Abstract
In this article a convenient method for the synthesis of novel piperazine based bis(4-hydroxy-2H-chromen-2-one) derivatives using pyrazine-1,4-diium tricyanomethanide {[1,4-DHPyrazine][C(CN)3]2} as a new nanostructured molten salt (NMS) catalyst has been described. These compounds were synthesized via Mannich type reaction between several aromatic aldehyde, piperazine and 4-hydroxycoumarin under solvent-free condition at room temperature. The NMS catalyst was fully characterized via Fourier transform infrared (FT-IR), nuclear magnetic resonance (1H NMR and 13C NMR), mass spectrometry, thermal gravimetric, derivative thermal gravimetric, differential thermal analysis, X-ray diffraction patterns, scanning electron microscopy and transmission electron microscopy analysis. The new compounds synthesized by using this NMS catalyst were also characterized by FT-IR, 1H NMR and 13C NMR, high-resolution mass spectrometry techniques. The new NMS catalyst simply recovers and can be reused several times without significant loss of catalytic activity. The major advantages of the described method in comparison to the classical reactions are low catalyst loading, short reaction time, high yields, simple isolation of product and reusability of the NMS catalyst.
Graphical Abstract
Pyrazine-1,4-diium tricyanomethanide as a nano molten salt catalyst was designed, synthesized and used for the synthesis of novel biological piperazine based bis(4-hydroxy-2H-chromen-2- one) derivatives as bioactive and drug candidates.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Among heterocyclic compounds, piperazine is a very important substrate in the pharmaceutical industry. Recently, attention has been drawn to considerable benefits of piperazine moieties for their versatile properties in pharmacology and chemistry [1,2,3,4,5,6,7,8,9,10]. Because of the derivatives’ close link to numerous types of biological properties, for example anticancer [11], antiviral [12], anti-HIV [13], antibacterial [14], antifungal [15] and antimalarial [16], piperazine moieties have been investigated widely by the organic chemists.
Molten salts (MSs) and ionic liquids (ILs) are both compounds solely composed of ions. MSs in the narrow concept are classified as high-temperature ILs but both definitions are often used interchangeably. The field of MSs and ILs has attracted an increasing amount of attention in the past decade generally because of their unique and tunable physicochemical properties and their flexibility in numerous uses [17,18,19]. As a unique class of solvents, MSs and ILs have many advantages including negligible vapor pressure, broad liquid ranges and excellent thermal stability. Furthermore, MSs and ILs are often described as ‘designer solvents’ since the combinations of cations and anions can be simply changed, and the resulting MSs and ILs can show preferred physicochemical and solvation properties [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38].
In view of the need to the effective and eco-friendly chemicals and in continuation of our studies on the development of designing, synthesis and application of nanostructured molten salts (NMSs) and ionic liquids (NILs) as a multi-role materials, for extractive desulfurization [39], catalytic organic synthesis [40,41,42], nitration [43, 44], sulfunation [45], anomeric based oxidation [46,47,48] and etc., we have described a convenient method for the synthesis of piperazine based bis(4-hydroxy-2H-chromen-2-one)s under mild conditions (Scheme 1).
2 Experimental
2.1 General Information
All chemicals and solvents were purchased from Merck, Fluka, Sigma-Aldrich and Across Organic Chemical Companies. All reagents were applied without any further purification. Solvents were dried, distilled and stored over molecular sieves. The mass spectra were recorded on MS Model: 5975C VL MSD with Tripe-Axis Detector. 1H NMR and 13C NMR spectra were attained using a Bruker DRX-400 spectrometer at 400 and 100 MHz, respectively by TMS as an internal standard in DMSO-d6. TGA was performed with a Perkin–Elmer TGA at a heating rate of 10 °C min−1 from room temperature to 600 °C. TLC was carried out on UV-active aluminium-backed silica gel plates F254. Melting points were measured with a thermo scientific apparatus and are uncorrected. FT-IR spectra were recorded with a Bruker spectrophotometer in KBr pellets. XRD patterns were recorded with a Bruker D8 ADVANCE diffractometer by Cu Kα radiation. SEM images were obtained with a KYKY-EM3200 and a maximum acceleration voltage of the primary electrons between 20 and 40 kV. TEM images were attained using a Zeiss-EM10C microscope with an accelerating voltage of 100 kV.
2.1.1 General Procedure for the Synthesis of Pyrazine-1,4-diium tricyanomethanide {[1,4-DHPyrazine][C(CN)3]2} as a Nanostructured Molten Salt Catalyst
To 5 mL of an aqueous solution of tricyanomethane (3 mmol: 0.273 g), pyrazine (3 mmol; 0.240 g) was added and the resulting mixture was stirred for 120 min at room temperature. Then the solvent was evaporated under reduced pressure. The white powder was dried under vacuum at 100 °C for 120 min. The achieved white solid was washed and filtered repeatedly with diethyl ether to remove any unreacted starting materials for attaining high purity, and then dried under vacuum. {[1,4-DHPyrazine][C(CN)3]2} was identified via FT-IR, 1H NMR, 13C NMR, mass, TG, DTG, DTA, XRD, SEM and TEM analysis (Scheme 1).
2.1.1.1 Pyrazine-1,4-diium tricyanomethanide {[1,4-DHPyrazine][C(CN)3]2}
White solid; M.p: >350 °C; Yield: 98% (0.771 g); IR (KBr): υ 3470, 2967, 2932, 2864, 2204, 2093, 1662, 1589, 1447, 1071 cm−1; 1H NMR (400 MHz, DMSO-d 6 ): δppm 7.48 (s, 2H, ArH), 8.88 (s, 1H, ―NH); 13C NMR (100 MHz, DMSO-d 6 ): δppm 130.1, 142.0, 152.6; MS: m/z = 262.2 [M]+.
