Abstract
The aim of the investigation was to assess histologically the effect of laser photobiomodulation (LPBM) on a repair of defects surgically created in the femurs of rats. Forty-five Wistar rats were divided into four groups: group I (control); group II (LPBM); group III (hydroxyapatite guided bone regeneration; HA GBR); group IV (HA GBR LPBM). The animals in the irradiated groups were subjected to the first irradiation immediately after surgery, and it was repeated every day for 2 weeks. The animals were killed 15 days, 21 days and 30 days after surgery. When the groups irradiated with implant and membrane were compared, it was observed that the repair of the defects submitted to LPBM was also processed faster, starting from the 15th day. At the 30th day, the level of repair of the defects was similar in the irradiated groups and those not irradiated. New bone formation was seen inside the cavity, probably by the osteoconduction of the implant, and, in the irradiated groups, this new bone formation was incremental. The present preliminary data seem to suggest that LPMB therapy might have a positive effect upon early wound healing of bone defects treated with a combination of HA and GBR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone loss may be a result of several pathological conditions, trauma, or as a consequence of surgical procedures. This aspect has led to extensive studies on the process of bone repair worldwide. Several techniques for the correction of bone defects have been proposed. Amongst them is the use of several types of grafts and membranes, and the combination of both techniques [1].
Of all of the biomaterials used to improve bone healing, hydroxyapatite (HA) is the most investigated one on both clinical and histological grounds [2–6]. This biomaterial has been found to be effective in improving bone formation [7, 8].
Guided bone regeneration (GBR) is a procedure based upon guided tissue regeneration (GTR), which is a periodontal surgical procedure that has been in clinical practice for more than a decade [9, 10]. The latter procedure is used as a way to encourage wound healing that favors regeneration of the tooth-supporting structures [11]. Its principles are based on the selective permeability provided by the membranes for the isolation of tissues not essential for bone repair. Previous studies on human histology have demonstrated that such regeneration can occur following this procedure [9, 12, 13]. The use of GBR with biomaterials such as HA was considered to be beneficial for the healing of bones [1].
Despite the growing successful application of laser photobiomodulation (LPBM) to bone repair, there are few studies assessing the association of laser light with biomaterials [1–8]. Although several reports have suggested benefits from the isolated or combined use of HA, GBR and LPBM on the repair of bone defects, the use of all these techniques in combination have not been studied as yet.
Materials and methods
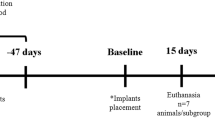
This study was approved by the Animal Ethics Committee of the School of Dentistry of the Federal University of Bahia. Forty-five healthy male and female young adult Wistar rats weighting 270–320 g were kept at the animal house during the experimental period. The animals were fed on a pelleted laboratory diet and had water ad libitum. They were kept in plastic cages and bedded on sterilized wood chips; they were kept in a day/night light cycle and in a controlled temperature.
Under intraperitoneal general anesthesia (10% chloral hydrate, 0.4 ml/100 g) the animals’ left legs were shaved and the femurs exposed. A special titanium bur was devised to produce a standard 3 mm2 cavity on the superior third of the lateral side of the bone. The animals were randomly distributed into four groups. Each group was then divided into three subgroups (Table 1).
In group I, the periosteum was repositioned and sutured with catgut (4.0), and the skin was closed with nylon (3.0). In the rats of groups II and IV, the wound margins were tattooed with nankin ink at four points. In groups (III and IV) synthetic micro-granular HA implants (Gen-Phos, Baumer S.A, Mogi Mirim, SP, Brazil) completely filled the bone defects, as recommended by the manufacturer. In groups III and IV, guided bone regeneration was performed, and a dehydrated bovine collagen membrane (Gen Derm, Baumer S.A) was placed on top of the defect. Laser photobiomodulation (Thera Lase, λ 830 nm, 40 mW, ϕ ∼ 0.60 mm, CW, DMC Equipamentos, São Carlos, SP, Brazil) was used on the rats in groups II and IV. LPBM was started immediately after the sutures had been placed and consisted of a transcutaneous application at four points around the surgical site repeated every other day for 15th days. The dose per point was 4 J/cm2; the session dose was 16 J/cm2, and the treatment dose 112 J/cm2. The animals were killed 15 days, 21 days and 30 days after the surgical procedure with an intraperitoneal overdose of 10% chloral hydrate. Specimens were routinely taken from the center of the defect and divided into two. They were kept in 4% formalin solution for 5 days, decalcified in 10% nitric acid, cut (5 μm) axially, and embedded in wax. They were stained routinely with hematoxylin and eosin and Sirius Red at the Oral Pathology Department of the School of Dentistry of the Federal University of Bahia. The slides were examined under light microscopy (Table 2) (Motic B5 Professional Series) by one experienced pathologist as previously described [2–5, 14, 15]. Descriptive and semi-quantitative histological analyses were performed, based on the reabsorption of the cortical plate and of the biomaterial inserted into the bone defect, the presence of medullar tissue and/or granulation tissue, inflammatory reaction, presence of giant cells, collagen fibers, and amount and quality of the newly formed bone.
