Abstract
Nanofluids can be utilized as efficient heat transfer fluids in many thermal energy systems to improve the system’s thermal efficiency. This survey reviews and summarizes the experimental and numerical studies performed to determine the effect of nanofluids on the performance of condensing and evaporating systems. Advantages and disadvantages of nanofluid implementation in condensing and evaporating systems are evaluated and summarized. Moreover, some suggestions and recommendations are presented for future studies. This review shows that the nanoparticle deposition and nanoparticle suspension are two important factors affecting the thermal system’s efficiency. These factors should be considered when using different nanofluids in condensing and evaporating systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thermal management of devices at high temperatures is a significant challenge in today’s industrial applications including quick cooling in materials processing, combustion, nuclear reaction, and electronic equipment cooling. Researchers have used various techniques to respond to the effective thermal management demanded by industry. Among the various tested methods, boiling, condensation, and evaporation are recognized as the most promising techniques used passively or actively in various systems. Active thermal management system methods rely on external energy introduced to the system to augment the heat transfer process. These methods typically have the highest heat transfer rate compared to passive methods that rely solely on thermodynamics of heat transfer modes to perform the heat transfer process. Among various methods, electrohydrodynamics [1], the cold spray technique [2] and acoustic actuation [3] are popular examples of active techniques for nanofluid utilization in thermal systems [4]. Controlled micro/nanostructured surfaces [5]; surface modification [6]; incorporating of turbulators or swirl flow devices [7] are examples of passive techniques. Kim et al. [5] reviewed the published papers on micro/nanostructured walls used to enhance the boiling heat transfer. They concluded that the micro-porous and tiny size roughness structures on a wall have a significant role in enhancing the boiling heat transfer rate.
One of the attractive techniques for improving heat transfer during a phase change process is the use of nanofluids. Some researchers reviewed papers published toward this regard. Fang et al. [8] reviewed published papers about heat exchange and critical heat flux of nanofluid boiling. Their review showed that most studies on nanofluid boiling are for pool boiling, while only about 20% are performed for flow boiling. Moreover, they concluded that novel technologies are required to improve the stability of nanofluids for boiling systems with nanofluids. Ciloglu and Bolukbasi [9] reviewed pool boiling of nanofluids. They reported that the volume fraction of nanoparticles has an important effect on the boiling heat transfer coefficient and the critical heat flux. The boiling heat transfer coefficient and the critical heat flux are enhanced as the volume fraction of nanoparticles increases to a specific point, beyond which extra enhancement deteriorates the boiling heat transfer coefficient and has no influence on critical heat flux. This can be interpreted as saying that there is an optimum volume fraction that generates the maximum value of critical heat flux but does not decrease the boiling heat transfer coefficient. Kamatchi and Venkatachalapathy [10] reviewed research on the applications of nanofluids for improvement of critical heat flux during pool boiling heat transfer. They recommended employing nanoparticles with smaller diameters as they are more efficient for enhancing the critical heat flux. Moreover, they stated that to decrease particle clustering, it is necessary to investigate the influence of relative size between nanoparticles in fluid phase. Celen et al. [11] reviewed flow behaviours and applications of nanorefrigerants. They reported the lack of a general analytical model for predicting the physical properties of nanorefrigerants. Cai et al. [12] performed a literature review on the latest progress in aggregating fractal-based models to nanoparticles in fluid. They reported that by considering nanoparticles as moving liquids, three new fractal approaches for heat exchange of nanofluids containing convective heat exchange, critical heat flux, and subcooled pool boiling heat exchange can be presented. Bashirnezhad et al. [13] reviewed the latest experimental research on thermal conductivity of nanofluids. They reported that there is a direct and two-way relationship between the thermal conductivity and the temperature of the nanofluids.
The literature review showed that the available papers in this field cover only the applications of nanofluids in the boiling process, and they do not cover other processes with condensing and evaporating. Therefore, this review paper focuses on the experimental and numerical research performed to improve the efficiency of condensing and evaporating systems using nanofluids. Advantages and disadvantages of nanofluid implementation in condensing and evaporating systems are evaluated and summarized. Moreover, some suggestions and recommendations are presented for future studies.
Applications of nanofluids
Applications of nanofluids in condensing systems
Condensation refers to the vapor-to-liquid phase change in fluids that involves a considerable amount of heat release due to the fluid’s high internal energy differences between the liquid and gas states. Therefore, the condensation process is ideal for high-power cooling systems such as air-cooled power station condensers [14], biotechnology [15], food technology [16], and industrial automations [17].
Dalkilic and Wongwises [18] reviewed the condensation process, including condensation in all horizontal, vertical and inclined tubes, within smooth and enhanced tubes. They reported that replacing the working liquids in the condensing system is necessary for addressing the challenges from the ozone substrate’s evacuation and global warming generated by new chemical compounds. Much research has focused on condensation improvement through diverse active or passive techniques. Usually, thermal energy is exchanged inside the condensate film by conduction. Accordingly, adding materials with high values of thermal conductivity is useful for improving the thermal efficiency of a condensing system. Utilizing nanotechnology as a passive technique has the strong potential for enhancing the performance of condensing systems. Some researchers used this technique and investigated the influence of nanoparticles on condensation phenomena.
