Abstract
The concentration of heavy metals in the natural waters of the area surrounding Dereli (Giresun), a mineralized area, was determined. In addition, the physicochemical parameters (such as pH, Eh, and HCO3−) influencing the concentration of dissolved metals in waters were evaluated. Samples were collected from stream, spring, and main river waters, some of which are used by local residents for drinking, fish farming, and animal husbandry. The present data indicate that the water-rock interaction is the main process controlling the chemical composition of waters in the area. The waters are predominantly CaMgHCO3-type waters with alkaline pH values. Heavy metal concentrations (Pb, Zn, Cu, Fe, Mn, U, Cd, As, Hg, Sb, Ni, Co, and Ba) in waters were generally low. Relatively high values of certain metals were determined in waters of the Kotana and Kurtulmus sites of Dereli. The levels of Pb in the river, stream, and spring water samples in these sites were somewhat higher than the Pb values reported in the literature for natural waters. However, these values only slightly exceeded the Pb water limit values proposed by the USEPA and the WHO. In addition, the concentrations of other heavy metals, except Pb, were fairly similar to those of other natural waters and these values were also under the drinking water limits set by the WHO and the USEPA. This study shows that waters in the area of Dereli were not significantly affected by mineralization. This is most likely caused by the bicarbonate-rich waters affecting the dissolved metal concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
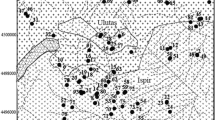
Surface waters (rivers and streams in particular) and springs are the most important natural water resources. Trace metals are present at various concentrations in these waters. These metals enter the waters by either natural sources or human activities (Kabata-Pendias 2010; Ciarkowska et al. 2019; Mondal et al. 2010). The concentration of metals in natural waters is closely connected with the geology of the region. In particular, mineralized areas have high concentrations of metals due to their natural characteristics. Hence, concentrations of metals in the water of mineralized areas are expected to be higher than in nonmineralized regions, even in the absence of mining (Runnells et al. 1992). Heavy metals are likely to be released into natural waters due to mineral deposit-water interactions. This may cause increasing metal levels in the water and therefore the deterioration of the water quality in the surrounding areas. For instance, Runnells et al. (1992) noted that many streams, springs, and deeper groundwaters in mineralized areas unaffected by mining contained highly elevated metal concentrations. Verplanck et al. (2009) reported that the waters in mineralized areas of the Southern Rocky Mountains were naturally acidic and metal rich. Similarly, Dames and Moore (1983) revealed that stream waters draining the undisturbed Red Dog deposit (AK) were acidic and contained high levels of cadmium, lead, and zinc. Giresun is located in the metallogenic province of the Eastern Black Sea region in Turkey. Previous studies on the region reported that Upper Cretaceous age acidic volcano-sedimentary rocks and Tertiary age (granitoid) intrusions are present. The region hosts a great number of mineralizations of various types and sizes. They are mainly characterized by volcanogenic massive sulfide (VMS-Kuroko type) and vein-type copper-lead-zinc (Cu-Pb-Zn) mineralizations. The main ore minerals reported are pyrite (FeS2), chalcopyrite (CuFeS2), sphalerite (ZnS), and galena (PbS). Barite (BaSO4), quartz, carbonate minerals (calcite and dolomite), gypsum (CaSO4.2H2O), anhydrite (CaSO4), and clay minerals are the commonly occurring gangue minerals. Additionally, iron-skarn and volcano-sedimentary manganese mineralization were noted in this area (Gokce and Bozkaya 2003; General directorate of mineral research and exploration (MTA) 2010; Karakaya and Karakaya 2014; Ciftci et al. 2015).
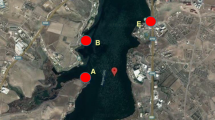
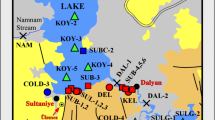
Dereli is a district of Giresun Province. Some of these mineralizations in Giresun are located in the vicinity of Dereli (Fig. 1). However, they have mostly not been mined, presumably due to their economic insignificance. The area has a humid and rainy climate and a very steep topography. This steep topography makes access to many locations within the area quite difficult. The area receives a high amount of precipitation throughout the year. Precipitation is generally in the form of rain, and the highest rainfall occurs in autumn (especially in October). High water flow makes access to the river difficult and dangerous during a period of high rainfall. The driest month is July. The average annual rainfall is 867 mm. The average temperature in the area is 12.7 °C. With an average temperature of 21.5 °C, August is the warmest month. January has the lowest average temperature of the year at 4.3 °C (climate-data.com/). The Aksu River is the main river passing through the Dereli area (see Fig. 1). Many streams merge with the Aksu River, and all of the water eventually flows into the Black Sea. Some streams and parts of the river are generally used by local residents for fish farming and animal husbandry. Spring waters are also used as potable water.
