Abstract
Objective
The aim of this study was to investigate solubility, pH value, chemical structure, radiopacity, and cytotoxicity of AH Plus BC, TotalFill BC, AH Plus, and AH Plus Jet sealers.
Materials and methods
Cytotoxicity analysis with direct and extraction tests at 3 different concentrations (1:1, 1:2, 1:4 v/v%) and time (24 h, 48 h, and 72 h) on Saos-2, PdLF, and THP-1 cell lines, chemical structure with scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) analysis, solubility, pH, and radiopacity values of AH Plus BC, TotalFill BC, AH Plus, and AH Plus Jet were evaluated. For statistical analyses of the groups, repeated measures, factorial, and one-way ANOVA tests were used. The statistical significance level was set at p < .05.
Results
Resin-based sealers showed higher cytotoxicity values than the bioceramic-based sealers (p < 0.05). Time and concentrations were effective on the cell viabilities for cell lines. Higher peaks of calcium were detected bioceramic-based sealers and higher amount of zirconium was detected in AH Plus BC (p < 0.05). AH Plus BC showed similar radiopacity value with AH Plus, AH Plus Jet, whereas TotalFill BC showed the lowest radiopacity (p < 0.05). Bioceramic-based sealers had higher pH values in all experiment periods, and the difference between resin- and bioceramic-based sealer groups was significant (p < 0.05). However, the solubility values of the tested root canal sealers revealed no differences (p > 0.05).
Conclusions
The newly produced AH Plus BC Sealer showed similar properties with TotalFill BC, and their biological properties were better than AH Plus and AH Plus Jet.
Clinical relevance
AH Plus BC could be a possible alternative to other bioceramic- or resin-based sealers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, significant progress has developed in bioceramic sealers, especially in hydraulic calcium silicate-based (HCS-based) bioceramic sealers which have a wide area of use in endodontics because of their high biocompatibility properties. HCS-based bioceramic sealers are mostly used in combination with gutta-percha cones and are preferred for root canal obturation [1]. Considering that the materials used in root canal treatment are in direct contact with the periapical tissues, biocompatibility is an important parameter in the choice of materials for root canal treatment. The ideal root canal sealer should not harm the periapical tissues and not prevent the repair process, and even should have healing-stimulating properties [2]. Compared to conventional endodontic root canal sealers [3] HCS-based bioceramic sealers provide essential advantages such as higher biocompatibility and reducing the inflammatory reaction [4, 5].
In resin-based systems, in order to overcome the complications of the two-tube system AH Plus, AH Plus Jet (Dentsply De Trey, Konstanz, Germany) was introduced with a device capable of automatic proportioning and mixing [6, 7] which provides homogenous distribution of the organic and inorganic components and improves the physicochemical properties of this sealer such as radiopacity, flow, and solubility [6, 7].
TotalFill BC sealer (FKG Dentaire, La Chaux-de-Fonds, Switzerland) is one of the new calcium silicate-based canal sealers and contains dicalcium silicate, tricalcium silicate, calcium hydroxide, monobasic calcium phosphate, zirconium oxide, tantalum oxide, filler, and thickening agents [8]. It is ready to use in a single syringe and self-cures in the root canal without pre-mixing. Biocompatibility, bond strength, and dentin penetration ability of TotalFill BC Sealer have been reported previously [9, 10].
The recently introduced AH Plus Bioceramic (AH Plus BC) sealer (Dentsply Sirona, Switzerland) is a premixed form of the tricalcium silicate bioceramic sealer. Until today, to the authors' knowledge, only two studies investigated the biological and physical properties of the newly produced AH Plus BC sealer [11, 12]. However, in these studies, biocompatibility was assessed on periodontal ligament stem cells, but not on osteoblast and macrophage cell-lines. The present study aimed to evaluate and compare the chemical structure, solubility, pH values, cytotoxicity on human osteoblast cell line (Saos-2), human peripheral blood monocytes cell line (THP-1) and human periodontal ligament fibroblast cell line (PdLF), and to evaluate and compare the radiopacity of AH Plus BC sealer, TotalFill BC sealer, which is another branded bioceramic-based sealer, and conventional root canal sealers. The null hypothesis was that the root canal sealers tested have not equivalent solubility, pH value, chemical structure, radiopacity, and cytotoxicity on human osteoblast cell line (Saos-2), human peripheral blood monocytes cell line (THP-1), and human periodontal ligament fibroblast cell line (PdLF) in vitro laboratory studies.
Materials and methods
In this study, two calcium silicate-based and two resin-based sealers were evaluated: AH Plus BC (Manufactured by Maruchi Distributed by Dentsply Sirona, Ballaigues, Switzerland), TotalFill BC (FKG Dentaire SA, La Chaux-de-Fonds, Switzerland), AH Plus (Dentsply, Konstanz, Germany) and AH Plus Jet sealers (Table 1). All materials were prepared under aseptic conditions according to the manufacturer's instructions and placed in standard molds with 5 mm of diameter and 2 mm of height and stored in an incubator at 37 °C and 95% humidity for 48 h to allow cure [12, 13]. Each of the sealer specimens was sterilized by UV for 15 min on each side before the cytotoxicity tests.
Evaluation of cytotoxicity
Root canal sealer samples preparations
Twelve samples were prepared and the cytotoxicity potentials were investigated on human osteoblast cell line (Saos-2), human peripheral blood monocytes cell lines (THP-1) and human periodontal ligament fibroblast cell line (PdLF) with (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) (MTT) assay, which was performed in triplicate for each of the tested sealers (AH Plus BC, Total Fill BC, AH Plus, and AH Plus Jet).
