Abstract
Recent studies have established the controlling influence of rhizospheric biota, especially arbuscular mycorrhizal fungi (AMF), on colonization and spread of some alien plants in their introduced range. But how AMF from different geographical sources influence traits that contribute to invasiveness, particularly in presence of neighbouring plants of other species, has been rarely investigated. Thus, we compared the influence of some local (Kashmir Himalayan isolates) and non-local (isolates from Rajasthan, India) AMF isolates of Glomus moseae, G. fasciculatum and Gigaspora margarita on vegetative and reproductive attributes of Mayweed Chamomile (Anthemis cotula L.), a highly invasive species in the Kashmir Himalaya, India. We also examined whether or not the neighbouring plant species, namely Daucus carota L. (Apiaceae) alters the mutualistic interaction between the AMF and A. cotula. Pot experiments revealed greater positive impact of the local than the non-local AMF on vegetative as well as reproductive attributes of A. cotula. Experimental field studies showed that the incidence of highly prevalent Arum-type mycorrhizal colonization in natural populations of A. cotula was reduced in presence of D. carota. Besides, the local AMF significantly promoted growth of A. cotula more than D. carota under mixed-culture conditions. These results suggest that the facilitation of some alien plant invasions by AMF needs to be considered together with plant–plant interactions and invasion-induced changes in the soil microbial community.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological invasion, a major component of current global environmental change, leads to habitat deprivation, reduced biodiversity, alteration of ecosystem services and functions (Simberloff and von Holle 1999). These modifications bring about significant harmful ecological and economic impacts (Perrings et al. 2005; Pimentel et al. 2005). Many invasive plants are known to successfully establish mutualistic associations with AMF and the facilitative role of arbuscular mycorrhizas in plant invasions is largely through enhanced nutrient acquisition and transfer (Marler et al. 1999; Zabinski et al. 2002; Carey et al. 2004; Burns 2004), improved drought tolerance (e.g. Ruiz-Lozano et al. 1995), enhanced herbivore avoidance (Abigail et al. 2005), evasion of native enemies (Shah and Reshi 2007), and alteration of competitive interactions of invasive plants with native species (Shah et al. 2006; Casper and Castelli 2007). Degree of plant growth promotion by AMF, however, is context specific and depends on habitat conditions, identity of the alien plant species, and nature of AMF symbiont (Streitwolf-Engel et al. 1997; Eom et al. 2000; Stampe and Daehler 2003). For example, AMF inocula from different sources are known to have a differential effect on growth and performance of host species (Bever 1994), and also on the outcome of competitive interactions between invasive and native plant species (Scheublin et al. 2007). While considerable progress has been achieved in understanding the ecological role of mycorrhizal mutualisms in invasiveness of certain alien plant species (Reinhart and Callaway 2006; Fumanal et al. 2006), the underlying mechanisms that govern such novel interaction have not been fully explained and thus merit further research through robust field and experimental studies (Shah and Reshi 2007). Hence we studied the role of AMF isolates of different geographical origins in promotion of invasiveness of Anthemis cotula L. (Mayweed chamomile, Stinking mayweed), an annual, herbaceous member of sunflower family (Asteraceae), native to southern Europe–west Siberia (Erneberg 1999). It grows as a common weed of arable lands and farmyards in Scottish lowlands, Ireland, England and Wales (Kay 1971). Its success in disturbed habitats has been attributed to a protracted recruitment pattern, high population size even after seedling mortality (Allaie et al. 2005), allelopathic activity of its aqueous leaf leachate (Allaie et al. 2006), herbivore induced over-compensatory growth (Rashid et al. 2006), profuse production of achenes and synchrony between their germination and favourable environmental conditions (Rashid et al. 2007) and mutualistic facilitation by AMF (Shah and Reshi 2007). We also examined the influence of neighbouring plant species, Daucus carota, on the mutualistic interaction between the AMF and A. cotula. Daucus carota (Apiaceae) is one of the common co-associates of A. cotula in Kashmir Himalaya.
