Abstract
Background
Despite previous reports of robotic-assisted laparoscopic release for median arcuate ligament syndrome (MALS), the safety and efficacy profile of this approach has been difficult to establish due to the rarity of this diagnostic entity. We aim to present our experience from a tertiary minimally invasive surgery referral center.
Methods
A case series was performed whereby all patients who underwent robotic-assisted MAL release from July 2010 to July 2017 at our institution were included. Diagnosis of MALS was made based on consideration of symptom presentation, celiac artery duplex ultrasound, and corresponding findings on Computed Tomography (CT) or Magnetic Resonance Angiography (MRA). Outcomes up until the most recent clinic follow-up were reviewed.
Results
A total of 13 patients underwent robotic-assisted MAL release. Patients’ age ranged from 16 to 71 years (mean 38 years) and consisted primarily of females (76.9%). Most common presenting symptoms included postprandial pain (76.9%), weight loss (76.9%), nausea and vomiting (76.9%). Mean symptom duration was 3 years (range 1–10 years). No intraoperative complications. None required conversion to open surgery. One case required a conversion back to laparoscopy due to anatomical complexity. The mean operative time for successfully completed robotic cases was 94.6 min (range 52–120 min), and for all cases including converted case was 103.5 min (52–210 min). Mean follow-up duration was 19.7 months (range 1–77 months). During subsequent follow-up, a 30-day readmission rate of 23.1% was observed. All but one of the patients experienced prompt symptom improvement. Four patients had symptom recurrence during follow-up.
Conclusions
Our experience demonstrates that the robotic-assisted approach to MAL release may be safe and efficacious in selected patients. Prospective comparative studies are required to further evaluate its outcomes against conventional laparoscopic approach, the current gold standard.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Median arcuate ligament syndrome (MALS), or celiac artery compression syndrome, was first described in the 1960s by Harjola and Dunbar et al. [1,2,3]. Median arcuate ligament syndrome was hypothesized to arise from either a neurogenic etiology secondary to the compression of the celiac plexus or a vascular origin secondary to the compression of the celiac axis by the median arcuate ligament (MAL) [4, 5]. In a normal population, the median arcuate ligament typically crosses the anterior aspect of the aorta above the celiac origin between T11 and L1 level. However, an anatomic variant in which the ligament passes inferiorly, causing compression of the celiac artery and the celiac ganglion, has been found in 12–49% of the population [6, 7]. Because of the rich collateralization between the celiac and superior mesenteric arteries, the majority of these patients are asymptomatic [5]. Failure to compensate for this anatomic anomaly results in MALS, frequently presenting with a variety of symptoms including postprandial or post-exertional abdominal pain, weight loss, nausea, vomiting, bloating, diarrhea, and abdominal bruit [8,9,10,11].
Definitive treatment of MALS involves the surgical release of the MAL, either via an open, laparoscopic, or robotic-assisted laparoscopic approach. The feasibility of robotic-assisted MAL release was first reported by Jaik et al. in 2007 [12]. Due to the rarity of the disease, it remains an infrequently performed procedure, with only a handful of small case series in the literature [13,14,15,16]. Previous studies reported comparable outcomes between robotic and laparoscopic MAL release; however, early case series frequently reported a significantly longer operative time [14]. Despite hypothesized advantages of the robotic platform such as magnified stereoscopic visualization, improved dexterity, and increased range of motion, its true clinical benefits are still under investigation. We hereby aim to add our experience from a tertiary minimally invasive surgery center to the literature.
Materials and methods
Data collection and statistical analysis
A case series was performed including all patients that underwent robotic-assisted MAL release from July 2010 to July 2017 at UCLA Medical Center. Patients were identified from electronic medical record by Common Procedural Terminology (CPT) codes for decompression of the celiac artery and laparoscopic-assisted robotic-procedure. Variables regarding patient demographics, disease presentation, perioperative outcomes, and subsequent recovery during clinic follow-up were retrospectively reviewed.
The primary outcome of interest was symptom improvement, while secondary outcomes included operative time, perioperative complications, radiographic improvement, and symptom recurrence. Symptom resolution was subjectively categorized by the patients as not improved, improved, resolved, or transiently improved but subsequently recurred. Lack of improvement was defined as experiencing the same level of symptoms as prior to surgery. Improvement was defined as a greater than 80% reduction in pain and other symptoms, with the residual pain not affecting daily living functions while under oral pain medication. Symptom resolution was defined as complete disappearance of symptoms. Follow-up information including changes in symptomatology was retrieved from medical records as documented by the operative surgeons, as well as patient self-report. Patients were evaluated by the same attending surgeons, and the same assessment of symptoms was used throughout the study period. No surveys or questionnaires were used.