Results
The animals that had not been treated showed on day 15 that the cortical defect remained open and was partially filled with medullar tissue. On day 21 sparse and delicate bone trabeculae were seen within the cavity, close to the remaining cortical plate. Despite new bone formation being detectable, it was incipient, and the cortical plate was thin, with no bridging. At the end of the experiment the defect remained filled by medullar tissue and, on most specimens, cortical repair was seen. However, the cortical plate was still thinner than that in the areas that had not been wounded (Fig. 1).
When LPBM had been used, it was seen on day 15 that the defect was filled with medullar tissue and there was new bone formation, starting from the cortical plate towards the center of the cavity. Although the cortical plate had been restored in all specimens, it was thinner than that in the areas that had not been operated on. No significant changes were seen up to day 21. There was increased deposition of newly formed bone close to the remaining cortical plates. Collagen fibers were also seen, encircling the newly formed bone. At the end of the experiment the newly formed cortical plate could not be differentiated from that of the areas not operated on; medullar tissue and delicate bone trabeculae could be seen in the repaired area (Fig. 2).
Photomicrograph of specimen from rat submitted to LPBM 30 days after surgery, showing complete cortical repair. The cortical plate is similar to that in the untreated areas. The cavity shows medullar tissue and delicate trabecular bone originating from the cortical plate. Sirius Red, approximately × 100
When HA and GBR had been used, on day 15 cortical repair was seen in all subjects, and it was associated with the remaining cortical area and was thinner than that in the non-treated areas. The bone was trabecular and spongy, and intense osteoblastic activity was noticed around fragments of HA within the defect, as well as the presence of giant cells. Collagen fibers seen on the granulation tissue were immature and disorganized. On day 21 there was increased reabsorption of the HA; increased cortical bone deposition and maturation were seen, the defect was filled with medullar tissue, and newly formed bone was seen encircling remnants of HA. At the end of the experiment, the cortical area was completely restored, and the defect was filled by medullar tissue. Delicate fragments of newly formed bone and vestiges of HA particles were also seen (Fig. 3).
a Photomicrograph of specimen grafted with HA and submitted to GBR 30 days after surgery showing complete cortical repair similar to that in untreated areas. Sirius Red, × 100. b Photomicrograph of specimen grafted with HA and submitted to GBR 30 day after surgery showing new bone formed within the cavity. Sirius Red, approximately × 200
The combination of HA, GBR and LPBM showed cortical repair on day 15, and the plate at the wounded site was thinner than that of the non-treated areas; the bone was spongy; intense osteoblastic activity was evident, large amounts of newly formed bone could be seen, and giant cell ingesting particles of HA were also observed. On day 21 increased bone deposition was seen, and most of the defect had been filled by newly formed bone; remnants of HA could be seen within the newly formed bone. Cortical repair had improved, but the cortical area was not yet similar that of to non-wounded areas. At the end of the experiment, cortical repair was complete and the cortical plate was similar to that of the area that had not been operated on; large amounts of newly formed bone and particles of HA could still be seen filling the defect (Fig. 4).
