engineering and Technology, 5, 11, 114–118.
Nawaz, H., Hanif, M. A., Ayub, M. A., Ishtiaq, F., Kanwal, N., Rashid, N., Saleem, M., & Ahmad, M. (2017). Raman spectroscopy for the evaluation of the effects of different concentrations of Copper on the chemical composition and biological activity of basil essential oil. Spectrochim. Acta Part A Mol. Biomol. Spectrosc., 185, 130–138. https://doi.org/10.1016/j.saa.2017.05.049
Ngamakeue, N., & Chitprasert, P. (2016). Encapsulation of holy basil essential oil in gelatin: Effects of palmitic acid in carboxymethyl cellulose emulsion coating on antioxidant and antimicrobial activities. Food and Bioprocess Technology, 9, 1735–1745. https://doi.org/10.1007/s11947-016-1756-4
Nguyen, P. M., Kwee, E. M., & Niemeyer, E. D. (2010). Potassium rate alters the antioxidant capacity and phenolic concentration of basil (Ocimum basilicum L.) leaves. Food Chemistry, 123, 1235–1241. https://doi.org/10.1016/j.foodchem.2010.05.092
Nurzyńska-wierdak, R., Borowski, B., Dzida, K., Zawiślak, G., & Kowalski, R. (2013). Essential oil composition of sweet basil cultivars as affected by nitrogen and potassium fertilization. Turkish Journal of Agriculture and Forestry, 37, 427–436. https://doi.org/10.3906/tar-1203-43.
Oliveira, J. L., Campos, E. V. R., Bakshi, M., Abhilash, P. C., & Fraceto, L. F. (2014). Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: Prospects and promises. Biotechnology Advances, 32, 1550–1561. https://doi.org/10.1016/j.biotechadv.2014.10.010
Onofrei, V., Benchennouf, A., Jancheva, M., Ouaret, W., Burducea, M., Lobiuc, A., Teliban, G., & Robu, T. (2018). Ecological foliar fertilization effects on essential oil composition of sweet basil (Ocimum basilicum L.) cultivated in a field system. Scientia Horticulturae, 239, 104–113. https://doi.org/10.1016/j.scienta.2018.05.021
Onyebuchi, C., & Kavaz, D. (2019). Chitosan and N, N, N-trimethyl chitosan nanoparticle encapsulation of Ocimum gratissimum essential oil: Optimised synthesis, in vitro release and bioactivity. International Journal of Nanomedicine, 14, 7707–7727. https://doi.org/10.2147/ijn.s220202
Ottai, M. E. S., Ahmed, S. S., & Din, M. M. E. (2012). Genetic variability among some quantitative characters, insecticidal activity and essential oil composition of two Egyptian and French sweet basil varieties. Australian Journal of Basic and Applied Sciences, 6, 185–192.
Padalia, R. C., Verma, R. S., Upadhyay, R. K., Chauhan, A., & Singh, V. R. (2017). Productivity and essential oil quality assessment of promising accessions of Ocimum basilicum L. from north India. Industrial Crops and Products, 97, 79–86. https://doi.org/10.1016/j.indcrop.2016.12.008
Pandey, A. K., Singh, P., Tripathi, N. N. (2014). Chemistry and bioactivities of essential oils of some Ocimum species: an overview. Asian Pacific Journal of Tropical Biomedicine, 4, 682–694. https://doi.org/10.12980/APJTB.4.2014C77
Pereira, I., Severino, P., Santos, A. C., Silva, A. M., & Souto, E. B. (2018). Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surfaces B Biointerfaces, 171, 566–578. https://doi.org/10.1016/j.colsurfb.2018.08.001
Predoi, D., Iconaru, S. L., Buton, N., Badea, M. L., & Marutescu, L. (2018). Antimicrobial activity of new materials based on lavender and basil essential oils and hydroxyapatite. Nanomaterials, 8, 291. https://doi.org/10.3390/nano8050291
Raut, J. S., & Karuppayil, S. M. (2014). A status review on the medicinal properties of essential oils. Industrial Crops and Products, 62, 250–264. https://doi.org/10.1016/j.indcrop.2014.05.055
Rodenak-Kladniew, B., Castro, A., Stärkel, P., De Saeger, C., García de Bravo, M., & Crespo, R. (2018). Linalool induces cell cycle arrest and apoptosis in HepG2 cells through oxidative stress generation and modulation of Ras/MAPK and Akt/mTOR pathways. Life Sciences, 199, 48–59. https://doi.org/10.1016/j.lfs.2018.03.006
Rodklongtan, A., & Chitprasert, P. (2017). Combined effects of holy basil essential oil and inlet temperature on lipid peroxidation and survival of Lactobacillus reuteri KUB-AC5 during spray drying. Food Research International, 100, 276–283. https://doi.org/10.1016/j.foodres.2017.07.016