Map of the study area, showing locations of water samples (left) and the distribution of known mineral deposits in the vicinity of the Dereli area (right). The map on right was adapted from MTA (2010)
To date, various studies have been carried out on the water quality in different parts of Giresun Province. These are mostly relevant to waters in areas impacted by mining (Karakaya and Karakaya 2014; Saglam et al. 2016). However, there are no studies regarding the heavy metal contents in natural waters (springs, streams, and the main river) of the Dereli (Giresun) area. This study focused on determining the concentration of heavy metals in the natural waters of Dereli and investigating whether heavy metal contamination is present. Therefore, the levels of heavy metals in waters were compared with the levels in natural waters and international water quality standards such as those set by the USEPA and the WHO. Furthermore, some geochemical characteristics of the waters were studied.
Materials and methods
The examined area, Dereli, is located in the south part of Giresun Province (Fig. 1). A total of 18 water samples were collected from the Dereli area in late August 2017. Sixteen of these samples were taken from accessible water points along the Aksu River and some streams. The other 2 samples were taken from springs.The sampling locations were recorded using a global positioning system (GPS), and the coordinates were plotted on a map (Fig. 1). All water samples collected were filtered using a 0.45-μm membrane filter and then divided into two polyethylene bottles of 100 mL. One sample was acidified to pH < 2 with ultrapure HNO3 and stored in a refrigerator at 4 °C until analysis. The other was not acidified and kept for anion analysis. The acidified samples were sent to an internationally accredited laboratory (ACME Lab, Canada) for chemical analyses by inductively coupled plasma mass spectrometry (ICP-MS). The method detection limits (MDL) for each studied metal are given in Table 2. The other, unacidified samples were used for anion analysis. The redox potential (Eh), pH, and temperature (T) values were measured in situ by using a portable meter. Alkalinity was determined by titration with hydrochloric acid and expressed as bicarbonate. The chemical composition of the water samples was identified and classified using a Piper diagram (Piper 1944). Gibbs (1970) plot was used to determine the process(es) controlling the chemistry of the waters. The saturation index (SI) was calculated for certain mineral phases by using the PHREEQC computer program (Parkhurst and Appelo 1999). To find the relationships among certain chemical parameters, correlation coefficients (r) were calculated using the IBM SPSS Statistics 24 software. Because the concentrations of some metals (Fe, Cd, As, Hg, Sb, and Ni) are under the detection limit of the method, these elements were not included in the correlation calculation.
Results and discussion
Physicochemical characteristics of water samples
The major ion concentrations and significant physicochemical parameters of all water samples (main river, streams, and springs) are presented in Table 1. The measured temperature values in the water samples were between 18 and 25.2 °C. The lowest T values were in the spring waters. The redox potentials of the water samples indicate an oxidizing environment with Eh values ranging from + 160 to + 222 mV. The calculated total dissolved solids (TDS) concentration in the water samples was in the range of 178–575 ppm, and the water samples had higher TDS values than the median TDS value of 120 mg/L noted for surface water (Langmuir 1997). However, the most of values are below the permissible limit (500 mg/L) set by the USEPA. This shows that the waters of the study area are fresh. As shown in Table 1, the water samples had pH values varying between 7.60 and 8.35. The USEPA (2018) and WHO (2008, 2011) standards stated that the pH of drinking water should be in the pH range of 6.5–8.5. The pH values of all water samples, including spring water, fell within the recommended range determined by the USEPA and WHO (Table 1). The concentration of Si in the waters was generally low. Its minimum and maximum values were 6.64 mg/L and 14.52 ppm, respectively. The amount of aluminum (Al) varied, ranging from 12 to 69 ppb.
Calcium (Ca2+) and magnesium (Mg2+), which are commonly present in natural waters, were the most abundant cations in the studied waters. The concentration of Ca ranged from 27.8–95.8 ppm. The highest value of 95.8 ppm was recorded at sample number 15 taken from river water. Ca was also a dominant cation in all of the waters. The Mg concentration was in the range of 5.7–40.3 ppm. The concentration of sodium (Na+) in nearly all of the samples was under the health-based value of 20 mg/L proposed by the USEPA for drinking water. The concentration of the potassium ion (K+) in natural fresh waters is generally lower than 10 mg/L (Hydrology Project 1999). The minimum and maximum concentrations of K+ in the studied waters are 0.83 ppm and 5.27 ppm, respectively, and K+ has the lowest concentration compared with other cations.