The Human Periodontal Ligament Fibroblast cell line (PdLF) was kindly granted by Dr. Sema Çınar. The Saos-2 and THP-1 cell lines were purchased from ATCC (Manassas, VA, USA). Cell lines were propagated in Dulbecco's Modified Eagle F12 (DMEM/F12) medium containing 10% fetal bovine serum (FBS), 1% L-glutamine, 100 μg/ml streptomycin, 100 U/ml penicillin (Gibco, NY, USA), and maintained. Cells were cultured in 75 cm2 flasks at a volume of 10 ml per flask and incubated at 37 °C in a humidified atmosphere of 5% CO2 following the International Standard ISO 10993-5 guidelines [14].
Determination of cell viability
Cytotoxicity of the materials was assessed both via direct contact and the extraction methods by the MTT analysis, which is a proliferation test based on the metabolic activity of the cells [15]. The principle of the test is based on the conversion of tetrazolium salt with increased dehydrogenase enzyme activity into a colored formazan by the mitochondrial activity of living cells. MTT solution which was diluted from stock solution (2.5 mg/ml stock) was used to measure the percent viability of the samples. 100 μl MTT solution was added for “direct contact test” and 20 μl MTT solution was added for “extraction test,” separately. After 4 h of incubation of the cells with MTT, 150 μl DMSO was added to dissolve the formazan crystals for both test and the absorbance of the samples was measured at 570 nm with a spectrophotometer (Thermo Fisher Scientific). In both “direct contact test” and “extraction test,” doxorubicin was used as the positive control group.
a. Evaluation of the cytotoxicity by direct-contact test
Saos-2, PdLF, and THP-1 cells and sealers in solid form were used in direct contact test analyses in 24-well microplates [14]. Cell counting was performed by trypan blue destaining-based cell count using hemocytometry and the cells were inoculated in 24-well microplates with an initial concentration of 8 × 104 cells/well at a total volume of 500 μl. Cells were incubated overnight at 37 °C in a humidified atmosphere of 5% CO2. At the end of the incubation, each solid sealer sample was placed in the middle of the 24-well microplates with sterile forceps. All microplates were incubated at 37 °C with 5% CO2 for 24 h [16]. The morphology of the cells was examined under an inverted microscope at the 1st and 24th hours. Cell viability was determined by MTT assay as mentioned above. After the incubation, 100 μl MTT solution was added each well and incubated for 4 h. At the end of the procedure, 150 μl DMSO was added to dissolve the formazan crystals and the absorbance of the samples was measured at 570 nm.
b. Evaluation of the cytotoxicity by extraction test
For all the tested root canal sealers, the cytotoxicity effects of the samples were evaluated according to time (24 h, 48 h, and 72 h) and concentration (1:1, 1:2, and 1:4v/v%). Each of the UV-sterilized sealer samples was extracted during 24 h in 1 ml of serum and antibiotic-free DMEM/F12 medium in sterile Eppendorf's. Also, Saos-2, THP-1, and PdLF cells were inoculated in 96-well microplates at an initial concentration of 5 × 104 cells/well at a total volume of 100 μl and were incubated overnight at 37 °C in a humidified atmosphere of 5% CO2. In order to provide 3 different concentrations (1:1, 1:2, 1:4 v/v%) at 24th, 48th, and 72nd hours, the determined amount of nutrient medium was drawn from the wells after extraction and the extraction medium was added to each well of 96-well microplates [14]. Serum and antibiotic-free DMEM/F12 medium were used as the control group, whereas Doxorubicin was used as the positive control group [17]. The samples were incubated for 24 h, 48 h, and 72 h. At the end of the incubation period for each microplate, 20 μl MTT solution was added to each well and incubated for 4 h. After the incubation, 150 μl DMSO was added to dissolve the formazan crystals, and the absorbance of the samples was measured at 570 nm. Samples were examined under an inverted microscope at 10× magnification at 24, 48, and 72 h during the extraction test. In addition, at the end of the 24th, 48th, and 72nd hours, the percent viability test was performed on the extraction samples using the MTT method.
Scanning electron microscopy (SEM) and energy-dispersive x-ray spectroscopy (EDX) analysis
Five standard samples were prepared for each group (n = 20). The samples were coated with gold and examined in a scanning electron microscope (SEM) (Thermoscientific Apreo S, Waltham, MA, USA) without any preparation or manipulation, and the chemical content of each sealer sample was analyzed using energy dispersive x-ray spectroscopy (EDX) (Thermoscientific Apreo S, Waltham, MA, USA) analysis. The metals used to sputter coat the specimens were excluded from the EDX data. EDX analyses to characterize the elemental composition were performed from the full area of each root canal sealer specimen (n = 20) by using EDAX Team software (EDAX., Mahwah, NJ, USA) under 20Kv. SEM surface analyses were performed at 65×, 150×, 300×, 1500×, and 5000× magnifications to determine the surface morphology and particle shape.
Evaluation of pH change
Initial pH value of the distilled water was determined as 7.09 in all groups. Five samples for each tested sealer group were placed in glass vials containing 10 mL mili-Q-water. They were kept in an incubator at 37 °C for 1, 2, 3, 7, 14, 21, and 28 days. The pH was measured after each period using a pre-calibrated pH meter (OrionFive Star; Thermo Scientific, MA, USA) [18, 19].