Descriptive analysis was used for baseline characteristics and outcome parameters. Data were presented in means or medians for those that were not normally distributed, along with standard deviation (SD) or range. Percentages were expressed along with the number of patients. Statistical analysis was performed using Microsoft Excel (Microsoft Corp., Redmond, WA). This study was approved by the UCLA Institutional Review Board.
Perioperative management
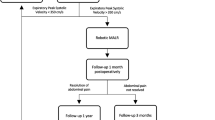
Patients with a presumptive diagnosis of MALS who were referred to UCLA Medical Center were evaluated by a multidisciplinary team of vascular and minimally invasive surgeons. After excluding other common differential diagnoses, patients underwent mesenteric duplex ultrasound and computed tomography (CT) or magnetic resonance (MR) angiography. Imaging findings consistent with MAL release included a focal narrowing in the proximal celiac axis or a characteristic hooked appearance on CT or MR angiography [17]. Diagnosis of MALS was made based on consideration of symptom presentation, celiac artery duplex ultrasound, and corresponding findings on Computed Tomography (CT) or Magnetic Resonance Angiography (MRA). An increase in celiac artery peak systolic velocity (PSV) during expiration to greater than 200 cm/s on duplex ultrasound was considered highly suggestive of MALS [18]. All patients with significant celiac artery compression with a history of postprandial abdominal pain or pain related to exercise, nausea, vomiting, weight loss, and an abnormal PSV on duplex ultrasound were offered a robotic-assisted laparoscopic MAL release. We excluded any patients who had previously undergone surgical MAL release, celiac artery angioplasty, and stenting and patients who had atherosclerosis of the celiac axis and/or celiac artery aneurysm. No routine postoperative imaging was performed unless patients complained of persistent or recurrent symptoms.
Operative technique
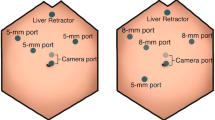
The patient was placed supine on the operating table and general anesthesia induced. An optical trocar was introduced through a peri-umbilical incision. Following establishment of pneumoperitoneum, additional trocars were placed (see Fig. 1). The patient was then positioned in steep reverse Trendelenburg in order to allow displacement of the small intestines from the supramesocolic area by gravity. Following insertion of the liver retractor, the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) was docked from the head of the bed. The gastrohepatic omentum was sharply incised to allow entry into the lesser sac. Continued dissection in a posterior fashion allowed visualization of the fibers of the right crus at the base of the crossing of the left and right crura. Hook cautery was used to divide these fibers posteriorly until the aorta above the celiac trunk was clearly visualized. Sequential division of these muscle fibers was carried out in a distal direction until the median arcuate ligament was encountered. The ligament was then divided using hook cautery resulting in very mild but visible anterior displacement of the celiac trunk. Adjacent adhesions were lysed until the proximal celiac axis along with its post-stenotic dilatation were widely exposed. At this point, the procedure was deemed complete. The trocars were removed under direct visualization and the incisions were closed in layers. (A video of the operative technique was made available for the online version.)
Results
Preoperative patient characteristics and imaging
A total of 13 patients underwent robotic-assisted MAL release during the study period. Patient characteristics are shown in Table 1. Patients’ age ranged from 16 to 71 years (mean 38 years) and consisted primarily of females (76.9%). Besides two young female patients with Body Mass Index (BMI) of 12.7 and 14.0 kg/m2, the majority of patients were either normal weight or obese, with BMI ranging between 20.0 and 32.7 kg/m2. Most of the patients had no other significant comorbidities, with a median Charlson comorbidity index of 0 (range 0–3) and a median ASA score of 2 (range 1–3). Besides one patient who had a previous Nissen fundoplication, none of the included subjects had prior foregut surgery.
The most common presenting symptoms included postprandial pain (76.9%), weight loss (76.9%), nausea and vomiting (76.9%), amongst others (tabulated in Table 2). Mean symptom duration was 3 years (range 1–10 years). Symptom onset timing was frequently not distinct and may be related to other ailments. For example, one of the patients had a history of portal venous thrombosis and had experienced chronic abdominal pain ever since an episode of hypertriglyceridemia-related acute pancreatitis.