Liu et al. [19] performed an experimental study on the heat transfer involved in vapor condensation of nanofluids in inclined and horizontal grooved heat pipes. They reported that the thermal efficiency of an inclined grooved heat pipe can be improved by applying nanofluids. They used 50 nm copper oxide nanoparticles for a 1% concentration nanofluid. Adding CuO nanoparticles to water causes the maximum heat flux of the heat pipe to increase. Huminic and Huminic [20] experimentally investigated the heat exchange in the condensation of nanofluid vapor in a thermosyphon system. They used iron oxide nanoparticles with the maximum concentration of 5.3%. Their results showed that the thermal resistance of the thermosyphon with the nanoparticle solution is lower compared to the pure water. Reis Parise [21] presented a simulation model that used nanofluids as condenser coolants in heat pumps. The results of their research showed that an increase in the thermal conductivity of the condenser coolant positively affects the efficiency of the heat pump. The author observed about a 5.4% enhancement in the heating coefficient of efficiency when using a nanoparticle concentration of 2%. Huminic and Huminic [22] investigated numerically the heat exchange in the condensation of the nanofluid vapor in thermosiphons. They considered three values for nanoparticle concentrations of 0, 2.0, and 5.3%. They concluded that the volume fraction of nanoparticles has an important influence in decreasing the temperature difference created between the evaporator and condenser. Avramenko et al. [23] presented an analytical model to investigate the flow characteristics and heat exchange in film condensation of stationary nanofluid vapor around a flat wall. They included the Brownian and thermophoretic diffusion mechanisms in their model and concluded that the Brownian diffusion decreases heat transfer, while the thermophoretic diffusion increases the heat transfer of the condensate film. In other research, Avramenko et al. [24] studied on vapor that flowed with a constant speed over a flat wall and achieved similar results. El Mghari et al. [25] numerically studied the nanofluid condensation within a horizontal smooth square microchannel. They reported that the condensation heat transfer coefficient is enhanced about 20% as the nanoparticle concentration increases from 0 to 5%. Moreover, they found that the condensation heat transfer increases with a decrease in the microchannel hydraulic diameter. This enhancement in condensation heat transfer by decreasing hydraulic diameter was more important for water than nanofluids. Turkyilmazoglu [26] performed an analytical study to investigate the film condensation of nanofluids over a perpendicular wall. They used both single and multi-phase models and various nanoparticles, including copper, copper oxide, silver, alumina, and titanium oxide, to simulate the nanofluids. The author found that the condensate film width decreased as the concentration of nanoparticles increased due to the acceleration of the fluid flow within the hydrodynamic boundary layer. Moreover, it was concluded that Ag nanoparticles have the maximum influence on cooling the condensate film, while TiO2 nanoparticles have the minimum effect due to their thermal conductivities. As a result, the thermal conductivity of nanoparticles is the most important factor affecting condensation. In other research, Turkyilmazoglu [27] performed the same problem on a curved perpendicular plate with a convex/concave configuration. The author observed a higher heat exchange due to the smaller thicknesses of hydrodynamic and thermal boundary layers for the condensation of nanofluids around the curved walls in comparison to flat ones. Malvandi et al. [28] used an analytical method to study the influences of alumina and titania nanoparticles’ migration on improving heat transfer of a film during condensation around a perpendicular cylinder. It is worth mentioning that changing the volume fraction and direction of nanoparticle migration can control the thermophysical attributes of nanofluids [29, 30]. This dynamic is effective as it can enhance the cooling efficiency by arranging the flow and heat transfer rate. Malvandi et al. [28] found that the migration of nanoparticles toward the cold wall enhances the nanoparticle concentration. This enhancement in nanoparticle concentration causes an increase in the thermal conductivity of the condensate film and improves the thermal efficiency of the system. Malvandi et al. [31] analytically studied the film-wise condensation of nanofluids around a perpendicular wall in a laminar regime. They included the nanoparticle migration in their model. Their results showed that the heat transfer rate and nanoparticle concentration within the condensate film increase as the saturation nanoparticle volume fraction increases. Malvandi et al. [32] investigated the influences of magnetic field on thermal conductivity of magnetic nanofluids in film-wise condensation around a perpendicular cylinder. They found that the speed of magnetic nanofluids through the film decreases as the magnetic field intensity increases. Moreover, their results showed that the nanoparticle migration is enhanced within the condensate film by increasing the nanoparticle size, which leads to an increase in the nanoparticle concentration near the cold surface. Heysiattalab et al. [33] investigated the anisotropic characteristic of magnetic nanofluids in film-wise condensation around a perpendicular wall under the influence of a uniform changeable-directional magnetic field.
Figure 1 shows the nanoparticle concentration for different values of the Brownian to thermophoretic diffusivity (N BT) ratio versus dimensionless temperature (T*). It can be seen that the nanoparticle concentration is non-uniformly distributed from the interface between the vapor and liquid phases (T* = 1) toward the cold wall (T* = 0) as the thermophoresis forces the nanoparticles to move from the hotter to the colder zone. Moreover, the migration of nanoparticles decreases with an enhancement in N BT. This leads to a decrease in the nanoparticle concentration within the condensate film. It should be noted that enhancing N BT can be interpreted as enhancing the thermophoresis versus the Brownian diffusion [33].
Nanoparticle volume fraction of the Brownian to thermophoretic diffusivity (N BT) ratio versus dimensionless temperature (T*).
Figure is reprinted from Heysiattalab et al. [33] with permission from the publisher
Lee et al. [34] experimentally studied the influence that nanoparticles walls modified by reticulated humidity had on condensation heat exchange. They reported that the nanoparticles—walls modified by reticulated humidity provided superior efficiency in condensation heat exchange, especially when the droplet-sweeping influence of gravity was not considered. Famileh et al. [35] numerically investigated the influence of nanoparticles on condensation of moist air in the presence of a large amount of air inside a perpendicular channel. Moist air condensation has various applications in heating, ventilation, and air conditioning (HVAC) devices including air conditioners, boilers, chillers, heat pumps, dehumidifiers, humidifiers, and radiant systems. They concluded generally that applying nanoparticles enhances the condensation in 12.3% on average. Moreover, their results showed that for small values of relative moistures, applying nanoparticles has an inconsiderable influence on the condensation phenomenon, while, for saturated moist air, nanoparticles have an important influence on this phenomenon.
