Abstract
Button mushroom have a short postharvest shelf life compared to most vegetables, due to a very high metabolic activity and high water content. This makes them prone to microbial spoilage and to exhibit enzymatic browning. In this research, the effects of aloe vera, gum tragacanth, and combination of both as edible coatings on the shelf life and postharvest losses of mushrooms were studied. Physical characteristics, general appearance (color and texture), weight loss, and carbohydrate percentage were evaluated during storage. Mushrooms were stored at 4, 10, and 15 °C for 13 days and physicochemical characteristics were analyzed after 2, 4, 6, 8, 10, and 13 days of storage. During cold storage, the uncoated mushrooms showed rapid weight loss, color changes, and accelerated softening while mushrooms treated with aloe vera gel, gum tragacanth, and the combination of both significantly delayed these phenomena. Among different coatings, the combination of aloe vera and gum tragacanth was more effective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The button mushroom (Agaricus bisporus) is the most widely grown and consumed mushroom in the world, and it includes about 40% of the total world production of mushroom (Giri and Prasad 2005). Mushrooms are the excellent source of some essential amino acids, vitamins (B2, niacin, and folates), and minerals (potassium, phosphorus, zinc, and copper) (Tao et al. 2006). Moreover, mushrooms contain various polyphenolics and flavonoids which are recognized as excellent antioxidants (Singla et al. 2010). On the other hand, button mushrooms have a short postharvest shelf life of less than 3 days at ambient temperature (Kim et al. 2006). So, mushrooms require special attention to preserve their freshness.
There are various methods to extend the shelf life of mushrooms including modified atmosphere packaging (Kim et al. 2006), controlled atmosphere storage (Lopez-Briones et al. 1992), vacuum cooling technology (McDonald and Sun 2000), coating (Nussinovitch and Kampf 1993), and refrigeration (Mau et al. 1993). Among these, coating is the most likely method of vegetable and fruit preservation. Edible coatings are customarily used for better food appearance and protection. They could help to decrease moisture loss and slow respiration by reducing oxygen uptake from the environment (Donhowe and Fennema 1994). Modification of fruits tissue metabolism by affecting respiration rate, extension of storage life, firmness retention, transportation of antimicrobials, antioxidants, and other preservatives and microbial growth control are the main functional advantages attributed to the use of edible films and coatings (García et al. 2010).
In addition to prolonging shelf life and delaying senescence, coatings add sheen and luster to products and make them more attractive and appealing to consumers. Fruits or vegetables are usually coated by dipping in or spraying with a range of edible materials. Therefore, a semipermeable membrane is formed on the surface for suppressing respiration, controlling moisture loss, and providing other functions (Thompson 2003b).
In recent years, the use of aloe vera (Aloe barbadensis Miller) in the formulation of various cosmetic and food products has increased substantially (Simal et al. 2000). It is used as a source of functional ingredients in drinks, beverages, and ice creams and also applied as an edible coating according to a patent (Martínez-Romero et al. 2003). This gel has a potential to be used for food maintenance. Leaves parenchyma cells include mucilaginous clear gel, which is applied as aloe vera gel. The raw pulp of aloe vera contains about 98.5% water, while the mucilage or gel consists of about 99.5% water (Eshun and He 2004). Furthermore, it involves a number of nutrients such as vitamins, fatty acids, amino acids, sugars, minerals, and enzymes. Therefore, it can be used in different formulations as a functional ingredient for health benefits. The gel works as a barrier to O2 and CO2 and acts as moisture barrier, and thus reduces weight loss, browning, softening, and growth of yeast and molds. The material contains antimicrobial compounds and thus prevents decay (Valverde et al. 2005). Aloe vera contains malic acid-acetylated carbohydrates (including β-1, 4-g1ucomannans) with anti-inflammatory activity (Esua and Rauwald 2006). The sensory examinations of sweet cherry coated with aloe vera gel showed useful effects in terms of delaying stem browning and dehydration and maintenance of fruit visual characteristic without any damaging effect on taste, aroma, or flavors (Martínez-Romero et al. 2006).
