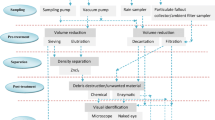
Abstract
Microplastics (MPs) in the air and indoor environments are of growing concern and have led to increased testing for MPs. This study draws attention to the quality and quantitative measures of MP studies by conducting laboratory experiments (on solutions, filters, and blank samples) that were rarely or were not adopted in the airborne and indoor MP literature. Experiments have been conducted to identify contaminations that may come from experimental procedures while determining MPs in the air samples. MPs in different matrices during experiments were counted and categorized by their shapes. Chemical characterization was performed by Raman Spectroscopy. Results showed that laminar flow is the best option over a fume hood or standard laboratory environment for detecting air MPs to reduce blank levels. Blue-green and Black-Grey were the dominant colors; fiber was the predominant type of MPs seen, and most of them fall under the size range from (1—1000 µm) in different indoor environments and blanks. Common MPs seen were PP, PVA, PTFE, PVC, and HDPE. Thermal treatment of fresh unused filters at 450 °C for 4 h was effective as it reduced the MP count by 50%. Working solutions are mainly contaminated, and their pre-filtration is essential. The average deposition of MPs in blank samples during seven days was around 55 MPs. There is an urgent need for studies on developing quality control and quality assurance of airborne and indoor MPs. Hence, a standard protocol needs to be accepted; by harmonizing procedures, comparable results can be found, uncovering the correct levels of MP contamination, as required for risk assessment.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastics have become the predominant element of the modern economy and combine unparalleled practical characteristics with low cost. In the last half-century, their use has grown 20-fold; today, almost everyone is in touch with plastics daily. Nowadays, it is not easy to imagine a world without plastics: also, the disposal and breakup of consumer products and industrial waste in environmental areas can result in tiny pieces of plastic debris, called microplastics (MPs), ranging from a few microns to five millimeters in length (Thompson et al., 2004). In the last decade, environmental problems caused by MPs have been at the center of scientific interest (Bhat et al., 2022, 2023a, 2023c; Eraslan et al., 2023). MPs have been detected globally in a wide range of environmental compartments. The number of studies in this area is rapidly increasing, especially regarding aquatic and marine environments (Bhat et al., 2023b; Thacharodi et al., 2024; Yu et al., 2020). Still, the knowledge gap on the contribution of MP to outdoor and indoor air contamination or quality remains significant compared to oceans, seas, lakes, rivers, and estuaries (Bhat et al., 2023a, 2023b, 2023c; Bhat, 2023b; Wright et al., 2021). Currently, MPs are considered a substantial environmental threat due to their overuse, omnipresence, permanence, and toxic potential (Bhat et al., 2021, 2023b; Engler, 2012; Eraslan et al., 2021; Thacharodi et al., 2024).
Most environmental plastics research focuses on MPs. Moreover, there can be no exclusion of the presence of smaller particles (nanoplastics). Analyses show that field investigations are not easy to conduct due to the vast array of samples and analytical procedures available to identify and quantify plastics. The wide variety of sampling approaches makes it difficult to meet the need for more and higher-quality data. There is no standardization of procedures for MP sampling in airborne and indoor environments, causing significant problems in comparing data (Bhat, 2023a, 2023b; Enst, 2021; Wright et al., 2021). Plastics are classified by shape (fragments, pellets, cosmetic beads, lines, fibers, films, foams) and type of polymer (polypropylene (PP), polyethylene, polystyrene, etc.). While the latter is often well determined, the shape characterization criteria are not always obvious. Hence, there is no uniformity in collecting, processing and analyzing samples and data. This makes a direct comparison between studies challenging and even leads to ambiguity between results. A considerable challenge remains in developing and implementing procedures for collecting, analyzing, and characterizing MPs following sound quality control (QC)/quality assurance (QA) procedures. This is because MPs are a vast group of contaminants that vary significantly in morphology, chemical properties, texture, color, density, and size.
Since MPs are everywhere, including indoor air (Catarino et al., 2018; Dris et al., 2017) and outdoor air (Abbasi et al., 2019; Dris et al., 2016; Tunahan Kaya et al., 2018), if no substantial contamination control procedures are taken, they can potentially contaminate samples, resulting in overestimated data. Several possible sources of contamination with microfibers might result from synthetic clothing abrasions, improper cleaning of lab equipment, plastic instruments used in treatment, bad sealing specimens, or ambient air. Different researchers have focused on the importance of blanks, like Hermsen et al., who visually evaluated samples from an artificial source under a microscope for the presence of particles (Hermsen et al., 2017). As this step could not be done in a laminar flow chamber, two clean Petri dishes were put on the opposite side of the sample as pollution controls during a visual examination. Su et al. investigated MP incidence in fish intestines and gills (Su et al., 2019). Hung et al. examined the possibility of introducing micro particles due to sampling collection, transportation, extraction, and quantification by collecting field and laboratory blanks (Hung et al., 2021). Recently, Paiva et al. highlighted the importance of airborne contamination control protocols in laboratory analyses of MPs (Paiva et al., 2022).
Air samples are usually collected by active sampling methodology. Active samplers draw air through a filter or other collection medium, such as sorbent tubes, using a pump. These samplers can collect particulate matter and gaseous pollutants (Habibi et al., 2022; Seethapathy et al., 2008; Woolfenden, 2010). Separating MPs from atmospheric samples requires careful sample preparation. When a filter/substrate is used for aerosol collection, it becomes more vital. The aerosol is a complex mixture of inorganic (mineral), organic (pollen, fungi, microorganisms, etc.), and MPs (Habibi et al., 2022). The removal of MPs from this complex composition often necessitates chemical processing, including the digestion of organic matter using potassium hydroxide, sodium hydroxide, and hydrogen peroxide (Allen et al., 2019; Dris et al., 2016; Renner et al., 2018). Different studies have used various solutions, such as sodium chloride (NaCl), sodium iodide, and zinc chloride, for the density separation of MPs in the aerosol (Habibi et al., 2022). Typically, the MP concentration in atmospheric samples is low, making sample preparation challenging and involving a multi-step process. Consequently, the risk of contamination is extremely high. Accordingly, handling these samples with extreme care and accuracy is essential. Although research has shown the presence of MPs in the air, the amounts identified are typically minimal. Dris et al. found 1—60 fibers m−3 in apartments and offices (Dris et al., 2017), Vianello et al. 1.7—16.2 particles m−3 in apartments (Vianello et al., 2019), Prata et al. found six fibers m−3 in the house (Prata et al., 2020), Gaston et al. found 3.3 ± 2.9 fibers and 12.6 ± 8.0 fragments m–3 in universities and hospitals (Gaston et al., 2020), Xie et al. found 16—93 MP m−3 in living and office rooms (Xie et al., 2022), Chen et al. found 46 ± 55 MPs m−3 in the nail salon (Chen et al., 2022), and Choi et al. found 0.49—6.64 MPs m−3 in the house (Choi et al., 2022). The low concentration of MPs in atmospheric samples demonstrates the need for adequate QC in atmospheric research.