As mentioned earlier, thermal energy is exchanged by conduction mode inside the condensate film; hence, adding materials with high values of thermal conductivity such as nanoparticles can be effective in improving the thermal efficiency of a condensing system. The thermophysical attributes of nanofluids can be controlled by changing the volume fraction and direction of nanoparticle migration. Researchers used this technique successfully to increase the thermal conductivity of the condensate film and improve the thermal efficiency of the condensing systems. The results showed that for small values of relative humidity, applying nanoparticles has an inconsiderable influence on the condensation phenomenon, while, for saturated humid air, nanoparticles have an important influence on this phenomenon. Table 1 summarizes the research performed on applying nanofluids in condensing systems. The influence of nanoparticles deposition and suspension type on phase change during condensation has not been studied in previous research. However, the importance of particle deposition and suspension type can affect the thermal performance of a condensing system. Numerical simulations and theoretical investigations accounted for about 85% of nanofluid condensing studies, and experimental research is rare. The experimental investigations deserve more attention in this regard. Most of the previous research in this field has focused on external condensation over a wall while internal condensation has been studied less. Note that internal condensation of refrigerants has occurred in many applications containing condensers employed in air conditioning, refrigeration, and heat pumps.
Applications of nanofluids in evaporating systems
Evaporation is a phenomenon in which a liquid absorbs thermal energy in changing to a vapor state. To enhance the efficiency of compact heat exchangers applied in heat pumps, these devices need to operate with their lowest temperature. One method to achieve this is to transfer thermal energy with a working liquid that leads to a phase change such as evaporation. Applying nanofluids is recognized as a novel technique for improving evaporation rate. Some researchers have performed experimental and numerical studies in this area.
Chon et al. [36] investigated the influence of nanoparticle diameters on the evaporation behaviors of nanofluid droplets. Figure 2 shows the nanofluid droplet evaporation for nanoparticles with small and large diameters. As shown in this figure, more smaller nanoparticles with their larger viscosity values causes the thermally driven nanoparticle movement to be suppressed, which leads to a fatter and more uniform evaporation in the core zone with a loosely defined broader ring in the edging zones. However, bigger nanoparticles with small viscosity values cause a faster movement toward the edge due to the more active distillation. The leading evaporation leaves a very specific ring-shaped stain.
Nanofluid droplet evaporation: a smaller nanoparticles (2-nm Au); b bigger nanoparticles (47-nm Al2O3).
Figure is reprinted from Chon et al. [36] with permission from the publisher
Sefiane and Bennacer [37] conducted experiments to evaluate the effect of Al nanoparticles on the evaporation and soaking dynamics of ethanol sessile droplets on a heated wall. Figure 3 shows TEM micrographs of aluminum nanoparticles. These micrographs were obtained by using Atomic Force Microscopy analysis. Note that Atomic Force Microscopy analysis was performed to show dried solutions and nanoparticle fouling. Sefiane and Bennacer [37] found that nanofluid droplets create larger primary contact angles in comparison to the pure fluid. Note that primary contact angles have an important influence on droplet evaporation rate.
a TEM micrographs of the Al nanoparticles; b Atomic Force Microscopy analysis.
Figure is reprinted from Sefiane and Bennacer [37] with permission from the publisher
Chen et al. [38] added different nanoparticles containing Laponite, Fe2O3, and Ag nanoparticles to deionized water to investigate their influence on droplet evaporation. They applied Polyvinylpyrrolidone to stabilize the nanoparticles and make the following conclusions:
-
Adding Laponite somewhat decreases the evaporation rate constant and enhances the apparent heat of evaporation.
-
The Ag nanofluid with Polyvinylpyrrolidone considerably increases the evaporation rate in comparison to the deionized water.
-
The Fe2O3 nanofluid with Polyvinylpyrrolidone decreases the evaporation rate and enhances the apparent heat of evaporation compared to the deionized water.
Chen et al. [39] repeated this problem and measured surface tension of nanofluid droplet evaporation. They concluded that Laponite and Ag nanoparticles are suitable for decreasing the surface tension by a factor of 3, when the solid volume fraction of the particle is enhanced by a factor of 10. Zhao et al. [40] analytically investigated the influence of nanoparticles on narrow film evaporation in microchannels. They concluded that a narrow porous coating substrate is formed near the walls by the nanoparticle fouling. This substrate recovers the surface humidity, while considerably decreasing the narrow film evaporation when the substrate thickness is augmented due to the substrate’s thermal resistance. Gan and Qiao [41] added nanoparticles to fuel droplets and experimentally investigated their effects on the evaporation behavior of the fuel. They numerically modelled the particle agglomeration process to find the influence of particle agglomeration on droplet evaporation rate. Figure 4 shows the particle agglomeration model in a vaporizing droplet. Note that particle agglomeration is created by collisions between particles where they simulated the dynamic agglomeration process of Al nanoparticles (80 nm) in a vaporizing fuel droplet (ethanol and n-decane). The particle agglomeration can be created in nanofluids by three main transport mechanisms containing Brownian diffusion, fluid movement, and differential setting. A droplet with sphere geometry and diameter of 1 mm was used in this model and Al nanoparticles were uniformly distributed inside the droplet at the initial time. More details about this model can be found in Gan and Qiao [41]. They found that when the droplet lifetime is greater than the characteristic agglomeration time, the particle agglomeration process has an influence on the evaporation. Otherwise, particle agglomeration has a negligible effect on droplet evaporation.