Gum tragacanth (GT) is a dried exudate which is obtained from slashing the stems of Asiatic species of Astragalus (Leguminosae). GT includes water soluble (30–40% of GT) and non-water-soluble (60–70% of GT) fractions, which are called tragacanthin and bassorin (Azarikia and Abbasi 2010). Gum tragacanth is widely used as a natural emulsifier and thickener in the food, drug, and allied industries. Its wide use is due to its stability in a wide range of temperature and pH and its effectiveness as an emulsifying agent with extremely long shelf life (Mohamadnia et al. 2008). As GT is a natural polymer, it is nontoxic and biocompatible (Otady et al. 2005). Development of natural preservative coatings to reduce respiration rate and inhibit enzymatic browning with antimicrobial agents is expanding due to the riskiness aspects of chemical preservatives (Lee et al. 2003).
The main procedures which lead to loss in quality after harvest are (a) discoloration, (b) browning, (c) loss of closeness, (d) weight loss, and (e) texture changes (Burton and Noble 1993). The color and the shape of the cap are the main parts of fresh mushrooms because these are the first characteristics which attract consumers (Brosnan and Sun 2004). This study aimed to assess the suitability of aloe vera, gum tragacanth, and combination of both as edible coatings for mushrooms and to determine the influence of the coating on the mushroom’s physicochemical changes during storage.
Materials
Plant Material and Experimental Design
White mushrooms (A. bisporus) used in this study were harvested from a commercial farm in Mashhad belonging to Pooya pazh company. The mushrooms were carried into the laboratory in 1 h after harvest. Mushrooms with homogeneous color and size and also have absence of injuries were selected and separated into four batches. Then, the mushrooms were treated with aloe vera gel (30% w/w), gum tragacanth (10% w/w), and aloe vera–gum tragacanth (50% w/w). As control, some of the mushrooms were immersed in distilled water.
Preparation of aloe vera-coating solution
Aloe vera gel was extracted from aloe vera leaves (Herbs Company, Mashhad, Iran). Each aloe leaf is made up of four layers. The first layer is the rind. This is the hard greenish gray outer protective layer of the leaf. The second layer consists of a bitter liquid called sap. This is located under the rind and surrounds the gel. The third layer contains mucilage gel which is known as the inner leaf area. And finally, the fourth layer is where the aloe vera is located. Extraction procedure of aloe vera gel from the leaves follows six steps: Cutting the leaves from plant, standing them upright for 15 min to drain the sap, slicing off the top skin layer of the leaf, cutting away the two side pieces of the leaf, slicing away the gel from the bottom layer of the skin and to prevent spoilage, putting aloe vera gel in a dark colored jar, and storing it in the refrigerator. The coating treatment was performed at 20 °C by immersing the mushrooms in a solution of diluted aloe vera with distilled water (1:3) for 5 min (pH was 1.87).
Preparation of gum tragacanth-coating solution
GT powder was purchased from a grocery in Mashhad and the ratio of 10 to 100 ml (w/w) was mixed in water (pH was 1.70). The solutions were stirred vigorously with a magnetic stirrer on a hotplate for 40 min and were kept in the refrigerator for 24 h.
Preparation of aloe vera and gum tragacanth-coating solution
Both the coating solutions were prepared with the above methods after 50/50 ratio of each solution added (pH was 1.77).
The ratio of mushroom to edible solutions was 1:20. In order to disinfect and create a strong texture, 2 g/100 ml calcium chloride and 40 g/l citric acid as anti-browning were added to all coating solutions and distilled water. At the end, the mushrooms were air-dried and stored at 5, 10, and 15 °C and 85% RH for 13 days. After 2, 4, 6, 8, 10, and 13 days of storage, physicochemical characteristics of the mushrooms were evaluated.
Weight Loss Analysis
The mushrooms’ weight was measured by a balance (SartoriusAGGöttingen, Germany). Weight loss was determined using the following relationship:
where W 0 is the weight on the first day and W f the weight on final storage day.
Hardness Determination
A penetration test was performed on the mushrooms’ cap using a texture analyzer (QTS25 CNS Farnell, UK) interfaced to a personal computer. The hardness was determined using a 5-mm diameter cylindrical probe. The probe was attached to the texture analyzer and the speed of loading head was set at 2.0 mm s−1, trigger force at 10 g, and the travel distance of the probe at 5 mm. Hardness was defined as the peak force for this penetration.
Color
In order to investigate the effect of the treatments and storage time on color changes of the mushrooms, the following procedure was applied:
-
1.