2.1.2 General Procedure for the Synthesis of 3,3′-(piperazine-1,4-diylbis(arylmethylene))bis(4-hydroxy-2H-chromen-2-one) Derivatives
Pyrazine-1,4-diium tricyanomethanide {[1,4-DHPyrazine][C(CN)3]2} as a NMS catalyst (1 mol%; 0.0026 g) was added to a mixture of various aldehyde (1 mmol), 4-hydroxycoumarin (1 mmol; 0.162 g) and piperazine (1 mmol; 0.086 g) under solvent-free conditions at room temperature for an appropriate time. After completion of the reaction which was checked by TLC (n-hexane/ethyl acetate: 5/2), the resulting mixture was washed with water (10 mL) and filtered to separate catalyst from the other materials (the reaction mixture was insoluble in water and catalyst was soluble in water). The water was removed and the crude product was purified by recrystallization from ethanol/water (5:1) to yield pure products.
2.1.3 Spectral Data Analysis for Compounds
2.1.3.1 3,3′-(Piperazine-1,4-diylbis((4-chlorophenyl)methylene))bis(4-hydroxy-2H-chromen-2-one) (Table 4a, 5)
White solid; M.p.: 253–255; Yield: 96%; IR (KBr): υ 3245, 3070, 2972, 2923, 1672, 1610, 1535, 1488, 1399, 1181 cm−1; 1H NMR (400 MHz, DMSO-d6) δppm 8.76 (s, 1H, –OH), 7.91 (d, J = 8.4 Hz, 0.5H, ArH), 7.81 (d, J = 7.7 Hz, 1H, ArH), 7.65 (d, J = 8.4 Hz, 0.5H, ArH), 7.49 (t, J = 7.7 Hz, 2H, ArH), 7.25 (d, J = 9.0 Hz, 1.5H, ArH), 7.20 (d, J = 9.4 Hz, 1.5H, ArH), 7.10 (d, J = 7.7 Hz, 1H, ArH), 6.24 (s, 1H, –CH aliphatic), 3.43 (s, 4H, –CH2); 13C NMR (100 MHz, DMSO-d6) δppm 170.7, 167.6, 155.6, 144.3, 134.2, 132.4, 131.6, 130.7, 127.2, 126.1, 122.8, 118.6, 106.2, 38.8, 21.6; HRMS (ESI): m/z = 654.13244 [M]+.
2.1.3.2 3,3′-(Piperazine-1,4-diylbis((2,5-dimethoxyphenyl)methylene))bis(4-hydroxy-2H-chromen-2-one) (Table 4b, 5)
White solid; M.p.: 244–246; Yield: 93%; IR (KBr): υ 3437, 3354, 3084, 2947, 1681, 1608, 1488, 1399, 1277 cm−1; 1H NMR (400 MHz, DMSO-d6) δppm 7.78 (d, J = 7.9 Hz, 1H, ArH), 7.44 (t, J = 7.7 Hz, 2H, ArH), 7.19 (d, J = 7.9 Hz, 2H, ArH), 7.16 (s, 1H, –OH), 6.77 (s, 1H, ArH), 6.69 (d, J = 8.7 Hz, 0.5H, ArH), 6.61 (d, J = 8.7 Hz, 0.5H, ArH), 6.16 (s, 1H, –CH aliphatic), 3.57 (s, 3H, –OCH3), 3.44 (s, 3H, –OCH3), 2.87 (s, 4H, –CH2); 13C NMR (100 MHz, DMSO-d6) δppm 170.2, 167.0, 155.6, 155.4, 154.7, 135.9, 133.6, 127.0, 125.8, 123.2, 119.8, 118.4, 114.9, 112.1, 106.6, 59.3, 58.1, 46.3, 35.9; HRMS (ESI): m/z = 706.25265 [M]+.
2.1.3.3 3,3′-(Piperazine-1,4-diylbis(phenylmethylene))bis(4-hydroxy-2H-chromen-2-one) (Table 4c, 5)
White solid; M.p.: 260–262; Yield: 91%; IR (KBr): υ 3445, 3010, 1681, 1634, 1612, 1562, 1402, 1199 cm−1; 1H NMR (400 MHz, DMSO-d6) δppm 8.50 (s, 1H, –OH), 7.79 (d, J = 7.6 Hz, 1H, ArH), 7.48 (t, J = 7.0 Hz, 2H, ArH), 7.24 (d, J = 8.7 Hz, 2H, ArH), 7.20 (d, J = 7.6 Hz, 1H, ArH), 7.14 (t, J = 7.6 Hz, 1H, ArH), 7.07 (t, J = 7.0 Hz, 2H, ArH), 6.25 (s, 1H, –CH aliphatic), 3.22 (s, 4H, –CH2); 13C NMR (100 MHz, DMSO-d6) δppm 170.8, 167.7, 155.6, 145.4, 134.0, 130.7, 129.7, 127.9, 127.2, 125.9, 123.0, 118.5, 106.5, 43.7, 39.2; HRMS (ESI): m/z = 586.21039 [M]+.
