Abstract
Raman spectroscopy (RS) was used to study the phase transformations of nanocrytalline TiO2 thin films. The films were grown by a vertical-flow cold-wall metal organic chemical vapour deposition system, using Ti(C10H14O5) as the source reagent, at different substrate temperatures. The results indicate that the anatase phase is present at around 550 °C and the rutile phase starts to form at 620 °C. The anatase phase completely transforms into the rutile phase at 680 °C. We have demonstrated that RS can be used as a powerful nondestructive technique for a quick and efficient determination of the phase of TiO2 thin films.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Titania (TiO2) has been widely studied because of it unique optical and chemical properties in catalysis [1], photocatalysis [2], and nonlinear optics [3]. The material crystallizes mainly in anatase or rutile phase and exhibits different physical and chemical properties. It is well-known that the anatase phase is suitable for catalysts and supports [4], while the rutile phase is used for optical and electronic purposes because of its high dielectric constant and high refractive index [5]. Although at ambient pressure and temperature the rutile phase is more thermodynamically stable than the anatase phase [6], the latter is the common phase for the formation of nanocrystalline TiO2 at relatively lower temperature because it is kinetically more stable than the rutile phase [7]. It has been reported that the anatase phase transforms to the rutile phase at some higher temperatures. However, the temperature of phase transition may vary according to the methods of synthesis. Therefore, understanding and controlling of the crystalline phase and the process of phase transformation of TiO2 are important.
Raman spectroscopy (RS) is an important nondestructive structural characterization tool that can be used to investigate amorphous to crystalline phase transitions, oxygen defects, stress states and quantum size effects in transition metal oxides [8, 9]. This non-invasive method offers quantitative microanalysis without damaging the sample which is the trend for electronic materials characterization. In the case of TiO2, Raman spectroscopy can identify its naturally occurring crystalline phases such as anatase, rutile and brookite [10].
In this study, we report the investigation of structural transformations in nanocrystalline TiO2 thin films by Raman spectroscopy. The films were grown by a vertical-flow cold-wall metal-organic chemical vapour deposition (MOCVD) system on sapphire (SA)(100) substrates, using titanium (IV) oxide bis(acetylacetonate) [Ti(C10H14O5)], as the source reagent, at different substrate temperatures. Combined with X-ray diffraction (XRD), RS is used to extract the structure and phase information of the as-deposited TiO2 films. The usefulness of RS as a nondestructive phase transformation characterization technique for nanostructural TiO2 thin films will be demonstrated.
2 Experimental details
A vertical-flow cold wall MOCVD system was utilized for the growth of the samples on sapphire(100) substrates. The titanium (IV) oxide bis(acetylacetonate) as the source reagent supplied by Strem Chemicals was used for chemical vapor deposition of nanostructural TiO2 films. High purity argon and oxygen were used as the carrier and reactive gas with flow rate fixed at 300 and 200 sccm, respectively. The argon flow carried the metalorganic vapor through the precursor reservoir and the heated transport line, which were maintained at 170 °C to prevent vapor condensation during the transportation. The oxygen passed through a gas ring set above the substrate and reactive with the vapor came out from the shower head. The chamber pressure was kept at 3 mbar. The temperature of the substrates, Ts, was set between 550 and 680 °C for different targeted growth.
The morphology of the as-deposited samples was studied with a JEOL-JSM6500F field-emission scanning electron microscopy. X-ray diffraction patterns taken on a Rigaku RTP300RC X-ray diffractometer equipped with a Cu Kα radiation source and a Ni filter were used to examine the crystallographic characteristics of the samples over a large area of the surface. Raman spectroscopy was used to extract the phase information of the TiO2 films. Raman spectra were recorded at room temperature utilizing the backscattering mode on a Renishaw inVia micro-Raman system with 1,800 grooves/mm grating and an optical microscope with a 50× objective. The Ar-ion laser beam of the 514.5 nm excitation line with a power of ∼1.5 mW was focused into a spot size ∼5 μm in diameter. Prior to the measurement, the system was calibrated by means of the 520 cm−1 Raman peak of a polycrystalline Si.