Materials and methods
Field studies
We carried out field studies at five study sites (Table 1) located in different parts of the Kashmir Himalaya (32° 20′ to 34° 50′ North latitude and 73° 55′ to 75° 35′ East longitude). All the study sites experienced sub-Mediterranean type of climate and average temperature during the study period (April to September, 2006) ranged from 7.2°C to 30.9°C. Total annual rainfall at each of the study sites was about 1,000 mm. At each site, we established replicate monospecific patches of A. cotula by continuously removing the individuals of its co-associates. We also established replicate heterospecific patches wherein individuals of A. cotula and D. carota were allowed to grow together. We regularly eliminated any species other than the study species that tended to grow in these patches throughout the course of the experiment and maintained constant density of plants across different study sites. We studied three patches per site and patch area was maintained uniformly at 5 m2. Average distance between patches in a study site was about 2 m. Each patch was divided into a central zone of 2.5 m2 and a surrounding buffer zone to reduce edge effects from surrounding vegetation. We collected 15 plants from the central zone of each patch by excavating the whole root systems which were pooled together for assessment of mycorrhizal colonization. Randomly selected 100 root pieces (1 cm long) were cleared in 15% KOH, acidified in 1 N HCl and stained with trypan blue followed by destaining in 50% Lactic acid in accordance with the modified procedure of Phillips and Hayman (1970). We used the modified line intersection method (McGonigle et al. 1990) and frequency distribution method of Bierman and Linderman (1981) to determine the percentage of root length colonized by AMF.
Pot trial
We established a pot trial in a randomized block design with three replications per treatment in the Botanical Garden of the University of Kashmir, Srinagar, J&K, India, to test the effect of AMF source (local vs. non-local), and neighbour (monoculture vs. mixed culture) in invasiveness of A. cotula. We transferred seedlings of the test species to earthen pots (22 cm × 28 cm) filled with sterilized garden soil (clay = 28%, silt = 50%, sand = 22%, pH = 7.5 and organic carbon = 1.6%) mixed with sand in a ratio of 2:1. The soil was sterilized by autoclaving three times at 85°C for 90 min with a 12 h interval between each autoclaving. The non-local AMF isolates, originally collected from Rajasthan—a region more than 600 km away from the Kashmir Himalaya, were obtained from School of Life Sciences, Jawahar Lal Nehru University, New Delhi, India. The native AMF were generated from the soils invaded by A. cotula using trap cultures. We used spore size, colour, and ornamentation for identification of AMF isolates using original descriptions of Schenck and Perez (1990) and the INVAM (http://invam.caf.wvu.edu). We placed a mixed mycorrhizal inoculum (50 g) of Glomus moseae, G. fasciculatum and Gigaspora margarita 3 cm below the surface of soil so as to ensure contact of seedling roots with the inoculum. The AMF inoculants were chosen on the basis of their abundant association with natural populations of A. cotula (Shah and Reshi 2007). Two seedlings per pot both in case of mono- and mixed cultures were maintained under natural light conditions (12 to 14 h), relative humidity (58.5–79.3) and temperature (7.2–30.9°C) and the pots were watered to field capacity on alternate days.
Plant growth analyses
We harvested the pot grown individuals of A. cotula from each of the above mentioned treatments at maturity and recorded the shoot and root length, dry mass, number of branches and capitula per plant. Shoot and root length were measured with the help of a standard measuring tape. Root and shoot dry mass was determined after drying the above- and belowground plant parts at 80°C for 48 h to constant weight. Since we were interested only in elucidating the effect of AMF on invasive attributes of A. cotula in mono- and mixed cultures, the data on growth characteristics of D. carota are not given.
We calculated AMF dependency of the target plant species in monoculture or in mixed-culture following Van’der Heijden (2002):
where ‘a’ is the average dry mass of AMF treated plants and ‘b’ is the dry mass of the untreated plants. Basic statistics, such as trait means and variances were obtained using SPSS 10. Multiple analysis of variance (MANOVA) was used to study the response of attributes, including dry mass of shoots and roots (g), shoot length and root length (cm) and number of branches and number of capitula per plant by to different treatments of AMF (local vs. non-local), and neighbour (monospecific vs. heterospecific). We performed univariate analyses of variance and compared each treatment to the control by Tukey HSD.
Results
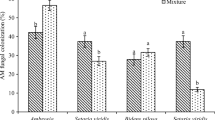
A. cotula, in monospecific patches at Zukura and Shikarghat, had more than 80% Arum-type root length colonization (RLC) and more than 60% RLC in other two study sites at Nagbal and Narbal (Table 1). This Arum-type AMF colonization was characterized by extensive intercellular hyphae and terminal arbuscles in root cortical cells of A. cotula. However, degree of root colonization of A. cotula was reduced in the neighbourhood of D. carota (Table 1) at all the study sites and highest reduction (24.5%) was recorded at Nagbal.