Solubility tests
The solubility amount of the sealers was calculated as a percentage with the formula specified in the ISO 6876 standards [20]. The sealers were mixed in accordance with the manufacturer’s recommendations and were placed in standard molds to prepare 5 samples for each group. The samples were kept in an incubator at 37 °C for during the test period. The initial weights of the samples were assessed with a precision scale and recorded (Denver Instrument GmbH, Gottingen, Germany). The solubility of the sealers was determined on the 1st, 2nd, 3rd, 7th, 14th, 21st, and 28th days. Samples were weighted and immersed in vials containing 10 mL mili-Q-water (Millipore Sigma, Burlington, MA). Both surfaces of the samples were dried several times with the help of filter paper before weighing. After making sure that there was no solvent left on the sample surface, the weighing process was started. For the drying process, different filter papers were used both on each surface and for each sample. The weight of the samples before (m1) and after (m2) immersion in distilled water was measured by means of a precision scale and the difference was recorded as the dissolution amount, which was divided by the initial weight of the samples and was multiplied by 100 to calculate the percentage of dissolution [20].
Radiopacity analysis
Five samples were utilized for each group (n = 20). A five-step 99% purity aluminum stepwedge (Al SW) with 2 mm difference between the steps was used as an internal radiographic standard in order to calculate the radiopacity of each root canal sealer and to compare the radiopacity of the samples. The samples and the aluminum stepwedge were positioned on a phosphor plate (Digora; Orion Corporation Soredex, Helsinki, Finland), and a radiograph was obtained using a dental X-ray machine (Gendex GX, Lake Zurich, IL, USA) under standard exposure conditions (60 kVp, 7 Ma, 0.32 s, 30 cm target to film distance). Exposed phosphor plates were scanned instantly after exposure by using the Digora plate scanner according to the manufacturer’s instructions. On the digital images, a region of interest (ROI) with 50 × 50 pixels was selected on every specimen and on every step of the Al SW. Utmost care was afforded to select regions without air bubbles inside the sealer material. The mean gray values (MGVs) of the ROIs of every test sample, each step of Al SW and dentin were evaluated using the histogram function of a computer graphics program (Adobe Photoshop 8.0, Adobe System, San Jose, CA, USA). The MGVs of all samples were measured three times and the mean MGV of each material was calculated. Density measurements of the materials were performed by a single operator who was blinded to the tested root canal sealers. In order to eliminate the variations between the digital images and to standardize the test conditions to assess the radiopacity, a regression curve equation was defined for each digital image by using the MGVs of each Al SW step. The radiopacity of the root canal sealer specimen was established in millimeters of equivalent Al (mm Al).
Statistical analyses
The Shapiro-Wilk and the Levene tests were used to evaluate the distribution of data within the groups. The Shapiro-Wilk and Levene tests revealed that the sample distribution was parametric. Descriptive statistics were calculated. The cell viability with direct tests and radiopacity were analyzed using one-way ANOVA and Tukey’s post hoc tests. Extraction tests (time and concentration effect on cell viability) were analyzed with factorial ANOVA and pH and solubility tests were analyzed using repeated measures ANOVA. Kruskal-Wallis tests were performed for elemental analysis. The statistical significance level was set at p < .05.
Results
Determination of cell viability
a. Cytotoxicity by direct-contact test results
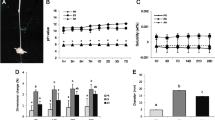
After 24 h of incubation of the direct-contact test samples, no difference was observed between the cell viability values of AH Plus BC and Total Fill BC sealers in Saos-2, PdLF, and THP-1 cell-lines (p > 0.05). AH Plus sealer presented lower cell viability value than the other groups in all of the cell-lines with statistically significant differences between AH Plus and AH Plus BC and TotalFill BC (p < 0.05). Doxorubicin was used as the positive control and showed the highest cytotoxicity value in all cell line groups compared with the other tested root canal sealers (p < 0.001) (Fig. 1).
b. Cytotoxicity by extraction test results
The analysis revealed that the cell viability values of all tested materials were significantly different between Saos-2 and THP-1 cells, and between PdLF and THP-1 cells (p < 0.05). The highest cell viability of the test materials was observed in Saos-2 cells and was followed by PdLF and THP-1 cells.
Considering the effect of sealers on the viability of Saos-2 cell lines, there were no significant differences at the end of 24 h. For all tested root canal sealers, the time was effective on the cell viability and statistically significant between 24 h and 48 h (p < 0.05) and 24 h and 72 h (p < 0.001). At the end of 72 h, the highest percent viability was observed with 1:4 concentration of AH Plus BC group, and the lowest percent viability was with 1:1 concentration of AH Plus group at the end of the 72 h (p < 0.05). Doxorubicin showed higher cytotoxicity at 1:1 concentration and 72 h (p < 0.001) (Fig. 2a, b, c).
The percent viability values were lower in PdLF cell lines than in Saos-2 cells. For all the tested root canal sealers, the cytotoxicity of the samples increased with time and high concentration, and the time (24 h, 48 h, and 72 h) and the concentration (1:1, 1:2, and 1:4) were effective on the cell viability (p < 0.001). After 24 h, the highest percent viability in PdLF cells was observed at the concentration of 1:4. At this concentration, the highest percentage viability was found in the TotalFill BC group and the lowest percentage viability was found in AH Plus group (Fig. 2d, e, f). In the positive control group, both concentrations and time were effective on the cell viability (p < 0.001).
Higher cytotoxicity values were found in THP-1 cell lines compared to other cells (p < 0.05). Percent viability values decreased significantly depending on time (24 h, 48 h, and 72 h) and concentration (1:1, 1:2, and 1:4) (p < 0.05). In Total Fill BC group, both the time and concentrations were effective and there were statistically significant differences between 1:1 and 1:4 concentrations at all time periods (24 h, 48 h, and 72 h) (p < 0.001). Similarly, in AH Plus Jet group, both the time and concentrations were effective and significant differences were observed between 1:1–1:2 and 1:1–1:4 concentrations at all time periods (24 h, 48 h, and 72 h) (p < 0.001). In AH Plus BC and AH Plus groups, the time and concentration were effective on the results, and there were significant differences between all-time periods and concentrations (p < 0.001) (Fig. 2g, h, i).