Preoperative duplex ultrasound was done in 6 patients, among whom the mean PSV and standard deviation were 470.5 ± 88.9 cm/s during expiration and 236.2 ± 29.9 cm/s during inspiration. CTA and/or MRA were obtained in all patients, the results of which are shown in Table 3. High-grade stenosis was observed in 8 of the 13 patients and post-stenotic dilatation was observed in all patients who eventually received surgery.
Intraoperative outcomes
No conversion to laparotomy or intraoperative complication occurred in any of the included cases. However, there was one instance of conversion to conventional laparoscopic approach due to technical difficulties arising from the patient’s anatomy. Patient no. 3 with an exceptionally narrow body habitus, which necessitated unusually close placement of the working ports. This leads to frequent instrument clashing and compromised visualization occurred throughout the case. In addition, due to the long-standing nature of the patient’s disease, matted lymph nodes and chronic inflammation surrounding the origin of the celiac artery were encountered. Decision was initially made to utilize the robot’s articulated instruments for dissection around the celiac trunk and the supraceliac aorta. However, it became evident during the dissection that instrument clashing was impeding the progress of the procedure. The decision was finally made to convert to the conventional laparoscopic approach. An additional camera trocar placed closer to the operative field in order to circumvent a pendulous falciform ligament, which intermittently obstructed the robotic camera view. Besides the prolonged operative time in this particular instance (210 min), mean operative time for successfully completed robotic cases was 94.6 min (52 and 120 min). Mean operative time for all cases including converted case was 103.5 min (52–210 min).
Short-term outcomes
There was only one postoperative complication which required further intervention. One patient presented with shortness of breath, pain and decreased serum hemoglobin level on postoperative day 2, whereby a massive left hemothorax was found. The hemothorax resolved with tube thoracostomy without hemodynamic instability or any further intervention. The cause of the hemothorax was undetermined. Mean length of hospital stay was 2.5 days (range 2–6 days). The 30-day readmission rate was 23.1% (3 cases). Two of the readmissions were due to poorly controlled pain and dehydration while the third was related to a fever of unknown origin.
Long-term efficacy
The mean follow-up duration was 19.7 months (range 1–77 months). Clinical and radiographic improvements are summarized in Table 4. Following surgery, twelve patients (92.3%) endorsed immediate symptomatic improvement. There was one patient who never experienced symptom improvement. During subsequent follow-up, six of the thirteen patients had complete resolution of symptoms while two endorsed only partial improvement. Symptom recurrence occurred in four patients at variable intervals from surgery, ranging from 1 month to 2 years. Persistent high-grade focal stenosis was found on follow-up imaging in the patient who experienced symptom recurrence within 1 month, although further intervention was refused. Two of the patients with symptom recurrence underwent adjunctive balloon angioplasty after the robotic MAL release due to observation of persistent celiac artery narrowing on postoperative imaging. One patient had satisfactory resolution of symptoms but the other remained symptomatic despite undergoing a total of three adjunctive balloon angioplasties, even with post-procedural CT angiogram demonstrating a patent stent and no evidence of in-stent stenosis.
Discussion
Our experience represents one of the largest case series of robotic-assisted MAL release in the literature. Besides one intraoperative conversion to laparoscopic approach due to patient body habitus and one postoperative hemothorax, we have encountered no complications. Mean operative time was 94.6 min. All but one patient experienced immediate postoperative symptomatic improvement. Complete symptom resolution rate following MAL release was 38.5% while overall clinical improvement rate was 76.9%.
Since the introduction of the robotic surgical platform to foregut surgeries like such as MAL release [12], several case series have been published. However, due to the rarity of the condition, and incomplete understanding of its pathophysiology, accumulation of experience on robotic-assisted MAL release has been gradual. In 2011, Relles et al. reported a robotic-assisted MAL release case series consisting of three patients utilizing operative techniques described by Jaik et al. [12, 13]. Two of the three patients had symptom resolution at 11-month follow-up. Similar small case series were described by Meyer et al. and You et al. [15, 19]. The largest robotic-assisted case series was that reported by Thoolen et al., in which nine patients who underwent robotic-assisted MAL release all experienced symptom improvement at 25-week follow-up, although only a third had complete symptom resolution [16]. In 2013, Do et al. performed a retrospective comparative study comparing MAL release outcomes between robotic-assisted and conventional laparoscopic approach (4 vs. 12 patients). Two of the four patients in the robotic group had complete resolution of abdominal pain [14]. In our study, similar efficacy has been found whereby complete resolution of the symptoms was observed in 6 (46.2%) patients, 2 (15.4%) patients had partial improvement, 4 (30.8%) patients experienced symptom recurrence, and 1 (7.7%) patient never experienced symptom improvement.