Particle agglomeration model in a vaporizing droplet.
Figure is reprinted from Gan and Qiao [41] with permission from the publisher
Javed et al. [42] investigated the effects of Al nanoparticles on the evaporation characteristics of heptane droplets. They found that the evaporation rate increases by adding nanoparticles at high temperatures due to formation of a highly porous shell. This leads to small aggregations, while at lower temperatures, the formation of large aggregations leads to a decrease in evaporation rate. Gerken et al. [43] investigated the nAl/ethanol nanofluid pendant droplet evaporation. They concluded that at longer periods, the packing of accumulates near the droplet boundary causes the creation of porous shell structures, which modify the droplet boundary and configuration. Shin et al. [44] investigated the positional aggregation behaviors of a nanofluid droplet during evaporation. Figure 5 shows images of the pure fluid droplet and nanofluid droplets at different views. It can be seen that the total evaporation time and initial equilibrium contact angles are reduced by adding the nanoparticles and enhanced nanoparticle concentration.
Images of the pure fluid droplet and nanofluid droplets: a the front view at the initial states; b and c the forepart and top view images after completing the natural evaporation, respectively.
Figure is reprinted from Shin et al. [44] with permission from the publisher
Tso and Chao [45] investigated enthalpy of evaporation and evaporation rate of aqueous nanofluids, using Al2O3 and TiO2 as nanoparticles. Their results showed that the enthalpy of evaporation is reduced by increasing the nanofluid volume fraction. Moreover, they concluded that the evaporation rate of nanofluids could be enhanced or reduced, depending on their volume fraction and the type of nanofluid. Chen and Lin [46] analytically investigated the narrow film evaporation of nanofluids using Cu, CuO, Al2O3, and SiO2 as nanoparticles. They found that increasing the concentration of nanoparticles causes a reduction in the liquid’s viscosity and an increase in the thermal conductivity, which enhances the evaporation in the narrow film. Moreover, they reported that the Cu-water nanofluid has a lower narrow film thickness in comparison with the pure water. Rudolf Eggers et al. [47] investigated particle motion in flash evaporation of nanofluids. They found that the flash evaporation of nanoparticles causes a full-phase separation in the evaporating process. Wei et al. [48] studied the influence of insoluble nanoparticles on droplet evaporation.
Evaporation of liquid droplets has extensive applications in food processing, spray drying, medicine, energy, and motive force fuels, among others. Studies show that the non-dimensional ratio of particle diffusion time to the droplet lifetime and initial particle concentration have a role in the evaporation characteristic prior to shell formation. Figure 6 is extracted from Wei et al. [48], which indicates the effect of particles on the morphology of waterless particles for two cases, i.e., Peclet numbers ≪ 1 and Peclet numbers ≫ 1. As shown in this figure, for Peclet numbers ≪ 1, the amount of evaporation of a droplet is adequately small. Accordingly, there is enough time for particles to redistribute themselves by diffusion all over the droplet. This creates a packed globular particle. For Peclet numbers ≫ 1, particles do not have adequate time for diffusing and are rapidly contained on the receding droplet boundary, causing vacant grains.
Effect of particles on the morphology of waterless particles, Wei et al. [48].
Figure is reprinted with permission from Elsevier
Wei et al. [49] analytically studied the influence of internal circulation on evaporation of nanofluid droplets and particle distribution inside the nanofluid droplet. Note that the internal circulation was created by viscous influences at the interface between liquid and vapour in the convective surroundings. They found that the particle distribution can be dominated by convection for stronger inner circulation. Fu et al. [50] experimentally investigated the evaporation of aluminium oxide–water nanofluids inside an evaporator, which was externally micro-grooved. Their results showed that the combination of nanofluids together with micro-grooves leads to a superior evaporation heat transfer performance. Mahian et al. [51] investigated the influence of nanofluids on the amount of evaporation inside a solar still coupled with a heat exchanger (see the schematic of the experimental set-up in Fig. 7). They tested SiO2 and Cu as nanoparticles with a maximum concentration of 4%. They concluded that nanofluids can enhance the amount of evaporation inside the solar still but the amount of effectiveness depends on the inlet temperature of the heat exchanger. For nanofluids with high inlet temperatures, the effect of nanoparticles on evaporation enhancement is marginal (about 1%). At low inlet temperatures of heat exchangers (i.e., 50 °C and less), nanofluids have a greater effect on the evaporation rate (about 10%), but in this case the use of the heat exchanger in the solar still is not beneficial since it reduces the productivity of the solar still in comparison with conventional ones.
Experimental set-up designed by Mahian et al. [51] to investigate the effect of nanofluids on the evaporation rate in solar still.
Figure reprinted with permission from Elsevier
Finally, evaporation heat transfer can be used in drying and food processors, compact heat exchangers, electronic cooling, etc. In all these applications, improving the evaporation rate is very essential to managing the thermal energy and efficiency of the system. Nanofluids are promising as future-generation heat exchange liquids for flow evaporating heat exchange. The results of previous research show that the evaporation rate of nanofluids can be enhanced or reduced with respect to their volume fraction and the kind of nanofluid. Many researchers in this field have focused on evaporation of nanofluid droplets. Table 2 summarizes research performed on applying nanofluids in evaporating systems. Nanofluid applications in mini/microchannel flow evaporating systems can be a new topic for future research.