This study aims to contribute to QC protocols for airborne and indoor MP research. Contamination of air samples for MP’s during sample preparation and analysis in the fume hood, laboratory, and laminar flow were investigated. Besides, the best indoor environment for MP sample preparation and analysis was also formulated. Meanwhile, different working solutions, filters, and laboratory equipment often used during sample preparation and analysis were also analyzed as to how they can contaminate MP samples and what measures should be taken to minimize them. The contamination of MPs in solutions, filters, and blank samples was analyzed first qualitatively under a stereo microscope and further verified and characterized under a Raman microscope.
Material and methods
The QC parameters and analytical methods adopted in this study include washing, thermal treatment, preparation of blank samples, drying filters in a desiccator overnight, and stereo and Raman microscopic analysis.
Washing
The purpose of washing is often to eliminate dirt, organic substances, and other impurities from the sample. This procedure aids in the precise isolation and analysis of the MPs by minimizing interference from other chemicals in the sample. During the experimental analysis, the use of plastic items was minimized as much as possible; only powder-free latex examination gloves and micropipettes were used. These gloves and micropipettes were washed three times with filtered Milli-Q-DI water to reduce the plastic contaminants if attached. However, mainly glass materials were used during the experimental process. Glass materials were washed three times with filtered Milli-Q-DI, then once with methanol (HPLC ≥ 99.9%), and thermally treated at 150 °C for 3 h. If needed, the materials were used only once in the analysis (forceps, spoon, spatula, and small glass stirring rods); they were washed at least three times with filtered Milli-Q-DI and once with methanol.
Thermal treatment of filters and filtration of working solutions
Thermal treatment of filters and filtration of working solutions play a pivotal role in reducing the risk of MP contamination during the production or handling of these materials. Whatman filters have been utilized in prior studies focusing on MPs (Dris et al., 2017; Gaston et al., 2020; Prata et al., 2020; Soltani et al., 2021). The Whatman glass microfiber filters (1.6 μm) were thermally treated at 450 °C for 4 h before use. The working solutions (100 mL Milli-Q-DI water and 20-g NaCl + 100 mL Milli-Q-DI water) were prefiltered through the thermally treated glass microfiber filters (1.6 μm). NaCl was easily available; other salts should also be analyzed for contamination. The main aim was to minimize plastic contamination from filters, materials, and working solutions. The solutions filtering was done through the same filter type with the same pore size (1.6 µm).
Preparation of blank samples
Due to microplastic contamination, blank samples are essential for any analytical method, especially MP analysis. Blank samples, which include all sample components except the analyte (MPs), serve numerous important purposes. During the sample analysis, seven petri dishes as a blank/control were kept open in the laboratory to check the MPs deposition in the laboratory for seven days. Petri dishes were also kept open in laminar flow, fume hood, laboratory, and stereo microscope laboratory to study the deposition of MPs during analysis. The supplementary file (SI) mentions detailed information about these workplaces. The filtration laboratory blanks were also taken to check the deposition of MPs during vacuum filtration from indoor air. The upper lid of the vacuum filtration was kept open to contact the filters with indoor air. The three thermally treated glass microfiber filters were put in the vacuum filtration device for 5 min at 20 kilopascals (kPa) to check the deposition of MPs during vacuum filtration. We used a Millipore filtering device in this study. All the materials were covered with aluminum foil and kept in laminar flow until the analysis and were taken out only when needed. Thus, all the experimental investigation was done in laminar flow. A cotton laboratory coat was worn throughout the research to prevent secondary contamination from clothes worn by researchers. All the Petri dishes were washed two times with 10 mL of filtered Milli-Q-DI water and vacuum filtered through thermally treated Whatman glass microfiber filters (1.6 μm) to collect the deposited MPs. The filters were stored in Petri dishes and dried in a desiccator (overnight). Filtration laboratory blanks were measured to check the contamination during the filtration processes.
Stereo microscopic analysis
For the identification (or confirmation) of MPs from filters, a stereo microscope (Carl Zeiss microscopy GmBh) Stemi 508 was used. The stereo microscope was operated by Axiovision SE64 Rel.4.9.1 software embedded with Axiocam 105 color camera. The microscope has different power of magnification like 0.63x, 0.8x, 1x, 1.25x, 1.6x, 2x, 2.5x, 3.2x, 4× and 5x. This microscope typically achieves a resolution that allows for the differentiation of particles down to around 10 µm in diameter. During the stereo microscope analysis, unwanted materials were removed with the help of tweezers if present without harming the filters. Under a stereo microscope, Petri dishes were closed immediately after the analysis to minimize air contact.
One petri dish was kept open close to the stereo microscope throughout the analysis to check the background contamination during analysis. The filter was divided into eight equal parts to count all the MPs correctly, and each part was counted at least three times to reduce human error, which might occur when taking a single photograph of the filter. Based on the samples, the selected magnification power was between 1.0x to 2.5x. MPs were identified according to Hidalgo-ruz et al. (Hidalgo-ruz et al., 2012). The MP fibers have to be equally thick throughout their entire length; Fibers should not be entirely straight, which indicates a biological origin; No cellular or organic structures should be visible to consider fiber as MP. Plastic pellets can have tablet-like, oblong, cylindrical, spherical, and disk shapes, mostly spherical to ovoid, with rounded ends: films (transparent and thin (thinner than fragments)) and foams (sponge-like texture). Fragments have sharp, broken edges and are round, subrounded, subangular, and angular. MPs were classified according to color as white-transparent, yellow-orange, red-pink, blue-green, or black-grey, and with the aid of ImageJ free version software (Bhat, 2023b, 2024), MPs were categorized according to size as follows: 1—100 μm; 101—250 μm; 251—500 μm; 501—1000 μm; 1001—4000 μm.