Effect of mycorrhizal inoculation on different attributes of A. cotula in mono- and mixed cultures using local and non-native AMF in comparison with control (no AMF used) is presented in Fig. 1. Perusal of results reveals that local AMF more than doubled its shoot length (Fig. 1b) and improved shoot dry mass by more than four times in comparison to uninoculated plants (Fig. 1d). However, both these attributes were only slightly improved in response to inoculation with non-local AMF. Even when A. cotula was grown in association with D. carota, local AMF had more positive influence on length and dry mass of shoot than non-native AMF (Fig. 1b, d). Root length of A. cotula did not show any significant change in response to inoculation either with local or non-local AMF (Fig. 1a), irrespective of whether grown under monoculture or mixed culture conditions, but roots were thicker and the dry mass was enhanced more by local than non-native AMF (Fig. 1c). Number of branches per plant in A. cotula was also improved more by local than non-local AMF (Fig. 1e). In comparison with untreated control plants, number of capitula in A. cotula grown in monoculture was over three times more in plants inoculated with local AMF and two times more in plants treated with non-local AMF. Under mixed-culture conditions, local and non-local AMF increased number of capitula in A. cotula by about three and two times, respectively, in comparison with uninoculated control treatment (Fig. 1f). The MANOVA (Table 2) showed that mycorrhizal inoculation significantly influenced the traits that contribute to invasiveness of A. cotula. However, the local and non-local AMF isolates differed significantly in their influence on various attributes of A. cotula. Irrespective of the geographical source of AMF, co-occurrence of D. carota also had a significant influence on the extent of growth promotion by AMF (Table 2). Analysis of variance revealed that AMF inoculation significantly (P < 0.001) influenced various vegetative and reproductive attributes of A. cotula both in mono- as well as in mixed-culture. All the investigated attributes, except length and dry mass of roots, were significantly influenced by the co-occurrence of D. carota with A. cotula. Mycorrhization, particularly with local AMF, positively influenced all the attributes of A. cotula, except root length and dry mass.
Means (±standard error) of various growth and fitness attributes of Anthemis cotula in monoculture (■) and in association with Daucus carota (□) under no (control), local, and non-local AMF treatments. Means that do not share a lower-case letter are significantly different following Tukey’s post hoc test
Irrespective of whether or not growing in association with D. carota, inoculation of A. cotula with local AMF decreased root: shoot ratio (Fig. 2) in comparison with non-local AMF. Mycorrhizal Inoculation Effect (MIE) in A. cotula due to local and non-native AMF in the absence of D. carota was 75.26 and 22.7, respectively and the same in presence of D. carota was reduced to 56.98 and 8.64, respectively upon inoculation with local and non-local AMF.
Discussion
Significant AMF colonization of A. cotula across different spatially separated populations (Table 1) together with improvement in its growth and fitness attributes upon AMF inoculation points towards possible contributory role of arbuscular mycorrhizal fungi in promoting the invasion of various habitats by this species in the Kashmir Himalaya, India. Higher incidence of AMF association with roots of some alien plant species have also been reported by Read et al. (1976) and Fumanal et al. (2006). Our study further established that mere presence of AMF mutualists does not necessarily confer advantage on the host; instead the source of AMF determines the extent of benefit. The present study demonstrated that local AMF had more positive influence on various traits of A. cotula than non-local AMF. However, the benefits derived by A. cotula from AMF association were significantly influenced by the co-occurring D. carota, as has also been observed by Callaway et al. (2003) while studying the effects of soil fungi on interactions between Centaurea melitensis, an exotic invasive weed in central California, and two co-occurring grasses, Nassella pulchra and Avena barbata. Mummey et al. (2005) also reported that the AMF community colonizing the roots of Dactylis glomerata was strongly controlled by neighbouring roots of an invasive forb, Centaurea maculosa. More recently, Hawkes et al. (2006) and Mummey and Rillig (2006) also demonstrated change in AMF assemblages in native plant roots in presence of invasive species. Instances of neighbouring plants significantly influencing invasive plants and vice-versa are also known (Stampe and Daehler 2003; Hallett 2006).
A positive effect of AMF inoculation on vegetative and reproductive attributes with consequent increases in fitness of A . cotula in the absence of co-occurring D. carota is similar to previous findings (Shah et al. 2006; Shah and Reshi 2007). We observed a more positive feedback from local than non-local AMF, both in mono- and mixed cultures. Despite favourable influences on root and shoot dry mass of A. cotula by local AMF inoculation, declines in root: shoot ratio (Fig. 2) is suggestive of mycorrhizal facilitation in uptake of mineral nutrients with possible implications for resource allocation. The trend in root: shoot ratio under different treatments, however, needs allometric analysis to validate whether or not the differences in the ratios are due to decreased growth and delayed development or reallocation in response to varying resources. The observed differences in growth and reproductive attributes of A. cotula in presence of D. carota could possibly be because of the interference of the latter with the belowground AMF mutualistic interactions. Similar inference has also been drawn by Stinson et al. (2006) who demonstrated that invasive plants can suppress growth of the native plants by disrupting their mutualistic associations with belowground AMF.