A computer vision system generally consists of four basic components: illumination, a camera, computer hardware, and software. In this research, sample illumination was achieved with four fluorescent lights (Opple, 8 W, model MX396-Y82; 60 cm in length) with a color index (Ra) close to 95%. The illuminating lights were placed in a dark wooden box, 45 cm above the sample and at the angle of 45º with sample plane to give a uniform light intensity over the mushroom sample (Quevedo et al. 2009). A color digital camera (Canon Power shot, Model A520, Japan) was located vertically at a distance of 25 cm from the sample. The iris was operated in manual mode, with the lens aperture of 4 and speed of 1/10 s (no zoom, no flash) to achieve high uniformity and repeatability.
-
2.
Image preprocessing: Improvement of background’s contrast of images and segmentation was performed using Adobe Photoshop (Adobe, v.8.0).
-
3.
Conversion of RGB chromatic space into L*a*b* units: Since the L*a*b* color is device independent and provides consistent color regardless of the input or output, the images taken were converted into L*a*b* units. In the L*a*b* space, the color perception is uniform, and therefore, the Euclidean distance between two colors is almost in agreement with the color difference perceived by the human eye (Pedreschi et al. 2007). The net color difference (ΔE) was calculated with the relation:
$$ \Delta E = \sqrt {{{{\left( {L_2^{*} - L_1^{*}} \right)}^2} + {{\left( {a_2^{*} - a_1^{*}} \right)}^2} + {{\left( {b_2^{*} - b_1^{*}} \right)}^2}}} $$where L* is referred to as the lightness or luminance, while a* is defined along the axis of red–green, and b* is defined along the axis of yellow–blue (Sun 2008). In this study, the image analysis was managed using ImageJ software (National Institutes Health, Bethesda, MD, USA) version 1.40g.
Determination of Sugars
Carbohydrates are important components of structural materials in plants and exist as free sugar and polysaccharide. The carbohydrate content can be calculated by hydrolyzing the polysaccharides into simple sugars by acid sulfuric and appraising the resultant monosaccharide. Since the level of mushroom sugar is low, total sugar was determined with Antherone reagent according to the procedure applied by Stewart et al. (1974).
According to this method, the mushrooms first were dried in an oven (Memret, Beschickung loading, model 100-800) then were crushed and 0.7 g of each treatment was weighed. The samples were hydrolyzed by keeping them in boiling water bath for 10 min with ethyl alcohol 96% and were made up 100 ml volume, were flattened with filter paper, and cooled to room temperature. For each filtrate, 1 ml of the sample solution was added to 2 ml Anthrone reagent (0.2% anthrone dissolved in concentrated sulfuric acid) and was heated for 10 min in boiling water bath until the reaction was completed. At the end, the sample was cooled rapidly and the green to dark green color was read at 620 nm wavelength of a spectrophotometer (JENWAY 6105 UV/Vis). This method determines both reducing and non-reducing sugars because of the presence of strongly oxidizing sulfuric acid. Total sugar concentrations of the samples were calculated using the calibration curve drawn for glucose standard solutions.
Statistical Analysis
The experimental design was a completely randomized factorial model with at least two replications for each treatment. Analysis of variance was performed to study the differences among the effects of coatings, storage temperature, and storage time on quality parameters of the mushrooms. Duncan’s multiple comparison tests were applied to determine the difference among the means. The level of statistical significance was determined at 95% probability.
Results and Discussion
Weight Loss
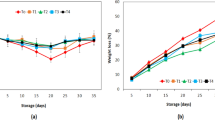
This quality parameter is crucial since every loss in weight can be translated into an economic loss. Additionally, the weight loss has a strong effect on the appearance, due to lack of a protective epidermal structure in mushrooms to prevent excessive moisture loss. Therefore, mushroom has a very high transpiration rate. The application of aloe vera gel, gum tragacanth, and combination of aloe vera and gum tragacanth coating retarded the weight loss of the button mushrooms (Fig. 1a–c). Compared with the control samples, the coated mushrooms showed a significant weight loss reduction during storage (Table 1). After 13 days of storage, GT-, aloe vera (A)–GT-, and A-coated samples showed 30%, 32%, and 35% weight loss at 4 °C, respectively. That was significantly different (p < 0.01) compared to 47% weight loss in the control mushrooms at this temperature (Table 1). As expected, larger changes of weight loss were observed at higher temperatures. The results showed that changes of weight loss (in percent) during storage for GT- and A–GT-treated mushrooms at 10 °C were approximately the same as samples stored at 4 °C. Totally, in this study, all coatings reduced weight loss in mushrooms compared with control. Several studies had dealt with the effect of various coatings based on polysaccharides in controlling the weight loss of several fruits and vegetables. Many researchers pointed to the use of commercial formulations including carboxymethyl cellulose and sucrose fatty acid esters. For example Semperfresh TM and TAL Pro-long exert a better weight loss control of many products such as cherries (Yaman and Bayoindirli 2002). Aloe vera gel, which mainly consists of polysaccharides (Ni et al. 2004), was highly effective as a moisture barrier without lipid incorporation. Weight loss is caused by respiration and evaporation of water from the mushroom. The main mechanism of weight loss is the evaporation of water activated by a gradient of water vapor pressure at different points in fruits and vegetables (Yaman and Bayoindirli 2002). The mechanism for these positive effects of the coatings is based on their hygroscopic properties, which enables the formation of a barrier to water diffusion between mushroom and environments, thus avoiding its external transference.