Raman microscopic analysis
A Renishaw inVia Raman microscope with a laser excitation wavelength of 532 nm, 1–5 mW laser power (mostly four mW and no obvious damage to MPs was seen), and 1—5 s acquisition time with a grating of 2400 l/mm and spectrum range 500—2400 was used. 50x (NA = 0.5; WD = 8.20 mm) objective lens was used. Raman spectra were recorded randomly from several points from the filters. For better illustration, smoothing, and baseline correction were done. The spectra obtained in the Raman microscope were compared with reference spectra from the Raman polymer database (Cowger et al., 2021). The spectrums were cross-checked with the previously published research (Cabernard et al., 2018; Käppler et al., 2016; Mark, 2009; Nava et al., 2021). The matching score of MPs was also analyzed; MPs with matching scores above 50% were considered MPs. Very few studies have discussed the matching score, and given it, in their results, their scores varied from 27 to 97% (Liu et al., 2019a; Song et al., 2021; Tunahan Kaya et al., 2018). Matching scores can be significantly boosted by using complete commercial libraries if the algorithm leverages multicomponent correlations in the matching process. Perhaps part of the difficulty is that commercial and custom libraries only include spectra from polymers that the environment has not degraded.
Statistical analysis
In the present study, Microsoft Excel, SPSS, and Origin were the computational tools for performing statistical analysis on the collected data and generating graphs based on those analyses. The study used methodological approaches, including applying an ANOVA single-factor analysis. To guarantee the highest possible levels of precision and dependability in the study findings, we used meticulously and methodically all of the most stringent analytical procedures.
Results and discussion
Contamination during sample preparation and analysis
The wind/airflow is a typical vector for spreading tiny particles, including plastics; consequently, air transport and deposition may be a direct and significant route of MPs from consumer items to the environment. The prevalent presence of airborne MPs certainly poses a novel challenge for the QC of MP detection in environmental matrices. It needs to be addressed to safeguard data consistency. Petri dishes were kept open for MP analysis to calculate the air contamination during the sample preparation and analysis in the fume hood, laboratory, and laminar flow. Petri dishes were also kept open during stereo microscope analysis to check background contamination during the filter analysis. Blanks were prepared and analyzed per the standard sample and examined qualitatively under a stereo microscope and chemically characterized by a Raman microscope. To mitigate air pollution, we have shifted our experiments from the laboratory, where access was restricted, and ventilation was regulated, to fume hoods and ultimately to laminar flow systems, intending to reduce airborne contamination. Such facilities are necessary for MP investigation; handling samples outside pure air is highly susceptible to airborne contamination. Most studies achieve this by performing all analysis stages in the laminar flow (Bordós et al., 2019; Hermsen et al., 2017; Scopetani et al., 2019; Wiesheu et al., 2016), fume hoods (Aliabad et al., 2019; Collard et al., 2015; De Witte et al., 2014; Santana et al., 2016; Van Cauwenberghe et al., 2013), and ultra-clean laboratories (Liu et al., 2019b; Wagner et al., 2017). Preventing air contamination is essential when dealing with MPs and, more precisely, microfibers. Shedding these fibers from clothes and home textiles with loose structures, such as knitted sweaters and fleece blankets, is prevalent (Bhat et al., 2021; Napper & Thompson, 2016). 100% cotton fabric is recommended with a single bright color. Identifying the presence of contamination in the real sample will be readily achievable.
The results of MP contamination found in the stereo microscope laboratory, laboratory blank, fume hood, and laminar flow based on their color, size, and shape are shown in Fig. 1. MPs deposition was higher in the stereo microscope laboratory (90), followed by laboratory blank (46), fume hood (29), and laminar flow (15). The presence of these MPs in the blank samples like stereo microscope laboratory, laboratory, fume hood, and laminar flow represents that contamination can occur during the analysis. These blank contaminations should be regarded to get the accurate number of MPs in the sample. It was seen that a fume hood reduces the contamination by 61.3% and laminar flow by 75.4% compared with a laboratory blank; however, the laminar flow reduces the contamination by 65.9% compared with a fume hood. Looking at the background contamination results, the laminar flow was the least contaminated compared to the other indoor environments. Both laminar flow and fume hood were present in the same laboratory where the analysis was done. The access to the laboratory was limited, and the doors and windows were kept closed to reduce external and secondary contamination. However, the stereo microscope laboratory was in the other department, where we could not limit human access. It is a common laboratory for use, and other instruments are also present; doors and windows were mostly open. This might be the reason for the higher deposition of MPs. These MPs had five colors: White-Transparent, Yellow-Orange, Red-Pink, Blue-Green, and Black-Grey (Fig. 1). Blue-Green and Black-Grey were the dominant colors of MPs.
Moreover, these MPs were identified as fibers, pellets, film, foam, and fragments; fiber was the dominant type of MP seen in all indoor environments. Fibers are ubiquitous; the clothes worn by researchers or other textiles in the laboratory can be a dominant source of MPs, especially fibers. Looking at MPs' size distribution, the abundance of MPs decreased as the size increased, indicating MPs lesser than 1000 µm were more abundant. This implies that small-size MPs should not be ignored during the analysis of MPs. There is a high chance that their number may surpass the bigger size MPs. In all the indoor environments, 11 MPs were found in (1—100 µm), 68 in (101—250 µm), 38 in (251—500 µm), 37 in (501—1000 µm), and 26 in (1001—4000 µm). In total, 154 MPs were seen under the size range from (1—1000 µm) while just 26 were in the size range of 1001—4000 µm (Fig. 1). Least number of MPs were seen in the size range of (1—100 µm); there is a chance that MPs (< 1.6 μm) can also come from the working solutions as the pore size of the filters through which the solutions were filtered (1.6 µm); this can contaminate the actual sample by increasing the number of small-size MPs (< 1.6 μm). The Anova single factor of the data for the size distribution of the identified MPs in the stereo microscope laboratory, lab blank, fume hood, and laminar flow does not show a significant difference (P = 0.27).
Contamination of filters
In some stages of sample preparation, the sample is filtered to separate MPs from other components, such as organic (digestion solutions) or inorganic matter (density separation solutions). Apart from filtering the sample, the filter choice also influences the limit of detection determined by its pore size. There are chances that these filters can get contaminated during the manufacturing processes or due to their production in non-controlled environments or from packaging materials (plastic bags).