a Photomicrograph of specimen grafted with HA and submitted to GBR and LPBM 30 days after surgery, showing complete cortical repair and delicate trabecular bone originating from the cortical plate. Sirius Red, approximately × 40. b Photomicrograph of specimen grafted with HA and submitted to GBR and LPBM 30 days after surgery, showing increased new bone formation within the cavity. Sirius Red, approximately × 40
Discussion
Bone loss may be a result of several pathological conditions, trauma, or as a consequence of surgical procedures. This aspect has led to extensive studies on the process of bone repair worldwide. Several techniques for the correction of bone defects have been proposed, amongst them the use of several types of grafts and membranes, and the combination of both techniques. It is accepted that, although HA has osteoconductivity, the repair of the defect may be slow because of the need of the graft to be reabsorbed, slowing down the process. It is clear that the use of a graft prevents fibrosis of the lesion and also protects the cavity and acts as a framework for the deposition of newly formed bone [1].
We have reported in this paper the assessment of the influence of LPBM (λ 830 nm) on the repair of bone defects treated or not treated with synthetic micro-granular hydroxyapatite implant (HA) and/or bovine bone membrane. The irradiated groups received seven irradiations every 48 h, the first being immediately after the surgical procedure. The dose was 16 J/cm2 per session, divided into four points of 4 J/cm2 around the defect (Ø∼0.6 cm, 40 mW). The results showed that all the experimental groups presented an increment in the repair of the bone defects in all the observation periods when compared with the control group, mainly in the groups with membrane and/or irradiation. When the groups irradiated and with implants and/or membranes were compared, it was observed that the repair of the defects of the rats submitted to LPBM was also processed faster, starting from 15 days and 21 days. On the 30th day, the level of repair of the defects was similar in the irradiated groups and in those not irradiated. New bone new formation was seen inside the cavity, by osteoconduction of the implant, and, in the irradiated groups, this new bone formation was incremental.
The results of this study showed that, in control specimens, new bone formation was limited to the area of the cortical defect. This was expected, as the cavity represented the medullar space where woven medulla and its cellular components prevail. However, it is worth pointing out that, in all the experimental groups, the repair of the cortical defect was more advanced in all the observation periods. In the experimental groups in which HA was associated with GBR and/or LPBM, besides the repair in the cortical area of the defect, there was new bone formation inside the cavity as a result of the osteoconductivity of the biomaterial [1–6].
Several reports have demonstrated the effectiveness of LPBM in bone repair in several experimental models, including association with organic or inorganic bovine bone, bone morphogenetic proteins (BMPs) and granular HA [1–6, 14], and also with the use of block HA [16].
Bone defects in the rats submitted to LPBM (group II) presented a more advanced repair than did controls. New bone formation in the area of the irradiated defect, as seen 15 days after surgery, was similar, due to the positive effect of LPBM on the repair of the bone defects. These results are aligned with previous results from our group [1–6, 14, 15, 17–19].
Previous studies in which LPBM was combined with other granular biomaterials have shown positive modulation of the bone repair, corroborating the results observed in our investigation in which LPBM was combined with granular HA [1–6].
The methodology when GBR was used was based on previous studies by our group, in which it was shown that there were no signs of interference of the membrane by the laser light [2–6].
Defects treated with HA, GBR and LPBM in comparison with those of non-irradiated subjects showed a positive effect of LPBM. It was evident that there was greater bone formation inside the defect around the particles of the HA implant. Bone formation in the presence of HA granules presented a spongy aspect. However, the thickness of the cortical area was similar to non-treated cortical areas from day 21. At the end of the experiment both groups were similar, and the cortical defect had been totally repaired. In the presence of HA remnants, there was strong evidence of osteoconduction [2–6].
The irradiation protocol used in this study, 4 J/cm2, has been successfully used in several studies by our group and is of clinical importance in cases in which a quick and efficacious bone repair is demanded [1–6, 9, 15, 17–19].
The initial requirement for correct experimental models is that the bony defects should not heal spontaneously during the animal’s lifetime. These defects are known as critical size defects and were initially described by Schmitz and Hollinger [20] and Hollinger and Kleinschmidt [21]. Therefore, whatever defect that has the capacity to heal spontaneously is called a non-critical size defect. Convention has established that, if bone regeneration has not been completed within the first 52 weeks, one could be certain that it would never occur [20, 21]. The regeneration of the critical size defect is difficult to achieve, and it nearly always requires autologous bone. Normally in humans, these are post-trauma defects, or defects caused by ablative oncological surgery. The immense majority of bony defects that we find in our daily clinical practice in oral, pre-prosthetic or implant surgery are small or medium sized defects; that is to say, non-critical size defects [22].