Positive Mycorrhizal Inoculation Effect (MIE) on A. cotula, especially by local AMF, points towards greater dependency of this alien invasive species on the mycorrhizal mutualism. Reduction in MIE in presence of D. carota could be due to alteration of the feedback between AMF and A. cotula. While plant species with more mycorrhizal dependency also exhibit greater mycorrhizal species sensitivity (Van’der Heijden 2002), such AMF specificity of A. cotula requires further investigation for its confirmation. Even being an inhabitant of ruderal habitats where disturbance impairs the mycorrhization incidence (Reeves et al. 1979), A. cotula appears to draw belowground AMF community to its advantage in the Kashmir Himalaya.
The present study allows us to conclude that local AMF have a more positive effect on almost all attributes that contribute to fitness and invasiveness of A. cotula as compared to non-local AMF. However, the extent and intensity of benefits accrued from AMF association with A. cotula are significantly influenced by the presence of neighbouring plant species, such as D. carota. We suggest future studies to further examine the tripartite (host–AMF–neighbour) interactive feedback in relation to alien plant invasions which would facilitate better understanding of factors promoting invasions.
References
Abigail ARK, Hartnett DC, Wilson GWT (2005) Effects of mycorrhizal symbiosis on tallgrass prairie plant–herbivore interactions. Ecol Lett 8:61–69
Allaie RR, Reshi Z, Wafai BA (2005) Demographic studies on alien invasive Anthemis cotula L. in Kashmir Himalaya. Trends Life Sci 20:49–56
Allaie RR, Reshi Z, Rashid I, Wafai BA (2006) Effects of aqueous leaf leachate of Anthemis cotula—alien invasive species on germination behaviour of some field crops. J Agron Crop Sci 192:186–191
Bever JD (1994) Feedback between plants and their soil communities in an old field community. Ecology 75:1965–1977
Bierman B, Linderman RG (1981) Quantifying vesicular arbuscular mycorrhizae: a proposed method towards standardization. New Phytol 87:63–67
Burns JH (2004) A comparison of invasive and non-invasive dayflowers (Commelinaceae) across experimental nutrient and water gradients. Divers Distrib 10:387–397
Callaway RM, Mahall BE, Wicks C, Pankey J, Zabinski C (2003) Soil fungi and the effects of an invasive forb on native versus naturalized grasses: neighbor identity matters. Ecology 84:129–135
Carey EV, Marler MJ, Callaway RM (2004) Mycorrhizae transfer carbon from a native grass to an invasive weed: evidence from stable isotopes and physiology. Plant Ecol 172:133–141
Casper BB, Castelli JP (2007) Evaluating plant–soil feedback together with competition in serpentine grassland. Ecol Lett 10:394–400
Eom AH, Hartnett DC, Wilson GWT (2000) Host plant species effects on arbuscular mycorrhizal fungal communities in tallgrass prairie. Oecologia 122:435–444
Erneberg M (1999) Effects of herbivory and competition on an introduced plant in decline. Oecologia 118:203–209
Fumanal B, Plenchette C, Chauvel B, Bretagnolle F (2006) Which role can arbuscular mycorrhizal fungi play in the facilitation of Ambrosia artemisiifolia L. invasion in France. Mycorrhiza 17:25–35
Hallett SG (2006) Dislocation from coevolved relationships: a unifying theory for plant invasion and naturalization. Weed Sci 54:282–290
Hawkes CV, Belnap J, D’Antonio C, Firestone MK (2006) Arbuscular mycorrhizal assemblages in native plant roots change in the presence of invasive exotic grasses. Plant Soil 281:369–380
Kay QON (1971) Anthemis cotula L. series: biological flora of the British Isles. J Ecol 59:623–636
Marler MJ, Zabinski CA, Callaway RM (1999) Mycorrhizae indirectly enhances competitive effects of invasive forbs on a native bunch grass. Ecology 80:1180–1186
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular–arbuscular mycorrhizal fungi. New Phytol 115:495–501
Mummey DL, Rillig MC (2006) The invasive plant species Centaurea maculosa alters arbuscular mycorrhizal fungal communities in the field. Plant Soil 288:81–90
Mummey DL, Rillig MC, Holben WE (2005) Neighbouring plant influences an arbuscular mycorrhizal fungal community composition as assessed by T-RFLP analysis. Plant Soil 271:83–90
Perrings C, Dehnen-Schmutz K, Touza J, Williamson M (2005) How to manage biological invasions under globalization. Trends Ecol Evol 20:212–215