Effects of different parameters on the thermal efficiency of nanofluids
Deposition of nanoparticle
Many authors found that deposition of nanoparticles on the evaporator surface is a main reason of improvement in thermal efficiency of condensing and evaporating systems. It should be stated that deposition of nanoparticle on evaporator surface increases surface wettability, capillary influence, condensate flow rate, and finally the thermal efficiency of condensing and evaporating systems. Moreover, deposition of nanoparticles on the surface creates an artificial layer that decreases the thermal resistance by bombarding the vapour bubbles during the bubble formation at the interface between solid and liquid surfaces [52–59]. Also, this layer increases the evaporator surface area and capillary force which enhances the evaporating heat transfer.
Surfactant of nanoparticle
The aggregation and sedimentation of nanoparticles in the refrigerant nanofluids can decrease the stability of them and limit the application of them in the refrigeration system containing air conditioning and heat pump systems. For stabilizing the nanoparticles in the refrigerant nanofluid, one efficient method is عusing the surfactant into them. The presence of surfactant additives has influences on the boiling heat transfer specifications and subsequently, affects the overall efficiency of evaporators in the refrigeration systems as they change the thermophysical attributes of working liquids containing surface tension, viscosity and so on. The researches performed by Peng et al. [60–63] and Prakash et al. [64, 65] indicated that the adding of surfactant materials considerably affect the efficiency of the pool boiling heat transfer of a nanofluid. The adding of surfactant increases the nucleate pool boiling heat transfer of nanofluid. Moreover, adding of surfactant directly affects microlayer evaporation process. The microlayer evaporation process is occurred in the thin liquid substrate created under a bubble during the bubble growth happening in an evaporating process.
Effects of base fluid
Base fluids are employed for suspending nanoparticles to form nanofluids. The base fluids, which mostly used in evaporating and condensing systems, are water, ethylene glycol, and refrigerants. Based on the available conventional thermal conductivity models (e.g. the Maxwell model), the thermal conductivity ratio enhances by reducing the thermal conductivity of base fluid in a mixture. Accordingly, poor conductive fluids provides superior results in comparison with highly conductive fluids. Moreover, when water is considered as the base fluid, the situation is more complex because the viscosity of the water affects the Brownian motion of nanoparticles and this subsequently, affects the thermal performance of the mixture [66].
Effect of particle
Effect of particle type on thermal conductivity of nanofluid
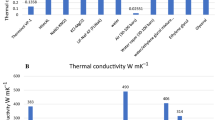
As mentioned earlier, the thermal conductivity of nanoparticles is the most important factor affecting condensation and evaporation. Many types of particle are employed for synthesising nanofluid used in evaporating and condensing systems containing Al2O3, CuO, TiO2, ZnO, SiO2, SiC, Ag, Au, Cu, and Fe3O4 nanoparticles. Previous researches indicated that particle type affect the thermal conductivity of nanofluids and thermal performance of system. The thermal conductivities of some materials are presented in Table 3. Note that the effects of particle type on thermal conductivity of nanofluid should be considered along with other parameters such as particle shape, particle diameter, particle concentration, base fluid thermal conductivity, and nanofluid temperature.
Effects of particle type on boiling characteristics
Generally, good understanding of the effects of nanoparticles on boiling characteristics helps to better understand mechanisms leading condensing and evaporating systems. Accordingly, the effects of particle type on boiling characteristics are discussed in this section. Table 4 presents boiling characteristics for different nanoparticles at three values of concentration. Critical heat flux, onset of nucleate boiling, and leidenfrost temperature are these characteristics presented in this table.
Critical heat flux is a momentous point on the boiling curve. Generally, the transition from nucleate boiling to film boiling can be achieved abruptly by enhancing heat flux. In this situation, the temperature difference is increased quickly. The point of transition from nucleate boiling to film boiling is recognized as the point of departure from nucleate boiling. The heat flux associated with departure from nucleate boiling is named as the critical heat flux. Critical heat flux is the thermal limit of a phenomenon where a phase change is occurred during heating, which suddenly reduces the performance of heat transfer, therefore leads to localize overheating of the heating surface. As presented in Table 4, the critical heat flux of the nanofluids is higher in comparison with water. Nanofluids create a buildup of a thin porous substrate of nanoparticles on the heater’s wall, which may considerably enhance the surface wettability and, consequently, leads to increase in critical heat flux. Moreover, the critical heat flux enhances by increasing the concentration of nanoparticles. It is worth mentioning that among all nanoparticles presented in this table Al2O3 has the maximum critical heat flux of onset of nucleate boiling, while TiO2 has the minimum one.
If the surface temperature rises adequately above the local saturation temperature, pre-existing vapor in surface sites can nucleate and grow. This temperature can be considered as the onset of nucleate boiling for this flow boiling situation. Note that heat and mass transfers during nucleate boiling has a considerable influence on the evaporative heat transfer rate. This heat transfer process helps quickly and efficiently to evaporate liquid from a surface and is therefore sometimes desirable. As presented in Table 4, the temperature of onset of nucleate boiling of the nanofluids is lower in comparison with water that means the nanofluids has a superior heat transfer performance than that of the water. In addition, the temperature of the onset of nucleate boiling decreases by increasing the volume fraction of nanoparticles. This means that the nucleate boiling heat transfer occurs faster for higher values of volume fraction of nanoparticles. It is worth mentioning that among all nanoparticles presented in this table Al2O3 has the minimum temperature of onset of nucleate boiling, while TiO2 has the maximum one.