SEM-EDX analysis results
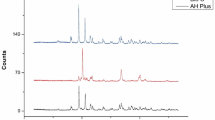
SEM-EDX analysis revealed surface element distributions of the root canal sealers (AH Plus BC, TotalFill BC, AH Plus, and AH Plus Jet). Chemical compositions and the amount of the elements of the root canal sealer specimens according to the EDX analysis are presented in Table 2 and Fig. 3. The elements O, Si, Zr, and, Ca were detected in all samples. A higher peak of calcium (Ca) was detected in AH Plus BC and Total Fill BC sealers compared to and AH Plus and AH Plus Jet, whereas a higher peak of zirconium (Zr) was observed in AH Plus BC compared with the other tested sealers. Tungsten (W) was detected in the AH Plus, AH Plus Jet and with a trace amount of in the AH Plus BC samples. A higher pick of carbon (C) was detected in AH Plus and AH Plus Jet, and at low level of in Total Fill BC. Scanning electron micrographs at 65×, 150×, 300×, 1500× and 5000× magnifications of the specimens are presented in Fig. 4. AH Plus BC and Total Fill BC exhibited irregular and rough surface structure, while AH Plus and AH Plus Jet presented smoother surfaces (Fig. 4).
pH analysis results
The measurements of pH values were performed at the end of the 1st, 2nd, 3rd, 7th, 14th, 21st, and 28th days with a pH meter are presented in Fig. 5. There was no statistically significant difference between AH Plus and AH Plus Jet and there was no statistically significant difference between AH Plus BC and Total Fill BC groups. Higher pH values were revealed in bioceramic-based sealer groups in all the experiment periods, and there was statistically significant difference between resin- and bioceramic-based sealer groups (p < 0.05). AH Plus BC and TotalFill BC showed a high pH throughout the test periods and almost kept the first day’s pH at the end of the 28th day (p > 0.05). There were statistically significant differences between initial (1st and 2nd days) and final pH measurement days both in in AH Plus (21st and 28th days) and AH Plus Jet (7th, 14th, 21st, and 28th days) groups (p <0.05) (Fig. 5).
Solubility tests results
In the present study, all groups met the ISO standards and did not show solubility higher than 3% (ISO 6876:2012). There was no statistically significant difference between AH Plus BC and TotalFill BC groups, and AH Plus and AH Plus Jet groups (p < 0.05). The time and material interaction were observed both in AH Plus and AH Plus groups (p < 0.05) (Fig. 6).
Radiopacity analysis results
The average radiopacity values of each sealer as millimeters of equivalent Al are presented in Fig. 7. All the analyzed sealers showed radiopacity values corresponding to at least 3 mm aluminum step [19]. There was no significant difference between the radiopacity values of AH Plus BC (12.75 mm Al), AH Plus (12.50 mm Al), and AH Plus Jet (12.02 mm Al). Total Fill BC showed significantly lower radiopacity value compared the other tested root canal sealers (p < 0.001). All test materials were more radiopaque than dentin (Fig. 7).
Discussion
Root canal sealers are placed inside the root canal and are expected to have minimal contact with periradicular tissues. However, during root canal treatment, they may protrude from apical or accessory foramens or lateral canals into the surrounding tissues in varying amounts [21]. Therefore, a sealer should not damage the periapical tissues or prevent the tissue healing process, especially at the cellular level [22, 23]. Within this context, the healing process after root canal filling is affected by the physicochemical properties of the sealer [3]. Understanding the physical and chemical properties of the material is important in clinical decision-making and the selection of the appropriate root canal sealer. This is even more crucial when a new endodontic sealer is introduced into endodontic practice. In the present study, solubility, pH value, chemical structure, radiopacity, and cytotoxicity properties of AH Plus, AH Plus Jet, Total Fill BC, and the newly produced AH Plus BC sealers were assessed. The null hypothesis of the present study was accepted, as the tested root canal sealers did not show equivalent cytotoxicity, radiopacity, pH, and solubility values according to the obtained results.
In vitro studies that comprise the evaluation of the cytotoxicity, radiopacity, pH, and solubility are required for acceptability of root canal sealers for clinical practice [24, 25]. Numerous cell lines, including human periodontal fibroblasts, osteoblasts, and monocytes, which have similar properties and phenotypes to dental and periapical tissues, have been used for cytotoxicity evaluation of dental materials [26,27,28,29,30,31]. Osteoblasts are an important cell group in the healing process of the teeth and the periapical tissues. Monocytes are the most important cells in many physiological and pathophysiological processes, and the first body reaction that occurs when the sealer encounters the periapical connective tissue with an attempt to phagocytize the sealer [32, 33]. Similar to monocytes, periodontal fibroblast cells (PDLFs) are in contact with root canal sealers in the periapical area. Since these cells play an important role in the healing process of periodontal ligament and implicitly existing periapical lesions [34], contact or extrusion of a root canal sealer should not damage these cells. Based on this information in the literature, all resin- or bioceramic-based root canal sealers are considered as foreign materials to the body if they remain in constant contact with the periapical tissues. Different immortalized cell lines have been used to evaluate the cytotoxicity of endodontic sealers, especially since they proliferate rapidly and have an indefinite lifespan, allowing higher reproducibility of results. Considering some of the expected differences in the responses of immortalized cells, the use of relevant human primary cells in the study of endodontic materials has previously been noted [35, 36]. Therefore, the THP-1, Saos-2, and PdLF cell lines were the closest to ideal cells for cytocompatibility experiments, as their direct interaction with biomaterials could play a critical role in a clinical setting [37, 38]. Thus, in the present study, human osteosarcoma cell line [39,40,41], monocytes cell line (THP-)1, and human periodontal fibroblast cell (PDLFs) lines were selected to evaluate the cytotoxic potential of the root canal sealers. Our results disclosed that resin-based AHP and AHP Jet sealers showed higher cytotoxicity than bioceramic-based sealers, resulting in significant reduction in cell viability of PDLF, Saos-2, and THP-1 cell lines with both tests.