In this study, thermal treatment of fresh unused glass microfiber filters (1.6 μm) 47 mm was done at 450 °C for 4 h, aiming to minimize plastic contamination from filters if present. Three fresh unused filters were assessed before and after thermal treatment. The average number of fibers and pellets present before thermal treatment was 4 ± 1 and 2.66 ± 0.57; however, after thermal treatment, an almost 50% reduction in the average number of MPs was 2 ± 1.73 and 1.33 ± 0.57 (Fig. 2). This indicates thermal treatment at 450 °C for 4 h can be an excellent option to reduce the MPs contamination of unused filters. A minimum of 2 fibers and 1.33 pellets should be considered blank background contamination after thermal treatment (450 °C for 4 h) of Whatman filters. Thermal treatment not only reduces the number of MPs but also the size of MPs. Before thermal treatment, MPs were quite visible at low magnification (1.0× to 1.2x); however, after thermal treatment, MPs were seen at high magnification at 2.5 × magnification, which indicates a decrease in MP size. Applying thermal treatments to fresh, unused filters can be a good option for cleaning the filters. Once the filters are combusted, they should be stored in washed glass petri dishes free from MPs. The petri dishes should be covered by aluminum foil or parafilm and stored in a laminar flow. The filters should only be taken out during the analysis. However, the lid of the Petri dishes should be covered as soon as possible whenever we take the filters out or store them in them to minimize contact with air, and all these steps should be done in laminar flow. Looking at the filter thermal treatment, two works on airborne MPs thermally treated their glass fiber filters at 450 °C for four hours (Li et al., 2019) or overnight. Prata et al. applied Nile Red staining to the new glass fiber filters, revealing various fibers and particles incorporated into the glass fiber matrix (Prata et al., 2021). These fibers and particles might cause the MPs in ambient samples to be overestimated. Prata et al. gave thermal treatment to these unused filters to resolve this problem (Prata et al., 2021). The results show that a 15-min treatment at 450 °C is inadequate, while a three-hour treatment at 450 °C may effectively eliminate plastics or fiber contamination from filters. Compared to lower-quality filters, high-quality glass fiber filters appear less contaminated. Failure to treat the filters might result in high blank levels.
Contamination of working solutions
Sample treatment regularly involves digestive and density separation reagents and distilled or Milli-Q-DI water used for rinsing materials and equipment, as mentioned previously. However, these solutions may be contaminated with MPs; they can be manufactured or processed in contaminated environments or packaged in plastic packaging, leading to sample overestimation. An effective way to overcome contamination in work solutions may be to prefilter all working solutions used during sample processing using clean vacuum filtering equipment and store the filtered solutions in tightly sealed sterile glass flasks.
In this study, contamination of filters, working solutions, vacuum filtration device, and glass materials were assessed in triplicate form, and their results are shown in Fig. 3. The washed and thermally treated glass materials (Petri dishes) were evaluated under the stereo microscope. None of the MPs were seen, which implies that washing and thermal treatment used simultaneously can be a good option for removing polymeric particles from the glass materials. The deposition of MPs during vacuum filtration from indoor air was also assessed (Fig. 3 A). The filtration laboratory blanks showed the presence of MPs. The maximum numbers of fiber (10) and pellets (9) were seen in filtration laboratory blank 1, while the minimum number of fiber (5) and pellets (4) was seen in filtration laboratory blank 2 and 3, respectively. The average number of fibers and pellets deposited during 5 min was 7.66 ± 2.51 and 6.33 ± 2.51. This implies that during vacuum filtration, contamination might occur from the indoor air; to minimize this contamination, the lid of the vacuum filtration device should be covered, or a blank sample should be taken to measure the background contamination.
100 mL of Milli-Q-DI water was also filtered, and the blanks in triplicate form were assessed (Fig. 3 B). A maximum of 9 fibers and pellets were found in 100 mL Milli-Q-DI water blank samples 3 and 2. However, a minimum of 6 fibers and five pellets were seen in 100 mL of Milli-Q-DI water blank samples 2 and 1, while the average number of fibers and pellets was seen in 100 mL of Milli-Q-DI water blank samples 7.66 ± 1.52 and 7.33 ± 2.08, respectively. Lastly, 20 g NaCl was diluted to 100 mL with Milli-Q-DI water and filtered; the filters were assessed under a stereo and Raman microscope. Results showed that a maximum number of fibers (34) and pellets (14) were seen in 20% NaCl blank samples 3 and 1, respectively, while the minimum number of fibers (23) and pellets (11) were seen in 20% NaCl blank sample 1 and 3. The average number of fibers and pellets was 29.33 ± 5.68 and 12.33 ± 1.52. Looking at all the cases, the number of fibers was predominantly more than pellets, and the 20% NaCl has the higher number of fibers and pellets (Fig. 3 C). There is a high chance that this background contamination may come from the indoor environment (laminar flow, fume hood, or laboratory). Filters, solutions, or reagents are commonly manufactured in uncontrolled conditions and packed in plastic materials, increasing the possibility of contamination. Apart from these working solutions, water may also be contaminated with MPs, so importance should be given to reducing the contamination from work solutions and filters in the future. Solutions should be filtered through thermally treated filters and then used. They should be analyzed for MP contamination to get accurate results. Working solutions should be stored in properly sealed, clean glass bottles, while the filters should be wrapped with aluminum foil and stored in laminar flow.
Pre-filtration of solutions is essential for MP analysis. For instance, for every kilogram of NaCl salt employed in the density separation of MPs from sediments, 50 to 280 MP particles were identified (Iñiguez et al., 2017). Solutions for density separation, such as NaCl, sodium iodide, zinc chloride, etc., should always be filtered. Ideally, filtering of working solutions should also be performed in a laminar flow or clean room with controlled airflow and human access, as mentioned before.
Cumulative deposition of microplastics in the laboratory
Seven Petri dishes were kept open in the air pollution laboratory during this study to check the cumulative deposition of MPs. The MPs were identified based on their color, type, size, and number (Fig. 4). The increasing trend was seen in the deposition of the MPs from day one to day seven. Day one had the lowest number of MPs (16), followed by day two (43), day three (46), day four (48), day five (56), and day six (80), while day seven had the highest (99). The average was 55.42 ± 26.94 MPs each day. Five different color MPs were seen: White-Transparent, Yellow-Orange, Red-Pink, Blue-Green, and Black-Grey. Blue-Green and Black-Grey were the dominant colors. However, fiber, pellet, film, foam, and fragment-shaped MPs were seen, and fiber was the predominant type of MPs, followed by pellets during all seven days (Fig. 4). Clothes worn by researchers and textiles present in the laboratory can be the source of fibers. However, the pellets might have formed by the breakdown of larger plastic materials. Size distribution of MPs seen in laboratory blanks showed that most of the MPs had a size lesser than 1000 µm like 64 MPs in (1—100 µm), 132 in (101—250 µm), 75 in (251—500 µm), 71 in (501—1000 µm) and just 46 in (1001—4000 µm). It was seen that most of the MPs (342) fall under the size range of (1—1000 µm) while just 46 were seen from (1001—4000 µm). The Anova single factor of the data for the size distribution of the identified MPs during one week showed a significant difference (P = 0.02).