Rodrigues, L. B., Martins, A. O. B. P. B., Castro, F. F., Albuquerque, T. R., Fernandes, M. N. M., Silva, B. A. F., Júnior, L. J. Q., Costa, J. G. M., Coutinho, H. D. M., Barbosa, R., & Menezes, I. R. A. (2016). Anti-inflammatory and antiedematogenic activity of the Ocimum basilicum essential oil and its main compound estragole: In vivo mouse models. Chemico-Biological Interactions, 257, 14–25. https://doi.org/10.1016/j.cbi.2016.07.026
Saggiorato, A. G., Gaio, I., Treichel, H., de Oliveira, D., Cichoski, A. J., & Cansian, R. L. (2012). Antifungal activity of basil essential oil (Ocimum basilicum L.): Evaluation in vitro and on an Italian-type sausage surface. Food and Bioprocess Technology, 5, 378–384. https://doi.org/10.1007/s11947-009-0310-z
Sefidkon, F., Abbasi, K., Jamzad, Z., & Ahmadi, S. (2007). The effect of distillation methods and stage of plant growth on the essential oil content and composition of Satureja rechingeri Jamzad. Food Chemistry, 100, 1054–1058. https://doi.org/10.1016/j.foodchem.2005.11.016
Sharma, S., Cheng, S., Bhattacharya, B., & Chakkaravarthi, S. (2019). Efficacy of free and encapsulated natural antioxidants in oxidative stability of edible oil: Special emphasis on nanoemulsion-based encapsulation. Trends in Food Science & Technology, 91, 305–318. https://doi.org/10.1016/j.tifs.2019.07.030
Sharma, T., & Jana, S. (2020). Boswellic acids as natural anticancer medicine: Precious gift to humankind. J. Herb. Med., 20, 100313. https://doi.org/10.1016/j.hermed.2019.100313
Shirazi, M. T., Gholami, H., Kavoosi, G., Rowshan, V., & Tafsiry, A. (2014). Chemical composition, antioxidant, antimicrobial and cytotoxic activities of Tagetes minuta and Ocimum basilicum essential oils. Food Science Nutrition, 2, 146–155. https://doi.org/10.1002/fsn3.85.
Shiwakoti, S., Saleh, O., Poudyal, S., Barka, A., Qian, Y., & Zheljazkov, V. D. (2017). Yield, composition and antioxidant capacity of the essential oil of sweet basil and holy basil as influenced by distillation methods. Chememical Biodiversity, 14,.
Shokri, S., Parastouei,. K., Taghdir, M., Abbaszadeh, S. (2020). Application an edible active coating based on chitosan - Ferulago angulata essential oil nanoemulsion to shelf life extension of Rainbow trout fillets stored at 4ºC. International Journal Biological Macromolecules, https://doi.org/10.1016/j.ijbiomac.2020.03.080
Sienkiewicz, M., Łysakowska, M., Pastuszka, M., Bienias, W., & Kowalczyk, E. (2013). The potential of use basil and rosemary essential oils as effective antibacterial agents. Molecules, 18, 9334–9351. https://doi.org/10.3390/molecules18089334
Sifola, M. I., & Barbieri, G. (2006). Growth, yield and essential oil content of three cultivars of basil grown under different levels of nitrogen in the field. Scientia Horticulturae, 108, 408–413. https://doi.org/10.1016/j.scienta.2006.02.002
Silvestre, W. P., Livinalli, N. F., Baldasso, C., & Tessaro, I. C. (2019). Pervaporation in the separation of essential oil components: A review. Trends in Food Science & Technology, 93, 42–52. https://doi.org/10.1016/j.tifs.2019.09.003
Singh, D., & Chaudhuri, P. K. (2018). A review on phytochemical and pharmacological properties of Holy basil (Ocimum sanctum L.). Industrial Crops and Products, 118, 367–382. https://doi.org/10.1016/j.indcrop.2018.03.048
Singh, K., Chand, S., & Yaseen, M. (2014). Integrated nutrient management in Indian basil (Ocimum basilicum). Industrial Crops and Products, 55, 225–229. https://doi.org/10.1016/j.indcrop.2014.02.009
Siva, S., Li, C., Cui, H., Meenatchi, V., Lin, L. (2020). Encapsulation of essential oil components with methyl-β-cyclodextrin using ultrasonication: Solubility, characterization, DPPH and antibacterial assay. Ultrasonics Sonochemistry, 104997.https://doi.org/10.1016/j.ultsonch.2020.104997
Stanojevic, L. P., Stanojevic, J. S., Savic, V. L., Cvetkovic, D. J., Kolarevic, A., Marjanovic-Balaban, Z., Nikolic, L. B. (2019). Peppermint and basil essential oils: Chemical composition, in vitro antioxidant activity and in vivo estimation of skin irritation. Journal Essential Oil Bearing Plants, 1–15.https://doi.org/10.1080/0972060x.2019.1661793