Cytotoxicity tests are basic and reproducible test methods that can be used in standard configurations and allow to assess the toxicity of a root canal sealer [42, 43]. There are three types of cytotoxicity test methods: extract dilution, direct contact, and indirect contact test that are sensitive to detect low to high levels of cellular toxicity [14, 43]. These tests can quickly produce results suitable for quantitative evaluation [43,44,45]. The direct contact test is the most sensitive for evaluating the cytotoxicity of the medical materials and the method produces direct contact of the solid materials with cultured cells in vitro. The direct contact test occurs by observing the morphological changes and detecting the changes in the number of cells; it can directly reflect the impact of testing the medical materials on the cells. The extract dilution test is applied to detect toxins leached from exposed surfaces [43]. Under our experimental conditions, AH Plus and AH Plus Jet sealers exhibited significantly higher cytotoxicity than AH Plus BC and Total Fill BC sealers with direct contact and extract dilution test methods. These results are in accordance with the literature which reported resin-based sealers have higher cytotoxicity levels than the hydraulic calcium silicate-based sealers [11, 12, 46].
In extraction analyses, 1:1, 1:2, and 1:4 concentrations were evaluated as described in the literature to simulate the root canal sealer leaching via accessory foramens for all types of cell lines [47]. Similar to direct contact testing results, the resin-based sealers were more cytotoxic than bioceramic-based sealers. During 24-, 48-, and 72-h measurements, the level of cell viability decreased over time for all types of root canal sealers. In all cell lines, AH Plus sealer was detected as the most cytotoxic sealer with 1:1 concentrations of the extracts. These results are in accordance with the literature [12].
Homogeneous surfaces of the AH Plus and AH Plus Jet fillings were observed with SEM examination. Large crystalline irregular structures were noticed on the surfaces of TotalFill BC and AH Plus BC sealers. Determining the elemental compositions of root canal sealers helps to understand their biological properties such as the cytotoxic effect on the cells they come into contact with, and to evaluate the effects of the elements in their contents [48,49,50]. Energy dispersive X-ray spectroscopy (EDX) is an analysis method used for this purpose, and determines the elements contained in and dispersed on the surfaces of the root canal sealers. As expected, both bioceramic-based sealers presented higher calcium and oxygen peaks than the resin-based sealers. The result of the present study was in accordance with the literature [1, 12]. The bioactivity and biocompatibility of root canal sealers are enhanced by the release of calcium ions. It has been shown in previous studies that the main components of AH Plus BC sealer, tricalcium silicate and a radiopacifier agent (zirconium oxides), are biocompatible [51]. Our EDX results confirmed that AH Plus BC contains 25.33 wt% of calcium and 43.12 wt% of zirconium. In the literature, endodontic sealers with ZrO2 as the radiopacifier have not presented unfavorable biological properties [52, 53].
The pH of root canal sealers affects their antimicrobial properties, osteogenic potential, and biocompatibility [54, 55]. The pH of all the tested root canal sealers showed alkaline properties throughout the experimental period. However, AH Plus BC and TotalFill BC had higher pH levels than AH Plus and AH Plus Jet sealers in all experimental periods, in line with their high calcium content [54,55,56]. The higher pH value of AH Plus BC and TotalFill BC may be attributed to their being more biocompatible, i.e., having more cell viability, than AH Plus and AH Plus Jet sealers [54, 55].
Dissolution of a root canal can affect the results of endodontic treatment by creating voids within the root canal filling [55,56,57]. All tested root canal sealers showed less than 3% resolution according to ISO standards [20]. No dissolution was detected in AH Plus, AH Plus Jet, and AH Plus BC sealers, and only a minimal amount of solubility was observed in the TotalFill BC group. However, the differences between the HCS-based sealer groups were not statistically significant, and this result was in accordance with the literature which reported similar solubility values between HCS-based sealer groups [11].
Radiopacity is a required property of endodontic sealers for their perception on radiographs [58]. In this study, all sealers met ISO standards and showed a radiopacity value above the specified minimum 3 mm Al radiopacity [20]. The radiopacity of AH Plus BC, AH Plus Jet, AH Plus, and Total Fill BC was descending, respectively. AH Plus BC sealer presented the highest radiopacity value, whereas there were no statistical differences between AH Plus Jet and AH Plus sealers. Our findings were in accordance with previous reports that showed that AH Plus was significantly more radiopaque than Endosequence BC [1, 55]. The energy-dispersive X-ray spectroscopic analysis confirmed these results. AH Plus BC sealer had higher percentage of zirconium and a trace amount of tungsten as radiopacifiers in comparison to AH Plus Jet and AH Plus sealer, whereas TotalFill BC only contained a low percentage of zirconium. Zirconium oxide has been proposed as an alternative radiopacifier to limit the content of heavy metals and substitute bismuth oxide in calcium silicate-based materials [59, 60]. The higher radiopacity value of AH Plus BC, AH Plus, and AH Plus Jet may be related to the higher zirconium and additional tungsten components of the sealers and the lower radiopacity value of the TotalFill BC may be related to only zirconium ingredient as a radiopacifier element. The higher zirconium component of the AH Plus BC than TotalFill BC was in accordance with the literature [11, 12] and was confirmed by our EDX analysis.