Bicarbonate (HCO3−) was the most dominant anion in almost all of the water samples. Its concentration varied between 65 and 415 mg/L. The spring water taken from point number 18 had the highest HCO3− concentration (415 mg/L). Bicarbonate values in the river and stream water samples were fairly high compared with the median value (58 mg/L) reported in Langmuir (1997) for surface waters. Chloride (Cl−) concentrations reached 33 ppm and were well below the USEPA (2018) secondary water standards of 250 mg/L. Sulfate (SO42−) was the next most abundant anion after bicarbonate in the investigated waters. Its concentration varied in the range of 6.0 to 69.0 ppm. The stream water samples taken from the Kolca site (samples 4 and 5) showed a slightly elevated SO42− concentration relative to the other samples. Furthermore, water sample 5 had a concentration of 69 ppm and was sulfate-dominated rather than bicarbonate. However, except for this sample, all water samples were bicarbonate-dominated, and the values recorded for sulfate were all below the USEPA permissible limit, as shown in Table 1. Based on the relative dominance of major cations and anions stated here, the waters in the study area could be classified mainly as Ca-Mg-HCO3- and Ca-HCO3-type waters with two exceptions. These two samples (4 and 5) are Ca-Mg-HCO3-SO4−- and Ca-Mg-SO4-HCO3-type waters, respectively. The data have been plotted in a Piper diagram (1944), which is represented in Fig. 2.
According to Gibbs (1970), the chemical composition of earth’s surface waters is controlled by three major mechanisms: atmospheric precipitation, rock dominance, and the evaporation-crystallization process. To determine the mechanism controlling the chemistry of the waters in the study area, the TDS versus Na/(Na+Ca) plot of Gibbs (1970) was used. All water samples fell within the rock dominance field (Fig. 3). This indicates that the water-rock interaction is the mechanism responsible for the chemical composition of the waters studied.
In the case of water-rock interactions, the chemical composition of natural water is mostly affected by the types of rocks and minerals that are in contact with it. At this stage, the calculation of the mineral SI is a useful method for predicting the presence of reactive minerals in water. If the calculated SI value for a mineral is less than zero (SI < 0), the water is undersaturated with respect to that mineral. This typically means that this mineral cannot precipitate from solution and should dissolve, if present, into solution to reach equilibrium concentrations. On the other hand, if the SI value for a mineral is greater than zero (SI > 0), the water is saturated with respect to that mineral, and thereby, the mineral will tend to precipitate (Deutsch 1997). The SI for some mineral species calculated by the PHREEQC computer program (Parkhurst and Appelo 1999) showed that the water samples, except two stream water samples, were saturated with respect to calcite (CaCO3) and dolomite [CaMg(CO3)2]. These two water samples (samples 4 and 5 with the highest SO42− levels) were near saturation with respect to these minerals. On the other hand, anhydrite, gypsum, and barite were identified as being undersaturated in the sampled waters. However, the waters containing a prominent amount of sulfate seemed to be weakly undersaturated with respect to barite, unlike the other water samples (Fig. 4). These data suggest that the waters are interacting with carbonate minerals and/or carbonate rocks present in the area. This suggestion is supported by the presence of carbonate minerals (calcite and dolomite) noted in the deposits, in particular, basement carbonate rocks (marbles) reported by Ciftci (2011) in the Kurtulmus and Kotana sites. In addition to carbonate minerals, the waters were saturated with respect to mineral quartz, K-feldspar, illite, kaolinite, gibbsite, diaspore, and boehmite. This means that silicate weathering is also present. Because most samples showed Fe concentrations below the detection limit of the method used (< 10 ppb), SI values for Fe in those samples could not be calculated. On the other hand, calculated SI values for four water samples (5, 8, 15, and 16) having higher iron concentration values than the detection limit show that these waters were saturated with respect to Fe-(hydr)oxides such as hematite, goethite, and magnetite (Fig. 4).