Bone regeneration in non-critical size defects has been widely studied under the concept of guided bone regeneration. Non-critical size defect has been defined as a bony defect that, under proper conditions, will heal spontaneously. These necessary bone conditions are the key to bone regeneration, and it is on this that the guided bone regeneration concept is based. Guided bone regeneration implies the provision of suitable conditions so that the spontaneous and natural repair process of small sized defects can take place by means of new bone formation. Bone cavities will always behave as non-critical size defects, providing that various walls are conserved. These defects have a great potential for spontaneous regeneration, provided that blood clots are stabilized, the space is maintained, and the area is not subjected to mechanical load [22]. In this study, we used a non-critical defect similar to one we have previously described, using several protocols to investigate bone healing [1–6, 14, and 17].
The results of our previous studies indicated that the effect of LPBM is more effective if the treatment is carried out in the early stages, when high cellular proliferation occurs. The mechanism that leads to a positive effect of laser light on different tissues remains not fully understood, as there are possibilities to be considered, such as stimulation by the laser light of porphyrins and cytochromes to increase cellular activity, increasing the concentrations of adenosine 5′-triphosphate (ATP) and alkaline phosphatase (ALP) and the release of calcium (Ca). Our experience also indicates that the magnitude of the biomodulative effect depends on the physiologic status of the cell at the irradiation time, or on the stimulant effect of the laser light during the initial phase of proliferation and initial differentiation of undifferentiated cells. However, this does not occur during more advanced stages [1].
It is known that the stimulant effect of laser light on bone occurs during the initial phase of proliferation of both fibroblasts and osteoblasts, as well as during the initial differentiation of mesenchymal cells. Fibroblastic proliferation and its increased activity have been detected previously on irradiated subjects and cell cultures, and these are responsible for great the concentration of collagen fibers seen within irradiated bone. The reason why the effect of LPBM was not very detectable until 30 days after treatment was due to the fact that, during early stages of bone healing, the cellular component is more prominent and more prone to be affected by laser light. Later, bone matrix is the main component of the healing tissue. This is why the frequency of application of the laser is effective when carried out during the cellular phase, when the number of osteoblasts is increasing. Later, the higher number of cells results in a larger deposition of bone matrix, which later incorporates Calcium hydroxyapatite (CHA), characterizing maturation of the bone. The treatment protocol used in our various studies is in agreement with our experience, as no existing parameters are universally accepted. A unique parameter that is able to produce, by itself, a photobiological response does not exist, but the conjugation of different parameters is in agreement with our experimental model. It still remains uncertain whether bone stimulation by laser light is a general effect, or if the isolated stimulation of osteoblasts is possible. It is possible that the effect of LPBM on bone regeneration depends not only on the total dose of irradiation, but also on the duration and mode of irradiation. The threshold parameters of energy density and intensity are biologically independent of one another. This independence accounts for both the success and failure of LPBM achieved at low-energy density levels [1].
This paper aimed to describe and semi-quantitatively assess bone repair. From the results observed it is fair to say that the use of LPBM was effective in improving bone repair, as the irradiated subjects showed not only the formation of bone from the cortical areas, as seen in the non-irradiated subjects, but also around the graft within the defect. On control specimens the defect was filled with medullar tissue, without evidence of osteoblastic differentiation. The present preliminary data seem to suggest that LPMB therapy might have a positive effect on early wound healing of bone defects treated with a combination of HA and GBR.