Phillips SJM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–160
Pimentel D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ 52:273–288
Rashid I, Reshi Z, Shah MA, Wafai BA (2006) Does herbivory promote invasiveness of Anthemis cotula in Kashmir Himalaya, India? In: International Symposium on Biology, Ecology and Management of world’s worst plant invasive species 10–14 Dec. CEMDE, University of Delhi, India
Rashid I, Reshi Z, Wafai BA (2007) Germination ecology of invasive alien Anthemis cotula L. helps it synchronize its successful recruitment with favourable habitat conditions. Ann Appl Biol 150:361–369
Read DJ, Koucheki HK, Hodgson J (1976) Vesicular arbuscular mycorrhiza in natural vegetation systems. I. The occurrence of infection. New Phytol 77:641–653
Reeves FB, Wagner D, Moorman T, Kiel J (1979) The role of endomycorrhizae in revegetation practices in the semi-arid west. I. A comparison of incidence of mycorrhizae in severely disturbed vs. natural environments.. Am J Bot 66:6–13
Reinhart KO, Callaway RM (2006) Soil biota and invasive plants. New Phytol 170:445–457
Ruiz-Lozano JM, Azcón R, Gomez M (1995) Effects of arbuscular–mycorrhizal Glomus species on drought tolerance: physiological and nutritional plant responses. Appl Environ Microbiol 61:456–460
Schenck NC, Perez Y (1990) Manual for the identification of VA mycorrhizal fungi. Synergistic, Gainesville
Scheublin TR, Van Logtestijn RSP, Van’ der Heijden MGA (2007) Presence and identity of arbuscular mycorrhizal fungi influence competitive interactions between plant species. J Ecol 95:631–638
Shah MA, Reshi Z (2007) Invasion by alien Anthemis cotula L. in a biodiversity hotspot: release from native foes or relief from alien friends. Curr Sci 92:21–22
Shah MA, Reshi Z, Rashid I, Wafai BA (2006) Effect of native and non-native arbuscular mycorrhizas on seedling establishment and competitive interactions of A. cotula in Kashmir Himalaya. In: International Symposium on Biology, Ecology and Management of World’s Worst Plant Invasive Species, 10–14 Dec. CEMDE, University of Delhi, India
Simberloff DS, von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown. Biol Inv 1:21–32
Stampe ED, Daehler CC (2003) Mycorrhizal species identity affects plant community structure and invasion: a microcosm study. Oikos 100:362–372
Stinson KA, Campbell SA, Powell JR, Wolfe BE, Callaway RM, Thelen GC et al (2006) Invasive plant suppresses the growth of native tree seedlings by disrupting belowground mutualisms. PLoS Biol 4:1–5
Streitwolf-Engel R, Boller T, Weimken A, Sanders IR (1997) Clonal growth traits of two Prunella species are determined by co-occurring arbuscular mycorrhizal fungi from a calcareous grassland. J Ecol 85:181–191
Van’der Heijden MGA (2002) Arbuscular mycorrhizal fungi as determinant of plant diversity: in search of underlying mechanisms and general principles. Ecol Stud 157:243–266
Zabinski CA, Quinn L, Callaway RM (2002) Phosphorus uptake, not carbon transfer, explains arbuscular mycorrhizal enhancement of Centaurea maculosa in the presence of native grassland species. Funct Ecol 16:758–765
Acknowledgements
We thank Stefano Benvenuti, ARI, University of Pisa, Italy, for providing information about A. cotula in its native range and C.C. Daehler, University of Hawaii, Manoa, USA, Ian Baldwin, Max Planck Institute, Germany, and R. Callaway, University of Montana, USA, for their suggestions and critical appraisal of the earlier draft of this manuscript and providing useful suggestions. First author thanks the International Foundation of Sciences (IFS) Sweden for sponsoring his visit to Montpllier, France to attend and present this paper in the international conference Rhizosphere 2. The authors are indebted to two anonymous reviewers for their valuable suggestions that helped considerably to improve quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Tim Simon George.
Rights and permissions
About this article
Cite this article
Shah, M.A., Reshi, Z. & Rashid, I. Mycorrhizosphere mediated Mayweed Chamomile invasion in the Kashmir Himalaya, India. Plant Soil 312, 219–225 (2008). https://doi.org/10.1007/s11104-008-9706-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9706-1