Small-size MPs should be taken into account when analyzing the MPs as their background contamination may be high. For example, a comprehensive study of MPs in blanks indicates higher average concentration in smaller size classes: 22 MPs in the blank in the size range of 20 and 50 μm, 11 MPs in the 50—100 μm class, 3 in the 100—500 μm class, and ultimately 1 in the 500—1000 μm class (Frei et al., 2019). Therefore, no contamination in the blanks will be detected when considering big MP-size classes. Since smaller MPs are more common in the environment, the rising evaluation of smaller particle sizes demands stringent contamination control methods to generate valid findings. This contamination in the samples can come from various places, including sample collecting equipment, field and laboratory personnel clothes, air and dust in the collection site, etc. Air deposition controls and procedural blanks should be conducted simultaneously and evaluated jointly. If laboratory procedural contamination is not thoroughly evaluated, sample results will likely be overstated. As a result, we highly encourage future research on airborne and indoor MPs to include laboratory blanks as a routine procedure. There is an urgent need to record standardized QC measures, including blank measurement recovery rate experiments. The typical MPs seen in the study are shown in Fig. 5.
Recent meta-analyses of MP quantification in several matrices, including sediments (Hanvey et al., 2017), surface water (Erni-Cassola et al., 2019), and marine biota (Erni-Cassola et al., 2019; Hermsen et al., 2018) found that many researchers did not identify procedural contamination correctly. Only 7 of the 43 studies examined by Havey et al. used laboratory blanks somehow (Hanvey et al., 2017). None of them had field blanks to account for contamination brought on by sampling collection, transportation, and analysis. Some of these studies do not mention the final concentration of MPs in controls, although the analysis is carried out (Lorenz et al., 2019; Scopetani et al., 2019; Wright et al., 2021). Others claim variable MP concentrations in blanks, while others claim no contamination (Aliabad et al., 2019; Fan et al., 2019; Zhang et al., 2019), while some average claim values up to 36 MPs per blank (Frei et al., 2019). A size restriction is also associated with the lack of MPs in the blanks. Contamination is typically produced by tiny polymeric particles that pass through materials or settle in the air.
Blank measurements enable us to quantify the MP concentration in the background via procedural contamination. For MP analysis, the sample should be proposed to allow the ambient count of particles. For each color morphology combination, or even for each color morphology polymer type combination, the level of procedural contamination may be evaluated if spectroscopy is employed on all particles. These particle classes may be thought of in the same manner as distinct congeners in the chemical classes, and concentrations in blanks can be removed from the sample counts. As a standardized quantification technique of MPs, those blank samples should be provided to verify that procedure contamination does not overstate ambient concentrations.
Raman microscope evaluation
Raman microscope analysis of samples has shown the presence of different MPs. Under the Raman microscope, 71 spectrums were taken randomly from all the samples, and 40 particles were identified as MPs. These 40 MPs were categorized into ten types of MPs like poly(trimethyl hexamethylene terephthalamide) (Nylon 6(3)T), polymethylpentene (PMP), polyacrylamide (PAM), PAM carboxy modified, PP, poly(vinyl alcohol) (PVA), polytetrafluoroethylene (PTFE), Varnish, polyvinyl chloride (PVC), and high-density polyethylene (HDPE). These 10 MPs are plotted in Fig. 6, while the rest, 30 MPs, are given in the supplementary file (Fig. S1-S2). The presence of these MPs shows that there were indoor sources present for these identified MPs. There might be many different sources of these polymers, including laboratory equipment, paper making, textiles, etc. (Chanda & Roy, 2008; Elias, 2009; Mark, 2009), and all of these were identified in these indoor environments. Identifying these MPs in different indoor environments, filters, and solutions shows a need to continuously take blanks (field and laboratory) during MP sampling, preparation, and analysis. Apart from taking the blank samples, strict precaution measures should be taken to minimize the contamination from solutions, filters, indoor environments, researcher clothes, etc.
Conclusions and recommendations
Washing of glass materials three times with the Milli-Q-DI water, then once with methanol (HPLC ≥ 99.9%), and thermally treated at 150 °C for 3 h can almost remove all MPs if present. New filters and working solutions can be highly contaminated with MPs. Prefiltering solutions using the same or smaller pore size of the filter used for analysis can significantly minimize MP count. In contrast, thermal treatment of fresh unused filters at 450 °C for 4 h reduced the fibers and pellets by 50%. This indicates that thermal treatment at 450 °C for 4 h can be an excellent option to reduce the MP contamination of unused filters. For all seven days, a total of (342) fall under the size range from (1—1000 µm), while just (46) MPs were seen between (1001—4000 µm); this indicates the calculation of small size MPs in samples can not be ignored. Raman analysis identified 40 MPs divided into ten different types of MPs like Nylon 6(3)T, PMP, PAM, PAM carboxy modified, PP, PVA, PTFE, Varnish, PVC, and HDPE.
Small-size classes of MPs should be more emphasized since they are more prevalent in the environment, and less or no contamination in samples and blanks should be recorded when considering big-sized classes of MPs. Future studies are needed on nanoplastic contamination. Hence, evaluating smaller particle sizes requires rigorous control methods to control contamination and obtain valid results. Laboratories, clean rooms, fume hoods, and laminar flow must be regularly maintained and cleaned to preserve a low pollution profile. MP technique guides should highlight the necessity of procedural checks. Fume hoods do poorly control the background air contaminants, while laminar flows are preferred and are a good option for minimizing background air contamination. Apart from laminar flow, isolated rooms with regulated airflow and access, like microscope rooms or working in laboratories with constant airflow and minimal individual circulation, can also be a good option for analyzing MPs.
Data availability
The authors confirm that the data supporting the findings of this study are available in the article.