Hardness
The texture of mushroom is often the first of many quality attributes judged by consumers, and therefore, is extremely important in overall product acceptance. In the present study, untreated (control) mushrooms gradually softened when stored at low temperatures, as evidenced by a gradual decrease in mushroom hardness during cold storage (Table 2). These results are in agreement with the report of Antmann et al. (2008) about shiitake (Lentinusedodes) mushrooms. In general, hardness of the control mushrooms declined from 19.06 ± 0.08 N at second day to 5.84 ± 1.26 N after 13 days of storage. In samples coated with GT, A–GT, and A formulations, hardness decreased from 27.40 ± 0.27, 23.66 ± 0.69, and 21.16 ± 0.24 N at second day to 12.30 ± 0.69, 9.04 ± 0.30, and 6.33 ± 0.54 N at 4 °C respectively at 13 days. It was clear that during storage, the texture of the mushrooms deteriorated and this difference increased; therefore, it is expected that hardness and fresh texture of mushroom can be maintained with coating. During the first days of storage, there were minimal differences between treated samples and the control for maintaining the initial quality of the samples’ texture, but with longer storage times, the positive effects of coatings on hardness changes, especially for GT-treated mushrooms, were observed.
These outcomes are in agreement with results of other works aimed to keep the hardness in fruits. For example, loss of hardness in strawberry was postponed by applying a pullulan-based edible coating (Diab et al. 2001) or delaying softening by shellac coating in apple (Bai et al. 2002) and chitosan coating in citrus fruit (Chien et al. 2007).
During storage, the texture of mushrooms is likely to soften considering various symbols, including loss in cell turgidity pressure, defeat of extracellular and vascular air and the decline of the cell wall, and consequent loss of water by the cell breakdown (Martinez-Ferrer et al. 2002). In spite of the hydrophilic character of polysaccharides, they can act as barriers to water transfer, delaying dehydration and therefore, extending the hardness of coated mushrooms and vegetables.
Color
Color is the most obvious indicator of quality to consumers. It is related to the age of the mushrooms, handling, and microbial spoilage and so color alone has been used as an indicator to quantify the shelf life. The color of fresh mushrooms is affected by the amount of oxygen presented by the way of suppressing the enzymatic browning reaction. In addition, microbial population could affect the color changes of fresh mushrooms.
Analysis of variance indicated that the use of the edible coatings and storage variables (temperature and time) in mushrooms had a significant effect in the color parameters (p < 0.01) (Table 1). Results of color changes in mushrooms coated with GT, A, and A–GT formulations and stored at different temperatures are shown in Fig. 2a–c.
As shown in Fig. 2a–c, L* changes decreased during storage while a sharp reduction is seen for the control. Significant differences were found between the control and treated samples (Table 1). According to Fig. 2a, ΔL of uncoated mushrooms declined from −7.04 ± 0.32 at second day to −15.81 ± 0.46 after 13 days of storage. For samples coated with GT, A, and A–GT formulations, ΔL decreased from −5.84 ± 1.14, −4.61 ± 0.75, and −5.51 ± 0.70 at second day to −10.47 ± 0.91, −8.36 ± 0.60, and −11.04 ± 0.13, at 4 °C, respectively at 13 days. The lowest change in ΔL was relevant to mushrooms coated with A–GT and approximately similar to changes in ΔL caused by GT, especially after 6 days (Table 3).