2.1.3.4 3,3′-(Piperazine-1,4-diylbis(naphthalen-1-ylmethylene))bis(4-hydroxy-2H-chromen-2-one) (Table 4d, 5)
Yellow solid; M.p.: 227–229; Yield: 96%; IR (KBr): υ 3442, 3049, 2955, 1668, 1605, 1527, 1397, 1032 cm−1; 1H NMR (400 MHz, DMSO-d6) δppm 8.00 (s, 1H, –OH), 7.80 (t, J = 7.6 Hz, 1H, ArH), 7.75 (d, J = 7.6 Hz, 1H, ArH), 7.66 (d, J = 8.1 Hz, 1H, ArH), 7.49 (d, J = 8.2 Hz, 1H, ArH), 7.45 (d, J = 8.3 Hz, 1H, ArH), 7.34 (t, J = 7.6 Hz, 2H, ArH), 7.23 (d, J = 8.2 Hz, 2H, ArH), 7.18 (t, J = 7.5 Hz, 2H, ArH), 6.72 (s, 1H, –CH aliphatic), 2.86 (s, 4H, –CH2); 13C NMR (100 MHz, DMSO-d6) δppm 171.4, 167.2, 155.4, 141.6, 136.7, 134.7, 133.9, 131.5, 129.1, 128.8, 128.4, 128.0, 127.9, 127.3, 127.1, 126.0, 123.0, 118.5, 107.2, 46.4, 38.0; HRMS (ESI): m/z = 686.24169 [M]+.
2.1.3.5 3,3′-(Piperazine-1,4-diylbis((4-nitrophenyl)methylene))bis(4-hydroxy-2H-chromen-2-one) (Table 4e, 5)
Cream solid; M.p.: 271–273; Yield: 98%; IR (KBr): υ 3443, 3082, 2909, 1676, 1603, 1516, 1406, 1347, 1181 cm−1; 1H NMR (400 MHz, DMSO-d6) δppm 8.05 (d, J = 7.8 Hz, 1H, ArH), 7.79 (d, J = 7.8 Hz, 1H, ArH), 7.51 (t, J = 7.7 Hz, 2H), 7.34 (d, J = 7.4 Hz, 1H, ArH), 7.27 (s, 1H, –OH), 7.25 (d, J = 5.1 Hz, 1H, ArH), 7.21 (d, J = 7.5 Hz, 2H, ArH), 6.33 (s, 1H, –CH aliphatic), 2.94 (s, 4H, –CH2); 13C NMR (100 MHz, DMSO-d6) δppm 171.0, 167.4, 155.6, 154.6, 148.3, 134.3, 130.9, 127.2, 126.2, 126.1, 122.8, 118.6, 105.8, 45.7, 39.8; HRMS (ESI): m/z = 676.18054 [M]+.
2.1.3.6 3,3′-(-piperazine-1,4-diylbis(2-methyl-3-phenylprop-2-ene-1,1-diyl))bis(4-hydroxy-2H-chromen-2-one) (Table 4f, 5)
Cream solid; M.p.: 203–205; Yield: 91%; IR (KBr): υ 3446, 3058, 2968, 1709, 1634, 1601, 1514, 1414, 1107 cm−1; 1H NMR (400 MHz, DMSO-d6) δppm 7.83 (d, J = 8.0 Hz, 0.5H, ArH), 7.73 (d, J = 7.0 Hz, 0.5H, ArH), 7.63 (d, J = 8.8 Hz, 0.5H, ArH), 7.57 (d, J = 8.6 Hz, 0.5H, ArH), 7.46 (s, 1H, –OH), 7.36 (t, J = 7.0 Hz, 1H, ArH), 7.29 (t, J = 7.6 Hz, 2H, ArH), 7.20 (t, J = 7.0 Hz, 2H, ArH), 7.12 (d, J = 8.0 Hz, 1H, ArH), 7.03 (d, J = 7.5 Hz, 0.5H, ArH), 7.00 (d, J = 7.8 Hz, 0.5H, ArH), 6.30 (s, 1H, –CH aliphatic), 3.75 (s, 1H, –CH), 2.81 (s, 4H, –CH2), 1.34 (s, 3H, –CH3); 13C NMR (100 MHz, DMSO-d6) δppm 162.5, 156.6, 155.6, 155.3, 135.3, 132.1, 131.8, 131.0, 130.9, 130.6, 127.7, 125.8, 125.2, 119.5, 118.5, 118.2, 115.6, 47.0, 22.3, 17.6; HRMS (ESI): m/z = 666.27299 [M]+.
2.1.3.7 3,3′-(Piperazine-1,4-diylbis((3-nitrophenyl)methylene))bis(4-hydroxy-2H-chromen-2-one) (Table 4g, 5)
Cream solid; M.p.: 238–240; Yield: 96%; IR (KBr): υ 3451, 3070, 1678, 1607, 1528, 1398, 1348, 1276, 1107 cm−1; 1H NMR (400 MHz, DMSO-d6) δppm 7.97 (d, J = 8.0 Hz, 0.5H, ArH), 7.87 (s, 1H, –OH), 7.80 (d, J = 7.8 Hz, 1H, ArH), 7.55 (t, J = 7.6 Hz, 1H, ArH), 7.50 (d, J = 7.0 Hz, 1H, ArH, ArH), 7.46 (d, J = 7.9 Hz, 0.5H, ArH), 7.27 (d, J = 8.2 Hz, 1H, ArH), 7.22 (t, J = 7.5 Hz, 2H, ArH), 6.34 (s, 1H, ArH), 5.73 (s, 1H, –CH aliphatic), 2.87 (s, 4H, –CH2); 13C NMR (100 MHz, DMSO-d6) δppm 171.0, 167.4, 155.6, 150.8, 148.2, 136.9, 134.3, 132.4, 127.2, 126.1, 124.1, 123.4, 122.7, 118.7, 105.7, 46.2, 39.3; HRMS (ESI): m/z = 676.18054 [M]+.