References
Abbasi, S., Keshavarzi, B., Moore, F., Turner, A., Kelly, F. J., Dominguez, A. O., & Jaafarzadeh, N. (2019). Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County Iran. Environmental Pollution, 244, 153–164. https://doi.org/10.1016/j.envpol.2018.10.039
Aliabad, M. K., Nassiri, M., & Kor, K. (2019). Microplastics in the surface seawaters of Chabahar Bay, Gulf of Oman (Makran Coasts). Marine Pollution Bulletin, 143, 125–133. https://doi.org/10.1016/j.marpolbul.2019.04.037
Allen, S., Allen, D., Phoenix, V. R., Le Roux, G., Durántez Jiménez, P., Simonneau, A., Binet, S., & Galop, D. (2019). Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nature Geoscience, 12, 339–344. https://doi.org/10.1038/s41561-019-0335-5
Bhat, M. A. (2023a). Indoor microplastics: A comprehensive review and bibliometric analysis. Environmental Science and Pollution Research, 30, 121269–121291. https://doi.org/10.1007/s11356-023-30902-0
Bhat, M. A. (2024). Unveiling the overlooked threat: Macroplastic pollution in indoor markets in an urban city. Case Studies in Chemical and Environmental Engineering, 9, 100558. https://doi.org/10.1016/j.cscee.2023.100558
Bhat, M. A., Gedik, K., & Gaga, E. O. (2023b). A preliminary study on the natural aging behavior of microplastics in indoor and outdoor environments. International Journal of Environmental Science and Technology. https://doi.org/10.1007/s13762-023-05319-4
Bhat, M. A., Gedik, K., & Gaga, E. O. (2023c). Atmospheric micro (nano) plastics: Future growing concerns for human health. Air Quality, Atmosphere & Health, 16, 233–262. https://doi.org/10.1007/s11869-022-01272-2
Bhat, M.A., Eraslan, F.N., Gedik, K., Gaga, E.O., 2021. Impact of Textile Product Emissions : Toxicological Considerations in Assessing Indoor Air Quality and Human Health, in: Malik, J.A., Marathe, S. (Eds.), Ecological and Health Effects of Building Materials. Springer Nature Switzerland, pp. 505–541. https://doi.org/10.1007/978-3-030-76073-1_27
Bhat, M.A., Gedik, K., Gaga, E.O., 2022. Environmental Toxicity of Emerging Micro and Nanoplastics: A Lesson Learned from Nanomaterials, in: Dar, A.H., Nayik, G.A. (Eds.), Nanotechnology Interventions in Food Packaging and Shelf Life. Taylor & Francis (CRC Press), pp. 311–337. https://doi.org/10.1201/9781003207641-18
Bhat, Mansoor Ahmad, Eraslan, F.N., Gaga, E.O., Gedik, K., 2023a. Scientometric Analysis of Microplastics across the Globe, in: Vithanage, M., Prasad, M.N.V. (Eds.), Microplastics in the Ecosphere: Air, Water, Soil, and Food. John Wiley & Sons Ltd, pp. 3–13. https://doi.org/10.1002/9781119879534.ch1
Bhat, M.A., 2023b. Identification and Characterization of Microplastics in Indoor Environments. Eskişehir Tech. Univ. Eskişehir Technical University. https://doi.org/10.13140/RG.2.2.22164.88960
Bordós, G., Urbányi, B., Micsinai, A., Kriszt, B., Palotai, Z., Szabó, I., Hantosi, Z., & Szoboszlay, S. (2019). Identification of microplastics in fish ponds and natural freshwater environments of the Carpathian basin, Europe. Chemosphere, 216, 110–116. https://doi.org/10.1016/j.chemosphere.2018.10.110
Cabernard, L., Roscher, L., Lorenz, C., Gerdts, G., & Primpke, S. (2018). Comparison of Raman and Fourier Transform Infrared Spectroscopy for the Quantification of Microplastics in the Aquatic Environment. Environmental Science and Technology, 52, 13279–13288. https://doi.org/10.1021/acs.est.8b03438
Catarino, A. I., Macchia, V., Sanderson, W. G., Thompson, R. C., & Henry, T. B. (2018). Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environmental Pollution, 237, 675–684. https://doi.org/10.1016/j.envpol.2018.02.069
Chanda, M., Roy, S.K., 2008. Industrial Polymers, Specialty Polymers, and Their Applications, 1st ed. CRC Press. https://doi.org/10.1201/9781420080599
Chen, E. Y., Lin, K. T., Jung, C. C., Chang, C. L., & Chen, C. Y. (2022). Characteristics and influencing factors of airborne microplastics in nail salons. Science of the Total Environment, 806, 151472. https://doi.org/10.1016/j.scitotenv.2021.151472
Choi, H., Lee, I., Kim, H., Park, J., Cho, S., Oh, S., Lee, M., & Kim, H. (2022). Comparison of Microplastic Characteristics in the Indoor and Outdoor Air of Urban Areas of South Korea. Water, Air, and Soil Pollution, 233, 1–10. https://doi.org/10.1007/s11270-022-05650-5
Collard, F., Gilbert, B., Eppe, G., Parmentier, E., & Das, K. (2015). Detection of Anthropogenic Particles in Fish Stomachs: An Isolation Method Adapted to Identification by Raman Spectroscopy. Archives of Environmental Contamination and Toxicology, 69, 331–339. https://doi.org/10.1007/s00244-015-0221-0
Cowger, W., Steinmetz, Z., Gray, A., Munno, K., Lynch, J., Hapich, H., Primpke, S., De Frond, H., Rochman, C., & Herodotou, O. (2021). Microplastic Spectral Classification Needs an Open Source Community: Open Specy to the Rescue! Analytical Chemistry, 93, 7543–7548. https://doi.org/10.1021/acs.analchem.1c00123
De Witte, B., Devriese, L., Bekaert, K., Hoffman, S., Vandermeersch, G., Cooreman, K., & Robbens, J. (2014). Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Marine Pollution Bulletin, 85, 146–155. https://doi.org/10.1016/j.marpolbul.2014.06.006
Dris, R., Gasperi, J., Saad, M., Mirande, C., & Tassin, B. (2016). Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Marine Pollution Bulletin, 104, 290–293. https://doi.org/10.1016/j.marpolbul.2016.01.006
Dris, R., Gasperi, J., Mirande, C., Mandin, C., Guerrouache, M., Langlois, V., & Tassin, B. (2017). A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environmental Pollution, 221, 453–458. https://doi.org/10.1016/j.envpol.2016.12.013
Elias, H.-G., 2009. Macromolecules: Applications of Polymers, 5th ed. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. https://doi.org/10.1002/macp.v210:22
Engler, R. E. (2012). The complex interaction between marine debris and toxic chemicals in the ocean. Environmental Science and Technology, 46, 12302–12315. https://doi.org/10.1021/es3027105
Enst, R.K.S., 2021. Plastics - Environmental aspects - State of knowledge and methodologies (ISO/TR 21960:2020).