Heavy metals in waters
The concentration of heavy metals in the water samples (main river, streams and springs) taken from the Dereli area and the limit values set by the WHO and USEPA for these metals are represented in Table 2. Additionally, the median contents of some metals in surface water and groundwater taken from Langmuir (1997) are given in this table. The concentration of cobalt (Co) in most of the waters studied was low, generally less than 1 ppb. Only the concentration values in some of the water samples (14, 15, and 16) taken from the Kurtulmus and Kotana sites were higher than those of the other water samples (up to 5.18 ppb). Concerning cobalt, there is no stated limit in the USEPA and WHO standards. However, all of the observed Co concentration values were similar to those of uncontaminated natural waters, stated by Hem (1985), generally not containing more than a few μg/L of Co. In all samples, the Ni and Hg concentrations were below the detection limit of the analytical method used here, and these values were below the limits recommended by the WHO/USEPA. Similarly, the concentration of Cd was under the detection limit in most of the water samples. In the rest of the samples, the values were lower than the WHO- and USEPA-recommended limits. Concentrations of Sb measured in the water samples ranged from nondetectable (detection limit 0.05 ppb) to 0.17 ppb (Table 2).
Barium (Ba) occurs naturally in trace amounts in most surface waters and groundwaters. Gaillardet et al. (2005) reported that river waters of the world contain Ba within the range from 4 to 73 μg/L, with an average of 23 μg/L. The maximum acceptable limits for Ba in drinking water given by the USEPA and WHO are 2000 and 700 μg/L, respectively. The concentrations of Ba in water samples ranged from 4.6 to 54.9 ppb, and these values were generally slightly higher in the waters taken around the Kotana and Kurtulmus sites. However, the concentrations of Ba in the water of the main river fell within the range reported by Gaillardet et al. (2005). None of the water samples contained Ba above the WHO and USEPA limits.
Arsenic (As) is one of the most hazardous and extremely toxic elements. Long-term exposure to high levels of As may cause cancer, including lung, bladder, and skin cancers (Kabata-Pendias and Szteke 2015). Because of its toxic nature, most drinking water standards limit the concentration of As. For instance, the USEPA and WHO standards give an upper limit of 10 μg/L in drinking water. The estimated global average concentration of As in river water is 0.62 μg/L, ranging from 0.1 to 9.5 μg/L (Gaillardet et al. 2005). The concentrations of As in the waters studied were generally very low, rarely exceeding 1 μg/L. The highest value of 3.2 ppb was found in the spring water of the Kurtulmus site (sample 14). However, the As concentrations were all below the limits of USEPA and WHO (Table 2).
Uranium (U) is a naturally occurring toxic heavy metal. It is present in concentrations between 0.1 and 10 μg/L in most natural waters (Hem 1985). The median concentration of U in surface water and groundwater is 0.5 μg/L (Langmuir 1997). The content of U in the surface and spring waters studied varied from 0.07 to 1.88 ppb, and the values were near those stated for natural waters. Water quality standards established by USEPA and WHO gave an upper limit of 30 and 15 μg/L for U, respectively. As shown in Table 2, the U concentration in the all of the water samples studied was below these limit values.
Manganese (Mn) is naturally ubiquitous and an essential element for human and animal life (Hem 1985). The median concentration of manganese in surface waters and groundwaters is approximately 15 μg/L (Langmuir 1997). Smith et al. (1987) reported a median dissolved manganese concentration of 24 μg/L in 286 samples from rivers and streams in the USA. No mandatory limit is defined for this element by the USEPA (2018). However, the secondary drinking water regulation (SDWR) limit for Mn is 0.05 mg/L, but this level is not legally enforceable by the USEPA. The concentrations of Mn in the waters studied ranged from 0.99 to 24 ppb, and these concentrations were below the abovementioned limit values.
Iron (Fe) is the second most abundant metal in the earth’s crust after aluminum (Cardarelli 2018). The amounts of dissolved iron in natural water are mainly related to the pH and Eh of the water. It occurs in two oxidation states, the ferrous form (Fe2+) and the ferric form (Fe3+). In most natural waters, the pH is not low enough to prevent hydroxides from forming, and under oxidizing conditions, practically all the iron is precipitated as ferric (Fe3+) hydroxide. Hence, the most common species of ferric iron in natural waters is ferric hydroxide, Fe(OH)3 (Hem and Cropper 1959). The concentrations of dissolved Fe in the waters of the study area were generally very low, and most of the samples had Fe contents lower than the MDL of 10 ppb (Table 2). This is most likely related to iron (III) (hydr)oxides precipitating in the alkaline pH (Table 1) and oxidation (Eh) range of the waters in the study area. Calculated SI values of four samples (5, 8, 15, and 16) revealed that these samples were saturated with respect to Fe-(hydr)oxides (Fig. 4). The waters taken from points 15 and 16, close to the Kotana and Kurtulmus sites, had slightly higher iron concentrations relative to most other samples (54 and 22 ppb, respectively). The higher iron values in these waters are possibly related to Fe-skarn mineralization, consisting chiefly of pyrrhotite (FeS) and magnetite (Fe3O4), also reported by Ciftci (2011) to occur within the marbles of the metamorphic basement. However, Fe concentrations did not exceed the water guideline value of 300 μg/L set by the USEPA (SDWR) in all waters.