In conclusion, in this in vitro laboratory study, resin-based sealer groups showed higher cytotoxicity values than the calcium silicate-based bioceramic sealer groups in both direct contact and extraction tests. Furthermore, the newly produced AH Plus BC sealer showed high radiopacity, high pH and low solubility values. Thus, this new calcium silicate- based bioceramic sealer can be proposed for clinical practice due to its similar properties with TotalFill BC, and better biological properties than AH Plus and AH Plus Jet. However, long-term clinical studies are needed to evaluate the effects of this newly produced sealer on the treatment outcome.
References
Mann A, Zeng Y, Kirkpatrick T et al (2022) Evaluation of the physicochemical and biological properties of EndoSequence BC Sealer HiFlow. J Endod 48:123–131. https://doi.org/10.1016/j.joen.2021.10.001
Al-Haddad AY, Kutty MG, Abu Kasim NH, Che Ab Aziz ZA (2017) The effect of moisture conditions on the constitution of two bioceramic-based root canal sealers. J Dent Sci 12:340–346. https://doi.org/10.1016/j.jds.2017.03.008
Rodriguez-Lozano FJ, Garcia-Bernal D, Onate-Sanchez RE, Ortolani-Seltenerich PS, Forner L, Moraleda JM (2017) Evaluation of cytocompatibility of calcium silicate-based endodontic sealers and their effects on the biological responses of mesenchymal dental stem cells. Int Endod J 50:67–76. https://doi.org/10.1111/iej.12596
Koch KA, Brave DG (2012) Bioceramics, part I: the clinician’s viewpoint. Dent Today 31:130–135
Prati C, Gandolfi MG (2015) Calcium silicate bioactive cements: biological perspectives and clinical applications. Dent Mater 31:351–370. https://doi.org/10.1016/j.dental.2015.01.004
Baldi JV, Bernardes RA, Duarte MA et al (2012) Variability of physicochemical properties of an epoxy resin sealer taken from different parts of the same tube. Int Endod J 45:915–920. https://doi.org/10.1111/j.1365-2591.2012.02049.x
De-Deus G, Scelza MZ, Neelakantan P et al (2015) Three-dimensional quantitative porosity characterization of syringe-versus hand-mixed set epoxy resin root canal sealer. Braz Dent J 26:607–611. https://doi.org/10.1590/0103-6440201300074
Donnermeyer D, Bürklein S, Dammaschke T, Schafer E (2018) Endodontic sealers based on calcium silicates: a systematic review. Odontology 107:421–436. https://doi.org/10.1111/iej.12662
Carvalho NK, Prado MC, Senna PM et al (2017) Do smear-layer removal agents affect the push-out bond strength of calcium-silicate based endodontic sealers? Int Endod J 50:612–619
Gokturk H, Bayram E, Bayram HM, Aslan T, Ustun Y (2017) Effect of double antibiotic and calcium hydroxide pastes on dislodgement resistance of an epoxy resin-based and two calcium silicate-based root canal sealers. Clin Oral Investig 21:1277–1282. https://doi.org/10.1007/s00784-016-1877-1
Chaves de Souza L, Teixeira Neves GS, Kirkpatrick T, Letra A, Silva R (2022) Physico-chemical and biological properties of AH Plus Bioceramic. J Endod 22:00715–00714. Advance online publication. https://doi.org/10.1016/j.joen.2022.10.009
Sanz JL, López-García S, Rodríguez-Lozano FJ et al (2022) Cytocompatibility and bioactive potential of AH Plus Bioceramic sealer: an in vitro study. Int Endod J 55:1066–1080. https://doi.org/10.1111/iej.13805
Kandemir Demirci G, Kaval ME, Kurt SM et al (2021) Energy-dispersive X-ray spectrometry analysis and radiopacity of five different root canal sealers. Braz Dent J 32:1–11. https://doi.org/10.1590/0103-6440202104638
International Organization for Standardization (ISO 10993-5) (2009) Biological evaluation of medical devices -Part 5: tests for in vitro cytotoxicity.