3 Results and discussion
3.1 Lattice dynamics of anatase and rutile phases in TiO2
Titania crystallizes mainly in anatase or rutile phase. Anatase is tetragonal and belongs to the space group \( D^{{19}}_{{4h}} \) [11]. The primitive unit cell contains two TiO2 chemical units. According to the factor group analysis, there are six Raman active modes (1A1g + 2B1g + 3Eg) [12]. The Raman spectrum for anatase single crystal was investigated by Ohsaka et al. [13] and six allowed bands in the first-order Raman spectrum were identified at 144 cm−1 (Eg), 197 cm−1 (Eg), 399 cm−1 (B1g), 516 cm−1 (A1g + B1g), and 639 cm−1 (Eg). The Raman band occurring at 516 cm−1 at room temperature is split into two peaks centred at 513 cm−1 (A1g) and 519 cm−1 (B1g) at 73 K [13]. Rutile is also tetragonal and belongs to the space group \( D^{{14}}_{{4h}} \) with two TiO2 molecules per unit cell. There are four Raman active modes with symmetry of A1g, B1g, B2g, and Eg [12]. These four Raman-active modes of rutile TiO2 were detected at 143 cm−1 (B1g), 447 cm−1 (Eg), 612 cm−1 (A1g), and 826 cm−1 (B2g) by Porto et al. [14]. Furthermore, the Raman spectra of anatase exhibit features different from those of rutile: second-order scattering in rutile is even more intense than one-phonon scattering, whereas in anatase only a few weak bands due to two-phonon scattering are observed.
3.2 Evolution of anatase phase in nanocrystalline TiO2
Figure 1 depicts the Raman spectra for the nanocrystalline TiO2 films deposited at (a) 550 °C, (b) 600 °C, and (c) 620 °C. As shown in curve 1(a), the S/N ratio of the Raman spectrum of TiO2 film deposited at 550 °C is rather large. This might be due to the low temperature of deposition which can result in thin and low film crystallinity. In addition to the substrate peaks indexed by S, a weak Raman peak at 152 cm−1 and three broader features in the high-wavenumber region located at around 405, 519 and 643 cm−1 are also detected. The Raman features observed in the spectra are close to those in the bulk anatase phase, except for their lower intensity and broader linewidth. Therefore, it is evident that the nanocrystalline TiO2 film possesses a certain degree of long-range order of the anatase phase. However, the weak overlapped broader peaks in the high wavenumber region indicate that the short-range order is poor and optical phonons may decay at the imperfect sites. Curve 1(b) is the Raman spectrum of the TiO2 film deposited at 600 °C. One significant observation of the spectrum is the intensification of the lowest wavenumber Eg mode. A very weak peak at around 196 cm−1 emerges at the higher wavenumber side of the Eg mode, which can also be assigned as the Eg mode of anatase phase. The 398, 516, and 638 cm−1 features are the Raman modes in anatase phase. With the increase in substrate temperature (550–600 °C), linewidths of the high wavenumber modes decrease and their intensities increase, indicating the enhancement of short-range order. As shown in curve 1(c), at substrate temperature increases to 620 °C, a small peak at around 448 cm−1 and a weak shoulder at around 608 cm−1 appeared. The features at 448 and 608 cm−1 are the Eg mode and A1g mode of the rutile phase, respectively. The presence of rutile Raman modes indicates that the high-temperature rutile phase starts to form at Ts = 620 °C.
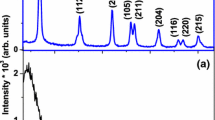
The formation of pure anatase phase of nanocrystalline TiO2 film at Ts = 600 °C is further confirmed by FESEM and XRD measurements. Figure 2(a–c) show the FESEM image, XRD pattern and Raman spectrum of the nanocrystalline TiO2 film grown on SA(100) substrate at 600 °C. The FESEM image reveals the formation of nanosized rough surface of the film. The XRD pattern shows the preferable orientation of [110] (2θ ∼ 70.4°) with anatase phase for the film grown on SA(100). The XRD and RS results indicate that the A-TiO2 NCs are (110) oriented on SA(100).