2.1.3.8 3,3′-(Piperazine-1,4-diylbis((4-hydroxyphenyl)methylene))bis(4-hydroxy-2H-chromen-2-one) (Table 4h, 5)
Yellow solid; M.p.: 215–217; Yield: 92%; IR (KBr): υ 3273, 3164, 3035, 2969, 1678, 1638, 1607, 1514, 1399, 1054 cm−1; 1H NMR (400 MHz, DMSO-d6) δppm 8.90 (s, 1H, –OH), 7.79 (d, J = 7.6 Hz, 1H, ArH), 7.47 (t, J = 7.4 Hz, 2H, ArH), 7.22 (d, J = 7.9 Hz, 2H, ArH), 7.19 (d, J = 7.6 Hz, 1H, ArH), 6.86 (d, J = 7.3 Hz, 1H, ArH), 6.53 (d, J = 6.1 Hz, 1H, ArH), 6.14 (s, 1H, –CH aliphatic), 4.33 (s, 1H, –OH), 2.90 (s, 4H, –CH2); 13C NMR (100 MHz, DMSO-d6) δppm 170.6, 167.6, 157.7, 155.5, 135.3, 133.8, 130.6, 127.1, 125.9, 123.1, 118.5, 117.5, 106.9, 46.0, 38.4; HRMS (ESI): m/z = 618.20022 [M]+.
2.1.3.9 3,3′-(Piperazine-1,4-diylbis((4-(dimethylamino)phenyl)methylene))bis(4-hydroxy-2H-chromen-2-one) (Table 4i, 5)
Pink solid; M.p.: 233–235; Yield: 93%; IR (KBr): υ 3425, 3167, 2968, 1677, 1631, 1610, 1513, 1391, 1181 cm−1; 1H NMR (400 MHz, DMSO-d6) δppm 7.79 (d, J = 7.8 Hz, 2H, ArH), 7.47 (t, J = 7.0 Hz, 1H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.18 (s, 1H, –OH), 6.88 (d, J = 8.4 Hz, 2H, ArH), 6.54 (d, J = 8.7 Hz, 2H, ArH), 6.15 (s, 1H, –CH aliphatic), 2.93 (s, 4H, –CH2), 2.48 (s, 6H, –CH3); 13C NMR (100 MHz, DMSO-d6) δppm 170.6, 167.7, 155.5, 151.3, 133.8, 133.2, 130.3, 127.1, 125.8, 123.1, 118.4, 115.5, 106.9, 45.7, 43.6, 38.2; HRMS (ESI): m/z = 672.29479 [M]+.
2.1.3.10 3,3′-(Piperazine-1,4-diylbis(furan-2-ylmethylene))bis(4-hydroxy-2H-chromen-2-one) (Table 4j, 5)
Brown solid; M.p.: 324–326; Yield: 90%; IR (KBr): υ 3437, 3064, 1674, 1605, 1519, 1415, 1212 cm−1; 1H NMR (400 MHz, DMSO-d6) δppm 7.79 7.85 (s, 1H, –OH), 7.81 (d, J = 7.9 Hz, 1H, ArH), 7.48 (t, J = 7.6 Hz, 1H, ArH), 7.33 (d, J = 7.7 Hz, 1H, ArH), 7.21 (t, J = 7.6 Hz, 2H, ArH), 7.15 (d, J = 8.9 Hz, 1H, ArH), 7.10 (d, J = 8.0 Hz, 1H, ArH), 5.87 (s, 1H, –CH aliphatic), 3.05 (s, 4H, –CH2); 13C NMR (100 MHz, DMSO-d6) δppm 181.0, 158.1, 155.5, 150.1, 143.6, 136.2, 134.0, 130.9, 127.2, 126.0, 118.5, 112.9, 107.8, 34.1, 21.4; HRMS (ESI): m/z = 566.16892 [M]+.
2.1.3.11 3,3′-(Piperazine-1,4-diylbis(thiophen-2-ylmethylene))bis(4-hydroxy-2H-chromen-2-one) (Table 4k, 5)
Brown solid; M.p.: 242–244; Yield: 90%; IR (KBr): υ 3445, 3094, 2923, 1673, 1608, 1557, 1400, 1183 cm−1; 1H NMR (400 MHz, DMSO-d6) δppm 7.82 (d, J = 7.8 Hz, 1H, ArH), 7.49 (t, J = 7.7 Hz, 2H, ArH), 7.24 (d, J = 3.3 Hz, 1H, ArH), 7.21 (d, J = 7.6 Hz, 1H, ArH), 7.11 (d, J = 5.0 Hz, 1H, ArH), 6.77 (t, J = 7.6 Hz, 1H, ArH), 6.56 (s, 1H, –OH), 6.39 (s, 1H, –CH aliphatic), 2.89 (s, 4H, –CH2); 13C NMR (100 MHz, DMSO-d6) δppm 170.9, 167.1, 155.5, 151.2, 134.1, 129.2, 127.3, 126.1, 126.0, 125.7, 122.9, 118.5, 106.8, 46.13, 36.0; HRMS (ESI): m/z = 598.12323 [M]+.