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Koulaouzidou EA, Roussou K, Sidiropoulos K et al (2018) Investigation of the chemical profile and cytotoxicity evaluation of organic components eluted from pit and fissure sealants. Food Chem Toxicol 120:536–543
Böke Sarıkahya N, Gören AC, Sümer Okkalı G, Çöven FO, Orman B, Kırcı D, Yücel B, Kışla D, Demirci B, Altun M, Önem AN, Nalbantsoy A (2021) Chemical composition and biological activities of propolis samples from different geographical regions of Turkey. Phytochem Lett 44:129–136. https://doi.org/10.1016/j.phytol.2021.06.008
Souza LC, Yadlapati M, Dorn SO, Silva R, Letra A (2015) Analysis of radiopacity, pH and cytotoxicity of a new bioceramic material. J Appl Oral Sci 23:383–389. https://doi.org/10.1590/1678-775720150065
Tanomaru-Filho M, Torres FFE, Chávez-Andrade GM et al (2017) Physicochemical properties and volumetric change of silicone/bioactive glass and calcium silicate-based endodontic sealers. J Endod 43:2097–2101. https://doi.org/10.1016/j.joen.2017.07.005
International Organization for Standardization (ISO 6876) (2012) Dental root canal sealing materials. Switzerland, Geneva
Aminoshariae A, Kulild JC (2020) The impact of sealer extrusion on endodontic outcome: a systematic review with meta-analysis. Aust Endod J 46:123–129. https://doi.org/10.1111/aej.12370
Poggio C, Arciola CR, Beltrami R et al (2014) Cytocompatibility and antibacterial properties of capping materials. ScientificWorldJournal 2014:181945. https://doi.org/10.1155/2014/181945
Ferreira GC, Pinheiro LS, Nunes JS et al (2022) Evaluation of the biological and physicochemical properties of calcium silicate-based and epoxy resin-based root canal sealers. J Biomed Mater Res B Appl Biomater 110:1344–1353. https://doi.org/10.1002/jbm.b.35004
Peters OA (2013) Research that matters-biocompatibility and cytotoxicity screening. Int Endod J 46:195–197. https://doi.org/10.1111/iej.12047
Sanz JL, Guerrero-Gironés J, Pecci-Lloret MP et al (2021) Biological interactions between calcium silicate-based endodontic biomaterials and periodontal ligament stem cells: a systematic review of in vitro studies. Int Endod J 54:2025–2043. https://doi.org/10.1111/iej.13600
Willershausen I, Wolf T, Kasaj A, Weyer V, Willershausen B, Marroquin BB (2013) Influence of a bioceramic root end material and mineral trioxide aggregates on fibroblasts and osteoblasts. Arch Oral Biol 58:1232–1237. https://doi.org/10.1016/j.archoralbio.2013.04.002
Bortoluzzi EA, Niu LN, Palani CD et al (2015) Cytotoxicity and osteogenic potential of silicate calcium cements as potential protective materials for pulpal revascularization. Dent Mater 31:1510–1522. https://doi.org/10.1016/j.dental.2015.09.020
Zordan-Bronzel CL, Tanomaru-Filho M, Rodrigues EM et al (2019) Cytocompatibility, bioactive potential and antimicrobial activity of an experimental calcium silicate-based endodontic sealer. Int Endod J 52:979–986. https://doi.org/10.1111/iej.13086
Pissiotis E, Spangberg LS (1991) Toxicity of Pulpispad using four different cell types. Int Endod J 24:249–257. https://doi.org/10.1111/j.1365-2591.1991.tb01150.x
Correa GT, Veranio GA, Silva LE, Hirata Junior R, Coil JM, Scelza MF (2009) Cytotoxicity evaluation of two root canal sealers and a commercial calcium hydroxide paste on THP1 cell line by Trypan Blue assay. J Appl Oral Sci 17:457–461. https://doi.org/10.1590/s1678-77572009000500020
Willershausen I, Callaway A, Briseno B, Willershausen B (2011) In vitro analysis of the cytotoxicity and the antimicrobial effect of four endodontic sealers. Head Face Med 7:15. https://doi.org/10.1186/1746-160X-7-15
Kapellos TS, Bonaguro L, Gemünd I, Reusch N, Saglam A, Hinkley ER, Schultze JL (2019) Human monocyte subsets and phenotypes in major chronic ınflammatory diseases. Front Immunol 10:2035. https://doi.org/10.3389/fimmu.2019.02035
Wang L, Zhu L, Duan C, Li L, Chen G (2020) Total saponin of Dioscorea collettii attenuates MSU crystal-induced inflammation via inhibiting the activation of the NALP3 inflammasome and caspase-1 in THP-1 macrophages. Mol Med Rep 21:2466–2474. https://doi.org/10.3892/mmr.2020.11035
Marchesan JT, Scanlon CS, Soehren S, Matsuo M, Kapila YL (2011) Implications of cultured periodontal ligament cells for the clinical and experimental setting: a review. Arch Oral Biol 56:933–943
Huang FM, Chang YC (2002) Cytotoxicity of resin-based restorative materials on human pulp cell cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 94:361–365
Silva EJ, Accorsi-Mendonça T, Pedrosa AC, Granjeiro JM, Zaia AA (2016) Long-term cytotoxicity, pH and dissolution rate of AH plus and MTA fillapex. Braz Dent J 27:419–423. https://doi.org/10.1590/0103-6440201600735
Zhu Q, Haglund R, Safavi KE, Spangberg LS (2000) Adhesion of human osteoblasts on root-end filling materials. J Endod 26:404–406
De-Deus G, Canabarro A, Alves GG, Marins JR, Linhares AB, Granjeiro JM (2012) Cytocompatibility of the ready-to-use bioceramic putty repair cement iRoot BP Plus with primary human osteoblasts. Int Endod J 45:508–513
Rodan SB, Imai Y, Thiede MA et al (1987) Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res 47:4961–4966
Pautke C, Schieker M, Tischer T et al (2004) Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res 24:3743–3748
Granchi D, Stea S, Ciapetti G, Cavedagna D, Stea S, Pizzoferrato A (1995) Endodontic cements induce alterations in the cell cycle of in vitro cultured osteoblasts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 79:359–366. https://doi.org/10.1016/s1079-2104(05)80230-6