Copper (Cu) is an essential trace metal, and a small amount of Cu is necessary for the proper functioning of organisms and for the activity of different enzymes. However, consuming an excessive amount of it may cause health problems. In natural waters, Cu appears during the dissolution of minerals and is in the range of 1–10 μg/L (Odobasic 2012). Due to the redox conditions of most natural waters, Cu2+ most commonly occurs in surface water and groundwater. In river water, the mean content of copper is 0.007 mg/L (Hitchon et al. 1999). Data from Langmuir (1997) suggest that the median value of Cu in surface water and groundwater is 3 μg/L. The value of Cu concentration in the studied waters varied from 1.5 to 7.7 ppb, and those values were fairly close to the abovementioned Cu values. Additionally, Cu concentrations in all water samples from the study area were below the recommended limits of the USEPA and WHO (Table 2). Like copper, zinc (Zn) is an essential element and has an important role in biological processes in organisms (Odobasic 2012). Only exposure to high levels may pose toxic effects, but intoxication by excessive exposure is rare (Plum et al. 2010). Levels of Zn in surface water and groundwater normally do not exceed 0.01 and 0.05 mg/L, respectively (WHO 2004). The mean value stated by Langmuir (1997) for the Zn concentration in water is 20 μg/L. Zn was determined in the waters in concentrations ranging from 1.9 to 16.3 ppb, and no significant difference was found among the Zn concentrations of spring, stream, and river waters. The concentration of Zn in all the waters of the study area was near that of the Zn concentration observed in natural waters. Additionally, the Zn concentration values do not exceed the USEPA (SDWR) of 5 mg/L.
Lead (Pb) is a toxic and quite common metal. It occurs in the environment mainly as Pb2+ (Kabata-Pendias and Mukherjee 2007). The average Pb2+ concentration of worldwide river water is estimated at 0.08 μg/L, within the range of 0.04–3.8 μg/L (Gaillardet et al. 2005). The median value of Pb noted for surface water and groundwater by Langmuir (1997) is 3 ppb. According to Dutta and Das (2015), both surface water and groundwater rarely contain Pb in excess of 10 μg/L. However, in a Pb mineralization area, the concentration of Pb may be ten-fold that of an unmineralized area. Additionally, depending on the proximity to sources, elevated Pb concentrations in river water are expected (Kabata-Pendias and Mukherjee 2007). The Pb concentration in water samples in Dereli varied from 0.4 to 41.4 ppb. The water samples (from springs, streams, and the main river) collected in the vicinity of the Kurtulmus and Kotana sites (samples 13, 14, 15, 16, 17, and 18) had relatively higher Pb concentrations than that in the other samples (Table 2). According to the typical surface water and groundwater, the waters of these sites were slightly enriched in Pb. Moreover, Pb concentrations in the stream waters of these locations were somewhat higher than the concentrations reported by Runnells et al. (1992) for Pb (between 0.0002 to 0.003 ppm) in stream waters from nonmineralized areas. In addition, the concentrations of Pb in those waters exceeded the limit values of the USEPA and WHO suggested as 15 and 10 μg/L, respectively. This increase could be attributed to the existence of Cu-Pb-Zn mineralizations located near the Kurtulmus and Kotana sites. A study by Monbeshora et al. (1983) reported that river water in Pb-mineralized areas contains Pb up to approximately 45 μg/L. Similarly, some stream and spring waters in the Espiye and Tirebolu areas where Cu-Zn-Pb deposits exist, located in the northeastern part of Dereli, have been reported to contain elevated amounts of Pb (Karakaya and Karakaya 2014).