2.1.3.12 3,3′-(Piperazine-1,4-diylbis((3-hydroxyphenyl)methylene))bis(4-hydroxy-2H-chromen-2-one) (Table 4l, 5)
Yellow solid; M.p.: 221–223; Yield: 90%; IR (KBr): υ 3379, 3069, 2963, 1663, 1610, 1559, 1534, 1401, 1107 cm−1; 1H NMR (400 MHz, DMSO-d6) δppm 8.88 (s, 1H, –OH), 7.80 (d, J = 7.7 Hz, 1H, ArH), 7.48 (t, J = 8.3 Hz, 2H, ArH), 7.34 (s, 1H, –OH), 7.23 (d, J = 7.9 Hz, 1H, ArH), 7.19 (d, J = 7.5 Hz, 1H, ArH), 6.90 (t, J = 7.8 Hz, 1H, ArH), 6.54 (s, 1H, ArH), 6.49 (d, J = 7.8 Hz, 0.5H, ArH), 6.43 (d, J = 7.9 Hz, 0.5H, ArH), 6.16 (s, 1H, –CH aliphatic), 2.87 (s, 4H, –CH2); 13C NMR (100 MHz, DMSO-d6) δppm 170.7, 167.6, 160.0, 155.6, 147.0, 133.9, 131.5, 127.2, 125.9, 123.1, 120.5, 118.5, 116.8, 114.9, 106.5, 46.2, 39.1; HRMS (ESI): m/z = 618.20022 [M]+.
2.1.3.13 3,3′-(Piperazine-1,4-diylbis(pyridin-4-ylmethylene))bis(4-hydroxy-2H-chromen-2-one) (Table 4 m, 5)
Yellow solid; M.p.: 254–256; Yield: 90%; IR (KBr): υ 3419, 3069, 2957, 1673, 1605, 1540, 1452, 1411, 1182 cm−1; 1H NMR (400 MHz, DMSO-d6) δppm 8.31 (d, J = 5.8 Hz, 2H, ArH), 7.79 (d, J = 7.0 Hz, 2H, ArH), 7.50 (t, J = 7.3 Hz, 1H, ArH), 7.25 (t, J = 7.6 Hz, 1H, ArH), 7.20 (s, 1H, –OH), 7.11 (d, J = 4.4 Hz, 2H, ArH), 6.25 (s, 1H, –CH aliphatic), 2.87 (s, 4H, –CH2); 13C NMR (100 MHz, DMSO-d6) δppm 170.9, 167.4, 155.6, 151.9, 134.3, 131.2, 127.2, 126.1, 125.5, 122.7, 118.6, 105.2, 46.2, 39.2; MS: m/z = 588.20 [M]+.
2.1.3.14 3,3′-(Piperazine-1,4-diylbis((2,4-dichlorophenyl)methylene))bis(4-hydroxy-2H-chromen-2-one) (Table 4n, 5)
Cream solid; M.p.: 218–220; Yield: 95%; IR (KBr): υ 3434, 3064, 2960, 1672, 1606, 1532, 1466, 1400, 1329, 1182 cm−1; 1H NMR (400 MHz, DMSO-d6) δppm 7.79 (d, J = 7.5 Hz, 1H, ArH), 7.48 (t, J = 7.3 Hz, 3H, ArH), 7.39 (s, 1H, ArH), 7.36 (s, 1H, –OH), 7.26 (d, J = 13.0 Hz, 2H, ArH), 7.20 (d, J = 8.1 Hz, 1H, ArH), 6.10 (s, 1H, –CH aliphatic), 2.88 (s, 4H, –CH2); 13C NMR (100 MHz, DMSO-d6) δppm 170.8, 166.8, 155.5, 142.7, 136.5, 134.7, 134.0, 133.6, 131.7, 129.2, 127.1, 126.0, 122.8, 118.5, 105.4, 46.2, 38.9; MS: m/z = 722.05 [M]+.
2.1.3.15 3,3′-(Piperazine-1,4-diylbis((3,4-dimethoxyphenyl)methylene))bis(4-hydroxy-2H-chromen-2-one) (Table 4o, 5)
White solid; M.p.: 269–271; Yield: 93%; IR (KBr): υ 3133, 3032, 2957, 1674, 1654, 1623, 1513, 1182 cm−1; 1H NMR (400 MHz, DMSO-d6) δppm 7.80 (d, J = 7.6 Hz, 1H, ArH), 7.47 (t, J = 7.6 Hz, 2H, ArH), 7.24 (d, J = 4.4 Hz, 1H, ArH), 7.21 (d, J = 3.7 Hz, 1H, ArH), 7.18 (s, 1H, ArH), 6.74 (d, J = 8.3 Hz, 0.5H, ArH), 6.64 (d, J = 4.2 Hz, 0.5H, ArH), 6.60 (s, 1H, –CH aliphatic), 3.66 (s, 3H, –OCH3), 3.50 (s, 3H, –OCH3), 2.95 (s, 4H, –CH2); 13C NMR (100 MHz, DMSO-d6) δppm 170.7, 167.6, 155.5, 151.3, 149.6, 138.0, 133.9, 127.1, 125.9, 123.0, 122.0, 118.5, 114.6, 114.5, 106.7, 58.6, 58.5, 46.0, 38.8; MS: m/z = 706.25 [M]+.
3 Results and Discussion
In this paper, we designed and synthesized a good range of novel 3,3′-(piperazine-1,4-diylbis(arylmethylene))bis(4-hydroxy-2H-chromen-2-one)s via condensation reaction between piperazine, 4-hydroxycoumarin and various aldehydes in the presence of pyrazine-1,4-diium tricyanomethanide {[1,4-DHPyrazine][C(CN)3]2} as a new nanostructured molten salt (NMS) catalyst under solvent-free conditions at room temperature (Scheme 1).
3.1 Characterization of Pyrazine-1,4-diium tricyanomethanide {[1,4-DHPyrazine][C(CN)3]2} as a Nanostructured Molten Salt Catalyst
The structure of {[1,4-DHPyrazine][C(CN)3]2} as a NMS catalyst was studied and fully characterized by FT-IR, 1H NMR, 13C NMR, mass, TG, DTG, DTA, XRD, SEM and TEM analysis.