Kangarloo A, Sattari M, Rabiee F, Dianat SO (2009) Evaluation of cytotoxicity of different root canal sealers and their effect on cytokine production. Iran Endod J 4:31–34
Li W, Zhou J, Xu Y (2015) Study of the in vitro cytotoxicity testing of medical devices. Biomed Rep. 3:617–620. https://doi.org/10.3892/br.2015.481
Candeiro GTM, Moura-Netto C, D'Almeida-Couto RS et al (2016) Cytotoxicity, genotoxicity and antibacterial effectiveness of a bioceramic endodontic sealer. Int Endod J 49:858–864. https://doi.org/10.1111/iej.12523
Srivastava GK, Alonso-Alonso ML, Fernandez-Bueno I, Garcia-Gutierrez MT, Rull F, Medina J, Coco RM, Pastor JC (2018) Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation. Sci Rep 23(8):1425. https://doi.org/10.1038/s41598-018-19428-5
Loushine BA, Bryan TE, Looney SW et al (2011) Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. J Endod 37:673–677. https://doi.org/10.1016/j.joen.2011.01.003
Zhang W, Li Z, Peng B (2010) Effects of iRoot SP on mineralization-related genes expression in MG63 cells. J Endod 36:1978–1982. https://doi.org/10.1016/j.joen.2010.08.038
Sari S, Duruturk L (2007) Radiographic evaluation of periapical healing of permanent teeth with periapical lesions after extrusion of AH Plus sealer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104:54–59. https://doi.org/10.1016/j.tripleo.2007.03.024
Teixeira L, Basso FG, Hebling J et al (2017) Cytotoxicity evaluation of root canal sealers using an in vitro experimental model with roots. Braz Dent J 28:165–171. https://doi.org/10.1590/0103-6440201701430
Oh H, Kim E, Lee S et al (2020) Comparison of biocompatibility of calcium silicate-based sealers and epoxy resin-based sealer on human periodontal ligament stem cells. Materials 20:5242. https://doi.org/10.3390/ma13225242
Campi LB, Torres FFE, Rodrigues EM, Guerreiro-Tanomaru JM, Tanomaru-Filho M (2022) Physicochemical and biological properties of new tricalcium silicate-based repair material doped with fluoride ions and zirconium oxide as radiopacifier. J Biomed Mater Res B Appl Biomater 110:862–870
Queiroz MB, Torres FFE, Rodrigues EM, Viola KS, Bosso Martelo R, Chavez-Andrade GM et al (2021a) Development and evaluation of reparative tricalcium silicate-ZrO2-Biosilicate composites. J Biomed Mater Res B Appl Biomater 109:468–476
Queiroz MB, Torres FFE, Rodrigues EM, Viola KS, Bosso Martelo R, Chavez-Andrade GM et al (2021b) Physicochemical, biological, and antibacterial evaluation of tricalcium silicate-based reparative cements with different radiopacifiers. Dent Mater 37:311–320
Zhou HM, Shen Y, Zheng W, Li L, Zheng YF, Haapasalo M (2013) Physical properties of 5 root canal sealers. J Endod 39:1281–1286. https://doi.org/10.1016/j.joen.2013.06.012
Lee JK, Kwak SW, Ha JH, Lee W, Kim HC (2017) Physicochemical properties of epoxy resin-based and bioceramic-based root canal sealers. Bioinorg Chem Appl:2582849. https://doi.org/10.1155/2017/2582849
Primus CM, Tay FR, Niu LN (2019) Bioactive tri/dicalcium silicate cements for treatment of pulpal and periapical tissues. Acta Biomater 96:35–54. https://doi.org/10.1016/j.actbio.2019.05.050
Al-Haddad A, Che Ab Aziz ZA (2016) Bioceramic- based root canal sealers: a review. Int J Biomater 2016:9753210. https://doi.org/10.1155/2016/9753210
Carvalho-Junior JR, Correr-Sobrinho L, Correr AB, Sinhoreti MA, Consani S, Sousa-Neto MD (2007) Radiopacity of root filling materials using digital radiography. Int Endod J 40:514–520. https://doi.org/10.1111/j.1365-2591.2007.01246.x
Touchefeu Y, Franken P, Harrington KJ (2012) Radiovirotherapy: principles and prospects in oncology. Curr Pharm Des 18:3313–3320. https://doi.org/10.2174/1381612811209023313
Walsh RM, He J, Schweitzer J, Opperman LA, Woodmansey KF (2018) Bioactive endodontic materials for everyday use: a review. Gen Dent 66:48–51
Acknowledgements
The authors thank Prof. Dr. Sema Çınar from Ege University School of Dentistry, Department of Periodontology for kindly donating HPDL cell lines for this study. AH Plus Bioceramic Sealer, AH Plus Sealer and AH Plus Jet Sealer to the study was provided by Dentsply Sirona (# E-2022-07).
The authors deny any conflicts of interest related to this study.
Funding
AH Plus Bioceramic Sealer, AH Plus Sealer, and AH Plus Jet Sealer to the study were provided by Dentsply Sirona (# E-2022-07).
Author information
Authors and Affiliations
Contributions
Conceptualization: Kandemir Demirci G, Çöven FO, Güneri P, Kaval ME. Data curation: Kandemir Demirci G, Güneri P, Köse T, Kaval ME. Formal analysis: Kandemir Demirci G, Köse T. Funding acquisition: Kandemir Demirci G. Investigation: Kandemir Demirci G, Çöven FO, Güneri P, Nalbantsoy A, Karavana SY, Kaval ME. Methodology: Kandemir Demirci G, Çöven FO, Güneri P, Kaval ME. Project administration: Kandemir Demirci G, Güneri P, Kaval ME, Nalbantsoy A, Karavana SY, Çöven FO. Resources: Kandemir Demirci G, Software: Kandemir Demirci G, Köse T. Supervision: Kandemir Demirci G, Güneri P, Kaval ME. Validation: Kandemir Demirci G, Güneri P, Çöven FO, Kaval ME. Visualization: Kandemir Demirci G, Güneri P, Çöven FO, Köse T. Writing - original draft: Kandemir Demirci G, Güneri P, Çöven FO, Kaval ME, Nalbantsoy A, Karavana SY. Writing - review & editing: Kandemir Demirci G, Güneri P, Çöven FO, Kaval ME, Nalbantsoy A, Karavana SY. All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kandemir Demirci, G., Çöven, F.O., Güneri, P. et al. The solubility, pH value, chemical structure, radiopacity, and cytotoxicity of four different root canal sealers: an in vitro study. Clin Oral Invest 27, 5413–5425 (2023). https://doi.org/10.1007/s00784-023-05160-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05160-6