Stanojevic, L. P., Stanojevic, J. S., Savic, V. L., Cvetkovic, D. J., Kolarevic, A., Marjanovic-Balaban, Z., Nikolic, L. B. (2019). Peppermint and basil essential oils: Chemical composition, in vitro antioxidant activity and in vivo estimation of skin irritation. Journal Essential Oil Bearing Plants, 1– 15. https://doi.org/10.1080/0972060x.2019.1661793
Taie, H. A. A., Salama, Z. A. E. R., Radwan, S. (2010). Potential activity of basil plants as a source of antioxidants and anticancer agents as affected by organic and bio-ornaic fertilization. Notulae botanicae Horti Agrobotanici Cluj-Napoca, 38, 119–127. https://doi.org/10.15835/nbha3813534
Talebi, M., Moghaddam, M., & Pirbalouti, A. G. (2018). Methyl jasmonate effects on volatile oil compounds and antioxidant activity of leaf extract of two basil cultivars under salinity stress. Acta Physiologiae Plantarum, 40, 1–11. https://doi.org/10.1007/s11738-018-2611-1
Tarchoune, I., Baâtour, O., Harrathi, J., Hamdaoui, G., Lachaâl, M., Ouerghi, Z., & Marzouk, B. (2013). Effects of two sodium salts on fatty acid and essential oil composition of basil (Ocimum basilicum L.) leaves. Acta Physiologiae Plantarum, 35, 2365–2372. https://doi.org/10.1007/s11738-013-1271-4
Tariq, S., Wani, S., Rasool, W., Shafi, K., Ahmad, M., Prabhakar, A., Hussain, A., & Rather, M. A. (2019). A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pthogenes., 134, 103580. https://doi.org/10.1016/j.micpath.2019.103580
Tavallali, V., Kiani, M., & Hojati, S. (2019). Iron nano-complexes and iron chelate improve biological activities of sweet basil (Ocimum basilicum L.). Plant Physiology and Biochemistry, 144, 445–454. https://doi.org/10.1016/j.plaphy.2019.10.021
Tongnuanchan, P., & Benjakul, S. (2014). Essential oils: Extraction, bioactivities, and their uses for food preservation. Journal of Food Science, 79(7), 1231–1249. https://doi.org/10.1111/1750-3841.12492
Vieira, P. R. N., Morais, S. M. D., Bezerra, F. H. Q., Augusto, P., Ferreira, T., Oliveira, Í. R., & Silva, M. G. (2014). Chemical composition and antifungal activity of essential oils from Ocimum species. Industrial Crops and Products, 55, 267–271. https://doi.org/10.1016/j.indcrop.2014.02.032
Wang, Y., Zhang, L., Feng, Y., Zhang, D., Guo, S., & Pang, X. (2019). Comparative evaluation of the chemical composition and bioactivities of essential oils from four spice plants (Lauraceae) against stored-product insects. Industrial Crops and Products, 140, 111640. https://doi.org/10.1016/j.indcrop.2019.111640
Wei, A., & Shibamoto, T., 2010. Antioxidant/lipoxygenase inhibitory activities and chemical compositions of selected essential oils. Journal of Agricultural and Food Chemistry, 58, 7218–7225. https://doi.org/10.1021/jf101077s
Xiong, Y., Li, S., Warner, R. D., & Fang, Z. (2020). Effect of oregano essential oil and resveratrol nanoemulsion loaded pectin edible coating on the preservation of pork loin in modified atmosphere packaging. Food Control, 114, 107226. https://doi.org/10.1016/j.foodcont.2020.107226
Yap, P. S. X., Yiap, B. C., Ping, H. C., & Lim, S. H. E. (2014). Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiology Journal, 8, 6–14. https://doi.org/10.2174/1874285801408010006.
Yousefi, M., Rahimi-Nasrabadi, M., Pourmortazavi, S. M., Wysokowski, M., Jesionowski, T., Ehrlich, H., Mirsadeghi, S. (2019). Supercritical fluid extraction of essential oils. Trends in Analytical Chemistry, 118, 182–193. https://doi.org/10.1016/j.trac.2019.05.038
Złotek, U., Michalak-majewska, M., & Szymanowska, U. (2016). Effect of jasmonic acid elicitation on the yield, chemical composition, and antioxidant and anti-inflammatory properties of essential oil of lettuce leaf basil (Ocimum basilicum L.). Food Chemistry, 213, 1–7. https://doi.org/10.1016/j.foodchem.2016.06.052
Funding
The authors were financially supported by CAPES (Finance Code 001″), CNPQ, and FAPERGS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva, W.M.F., Kringel, D.H., de Souza, E.J.D. et al. Basil Essential Oil: Methods of Extraction, Chemical Composition, Biological Activities, and Food Applications. Food Bioprocess Technol 15, 1–27 (2022). https://doi.org/10.1007/s11947-021-02690-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-021-02690-3