As shown in Fig. 3a–c, color difference (ΔE) increased during storage time. The results showed that (Fig. 3a) the mushrooms treated with A–GT had the lowest changes of ΔE from 8.02 ± 0.15 to 15.64 ± 0.69, and for uncoated mushrooms, changes of ΔE were from 11.69 ± 0.42 to 24.16 ± 0.54 at 4 °C after 13 days. As expected, more changes in ΔE were observed at higher temperatures. Studies by Choi and Sapers (1994) indicated that purple discoloration apparently resulted from a reaction involving a short-lived intermediate in the enzymatic browning of l-dopaquinone, a phenolic compound in mushrooms. Hunter L* measurement is the most commonly applied method for mushroom quality grading. According to Taghizadeh et al. (2009) mushrooms with an L* less than 69 would not be acceptable even at retail levels. L* in this experiment ranged from 76.80 to 56.77. L* generally decreased over the storage period, indicating a loss of luminosity in the samples over the storage period. Generally, mushrooms coated with aloe vera and gum tragacanth showed the highest L* in all experimental temperatures during the storage period.
Sugars
Aerobic respiration causes breaking sugars and other sources of energy. Therefore, sugar content of mushrooms during storage is reduced. Sugars are the major respiratory substrates (Tseng and Mau 1999). Total and soluble sugar concentrations in harvested plant products are considered important indicators of postharvest deterioration (Hammond and Nichols 1975). Hammond and Nichols (1975) found that trehalose and sugar alcohols such as mannitol and arabitol are the main carbohydrates in mushrooms that reduce quickly after harvest. This indicated that consumption of these compounds were sufficient to account for the postharvest CO2 production. The results in Table 1 show that total sugar of the mushroom samples during storage reduced significantly. This reduction in the control samples occurred faster. Bosporus mushrooms were stored at 12 °C for 12 days (Tseng and Mau 1999). According to Fig. 4, the total amount of sugar for the controls declined from 31.75 ± 0.80 mg at second day to 0.43 ± 0.25 mg, and for samples coated with GT, A–GT, and A formulations, total sugar decreased from 34.28 ± 1.04, 40.64 ± 2.01, and 35.63 ± 1.68 mg at second day to 7.929 ± 4.63, 10.63 ± 8.73, and 6.81 ± 4.40 mg at 15 °C respectively after 13 days of storage. During the first days of storage, there were minimal differences between the treated samples and the control, but with longer storage times, the positive effects of the coatings on total sugar samples, especially at temperatures 10 and 15 °C, were observed. Among the samples treated with A–GT, the best effect was found in delaying reducing sugar, although GT coating had a nearly similar effect with coating A–GT for reducing sugar.
Conclusions
As the result of increasing demand for fresh mushrooms by consumers and the need for technologists to keep the quality of the product, application of suitable edible coating has become a field of interest in recent years. Coating with natural compounds is a minimal process which is more accepted by consumers compared to other shelf life extension methods such as irradiation. Additionally, it is more economical and convenient in comparison with modified atmosphere packaging.
In this research, aloe vera gel, gum tragacanth, aloe vera, and gum tragacanth-based edible coating increased the shelf life of mushroom in comparison with uncoated mushroom. Furthermore, texture analysis showed that these coatings had a protective effect on mushrooms, reflected by the greater hardness of coated samples during storage. This could reduce economic losses due to spoilage as the result of mechanical damage during handling and transportation. Also, gum tragacanth had beneficial effects in retarding the ripening process. This treatment was effective as a physical barrier and thus reduced the weight loss during postharvest storage. Therefore, the use of gum tragacanth and combination of aloe vera and gum tragacanth for maintaining the quality of mushrooms during long-term storage could be recommended. Results showed that mushrooms treated with gum tragacanth and aloe vera and gum tragacanth had good quality at higher temperatures, such as 10 °C up to 10 days of storage, and the physicochemical characteristics at this temperature were nearly similar to the samples stored at 4 °C. Therefore, mushrooms treated with gum tragacanth and the combination of aloe vera and gum tragacanth could be stored at 10 °C. This could reduce energy consumption and warehousing costs during storage. Meanwhile, the application of different packaging such as MAP of such coated samples may lead to higher quality attributes during storage at elevated temperature and will be the next step for upcoming researches.