Factors affecting the heavy metal concentrations in waters of the sampled area
The concentration of metals in natural water is controlled by several factors (pH, Eh, and HCO3−). Among them, pH is a crucial chemical property showing the acidity or alkalinity of water, and it has a direct influence on the concentration of elements in natural waters. The pH of natural waters usually ranges from 6 to 9. However, in some geologic environments with an abundance of iron sulfide minerals (i.e., pyrite) present, sulfuric acid is produced when sulfide minerals come into contact with water and oxygen. Therefore, water existing in this area would be acidic (i.e., have a pH less than 5). Thus, these waters tend to contain higher concentrations of dissolved metals than alkaline water (Sullivan et al. 2005). Furthermore, the oxidation of iron sulfides can enhance the oxidation of other sulfide minerals (such as lead- and copper-bearing minerals), commonly associated with iron sulfides (Blowes et al. 2005). This process can lead to increased sulfate content in waters. Similar to pyrite, other sulfide minerals are capable of producing acid in water (Gray and Sanzalone 1996). However, if the waters are a calcium-bicarbonate type present in mineralized areas, the pH will be alkaline, limiting the water metal content (Miller and McHugh 1994).
A considerable amount of sulfate (up to 69 ppm) was present in the river and stream waters of the study area. Additionally, the concentrations of sulfate in these waters (see Table 1) were fairly higher than the median sulfate value of 3.7 mg/L reported by Langmuir (1997) for natural surface waters. The elevated values of sulfate in waters may be attributed to the oxidation of sulfide minerals (i.e., pyrite, chalcopyrite, etc.) and/or dissolution of sulfate-bearing minerals (such as barite, gypsum, and anhydrite) present naturally in the area. On the other hand, despite the relatively elevated concentrations of sulfate in the spring, stream, and river waters, the waters showed alkaline pH values ranging from 7.60 to 8.35 and do not have a high concentration of metals compared with that in other natural waters. In addition, no clear correlation was observed between pH and the concentrations of other metals (Table 3). Similarly, there was no significant positive or negative correlation between SO42− and concentrations of Pb, Cu, and Zn contained within the present sulfide minerals. In addition, although gypsum and anhydrite are often the source of dissolved sulfate in water (Younger 2007), the absence of any correlation between SO42− and Ca2+ suggests that dissolved sulfate might not be related to the dissolution of gypsum or anhydrite. This suggestion is consistent with the negative SI values calculated for gypsum and anhydrite (Fig. 4). On the other hand, a significant positive correlation (r = 0.71, p < 0.01) exists between SO42− and Ba concentrations. This means that the dissolved sulfate present in the waters is chiefly controlled by the solubility of barite (BaSO4), which is pH independent, as stated by Verplanck et al. (2010).
Meanwhile, in the area, Ca-Mg bicarbonate-type waters are prevalent and the alkalinity values of the water samples were fairly high (ranging from 65 to 415 ppm as HCO3−). In addition, all of the tested waters were saturated or were near saturation with respect to calcite and dolomite. Furthermore, analysis of the correlation (Table 3) between the HCO3− and major ions Mg and Ca revealed that HCO3− is strongly positively correlated with Ca2+ and Mg2+ (r = 0.91 and r = 0.86, respectively, p < 0.01). Moreover, there is a significant positive correlation between Ca2+ and Mg2+ (r = 0.71; p < 0.01), indicating a common source. This reflects that dissolution of carbonate minerals is the primary process controlling water chemistry in this area.
Likewise, from the heavy metals, Pb, Cu, and Mn show a prominent positive correlation with bicarbonate (r = 0.66, r = 0.54, and r = 0.56, respectively). This correlation suggests that bicarbonate ions in the waters play a significant role in controlling the concentrations of dissolved metals such as Pb, Cu, and Mn. Furthermore, a significant positive correlation of Pb with Cu, Mn, and Co (r = 0.59, r = 0.65, and r = 0.62, respectively, p < 0.01) is an indication that those metals could have originated from similar sources.
Conclusions
Elevated levels of heavy metals in natural waters have a negative effect on the quality of the water. The heavy metal concentrations in natural waters are closely associated with the geology of that region, and therefore the concentration of heavy metals in waters of mineralized areas can be naturally high.
In the present study, the heavy metal concentrations in natural waters of an area (Dereli-Giresun) containing mineral deposits were determined. These concentrations were then compared with the concentrations in natural waters and with international water quality standards. Some physical and chemical parameters (such as Eh, pH, and HCO3−) affecting dissolved metal concentrations and the quality of the waters were also investigated. The obtained data indicate that natural waters in the Dereli (Giresun) area are predominantly Ca- and Mg-rich bicarbonate-type waters with alkaline pH values. The chemical composition of waters in the area is mainly controlled by water-rock interactions. The chemistry of waters is considerably affected by carbonate minerals. The waters in the area of Dereli had low heavy metal contents (Pb, Zn, Cu, Fe, Mn, U, Cd, As, Hg, Sb, Ni, Co, and Ba), and heavy metal levels were, in general, quite similar to those of natural waters. The levels of heavy metals in the waters were below the WHO and USEPA permissible limits, except for Pb, which was detected in water samples of the Kotana and Kurtulmus sites. The results of this study indicate that there is currently no significant heavy metal contamination in waters of this area, probably because of the bicarbonate-type waters. This study is the first attempt at determining the levels of heavy metals in natural waters of the Dereli (Giresun) area. It also provides an overview of the geochemical characteristics of waters in this area. Thus, the data of this study can provide baseline information for future studies on the quality of waters in the area.