Eraslan, F.N., Bhat, M.A., Gaga, E.O., Gedik, K., 2021. Comprehensive Analysis of Research Trends in Volatile Organic Compounds Emitted from Building Materials : A Bibliometric Analysis, in: Malik, J.A., Marathe, S. (Eds.), Ecological and Health Effects of Building Materials. Springer Nature Switzerland, pp. 87–109. https://doi.org/10.1007/978-3-030-76073-1_6
Eraslan, F.N., Bhat, M.A., Gedik, K., Gaga, E.O., 2023. The Single-Use Plastic Pandemic in the COVID-19 Era, in: Vithanage, M., Prasad, M.N.V. (Eds.), Microplastics in the Ecosphere: Air, Water, Soil, and Food. John Wiley & Sons Ltd, pp. 65–75. https://doi.org/10.1002/9781119879534.ch4
Erni-Cassola, G., Zadjelovic, V., Gibson, M. I., & Christie-Oleza, J. A. (2019). Distribution of plastic polymer types in the marine environment; A meta-analysis. Journal of Hazardous Materials, 369, 691–698. https://doi.org/10.1016/j.jhazmat.2019.02.067
Fan, Y., Zheng, K., Zhu, Z., Chen, G., & Peng, X. (2019). Distribution, sedimentary record, and persistence of microplastics in the Pearl River catchment China. Environmental Pollution, 251, 862–870. https://doi.org/10.1016/j.envpol.2019.05.056
Frei, S., Piehl, S., Gilfedder, B. S., Löder, M. G. J., Krutzke, J., Wilhelm, L., & Laforsch, C. (2019). Occurence of microplastics in the hyporheic zone of rivers. Science and Reports, 9, 1–11. https://doi.org/10.1038/s41598-019-51741-5
Gaston, E., Woo, M., Steele, C., Sukumaran, S., & Anderson, S. (2020). Microplastics Differ Between Indoor and Outdoor Air Masses: Insights from Multiple Microscopy Methodologies. Applied Spectroscopy, 74, 1079–1098. https://doi.org/10.1177/0003702820920652
Habibi, N., Uddin, S., Fowler, S.W., Behbehani, M., 2022. Microplastics in the atmosphere: a review. J. Environ. Expo. Assess. 1, 1–20. https://doi.org/10.20517/jeea.2021.07
Hanvey, J. S., Lewis, P. J., Lavers, J. L., Crosbie, N. D., Pozo, K., & Clarke, B. O. (2017). A review of analytical techniques for quantifying microplastics in sediments. Analytical Methods, 9, 1369–1383. https://doi.org/10.1039/c6ay02707e
Hermsen, E., Pompe, R., Besseling, E., & Koelmans, A. A. (2017). Detection of low numbers of microplastics in North Sea fish using strict quality assurance criteria. Marine Pollution Bulletin, 122, 253–258. https://doi.org/10.1016/j.marpolbul.2017.06.051
Hermsen, E., Mintenig, S. M., Besseling, E., & Koelmans, A. A. (2018). Quality Criteria for the Analysis of Microplastic in Biota Samples: A Critical Review. Environmental Science and Technology, 52, 10230–10240. https://doi.org/10.1021/acs.est.8b01611
Hidalgo-ruz, V., Gutow, L., Thompson, R.C., Thiel, M., 2012. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 3060−3075. https://doi.org/10.1021/es2031505
Hung, C., Klasios, N., Zhu, X., Sedlak, M., Sutton, R., & Rochman, C. M. (2021). Methods Matter: Methods for Sampling Microplastic and Other Anthropogenic Particles and Their Implications for Monitoring and Ecological Risk Assessment. Integrated Environmental Assessment and Management, 17, 282–291. https://doi.org/10.1002/ieam.4325
Iñiguez, M. E., Conesa, J. A., & Fullana, A. (2017). Microplastics in Spanish Table Salt. Science and Reports, 7, 1–7. https://doi.org/10.1038/s41598-017-09128-x
Käppler, A., Fischer, D., Oberbeckmann, S., Schernewski, G., Labrenz, M., Eichhorn, K. J., & Voit, B. (2016). Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Analytical and Bioanalytical Chemistry, 408, 8377–8391. https://doi.org/10.1007/s00216-016-9956-3
Li, Q., Wu, J., Zhao, X., Gu, X., & Ji, R. (2019). Separation and identification of microplastics from soil and sewage sludge. Environmental Pollution, 254, 113076. https://doi.org/10.1016/j.envpol.2019.113076
Liu, K., Wang, X., Fang, T., Xu, P., Zhu, L., & Li, D. (2019a). Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Science of the Total Environment, 675, 462–471. https://doi.org/10.1016/j.scitotenv.2019.04.110
Liu, K., Wu, T., Wang, X., Song, Z., Zong, C., Wei, N., & Li, D. (2019b). Consistent Transport of Terrestrial Microplastics to the Ocean through Atmosphere. Environmental Science and Technology, 53, 10612–10619. https://doi.org/10.1021/acs.est.9b03427
Lorenz, C., Roscher, L., Meyer, M. S., Hildebrandt, L., Prume, J., Löder, M. G. J., Primpke, S., & Gerdts, G. (2019). Spatial distribution of microplastics in sediments and surface waters of the southern North Sea. Environmental Pollution, 252, 1719–1729. https://doi.org/10.1016/j.envpol.2019.06.093
Mark, J. E. (2009). Polymer Data Book (2nd ed.). Oxford University Press.