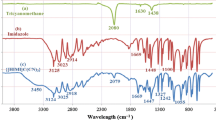
In the FT-IR spectrum of the new NMS catalyst, {[1,4-DHPyrazine][C(CN)3]2}, the absorption band at 1662 cm−1 is linked to vibrational modes of C=N bonds of tricyanomethanide counter ion. Moreover, the identified peak at 2204 and 2093 cm−1 related to C≡N stretching group on tricyanomethanide counter ion. Also, the known peak at 3470 cm−1 is assigned to N–H stretching of pyrazine-1,4-diium. The changes in FT-IR spectrum of {[1,4-DHPyrazine][C(CN)3]2} in comparison with tricyanomethane and pyrazine is a confirmation of production of NMS catalyst (Fig. 1).
The 1H NMR spectrum of the catalyst {[1,4-DHPyrazine][C(CN)3]2} shows two singlet peaks at 7.48 and 8.88 ppm that can be assigned to the aromatic ring protons of pyrazine-1,4-diium and N–H group on pyrazine-1,4-diium, respectively (Fig. 2).
Presence of three signals in the 13C NMR spectrum are also in agreement with the structure of {[1,4-DHPyrazine][C(CN)3]2} catalyst. The important peaks of 13C NMR spectrum of the NMS catalyst are the peak at δ = 152.6 which is related to the C≡N group of tricyanomethanide counter ion and the peak at δ = 130.1 ppm that is assigned to the carbon connected to the three cyanide groups (–C(CN)3). Furthermore, the known peak at 142.0 ppm is related to aromatic ring carbons of pyrazine-1,4-diium (Fig. 3). From all the changes in the carbon and proton NMR chemical shifts, the conclusion can be drawn that the new NMS catalyst has been successfully prepared (Figs .S1 and S2).
The mass spectrum (EI, 70 eV) of {[1,4-DHPyrazine][C(CN)3]2} catalyst was in good agreement with what we expected. The molecular ion peak (M+) was detected at m/z = 262.2 which is equivalent to the catalyst molecular weight. Additionally, the peaks at m/z = 90.0 and m/z = 82.1 corresponded to the tricyanomethanide and pyrazine-1,4-diium fragments, respectively (Fig. 4).
Figures 5 and 6 display the thermal behaviour of our new NMS catalyst. Thermal analysis (TGA/DTG/DTA) of the {[1,4-DHPyrazine][C(CN)3]2} shows two peaks (The DTA analysis diagram is downward because of the exothermic decomposition of NMS catalyst) between room temperature and 450 °C. The mechanism proposed in the thermal decomposition involves the breakdown of the compound in the range of 190 to 305 °C and the subsequent removal of the surface-adsorbed solvent and counter ion. The initial weight loss is about 45%. The second weight loss region between 360 and 450 °C is attributed to the continuing decomposition of organic compound. The weight loss in this step is about 55%. No higher weight loss was observed at higher temperatures.
The size, shape and morphology of {[1,4-DHPyrazine][C(CN)3]2} were characterized using XRD pattern, SEM and TEM analysis. X-ray diffraction pattern of {[1,4-DHPyrazine][C(CN)3]2} was obtained in solid state form (Fig. 7). The eight XRD characteristic peaks at 23.60°, 27.30°, 30.70°, 31.70°, 39.00°, 46.10°, 63.70° and 75.60°, indicate the crystallographic planes of the NMS catalyst, can be obviously detected. The corresponding XRD data are given in Table 1. Usually, solid materials are classified as being either crystalline or amorphous. In crystalline materials the ions occupy specific locations in a regular lattice. The results of investigations of XRD analysis display the NMS catalyst as nanometer-sized crystallites. The average crystallite diameter was calculated via the Scherrer equation [D = Kλ/(β cosθ)] by the peak broadening (FWHM) of the most intense diffraction peak (27.30°). The SEM and TEM images in Fig. 8 show that the NMS particles have a rather spherical morphology with good monodispersity. It can be noted that a fair uniform dispersion of small particles around 25–72 nm of NMS catalyst has been obtained through our synthesis method. An average crystallite size of 12.78 nm can be reported according to XRD pattern, SEM and TEM images and histogram of TEM analysis.
3.2 Application of {[1,4-DHPyrazine][C(CN)3]2} Catalyst in the Synthesis of 3,3′-(piperazine-1,4-diylbis(arylmethylene))bis(4-hydroxy-2H-chromen-2-one) Derivatives
In order to investigate the catalytic activity of {[1,4-DHPyrazine][C(CN)3]2}, the condensation reaction of 4-chlorobenzaldehyde, 4-hydroxycoumarin and piperazine as a model reaction was performed under solvent-free condition using different amounts of various catalysts (Table 2). As is obvious by the results, {[1,4-DHPyrazine][C(CN)3]2} is the most efficient catalyst in terms of yield of product, while the other catalysts lead to product with yields of 60–90%.

In order to optimize the reaction condition, different experimental parameters were tested, for the model reaction in the presence of various amount of the new NMS catalyst. The amount of the catalyst and temperature were optimized under solvent-free conditions. As seen from Tables 3 and 1 mol% (0.0026 g) of NMS was found to be the optimum amount of the catalyst (Table 3, entries 7 and 8). There was no significant change in the yield of products when larger amounts of NMS catalyst were applied (Table 3, entries 10–12). The effect of temperature was also investigated by performing the model reaction at various temperatures (room temperature, 50 and 100 °C) while the amount of NMS catalyst has been kept constant. The best result is achieved at room temperature (Table 3, entry 7). It is worth mentioning that under catalyst-free condition, such a synthesis was not possible (Table 3, entries 1–3).