References
Antmann, G., Ares, G., Lema, P., & Lareo, C. (2008). Influence of modified atmosphere packaging on sensory quality of shiitake mushrooms. Postharvest Biology and Technology, 49, 164–170.
Azarikia, F., & Abbasi, S. (2010). On the stabilization mechanism of Doogh (Iranian yoghurt drink) by gum tragacanth. Food Hydrocolloids, 24, 358–363.
Bai, J., Baldwin, E. A., & Hagenmaier, R. H. (2002). Alternatives to shellac coatings provide comparable gloss, internal gas modification, and quality for ‘delicious’ apple fruit. Hortscience, 37(3), 559–563.
Brosnan, T., & Sun, D. W. (2004). Improving quality inspection of products by computer vision—review. Journal of Food Engineering, 61, 3–16.
Burton, K. S., & Noble, R. (1993). The influence of flush number, bruising and storage temperature onmushroom quality. Postharvest Biology and Technology, 3, 39–47.
Chien, P. J., Sheu, F., & Yang, F. H. (2007). Effects of edible chitosan coating on quality and shelf life of sliced mango fruit. Journal of Food Engineering, 78, 225–229.
Choi, S. W., & Sapers, G. M. (1994). Purpling reaction of sinapic acid model system containing L-DOPA and mushroom tyrosinase. Journal of Agricultural and Food Chemistry, 42, 1183–1189.
Diab, T., Biliaderis, C. G., Gerasopoulos, D., & Sfakiotakis, E. (2001). Physicochemical properties and application of pullulan edible films and coatings in fruit preservation. Journal of the Science of Food and Agriculture, 81, 988–1000.
Donhowe, G., & Fennema, O. (1994). Edible films and coatings: characteristics, fonnation, definitions and testing methods. In J. M. Krochta, E. A. Baldwin, & M. O. Nisperos-Carriedo (Eds.), Edible coatings and films to improve food quality (pp. 1–24). Lancaster: Technomic.
Eshun, K., & He, Q. (2004). Aloe vera: a valuable ingredient for the food, pharmaceutical and cosmetic industries—a review. Critical Reviews in Food Science and Nutrition, 44, 91–96.
Esua, M. F., & Rauwald, J. W. (2006). Novel bioactive maloylglucans from aloe vera gel: isolation, structure elucidation and invitro bioassays. Carbohydrate Research, 341, 355–364.
García, L. C., Pereira, L. M., Sarantópoulos, C. I. G. L., & Hubinger, M. D. (2010). Selection of an edible starch coating for minimally processed strawberry. Food and Bioprocess Technology, 3, 834–842. doi:10.1007/s11947-009-0313-9.
Giri, S. K., & Prasad, S. (2005). Drying kinetics and rehydration characteristics of microwave-vacuum and convective hot-air dried mushrooms. Journal of Food Engineering, 78, 512–521.
Hammond, J. B. W., & Nichols, R. (1975). Changes in respiration and soluble carbohydrates during the post-harvest storage of mushrooms Agaricus bisporus. Journal of the Science of Food and Agriculture, 926, 835–842.
Kim, K. M., Ko, J. A., Lee, J. S., Park, H. J., & Hanna, M. A. (2006). Effect of modified atmosphere packaging on the shelf-life of coated, whole and sliced mushrooms. LWT-Food Science and Technology, 39, 364–371.
Lee, J. Y., Park, H. J., Lee, C. Y., & Choi, W. Y. (2003). Extending shelf-life of minimally processed apples with edible coatings and antibrowning agents. LWT-Food Science and Technology, 36, 323–329.
Lopez-Briones, G. L., Varoguaux, P., Chambroy, Y., Bouquant, J., Bureau, G., & Pascat, B. (1992). Storage of common mushroom under controlled atmospheres. International Journal of Food Science and Technology, 27, 493–505.
Martinez-Ferrer, M., Harper, C., Perez-Munoz, M., & Chaparro, M. (2002). Modified atmosphere packaging of minimally processed mango and pineapple fruits. Journal of Food Science, 67, 3365–3371.
Martínez-Romero, D., Serrano, M., Valero, D. & Castillo, S. (2003). Aplicación de Aloe vera como recubrimiento sobre frutas y hortalizas. SP Patent filed 200302937.
Martínez-Romero, D., Alburquerque, N., Valverde, J. M., Guillén, F., Castillo, S., Valero, D., et al. (2006). Postharvest sweet cherry quality and safety maintenance by aloe vera treatment: a new edible coating. Postharvest Biology and Technology, 39(1), 93–100.