The Leidenfrost influence is a physical phenomenon in which a fluid on a surface considerably hotter than the fluid’s boiling point, generates an insulating vapor substrate. This substrate keeps the fluid from boiling quickly. Accordingly, a droplet hovers over the surface rather than making physical contact with it. If the surface temperature is at or above the Leidenfrost point, the water moves across the surface and takes longer to evaporate than in a surface below the temperature of the Leidenfrost point. At temperatures above the Leidenfrost point, the bottom part of the water droplet vaporizes quickly on contact with the hot plate. As a result, Leidenfrost point has an important influence on the evaporating rate. As presented in Table 4, the Leidenfrost point of the nanofluids is higher in comparison with water. Usually, the Leidenfrost temperature for nanofluids are about 57–70 °C higher than that of water. Moreover, Leidenfrost temperature decreases as the nanoparticle volume fraction increases. Note that the deposited nanoparticles on the heater surface can increase the rewetting phenomena which lead to increase in Leidenfrost temperature.
Concluding remarks and directions for future work
This survey reviews and summarizes experimental and numerical research on improving the efficiencies of condensing and evaporating systems by using nanofluids. Some of the applications of condensing and evaporating systems are listed in Table 5.
Moreover, the following conclusions were made:
-
The thermophysical attributes of nanofluids can be controlled by changing the volume fraction and direction of nanoparticle migration. This dynamic is effective in enhancing the cooling and condensing efficiencies by arranging the flow and heat transfer rate.
-
The nanoparticle-modified wall with reticulated wettability characteristic is suitable for superior efficiency in condensation heat exchange, especially for the condition in which the droplet-sweeping influence of gravity is not considered.
-
For small values of relative humidities, applying nanoparticles has an inconsiderable influence on the condensation phenomenon, while, for saturated humid air, nanoparticles have an important influence on this phenomenon.
-
The evaporation rate of nanofluids can be enhanced or reduced, relative to their volume fraction and the kind of nanofluid.
-
Small agglomerates lead to evaporation rate increases after a highly porous shell is formed when adding nanoparticles at high temperatures. At lower temperatures, the formation of large agglomerates leads to decrease in evaporation rate.
The following recommendations are proposed:
-
Generally, the number of studies in heat exchange during condensation of the vapor nanofluids is rather limited, and this area requires more attention.
-
Nanoparticle deposition and nanoparticle suspension are two important factors that affect the efficiency of a thermal system. These factors should be considered when using different nanofluids in condensing and evaporating systems.
-
Numerical simulations and theoretical investigations accounted for about 85% of nanofluid condensing studies, and experimental research was rare. There is a need for additional experimental investigations and data.
-
Most of the previous research in the field of nanofluid condensation has focused on external condensation over a wall while internal condensation is less studied. Note that internal condensation of refrigerants has occurred in many applications containing condensers employed in air conditioning, refrigeration, and heat pumps.
-
New types of nanofluids and nanoparticles such as graphene nanoparticles and ionic liquid nanofluids with promising thermo-physics properties should be used in condensing and evaporating systems to evaluate their potential in these systems.
-
The pressure drop penalty added by nanofluids should be coupled with heat transfer improvement of these fluids. This helps to evaluate the system’s efficiency properly.
-
Checking nanofluid stability and using novel technologies to enhance the stability are required to make nanofluids efficient and reliable in condensing and evaporating systems.
References
Laohalertdecha S, Kaew-On J, Wongwises S. The effect of the electrohydrodynamic on the two-phase flow pressure drop of R-134a during evaporation inside horizontal smooth and micro-fin tubes. Heat Transf Eng. 2010;31:108–18.
Sohag FA, Becka FR, Mohanta L, Cheung FB, Segall AE, Eden TJ, Potter JK. Enhancement of downward-facing saturated boiling heat transfer by the cold spray technique. Nucl Eng Technol. 2017;49:113–22.
Boziuk TR, Smith MK, Glezer A. Enhanced boiling heat transfer on plain and featured surfaces using acoustic actuation. Int J Heat Mass Transf. 2017;108:181–90.
He Y, Li H, Hu Y, Wang X, Zhu J. Boiling heat transfer characteristics of ethylene glycol and water mixture based ZnO nanofluids in a cylindrical vessel. Int J Heat Mass Transf. 2016;98:611–5.
Kim DE, Yu DI, Jerng DW, Kim MH, Ahn HS. Review of boiling heat transfer enhancement on micro/nanostructured surfaces. Exp Therm Fluid Sci. 2015;66:173–96.
Mori S, Utaka Y. Critical heat flux enhancement by surface modification in a saturated pool boiling: a review. Int J Heat Mass Transf. 2017;108:2534–57.
Famileh IZ, Esfahani JA. Experimental investigation of wet flue gas condensation using twisted tape insert. Int J Heat Mass Transf. 2017;108:1466–80.
Fang X, Chen Y, Zhang H, Chen W, Dong A, Wang R. Heat transfer and critical heat flux of nanofluid boiling: a comprehensive review. Renew Sustain Energy Rev. 2016;62:924–40.
Ciloglu D, Bolukbasi A. A comprehensive review on pool boiling of nanofluids. Appl Therm Eng. 2015;84:45–63.
Kamatchi R, Venkatachalapathy S. Parametric study of pool boiling heat transfer with nanofluids for the enhancement of critical heat flux: a review. Int J Therm Sci. 2015;87:228–40.
Celen A, Çebi A, Aktas M, Mahian O, Dalkilic AS, Wongwises S. A review of nanorefrigerants: flow characteristics and applications. Int J Refrig. 2014;44:125–40.
Cai J, Hu X, Xiao B, Zhou Y, Wei W. Recent developments on fractal-based approaches to nanofluids and nanoparticle aggregation. Int J Heat Mass Transf. 2017;105:623–37.