To display the applicability of the synthesized NMS catalyst in different organic reactions, {[1,4-DHPyrazine][C(CN)3]2} was applied to the synthesis of 3,3′-(piperazine-1,4-diylbis(arylmethylene))bis(4-hydroxy-2H-chromen-2-one) derivatives at room temperature. Again the reaction between 4-chlorobenzaldehyde, 4-hydroxycoumarin and piperazine was chosen as a model, and this model reaction was examined in common organic solvents with varying polarities (Table 4). We found that the reaction proceeded readily in polar solvents (Table 4, entries 2 and 3), but the product yields decreased by decreasing the solvent polarities (Table 4, entries 5–8). The results show that the best efficacy and the highest yield are attained under solvent-free conditions as a green approach (Table 4, entry 1).

To study the efficacy and applicability of this NMS catalyst in the synthesis of 3,3′-(piperazine-1,4-diylbis(arylmethylene))bis(4-hydroxy-2H-chromen-2-one) derivatives, the reaction was extended to other substituted aldehydes at room temperature under solvent-free condition. The results are summarized in Table 5. In all cases, the reaction proceeds readily to give the corresponding products in good to excellent yields (90–98%) in short reaction time. The results show that aldehydes with electron-donating substituents afford lower yields as compared to the ones with electron-withdrawing substituents. Also, hetero-aromatic aldehydes took longer time than aromatic aldehydes and provide lower yield of corresponding products.

A possible mechanism for the production of 3,3′-(piperazine-1,4-diylbis(arylmethylene))bis(4-hydroxy-2H-chromen-2-one) derivatives (4) has been proposed in Scheme 2. The mechanism of this reaction is similar to a Mannich-based pseudo-five-component reaction, actually a bidirectional three-component reaction of type ABBCC [49,50,51,52]. Firstly, {[1,4-DHPyrazine][C(CN)3]2} as a NMS catalyst activates the carbonyl group of the aromatic aldehyde (1) to provide intermediate (5). The nucleophilic attack of piperazine (2) to intermediate (5) was carried out to form the intermediate (6). Then, 4-hydroxycoumarin (3) perform nucleophilic attack to intermediate (6) and gives the 3,3′-(piperazine-1,4-diylbis(arylmethylene))bis(4-hydroxy-2H-chromen-2-one) derivatives (4) via the elimination of two water molecule and tautomerization.
We further assessed the reusability of the NMS catalyst in the synthesis of 3,3′-(piperazine-1,4-diylbis(arylmethylene))bis(4-hydroxy-2H-chromen-2-one)s, because it is crucial to approve that the highly active catalyst is recyclable [53]. We checked the reusability of the catalyst by scaling up to 10 mmol of each substrate using 10 mol% of NMS catalyst at room temperature under solvent-free condition. The selected model reaction between 4-chlorobenzaldehyde, 4-hydroxycoumarin and piperazine was repeated as far as yield didn’t go lower than 90%. At the end of each repeated reaction, the catalyst is separated by filtration and then washed with ethyl acetate. As seen, from Fig. 9, the NMS catalyst can be reused up to six times without important loss of activity. The structure of reused NMS catalyst was also confirmed by FT-IR after its application in the reaction. Also, the size and morphology of reused catalyst was investigated by SEM and TEM analyses. These studies showed that the catalyst was recovered in nano size (Figs. S100–S102).
4 Conclusions
A novel nanostructured molten salt {[1,4-DHPyrazine][C(CN)3]2} (NMS) was produced. The molecular structure of NMSs was fully characterized by using various techniques including FT-IR, 1H NMR, 13C NMR, mass, TGA, DTG, DTA, XRD, SEM and TEM analysis. The described NMSs was used as an efficient and recyclable catalyst for the synthesis of novel 3,3′-(piperazine-1,4-diylbis(arylmethylene))bis(4-hydroxy-2H-chromen-2-one) derivatives. The NMS catalyst could be reused for six consecutive cycles without loss of its activity. The combination of advantages showed by NMS catalyst, for example stability, reasonable catalytic activity and reusability, means that this catalyst may be investigated as a viable alternative in organic reactions in environmental and economic fields.