3.3 Anatase to rutile phase transformations
Figure 3 depicts the Raman spectra of the nanocrystalline TiO2 films deposited at (a) 620 °C, (b) 650 °C, and (c) 680 °C. The spectra in Fig. 3 show the strong anatase Eg mode at 150 cm−1 begins to weaken when the rutile modes at around 448 and 608 cm−1 intensify with the increase in substrate temperatures. The event of anatase to rutile transformation is clearly illustrated by curve 3(b). As can be seen in curve 3(c), the anatase features disappear and the broad rutile peaks stand out in the Raman spectrum of the sample deposited at 680 °C. Curve 3(c) show that the Eg and A1g modes as well as the two-phonon bands at ∼242 cm−1 (marked as *) are the major features of rutile TiO2 film. The B2g mode is extremely weak and B1g mode is almost absent.
The formation of pure rutile phase of nanocrystalline TiO2 film at Ts = 680 °C is further confirmed by FESEM and XRD measurements. Figure 4(a–c) show the FESEM images, the XRD pattern and Raman spectrum of the nanocrystalline TiO2 film grown on SA(100) substrate at 680 °C. The FESEM images reveal the formation of nanosized rough surface of the film. The XRD pattern shows the uniquely single-directional growth of rutile phase TiO2 films along [001] for the film grown on SA(100). The single-directional growth of rutile phase TiO2 films along [001] on SA(100) can been explained as the combined effects of the minimization of the oxide sublatice structural mismatch and the c-directional growth mechanism [15]. The redshifts of peak positions and broadening of linewidths of the rutile TiO2 Raman features have been determined to be related to the phonon-confinement effect in the nanosized film and residual stress effects [16].
4 Summary
Raman spectroscopy can sensitively identify the anatase and rutile phases of TiO2 on the basis of their characteristic Raman bands. RS was used to study the phase transformations of nanocrystalline TiO2 thin films deposited by MOCVD. The results indicate that the anatase phase is present at around 550 °C and perfect anatase phase is formed at a temperature of 600 °C. The rutile phase starts to form at 620 °C, and the anatase phase completely transforms into the rutile phase at 680 °C. These results are further confirmed by X-ray diffraction. We have demonstrated that RS can be used as a powerful nondestructive technique for a quick and efficient determination of the phase of nanostructural TiO2.
References
L.E. Coulter, A.G. Sault, J. Catal. 154, 56 (1995)
A. Fujishima, K. Honda, Nature 238, 37 (1972)
M. Grätzel, Nature 353, 737 (1992)
K. Foger, J.R. Anderson, Appl. Catal. 23, 139 (1986)
F. Zhang, Z. Zheng, X. Ding, Y. Mao, Z. Chen, S. Yang, X. Liu, J. Vac. Sci. Technol. A. 15, 1824 (1997)
J. Muscat, V. Swamy, N.M. Harrison, Phys. Rev. B. 65, 224112 (2002)
J. Ovenstone, K. Yanagisawa, Chem. Mater. 11, 2770 (1999)
J.C. Parker, R.W. Siegel, J. Mater. Res. 5, 1246 (1990)
D. Bersani, P.P. Lottici, X.Z. Ding, Appl. Phys. Lett. 72, 73 (1998)
U. Balachandran, N.G. Eror, J. Solid State Chem. 42, 276 (1982)
R.W.G. Wyckoff, in Crystal Structures, vol. 1 (Interscience Publishers, New York, 1965), p. 253
R. Loudon, Adv. Phys. 13, 423 (1964)
T. Ohsaka, F. Izumi, Y. Fujiki, J. Raman Spectrosc. 7, 321 (1978)
S.P.S. Porto, P.A. Fleury, T.C. Damen, Phys. Rev. 154, 522 (1967)
R.S. Chen, A. Korotcov, Y.S. Huang, D.S. Tsai, Nanotechnology 17, R67 (2006)
A.V. Korotcov, Y.S. Huang, K.K. Tiong, D.S. Tsai, J. Raman Spectrosc. 38, 737 (2007)
Acknowledgements
The authors acknowledge the support of the National Science Council of Taiwan under the project Nos. NSC 95-2120-M-011-001 and NSC 95-2112-M-019-001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, C.A., Huang, Y.S., Chung, W.H. et al. Raman spectroscopy study of the phase transformation on nanocrystalline titania films prepared via metal organic vapour deposition. J Mater Sci: Mater Electron 20 (Suppl 1), 303–306 (2009). https://doi.org/10.1007/s10854-008-9595-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-008-9595-3