References
Blowes, D. W., Ptacek, C. J., Jambor, J. L., & Weisener, C. G. (2005). The geochemistry of acid mine drainage. In B. S. Lollar, H. D. Holland, & K. K. Turekian (Eds.), Environmental Geochemistry vol 9: treatise on geochemistry (pp. 149–204). Amsterdam: Elsevier.
Cardarelli, F. (2018) Materials handbook: a concise desktop reference, 3rd. edition, springer, 2254 pages.
Ciarkowska, K., Gambuś, F., Antonkiewicz, J., & Koliopoulos, T. (2019). Polycyclic aromatic hydrocarbon and heavy metal contents in the urban soils in southern Poland. Chemosphere, 229, 214–226.
Ciftci, E. (2011). Sphalerite associated with pyrrhotite-chalcopyrite ore occurring in the Kotana Fe-Skarn deposit (Giresun, NE Turkey): exsolution or replacement. Turkish Journal of Earth Sciences, 20, 307–320.
Ciftci, E., Lermi, A., & Yalçınalp, B. (2015). Ore mineral textures of late cretaceous volcanogenic massive sulfide deposits of Turkey: proposed Paragenetic sequence. In: Dong, F. (Ed) Proceedings of the 11th International Congress for Applied Mineralogy (ICAM). Springer Geochemistry/Mineralogy. Springer, Cham.
Dames and Moore (1983). Environmental baseline studies, Red Dog project. Water quality report, chapter 3, prepared by L.A. Peterson and Associates, Inc., for the Red Dog mine Project, Cominco, Alaska, Inc., Anchorage, Alaska.
Deutsch, W. J. (1997). Groundwater geochemistry: fundamentals and applications to contamination. USA: Lewis Publisher.
Dutta, S., & Das, A.K. (2015). Drinking water. In Guardia M. and Garrigues S. (Ed.), Handbook of mineral elements in Food, (pp 455–471).Wiley Blackwell.
Gaillardet, J., Viers, J., & Dupré, B. (2005). Trace elements in river waters. In Drever, J.I. (Ed.), H.D Holland, K.K. Turekian (executive Ed.), Surface and ground water, weathering, and soils, vol 5, Treatise on geochemistry (pp. 225–272). Netherlands: Elsevier.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science, 170, 1088–1090.
Gray, J.E. & Sanzalone, R.F. (1996). (Ed), Environmental studies of mineral deposits in Alaska: U.S. Geological Survey Bulletin 2156.
Gokce, A., & Bozkaya, G. (2003). Fluid-inclusion and stable-isotope characteristics of the Inler Yaylasi lead-zinc deposits, northern Turkey. International Geology Review, 45, 1044–1054.
Hem, J.D. (1985). Study and interpretation of the chemical characteristics of natural waters 3rd edition, US Geological Survey Water-Supply Paper 2254, University of Virginia, Charlottesville 263 p.
Hem, J. D., & Cropper, W. H. (1959). Chemistry of iron in natural water. A survey of ferrous-ferric chemical equilibria and redox potentials. U. S. Geological Survey Water-Supply Paper, 1459-A, 31.
Hitchon, B., Perkins, E. H., & Gunter, W. D. (1999). Introduction to ground water geochemistry. Sherwood Park, Alberta: Geosciene Publishing Ltd.
Hydrology Project-Technical Assistance (1999) Training module # WQ - 28: Major ions in water. Training notes of the World Bank and Government of Netherlands funded. http://nhp.mowr.gov.in/docs/HP2/MANUALS/WaterQuality/5014/-download-manuals-WaterQuality-WQManuals-28MajorIonsinWater.pdf. Accessed Feb 2016
Kabata-Pendias, A. (2010). Trace elements in soils and plants (4th ed.p. 505). Boca Raton: CRC Press.
Kabata-Pendias, A., & Mukherjee, A. B. (2007). Trace elements from soil to human. Berlin: Springer.
Kabata-Pendias, A., & Szteke, B. (2015). Trace Elements in Abiotic and Biotic Environments (p. 468). Boca Raton: CRC Press.