While large comparative studies are still pending, we have found comparable outcomes between our robotic-assisted MAL release experience to figures associated with conventional laparoscopic or open approaches in the literature. With regard to immediate postoperative improvement, immediate response rate following open and laparoscopic MAL release have been quoted as 85% [10], whereas 92.3% of our patients experienced immediate postoperative improvement. For open or laparoscopic MAL release, the symptom recurrence rate has been variably quoted as ranging from 38 to 50% [10], while 30.8% of our patients experienced symptom recurrence.
Despite the high incidence of immediate postoperative symptom improvement, near one-third of the patients experienced symptom recurrence during subsequent follow-up. It is unclear whether these arose due to disease progression or technical failure from the initial operation. In the absence of an established pathophysiologic mechanism for MALS, management of symptom recurrence is often challenging with unpredictable outcomes. Balloon angioplasty and stenting are typically reserved for patients who have residual stenosis on postoperative imaging after extrinsic celiac artery decompression. However, due to limited evidence of its efficacy, the absolute indication for angioplasty remains a matter of debate. Roseborough et al. reported 6 of 15 patient who successfully underwent an adjunctive intervention; 2 patients underwent angioplasties; 3 stenting; and 1 celiac bypass [20]. Columbo et al. reported 5 of 7 patients remained symptom-free at the 6 months following subsequent celiac stent placement [21]. In our case series, the two patients who underwent angioplasty for postoperative symptom recurrence had mixed outcomes. Future research is warranted to establish risk factors and mechanisms of postoperative MALS recurrence in order to develop more effective management algorithms.
One unique aspect of our experience is the significantly shorter operative time than previously published robotic-assisted MAL case series. Early reports of robotic-assisted MAL release cited operative times ranging from 132 to 145.8 min [13,14,15,16, 19] while the mean operative time in our case series was 103.5 min (including one converted case). While operative time is likely influenced by a myriad of patient-, technique-, equipment-, and surgeon-related issues, we believe that our shorter operative time is likely associated with the surgeons’ experience with the surgical robotic platform and familiarity with other foregut procedures such as Nissen fundoplication. As surgeons’ comfort level with the surgical robotic system increases, prolonged operative time may no longer be a significant concern when adopting conventional laparoscopic procedures for robotic-assisted approach.
In addition to the reduced operative time, we found that incorporating the robotic system to MAL release procedures has offered several technical advantages. The field of dissection during MAL release procedures is usually deep and its direct view from the umbilicus frequently obstructed by organs such as the stomach and the pancreas. The articulated joints afforded by the robotic Endowrist technology allows surgeons to operate in this difficult-to-reached surgical field with ease and minimizes the need for assistant retraction. Moreover, other advantages of the robotic surgical system included improved ergonomics, and tremor minimization [22] may prove beneficial when performing sharp dissection near critical structures like the aorta for prolonged periods of time. Disadvantages of the robotic approach include the complete loss of tactile feedback, the equipment’s high capital cost, and the need of an experienced bedside surgical assistant in addition to the console surgeon. Whether the reduced operative time and improved surgeon ergonomics associated with adoption of the robotic approach outweigh these disadvantages depends on unique surgeon- and institutional-factors.
We acknowledge several limitations in our study. First, due to the retrospective design, follow-up duration was frequently limited. No questionnaires or surveys were used. Further prospective follow-up may potentially reveal more symptom recurrence instances than documented. In addition, due to the lack of a comparative group of laparoscopic cases performed for the same indication under the same setting, it is difficult to extrapolate our findings to postulate the equivalence of efficacy between robotic and laparoscopic MAL release. However, in view of the rarity of the condition, randomized prospective trials may not be feasible and relatively large case series such as ours may be the best-level evidence available for clinicians to base their practice on.
Conclusion
The results of our single-institution robotic-assisted MAL release case series showed that the incorporation of the robotic surgical platform is safe and feasible in selected patients and may be associated with reduced operative times. Robotic assistance offers excellent visualization and improved instrument dexterity, both of which facilitate dissection in deep spaces surrounding the celiac axis. While randomized controlled trials comparing robotic and laparoscopic approaches for MAL release may not become feasible, our experience from a tertiary minimally invasive surgical center has demonstrated that the robotic approach may be a safe and effective option for the surgical management of MALS.