Bashirnezhad K, Rashidi MM, Yang Z, Bazri S, Yan WM. A comprehensive review of last experimental studies on thermal conductivity of nanofluids. J Therm Anal Calorim. 2015;122:863–84.
Fieg GP, Roetzel W. Calculation of laminar film condensation in/on inclined elliptical tubes. Int J Heat Mass Transf. 1994;37:619–24.
Capellas M, Caminal G, Gonzalez G, Lopez-Santin J, Clapes P. Enzymatic condensation of cholecystokinin CCK-8 (4–6) and CCK-8 (7–8) peptide fragments in organic media. Biotechnol Bioeng. 1997;56:456–63.
Sun DW, Zheng L. Vacuum cooling technology for the agri-food industry: past, present and future. J Food Eng. 2006;77:203–14.
Dutta A, Som SK, Das PK. Film condensation of saturated vapour over horizontal non-circular tubes with progressively increasing radius of curvature drawn in the direction of gravity. ASME J Heat Transf. 2004;126:906–14.
Dalkilic AS, Wongwises S. Intensive literature review of condensation inside smooth and enhanced tubes. Int J Heat Mass Transf. 2009;52:3409–26.
Liu ZH, Li YY, Bao R. Thermal performance of inclined grooved heat pipes using nanofluids. Int J Therm Sci. 2010;49:1680–7.
Huminic G, Huminic A. Heat transfer characteristics of a two-phase closed thermosyphons using nanofluids. Exp Therm Fluid Sci. 2011;35:550–7.
Reis Parise JA. A simulation model for the application of nanofluids as condenser coolants in vapor compression heat pumps. In: International refrigeration and air conditioning conference, 2012, Purdue University.
Huminic G, Huminic A. Numerical study on heat transfer characteristics of thermosyphon heat pipes using nanofluids. Energy Convers Manag. 2013;76:393–9.
Avramenko AA, Shevchuk IV, Tyrinov AI, Blinov DG. Heat transfer at film condensation of stationary vapor with nanoparticles near a vertical plate. Appl Therm Eng. 2014;73:389–96.
Avramenko AA, Shevchuk IV, Tyrinov AI, Blinov DG. Heat transfer at film condensation of moving vapor with nanoparticles over a flat surface. Int J Heat Mass Transf. 2015;82:316–24.
El Mghari H, Louahlia-Gualous H, Lepinasse E. Numerical study of nanofluid condensation heat transfer in a square microchannel. Numer Heat Transf Part A Appl. 2015;68:1242–65.
Turkyilmazoglu M. Analytical solutions of single and multi-phase models for the condensation of nanofluid film flow and heat transfer. Eur J Mech B Fluids. 2015;53:272–7.
Turkyilmazoglu M. Condensation of laminar film over curved vertical walls using single and two-phase nanofluid models. Eur J Mech B Fluids. 2017;65:184–91.
Malvandi A, Ghasemi A, Ganji DD, Pop I. Effects of nanoparticles migration on heat transfer enhancement at film condensation of nanofluids over a vertical cylinder. Adv Powder Technol. 2016;27:1941–8.
Malvandi A, Ganji DD, Kaffash MH. Magnetic field effects on nanoparticle migration and heat transfer of alumina/water nanofluid in a parallel-plate channel with asymmetric heating. Eur Phys J Plus. 2015;130:63.
Moshizi SA, Malvandi A. Different modes of nanoparticle migration at mixed convection of Al2O3–water nanofluid inside a vertical microannulus in the presence of heat generation/absorption. J Therm Anal Calorim. 2016;126:1947–62.
Malvandi A, Ganji DD, Pop I. Laminar filmwise condensation of nanofluids over a vertical plate considering nanoparticles migration. Appl Therm Eng. 2016;100:979–86.
Malvandi A, Heysiattalab S, Ganji DD. Effects of magnetic field strength and direction on anisotropic thermal conductivity of ferrofluids (magnetic nanofluids) at filmwise condensation over a vertical cylinder. Adv Powder Technol. 2016;27:1539–46.
Heysiattalab S, Malvandi A, Ganji DD. Anisotropic behavior of magnetic nanofluids (MNFs) at filmwise condensation over a vertical plate in presence of a uniform variable-directional magnetic field. J Mol Liq. 2016;219:875–82.
Lee YA, Kuo LS, Su TW, Hsu CC, Chen PH. Orientation effects of nanoparticle-modified surfaces with interlaced wettability on condensation heat transfer. Appl Therm Eng. 2016;98:1054–60.
Famileh IZ, Esfahani JA, Vafai K. Effect of nanoparticles on condensation of humid air in vertical channels. Int J Therm Sci. 2017;112:470–83.
Chon CH, Paik S, Tipton JB, Kihm KD. Effect of nanoparticle sizes and number densities on the evaporation and dryout characteristics for strongly pinned nanofluid droplets. Langmuir. 2007;23:2953–60.
Sefiane K, Bennacer R. Nanofluids droplets evaporation kinetics and wetting dynamics on rough heated substrates. Adv Colloid Interface Sci. 2009;147–148:263–71.
Chen RH, Phuoc TX, Martello D. Effects of nanoparticles on nanofluid droplet evaporation. Int J Heat Mass Transf. 2010;53:3677–82.
Chen RH, Phuoc TX, Martello D. Surface tension of evaporating nanofluid droplets. Int J Heat Mass Transf. 2011;54:2459–66.
Zhao IJ, Wang XD, Duan YY, Wang BX. Effect of nanofluids on thin film evaporation in microchannels. J Nanopart Res. 2011;13:5033–47.