Karakaya, N., & Karakaya, M. C. (2014). Toxic element contamination in waters from the massive sulfide deposits and wastes around Giresun Turkey. Turkish Journal of Earth Sciences, 23, 113–112.
Langmuir, D. (1997). Aqueous environmental geochemistry. Upper Saddle River NJ Prentice Hall, 600p.
M.T.A 2010. General directorate of mineral research and exploration, Mineral and energy resources in Giresun (http://www.mta.gov.tr). Accessed March 2015.
Miller, W. R., & McHugh, J. B. (1994). Natural acid drainage from altered areas within and adjacent to the upper Alamosa River basin, Colorado. U.S. Geological Survey Open-File Report, 94–144, 47.
Monbeshora, C., Osibanjo, O., & Ajayi, S. O. (1983). Pollution studies on Nigerian rivers: the onset of lead pollution of surface waters in Ibadan. Environment International, 9, 81–84.
Mondal, N. C., Singh, V. S., Puranik, S. C., & Singh, V. P. (2010). Trace element concentration in groundwater of Pesarlanka Island Krishna Delta, India. Environmental Monitoring and Assessment, 163, 215–227.
Odobasic, A. (2012). Water quality monitoring and assessment. In K. Voudouris & D. Voutsa (Eds.), Determination and speciation of trace heavy metals in natural water by DPASV (pp. 429–456). Intech: Croatia.
Plum, L. M., Rink, L., & Haase, H. (2010). The essential toxin: impact of zinc on human health. International Journal of Environmental Research and Public Health, 7, 1342–1365.
Parkhurst, D. L., & Appelo, C. A. J. (1999). User’s guide to PHREEQC (version 2.8)-a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. USGS water resource investigation report, 99–4259, 312.
Piper, A. M. (1944). A graphic procedure in geochemical interpretation of water analyses. Transactions of the American Geophysical Union, 25, 914–923.
Runnells, D. D., Shepherd, T. A., & Angino, E. E. (1992). Metals in water-determining natural background concentrations in mineralized areas. Environmental Science & Technology, 26, 2316–2323.
Saglam, E. S., Akçay, M., Çolak, D. N., Bektas, K. I., & Belduz, A. O. (2016). Generation of acid mine drainage around the Karaerik copper mine (Espiye, Giresun, NE Turkey): implications from the bacterial population in the Acısu effluent. Extremophiles, 20, 673–685.
Smith, R. A., Alexander, R. B., & Wolman, M. G. (1987). Water-quality trends in the nation’s rivers. Science, 235(4796), 1607–1615.
Sullivan, P. J., Agardy, F. J., & Clark, J. J. J. (2005). The environment science of drinking water (368p). Burlington: Elsevier.
USEPA (2018). Drinking water standards and health advisories tables. Office of Water U.S. Environmental Protection Agency Washington, DC March. https://www.epa.gov/dwstandardsregulations/2018-drinking-water-standards-and-advisory-tables
Verplanck, P.L., Nordstrom, D.K., Plumlee, G.S., & Walker, B. (2010). Estimating natural background groundwater chemistry, Questa molybdenum mine, New Mexico, In Morgan, L.A. and Quane, S.L. (Ed.) Through the generations: geologic and anthropogenic field excursions in the Rocky Mountains from modern to ancient, Geological Society of America field guide 18, p. 141–161.
Verplanck, P. L., Nordstrom, D. K., Bove, D. J., Plumblee, G. S., & Runkel, R. L. (2009). Naturally, acidic surface and ground waters draining porphyry-related mineralized areas of the Southern Rocky Mountains, Colorado and New Mexico. Applied Geochemistry, 24, 255–267.
Younger, P. L. (2007). Groundwater in the environment (p. 336). Oxford: An introduction; Blackwell Publishing Ltd..
WHO. (2004). Guidelines for drinking-water quality. In World Health Organization, third edition volume 1. Geneva: Recommendations.
WHO (2008) and (2011). Guidelines for drinking-water quality in third edition (2008) and fourth edition (2011) World Health Organization.
Acknowledgments
The author would like to thank Dr. Sibel Kacmaz of Giresun University for her contributions to the field work. The author would also like to thank the reviewers for their recommendations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kacmaz, H. Assessment of heavy metal contamination in natural waters of Dereli, Giresun: an area containing mineral deposits in northeastern Turkey. Environ Monit Assess 192, 91 (2020). https://doi.org/10.1007/s10661-019-8057-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-8057-0