References
Harjola PT (1963) A rare obstruction of the coeliac artery. report of a case. Ann Chir Gynaecol Fenn 52:547–550
Dunbar JD, Molnar W, Beman FF, Marable SA (1965) Compression of the celiac trunk and abdominal angina. Am J Roentgenol Radium Ther Nucl Med 95(3):731–744
Harjola PT, Lahtiharju A (1968) Celiac axis syndrome. Abdominal angina caused by external compression of the celiac artery. Am J Surg 115(6):864–869
Balaban DH, Chen J, Lin Z, Tribble CG, McCallum RW (1997) Median arcuate ligament syndrome: a possible cause of idiopathic gastroparesis. Am J Gastroenterol 92(3):519–523
Cornell SH (1971) Severe stenosis of the celiac artery. Analysis of patients with and without symptoms. Radiology 99(2):311–316
Bron KM, Redman HC (1969) Splanchnic artery stenosis and occlusion. Incidence; arteriographic and clinical manifestations. Radiology 92(2):323–328
Lindner HH, Kemprud E (1971) A clinicoanatomical study of the arcuate ligament of the diaphragm. Arch Surg 103(5):600–605
Harr JN, Haskins IN, Brody F (2017) Median arcuate ligament syndrome in athletes. Surg Endosc 31(1):476
Haskins IN, Harr JN, Brody F (2016) Exercise-related transient abdominal pain secondary to median arcuate ligament syndrome: a case report. J Sports Sci 34(13):1246–1249
Jimenez JC, Harlander-Locke M, Dutson EP (2012) Open and laparoscopic treatment of median arcuate ligament syndrome. J Vasc Surg 56(3):869–873
Desmond CP, Roberts SK (2004) Exercise-related abdominal pain as a manifestation of the median arcuate ligament syndrome. Scand J Gastroenterol 39(12):1310–1313
Jaik NP, Stawicki SP, Weger NS, Lukaszczyk JJ (2007) Celiac artery compression syndrome: successful utilization of robotic-assisted laparoscopic approach. J Gastrointestin Liver Dis 16(1):93–96
Relles D, Moudgill N, Rao A, Rosato F, DiMuzio P, Eisenberg J (2012) Robotic-assisted median arcuate ligament release. J Vasc Surg 56(2):500–503
Do MV, Smith TA, Bazan HA, Sternbergh WC 3rd, Abbas AE, Richardson WS (2013) Laparoscopic versus robot-assisted surgery for median arcuate ligament syndrome. Surg Endosc 27(11):4060–4066
You JS, Cooper M, Nishida S, Matsuda E, Murariu D (2013) Treatment of median arcuate ligament syndrome via traditional and robotic techniques. Hawaii J Med Public Health 72(8):279–281
Thoolen SJ, van der Vliet WJ, Kent TS, Callery MP, Dib MJ, Hamdan A et al (2015) Technique and outcomes of robot-assisted median arcuate ligament release for celiac artery compression syndrome. J Vasc Surg 61(5):1278–1284
Horton KM, Talamini MA, Fishman EK (2005) Median arcuate ligament syndrome: evaluation with CT angiography. Radiographics 25(5):1177–1182
Moneta GL, Lee RW, Yeager RA, Taylor LM Jr, Porter JM (1993) Mesenteric duplex scanning: a blinded prospective study. J Vasc Surg 17(1):79–84 (discussion 5–6)
Meyer M, Gharagozloo F, Nguyen D, Tempesta B, Strother E, Margolis M (2012) Robotic-assisted treatment of celiac artery compression syndrome: report of a case and review of the literature. Int J Med Robot 8(4):379–383
Roseborough GS (2009) Laparoscopic management of celiac artery compression syndrome. J Vasc Surg 50(1):124–133
Columbo JA, Trus T, Nolan B, Goodney P, Rzucidlo E, Powell R et al (2015) Contemporary management of median arcuate ligament syndrome provides early symptom improvement. J Vasc Surg 62(1):151–156
Maeso S, Reza M, Mayol JA, Blasco JA, Guerra M, Andradas E et al (2010) Efficacy of the Da Vinci surgical system in abdominal surgery compared with that of laparoscopy: a systematic review and meta-analysis. Ann Surg 252(2):254–262
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Usah Khrucharoen, Yen-Yi Juo, Yas Sanaiha, Yijun Chen, Juan C. Jimenez, and Erik P. Dutson have no conflicts of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 294447 KB)
Rights and permissions
About this article
Cite this article
Khrucharoen, U., Juo, YY., Sanaiha, Y. et al. Robotic-assisted laparoscopic median arcuate ligament release: 7-year experience from a single tertiary care center. Surg Endosc 32, 4029–4035 (2018). https://doi.org/10.1007/s00464-018-6218-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6218-9