Gan Y, Qiao L. Evaporation characteristics of fuel droplets with the addition of nanoparticles under natural and forced convections. Int J Heat Mass Transf. 2011;54:4913–22.
Javed I, Baek SW, Waheed K. An experimental investigation on effects of an electric field on bubble growth on a small heater in pool boiling. Combust Flame. 2013;160:170–83.
Gerken WJ, Thomas AV, Koratkar N, Oehlschlaeger MA. Nanofluid pendant droplet evaporation: experiments and modeling. Int J Heat Mass Transf. 2014;74:263–8.
Shin DH, Choi CK, Kang YT, Lee SH. Local aggregation characteristics of a nanofluid droplet during evaporation. Int J Heat Mass Transf. 2014;72:336–44.
Tso CY, Chao YH. Study of enthalpy of evaporation, saturated vapor pressure and evaporation rate of aqueous nanofluids. Int J Heat Mass Transf. 2015;84:931–41.
Chen W, Lin J. Thermal analysis of nanofluids on the thin film evaporation of meniscus. Heat Transf Asian Res. 2016;45:578–93.
Rudolf Eggers J, Matthias Lange E, Kabelac S. Particle migration in isobaric and flash evaporation of nanofluids. Forsch Ingenieurwes. 2016;80:101–9.
Wei Y, Deng W, Chen RH. Effects of insoluble nano-particles on nanofluid droplet evaporation. Int J Heat Mass Transf. 2016;97:725–34.
Wei Y, Deng W, Chen RH. Effects of internal circulation and particle mobility during nanofluid droplet evaporation. Int J Heat Mass Transf. 2016;103:1335–47.
Fu S, Tso C, Fong Y, Chao CYH. Evaporation of Al2O3-water nanofluids in an externally micro-grooved evaporator. Sci Technol Built Environ. 2016;23:345–54.
Mahian O, Kianifar A, Zeinali Heris S, Wen D, Sahin AZ, Wongwises S. Nanofluids effects on the evaporation rate in a solar still equipped with a heat exchanger. Nano Energy. 2017;36:134–55.
Wang GS, Song B, Liu ZH. Operation characteristics of cylindrical miniature grooved heat pipe using aqueous CuO nanofluids. Exp Therm Fluid Sci. 2010;34:1415–21.
Chiang Y, Kuo W, Ho C, Chieh J. Experimental study on thermal performances of heat pipes for air-conditioning systems influenced by magnetic nanofluids, external fields, and micro wicks. Int J Refrig. 2014;43:62–70.
Liu Z, Li Y, Bao R. Compositive effect of nanoparticle parameter on thermal performance of cylindrical microgrooved heat pipe using nanofluids. Int J Therm Sci. 2011;50:558–68.
Do KH, Jang SP. ‘‘Effect of nanofluids on the thermal performance of a flat micro heat pipe with a rectangular grooved wick. Int J Heat Mass Transf. 2010;53:2183–92.
Mehrali M, Sadeghinezhad E, Azizian R, Reza A, Tahan S, Mehrali M, Simon H, Metselaar C. Effect of nitrogen-doped graphene nanofluid on the thermal performance of the grooved copper heat pipe. Energy Convers Manag. 2016;118:459–73.
Kole M, Dey TK. Thermal performance of screen mesh wick heat pipes using water-based copper nanofluids. Appl Therm Eng. 2013;50:763–70.
Do KH, Ha HJ, Jang SP. Thermal resistance of screen mesh wick heat pipes using the water-based Al2O3 nanofluids. Int J Heat Mass Transf. 2010;53:5888–94.
Putra N, Septiadi WN, Rahman H, Irwansyah R. Thermal performance of screen mesh wick heat pipes with nanofluids. Exp Therm Fluid Sci. 2012;40:10–7.
Peng H, Ding G, Hu H. Effect of surfactant additives on nucleate pool boiling heat transfer of refrigerant-based nanofluid. Exp Therm Fluid Sci. 2011;35:960–70.
Peng H, Ding G, Hu H. Influences of refrigerant-based nanofluid composition and heating condition on the migration of nanoparticles during pool boiling. Part I: experimental measurement. Int J Refrig. 2011;34:1823–32.
Peng H, Ding G, Hu H. Influences of refrigerant-based nanofluid composition and heating condition on the migration of nanoparticles during pool boiling. Part II: model development and validation. Int J Refrig. 2011;34:1833–45.
Peng H, Ding G, Hu H, Jiang W. Effect of nanoparticle size on nucleate pool boiling heat transfer of refrigerant-oil mixture with nanoparticles. Int J Heat Mass Transf. 2011;54:1839–50.
Prakash NG, Anoop KB, Das SK. Mechanism of enhancement/deterioration of boiling heat transfer using stable nanoparticles suspensions over vertical tubes. J Appl Phys. 2007;102:1–7.
Prakash NG, Anoop KB, Sateesh G, Das SK. Effect of surface orientation on pool boiling heat transfer of nanoparticle suspensions. Int J Multiph Flow. 2008;34:145–60.
Zheng R, Gao J, Wang J, Chen G. Reversible temperature regulation of electrical and thermal conductivity using liquid–solid phase transitions. Nat Commun. 2011;2:289.
Hsieh SS, Liu HH, Yeh YF. Nanofluids spray heat transfer enhancement. Int J Heat Mass Transf. 2016;94:104–18.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rashidi, S., Mahian, O. & Languri, E.M. Applications of nanofluids in condensing and evaporating systems. J Therm Anal Calorim 131, 2027–2039 (2018). https://doi.org/10.1007/s10973-017-6773-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6773-7