References
Edlund C, Oh H, Nord CE (1999) Clin Microbiol Infect 1:51–53
Chaudhary P, Kumar R, Verma AK, Singh D, Yadav V, Chhillar AK, Sharma GL, Chandra R (2006) Bioorg Med Chem 14:1819–1828
Zhao HY, Prosser AR, Liottaa DC, Wilson LJ (2015) Bioorg Med Chem Lett 25:4950–4955
Kumar A, Gupta MK, Kumar M (2011) Tetrahedron Lett 52:4521–4525
Pallavi R, Saidulu K, Javed I, Srinivas O (2012) Tetrahedron Lett 53:5314–5317
Chhanda M, Sunil R, Ray J (2012) Synth Commun 42:3077–3088
Ghosh PP, Das AR (2012) Tetrahedron Lett 53:3140–3143
Hatnapure GD, Keche AP, Rodge AH, Birajdar SS, Tale RH, Kamble VM (2012) Bioorg Med Chem Lett 22:6385–6390
Beyeh NK, Valkonen A (2010) Org Lett 12:1392–1395
Long JZ, Jin X, Adibekian A, Li WW, Cravatt BF (2010) J Med Chem 53:1830–1842
Lee YB, Gong YD, Yoon H, Ahn CH, Jeon MK, Kong JY (2010) Bioorg Med Chem 18:7966–7974
Dou D, He G, Mandadapu SR, Aravapalli S, Kim Y, Chang KO, Groutas WC (2012) Bioorg Med Chem Lett 22:377–379
Patel RV, Kumari P, Rajani DP, Pannecouque C, De Clercq E, Chikhalia KH (2012) Future Med Chem 4:1053–1065
Patel RV, Kumari P, Rajani DP (2012) J Enzyme Inhib Med Chem 27:370–374
Xu J, Cao Y, Zhang J, Yu S, Zou Y, Chai X, Wu Q, Zhang D, Jiang Y, Sun Q (2011) Eur J Med Chem 46:3142–3148
Ibezim E, Duchowicz PR, Ortiz EV, Castro EA (2012) Chemometr Intell Lab 110:81–88
Johnson KE (2007) Electrochem Soc Interface 16:38–43
Adams DJ, McDonald IR (1974) J Phys C Solid State Phys 7:2761–2773
Wilkes JS, Mamantov G, Marassi R (1987) Vol 200. D Reidel Co, Dordrecht, p 217
Wasserscheid P, Keim W (2009) Angew Chem Int Ed 39:3772–3789
Taheri A, Lai B, Cheng C, Gu Y (2015) Green Chem 17:812–816
Taheri A, Liu C, Lai B, Cheng C, Pan X, Gu Y (2014) Green Chem 16:3715–3719
Taheri A, Pan X, Liu C, Gu Y (2014) ChemSusChem 7:2094–2100
García-Verdugo E, Altava B, Burguete MI, Lozano P, Luis SV (2015) Green Chem 17:2693–2713
Luska KL, Migowski P, Leitner W (2015) Green Chem 17:3195–3206
Zhu S, Wu Y, Chen Q, Yu Z, Wang C, Jin S, Ding Y, Wu G (2006) Green Chem 8:325–327
Van Rantwijk F, Lau RM, Sheldon RA (2003) Trends Biotechnol 21:131–138
Dupont J, Fonseca GS, Umpierre AP, Fichtner PFP, Teixeira SR (2002) J Am Chem Soc 124:4228–4229
Reichardt C (2007) Org Process Res 11:105–113
Sundermeyer W (1965) Angew Chem Int Ed Engl 4:222–238
Parvulescu VI, Hardacre C (2007) Chem Rev 107:2615–2665
Welton T (1999) Chem Rev 99:2071–2083
Lei Z, Dai C, Chen B (2014) Chem Rev 114:1289–1326
Hayes R, Gregory G, Warr GG, Atkin R (2015) Chem Rev 115:6357–6426
Amarasekara AS (2016) Chem Rev 116:6133–6183
Egorova KS, Ananikov VP (2014) ChemSusChem 7:336–360
Frade RFM, Afonso CAM (2010) Hum Exp Toxicol 29:1038–1054
Jastorff B, Störmann R, Ranke J, Mölter K, Stock F, Oberheitmann B, Hoffmann W, Hoffmann J, Nüchter M, Ondruschka B, Filser J (2003) Green Chem 5:136–142
Kianpour E, Azizian S, Yarie M, Zolfigol MA, Bayat M (2016) Chem Eng J 295:500–508
Zolfigol MA, Yarie M, Baghery S (2016) Synlett 27:1418–1422
Zolfigol MA, Mansouri N, Baghery S (2016) Synlett 27:1511–1515
Ghaderi H, Zolfigol MA, Bayat Y, Zarei M, Noroozizadeh E (2016) Synlett 27:2246–2250
Zolfigol MA, Khazaei A, Moosavi-Zare AR, Zare A, Kruger HG, Asgari Z, Khakyzadeh V, Kazem-Rostami M (2012) J Org Chem 77:3640–3645
Moosavi-Zare AR, Zolfigol MA, Zarei M, Noroozizadeh E, Beyzavi MH (2016) RSC Adv 6:89572–89577
Moosavi-Zare AR, Zolfigol MA, Noroozizadeh E (2016) Synlett 27:1682–1684
Zolfigol MA, Afsharnadery F, Baghery S, Salehzadeh S, Maleki F (2015) RSC Adv 5:75555–75568
Zolfigol MA, Khazaei A, Alaie S, Baghery S, Maleki F, Bayat Y, Asghari A (2016) RSC Adv 6:58667–58679
Zolfigol MA, Kiafar M, Yarie M, Taherpour A, Saeidirad M (2016) RSC Adv 6:50100–50111
Verkade JMM, Van Hemert LJC, Quaedflieg PJLM, Rutjes FPJT (2008) Chem Soc Rev 37:29–41
Hayashi Y, Urushima T, Shin M, Shoji M (2005) Tetrahedron 61:11393–11404
Zolfigol MA, Bahrami-Nejad N, Afsharnadery F, Baghery S (2016) J Mol Liq 221:851–859
Safaiee M, Zolfigol MA, Bahrami-Nejad N, Afsharnadery F, Baghery S (2015) RSC Adv 5:102340–102349
Yu B, Xie JN, Zhong CL, Li W, He LN (2015) ACS Catal 8:3940–3944
Acknowledgements
We thank Bu-Ali Sina University and Iran National Science Foundation (INSF) for financial support (Grant of Allameh Tabataba’i’s Award, Grant Number BN093), and National Elites Foundation to our research groups.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baghery, S., Zolfigol, M.A., Schirhagl, R. et al. {[1,4-DHPyrazine][C(CN)3]2} as a New Nano Molten Salt Catalyst for the Synthesis of Novel Piperazine Based bis(4-hydroxy-2H-chromen-2-one) Derivatives. Catal Lett 147, 2083–2099 (2017). https://doi.org/10.1007/s10562-017-2096-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-017-2096-3