Napper, I. E., & Thompson, R. C. (2016). Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Marine Pollution Bulletin, 112, 39–45. https://doi.org/10.1016/j.marpolbul.2016.09.025
Nava, V., Frezzotti, M. L., & Leoni, B. (2021). Raman Spectroscopy for the Analysis of Microplastics in Aquatic Systems. Applied Spectroscopy, 75, 1341–1357. https://doi.org/10.1177/00037028211043119
Paiva, B. O., de Souza, A. K. M., Soares, P. L., Palma, A. R. T., & Vendel, A. L. (2022). How to Control the Airborne Contamination in Laboratory Analyses of Microplastics? Brazilian Archives of Biology and Technology, 65, 1–9. https://doi.org/10.1590/1678-4324-2022210399
Prata, J. C., Castro, J. L., da Costa, J. P., Duarte, A. C., Rocha-Santos, T., & Cerqueira, M. (2020). The importance of contamination control in airborne fibers and microplastic sampling: Experiences from indoor and outdoor air sampling in Aveiro Portugal. Marine Pollution Bulletin, 159, 111522. https://doi.org/10.1016/j.marpolbul.2020.111522
Prata, J. C., Reis, V., da Costa, J. P., Mouneyrac, C., Duarte, A. C., & Rocha-Santos, T. (2021). Contamination issues as a challenge in quality control and quality assurance in microplastics analytics. Journal of Hazardous Materials, 403, 123660. https://doi.org/10.1016/j.jhazmat.2020.123660
Renner, G., Schmidt, T. C., & Schram, J. (2018). Analytical methodologies for monitoring micro(nano)plastics: Which are fit for purpose? Current Opinion in Environmental Science & Health, 1, 55–61. https://doi.org/10.1016/j.coesh.2017.11.001
Santana, M. F. M., Ascer, L. G., Custódio, M. R., Moreira, F. T., & Turra, A. (2016). Microplastic contamination in natural mussel beds from a Brazilian urbanized coastal region: Rapid evaluation through bioassessment. Marine Pollution Bulletin, 106, 183–189. https://doi.org/10.1016/j.marpolbul.2016.02.074
Scopetani, C., Chelazzi, D., Cincinelli, A., & Esterhuizen-Londt, M. (2019). Assessment of microplastic pollution: Occurrence and characterisation in Vesijärvi lake and Pikku Vesijärvi pond Finland. Environmental Monitoring and Assessment, 191, 652. https://doi.org/10.1007/s10661-019-7843-z
Seethapathy, S., Górecki, T., & Li, X. (2008). Passive sampling in environmental analysis. Journal of Chromatography A, 1184, 234–253. https://doi.org/10.1016/j.chroma.2007.07.070
Soltani, N.S., Taylor, M.P., Wilson, S.P., 2021. Quantification and exposure assessment of microplastics in Australian indoor house dust. Environ. Pollut. 283. https://doi.org/10.1016/j.envpol.2021.117064
Song, Z., Liu, K., Wang, X., Wei, N., Zong, C., Li, C., Jiang, C., He, Y., & Li, D. (2021). To what extent are we really free from airborne microplastics? Science of the Total Environment, 754, 142118. https://doi.org/10.1016/j.scitotenv.2020.142118
Su, L., Deng, H., Li, B., Chen, Q., Pettigrove, V., Wu, C., & Shi, H. (2019). The occurrence of microplastic in specific organs in commercially caught fishes from coast and estuary area of east China. Journal of Hazardous Materials, 365, 716–724. https://doi.org/10.1016/j.jhazmat.2018.11.024
Thacharodi, A., Meenatchi, R., Hassan, S., Hussain, N., Bhat, M. A., Arockiaraj, J., Ngo, H. H., Le, Q. H., & Pugazhendhi, A. (2024). Microplastics in the environment: A critical overview on its fate, toxicity, implications, management, and bioremediation strategies. Journal of Environmental Management, 349, 119433. https://doi.org/10.1016/j.jenvman.2023.119433
Thompson, R.C., Olsen, Y., Mitchell, R.P., Davis, A., Rowland, S.J., John, A.W.G., McGonigle, D., Russell, A.E., 2004. Lost at sea: where is all the plastic? Science (80-. ). 304, 838–838. https://doi.org/10.1111/sum.12058
Tunahan Kaya, A., Yurtsever, M., Çiftçi Bayraktar, S., 2018. Ubiquitous exposure to microfiber pollution in the air. Eur. Phys. J. Plus 133. https://doi.org/10.1140/epjp/i2018-12372-7
Van Cauwenberghe, L., Vanreusel, A., Mees, J., & Janssen, C. R. (2013). Microplastic pollution in deep-sea sediments. Environmental Pollution, 182, 495–499. https://doi.org/10.1016/j.envpol.2013.08.013
Vianello, A., Jensen, R. L., Liu, L., & Vollertsen, J. (2019). Simulating human exposure to indoor airborne microplastics using a Breathing Thermal Manikin. Science and Reports, 9, 1–11. https://doi.org/10.1038/s41598-019-45054-w
Wagner, J., Wang, Z. M., Ghosal, S., Rochman, C., Gassel, M., & Wall, S. (2017). Novel method for the extraction and identification of microplastics in ocean trawl and fish gut matrices. Analytical Methods, 9, 1479–1490. https://doi.org/10.1039/c6ay02396g
Wiesheu, A. C., Anger, P. M., Baumann, T., Niessner, R., & Ivleva, N. P. (2016). Raman microspectroscopic analysis of fibers in beverages. Analytical Methods, 8, 5722–5725. https://doi.org/10.1039/c6ay01184e
Woolfenden, E. (2010). Sorbent-based sampling methods for volatile and semi-volatile organic compounds in air. Part 1: Sorbent-based air monitoring options. Journal of Chromatography A, 1217, 2674–2684. https://doi.org/10.1016/j.chroma.2009.12.042
Wright, S. L., Gouin, T., Koelmans, A. A., & Scheuermann, L. (2021). Development of screening criteria for microplastic particles in air and atmospheric deposition: Critical review and applicability towards assessing human exposure. Microplastics and Nanoplastics, 1, 1–18. https://doi.org/10.1186/s43591-021-00006-y
Xie, Y., Li, Y., Feng, Y., Cheng, W., & Wang, Y. (2022). Inhalable microplastics prevails in air: Exploring the size detection limit. Environment International, 162, 107151. https://doi.org/10.1016/j.envint.2022.107151
Yu, Q., Hu, X., Yang, B., Zhang, G., Wang, J., & Ling, W. (2020). Distribution, abundance and risks of microplastics in the environment. Chemosphere, 249, 126059. https://doi.org/10.1016/j.chemosphere.2020.126059
Zhang, L., Zhang, S., Wang, Y., Yu, K., & Li, R. (2019). The spatial distribution of microplastic in the sands of a coral reef island in the South China Sea: Comparisons of the fringing reef and atoll. Science of the Total Environment, 688, 780–786. https://doi.org/10.1016/j.scitotenv.2019.06.178
Acknowledgements
The authors would like to thank Gül İpek Selimoğlu (Ph.D.) and Yasemin Çelik (Ph.D.) for giving access to stereo and Raman microscope instruments.
Funding
This study was supported by the Eskişehir Technical University Research Fund (project number 21DRP106), and we are thankful to the Presidency For Turks And Related Communities for providing the Ph.D. grant to Mansoor Ahmad Bhat.
Author information
Authors and Affiliations
Contributions
Conceptualization, data curation, investigation, methodology, writing of the original draft, validation, formal analysis, and writing – review & editing was conducted by Mansoor Ahmad Bhat; funding acquisition, project administration, writing – review & editing, supervision by Eftade O. Gaga, writing – review & editing by Kadir Gedik.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights.
• The lack of contamination control measures hinders comparisons of data. Therefore, contamination control measures were assessed for their significance, and without proper control measures, contamination may occur.
• Laminar flow should be used to minimize the impact of airborne contamination.
• Small-size MPs (1 - 1000 µm) should not be neglected as their contamination may be higher.
• Combining washing glass materials with Milli-Q-DI water, methanol, and thermal treatment can almost remove all MPs if present.
• Thermal treatment for fresh, unused filters (450°C for 4 hours) can reduce MP contamination by 50%.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhat, M.A., Gaga, E.O. & Gedik, K. How can contamination be prevented during laboratory analysis of atmospheric samples for microplastics?. Environ Monit Assess 196, 159 (2024). https://doi.org/10.1007/s10661-024-12345-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-024-12345-3