Mau, J. L., Miklus, M. B., & Beelman, R. B. (1993). The shelf life of Agaricus mushrooms. In C. Charalambous (Ed.), The shelf life of foods and beverages (pp. 255–288). Amsterdam: Elsevier.
McDonald, K., & Sun, D. W. (2000). Vacuum cooling technology for the food industry: a review. Journal of Food Engineering, 45(2), 55–65.
Mohamadnia, Z., Zohuriaan-Mehr, M. J., Kabiri, K., & Razavi-Nouri, M. (2008). Tragacanth gum-graft-polyacrylonitrile: synthesis, characterization and hydrolysis. Journal of Polymer Research, 15, 173–180.
Ni, Y., Turner, D., Yates, K. M., & Tizard, I. (2004). Isolation and characterization of structural components of Aloe vera L. leaf pulp. International Immunopharmacology, 4, 1745–1755.
Nussinovitch, A., & Kampf, N. (1993). Shelf life extension and conserved texture of alginate coated mushrooms (Agaricus bisporus). Journal of Food Technology, 26, 469–475.
Otady, M., Vaziri, A., Seifkordi, A. A., & Kheirolomoom, A. (2005). Gum tragacanth gels as a new supporting matrix for immobilization of whole-cell. Iranian Journal of Chemistry and Chemical Engineering, 24(4), 1–7.
Pedreschi, F., León, J., Mery, D., Moyano, P., Pedreschi, R., Kaack, K., et al. (2007). Color development and acrylamide content of pre-dried potato chips. Journal of Food Engineering, 79(3), 786–793.
Quevedo, R., Aguilera, J., & Pedreschi, F. (2009). Color of salmon fillets by computer vision and sensory panel. Food and Bioprocess Technology, 3, 637–643.
Simal, S., Femenia, A., Llull, P., & Rossello, C. (2000). Dehydration of aloe vera: simulation of drying curves and evaluation of functional properties. Journal of Food Engineering, 43, 109–114.
Singla, R., Abhijit, G., & Ghosh, M. (2010). Physicochemical and nutritional characteristics of organic acid-treated button mushrooms (Agaricus bisporous). Food and Bioprocess Technology. doi:10.1007/s11947-010-0457-7.
Stewart, C. R., Morris, C. J., & Thompson, J. F. (1974). Changes in amino acid content of excised leaves during incubation. II. Role of sugar in the accumulation of proline in wilted leaves. Planta, 120, 279–289.
Sun, D.-W. (2008). Computer vision technology for food quality evaluation (p. 608). San Diego: Academic Press/Elsevier.
Taghizadeh, M., Gowen, A., & O’Donnell, C. P. (2009). Prediction of white button mushroom (Agaricus bisporus) moisture content using hyperspectral imaging. Sensing and Instrumentation for Food Quality and Safety, 3, 219–226.
Tao, F., Zhang, M., Hangqing, Y., & Jincai, S. (2006). Effects of different storage conditions on chemical and physical properties of white mushrooms after vacuum cooling. Journal of Food Engineering, 77, 545–549.
Thompson, A. K. (2003). Postharvest treatments. In Fruit and vegetables (pp. 47–52). Ames: Blackwell.
Tseng, Y. H., & Mau, J. L. (1999). Contents of sugars, free amino acids and free 50-nucleotides in mushrooms, Agaricus bisporus, during post-harvest storage. Journal of the Science of Food and Agriculture, 79, 1519–1523.
Valverde, J. M., Valero, D., Martínez-Romero, D., Guillén, F., Castillo, S., & Serrano, M. (2005). Novel edible, coating based on aloe vera gel to maintain table grape quality and safety. Journal of Agricultural and Food Chemistry, 53(20), 7807–7813.
Yaman, Ö., & Bayoindirli, L. (2002). Effects of an edible coating and cold storage on shelf-life and quality of cherries. LWT-Food Science and Technology, 35, 146–150.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohebbi, M., Ansarifar, E., Hasanpour, N. et al. Suitability of Aloe Vera and Gum Tragacanth as Edible Coatings for Extending the Shelf Life of Button Mushroom. Food Bioprocess Technol 5, 3193–3202 (2012). https://doi.org/10.1007/s11947-011-0709-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-011-0709-1