References
Pinheiro ALB, Gerbi MEMM (2006) Photoengineering of bone repair processes. Photomed Laser Surg 24:169–178
Pinheiro ALB, Limeira Júnior FA, Gerbi MEMM et al (2003) Effect of low level laser therapy on the repair of bone defects grafted with inorganic bovine bone. Braz Dent J 14:177–181
Pinheiro ALB, Limeira Júnior FA, Gerbi MEMM et al (2003) Effect of 830nm laser light on the repair of bone defects grafted with inorganic bovine bone and decalcified cortical osseous membrane. J Clin Laser Med Surg 21:383–388
Limeira Junior FA et al (2003) Assessment of bone repair following the use of inorganic bone graft and membrane associated or not to 830 nm laser light. Proc SPIE 4950:30–36
Pinheiro ALB, Limeira Junior FA, Gerbi MEMM et al (2003) Assessment of bone repair following the use of inorganic bone graft Gen-ox inorganic and membrane or not with 830-nm laser light. Int Congr Ser 1248:445–447
Limeira Junior FA et al (2005) The biomodulative effect of low level laser therapy on the repair of bone defects submitted to xenografts. The 9th International Congress on Lasers in Dentistry, 2005, São Paulo. International Proceedings Division. Moduzzi Editore, Bologna, 99–102
Gross JS (1997) Bone grafting materials for dental applications: a practical guide. Compend Contin Educ Dent 18:1013–1038
Pinto LP, Brito JHM, Oliveira MG (2003) Avaliação Histológica do Processo de Reparo Ósseo na Presença da Proteína Morfogenética Óssea (Gen-pro) Associada com uma Membrana Biológica (Gen-derm). R Bras Implantol Prot Implant 10:25–32
Nyman S, Lindhe J, Karring T, Rylander H (1982) New attachment following surgical treatment of human periodontal disease. J Clin Periodontol 9:290–296
Gottlow J, Nyman S, Karring T, Lindhe J (1984) New attachment formation as the result of controlled tissue regeneration. J Clin Periodontol 11:494–503
Garrett S (19996) Periodontal regeneration around natural teeth. Ann Periodontol 1:621–666
Stahl SS, Froum S, Tarnow D (1990) Human histologic responses to guided tissue regenerative techniques in intrabony lesions. Case reports on 9 sites. J Clin Periodontol 17:191–198
Cortellini P, Clauser C, Prato GP (1993) Histologic assessment of new attachment following the treatment of a human buccal recession by means of a guided tissue regeneration procedure. J Periodontol 64:387–391
Gerbi MEMM, Pinheiro ALB, Marzola C et al (2005) Assessment of bone repair associated with the use of organic bovine bone and membrane irradiated at 830 nm. Photomed Laser Surg 23:382–388
Weber JBB, Pinheiro ALB, Oliveira MG, Oliveira FAM, Ramalho LMP (2006) Laser therapy improves bone healing of bone defects submitted to autologous bone graft. Photomed Laser Surg 24:38–44
Guzzardella et al (2003) Osseointegration of endosseous ceramic implants after postoperative low-power laser stimulation: an in vivo comparative study. Clin Oral Implants Res 14:226–232
Silva Júnior AN, Pinheiro ALB, Oliveira MG et al (2002) Computerized morphometric assessment of the effect of low-level laser therapy on bone repair. J Clin Laser Med Surg 20:83–87
Pinheiro ALB, Oliveira MAM, Martins PPM (2001) Biomodulação da cicatrização óssea pós-implantar com o uso da laserterapia não-cirúrgica: Estudo por microscopia eletrônica de varredura. Rev FOUFBA 22:12–19
Lopes CB, Pinheiro ALB, Sathaiah S et al (2005) Infrared laser light reduces loading time of dental implants: a Raman spectroscopic study. Photomed Laser Surg 23:27–31
Schmitz J, Hollinger J (1986) The critical size defect as an experimental model for craniomandibular non junction. Clin Orthop Relat Res 205:299–304
Hollinger JO, Kleinschmidt JC (1990) The critical size defect as an experimental model to test bone repair materials. J Craniofac Surg 2:237–243
Caicoya SO (2007) Relleno de cavidades óseas en cirugía maxilofacial con materiales aloplásticos. Rev Esp Cir Oral Maxilofac 29:21–32
Acknowledgments
The authors would like to thanks the Conselho Nacional de Desensenvolvimento Científico e Tecnológico (CNPq) for the grant that allowed the realization of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pinheiro, A.L.B., Martinez Gerbi, M.E., de Assis Limeira, F. et al. Bone repair following bone grafting hydroxyapatite guided bone regeneration and infra-red laser photobiomodulation: a histological study in a rodent model. Lasers Med Sci 24, 234–240 (2009). https://doi.org/10.1007/s10103-008-0556-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-008-0556-0