Abstract
Purpose
To describe the abdominal wall reconstruction technique with an Ultrapro mesh and outcome for the repair of postoperative ventral hernias after the use of a Mercedes incision during the initial abdominal operation.
Method
A retrospective review of all the patients undergoing elective postoperative ventral hernia repair between 2013 and 2019. The cohort of these patients that had an initial Mercedes incision was used for this study.
Results
Fourteen patients met the criteria for this study. Thirteen of the patients were transplant patients (10 liver transplant and 3 combined pancreas and kidney transplant), and one patient was after a hepatectomy. Fifty-seven percent of these hernias were multiple defects. All the patients underwent the same repair of a modified Rives–Stoppa, transversus abdominis release, and a bilateral transverse plication. A partially absorbable Ultrapro mesh was used for all the patients, with two of the patients needing an additional Symbotex mesh in order to bridge a portion of the posterior fascia. There were 6 minor early postoperative complications (hematoma, superficial wound infection, and seroma) that did not require reoperation. Two patients were readmitted for observation of a wound hematoma, and two patients (14.2%) had recurrence during the follow-up period. The average length of hospitalization was 5.6 days.
Conclusion
This technique, with the use of an Ultrapro mesh, was found to be safe and effective for the repair of a postoperative ventral hernia due to an initial Mercedes incision.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
A bilateral subcostal incision with a midline extension (the Mercedes incision or inverted-Y incision) has been used in abdominal surgery for decades [1], especially when needing to access the liver (including transplantation), pancreas, or other foregut visera [2]. It is difficult to determine when exactly this incision came into common practice. Since the beginning of the 20th century, there have been strong advocates for transverse upper abdominal incisions with variations of extensions to the initial incision, followed by the landmark description of an infracostal incision by Kocher [3, 4]. The anatomical and physiological benefits initially reported for these non-vertical incisions included less pain, more rapid healing, less wound disruption, shorter hospitalization, less ileus, and significantly fewer postoperative ventral hernias [3, 5]. More recent data has also concluded that based on “anatomical principles” the transverse abdominal incisions are associated with fewer complications—including pain and pulmonary complications—with no significant difference in postoperative ventral hernia (POVH) occurrence [6].
The incision and closure type continues to be a highly researched and important aspect of patients’ care. This is especially relevant in patients receiving a Mercedes incision, principally in the surgical specialties of pancreas-hepatobiliary and transplant surgery, in which the patients are highly vulnerable and wound complications carry a high morbidity. This patient population receiving a Mercedes incision usually has many of the identified risk factors for wound complications and POVH formation—such as age, obesity, poor nutritional status, cancer diagnosis, ascites, and/or the need for immunosuppressive therapy [7]. A wide range of POVH occurrences has been reported after a Mercedes incision, from 7.6 to over 31% [8].
In addition to the patient-related risk factors for a POVH mentioned above, there are also anatomical and technical aspects to a Mercedes incision that augment the risk for POVH occurrence and increase the difficulty of a POVH repair with acceptable short- and long-term outcomes. For example, reports have emphasized the potential increased vulnerability to ischemia at the trifucta point of the incision, the increased morbidity when having to injure both rectus abdominis muscles, and the higher rate of complications with a longer incision [7, 9, 10].
Once a POVH has formed, the repair must take into consideration the patient and anatomical characteristics in order to decrease morbidity and recurrence. There is a paucity of data reporting how to adequately repair these challenging POVH after a Mercedes abdominal incision. Here, we present our novel approach to repairing these hernias from the experience of our operative teams led by a single senior surgeon.
Materials and methods
A retrospective analysis was conducted from the medical records of patients admitted for elective POVH repair from 2013 to 2019. This patient population comes from Tel Aviv Sourasky Medical Center a tertiary hospital in central Israel, that is a hepatobiliary, transplant center, and referral hospital for complex abdominal wall defects. Of these patients, the cohort with a POVH secondary to a previous Mercedes incision was included for this study. Fourteen patients met the requirements. The patients excluded had emergent surgery, laparoscopic surgery, or a POVH not related to a Mercedes incision. All the patients operated on in this cohort were under the supervision of a single senior surgeon and different surgical residents. Patient demographics, comorbidities, and previous abdominal operational history were evaluated. Operative data, complications (intraoperative and postoperative), and outcomes were all analyzed. The study met all institutional ethical standards and requirements.
Surgical technique
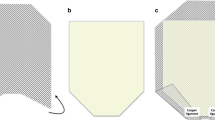
The surgery is performed under general endotracheal intubation with the patient in supine position. Depending on the extent of the hernia, the patients need for full anticoagulation, and anesthesias preference, an epidural catheter is placed for intraoperative and postoperative pain control. Preoperative antibiotics (first-generation cephalosporin) and venous thromboembolism prophylaxis were given. The basic principles of a modified Rives–Stoppa technique [11, 12] with transversus abdominis release (TAR) [13] were incorporated in all of these procedures. The key steps/principles we observed are as follows. Our operation always is conducted through a midline incision, even if the hernia is from one of the subcostal extensions. We always excise the previous scar together with the hernia sac (Fig. 1). The peritoneum is entered, and all adhesions to the anterior and lateral abdominal wall are taken down. We do not manipulate adhesions that are not involving the abdominal wall. Via the midline, we access the retrorectus plane and perform a myofascial release until the semilunaris line on each side with preservation of neurovascular bundles. Medial to the semilunaris line, we open the facia in order to access the fibers of the transversus abdominis muscle in order to perform a TAR. Where the subcostal incisions intersects with the midline, we use digital blunt dissection to form a plane where the scar tissue has formed at the intersection and free the entire length of the scars (right and left) from the underlying fascia (Fig. 2). Once the plane is established, and as previously described by Tastaldi et al., we perform the TAR above and below the right and left subcostal scars with full lateral mobilization [14]. After TAR, and in the retrorectus preperitoneal space, dissection is continued in standard form cranially and caudally. Cranially, we emphasized the importance of a generous retroxiphoid dissection in order to have maximal expansion of the mesh past the defect. Caudally, the dissection is continued into the retropubic space of Retzius past the pubic symphysis in order to have maximal expansion of the mesh and enough tissue for a tension-free closure of the fascia.
Once the entire plane is formed, we reinforce the left and right subcostal scars by transverse plication using a 2-0 nylon suture. This is done with or without the presence of a hernia defect. We believe this reinforces the inherently weaker scar tissue and reproximates the rectus abdominis for optimal healing. There is also critical injury of the posterior rectus sheath and transversus abdominis fascia due to the transverse incisions damaging the blood supply to the rectus abdominis muscles and corresponding fascias (as mentioned above). This plication with a permanent suture we believe also counters this traumatic injury (Fig. 3). Next, we recreate the midline by closing the posterior rectus sheaths also with a 2-0 nylon suture. If the posterior rectus sheaths are not able to be approximated without tension, we use a Symbotex composite mesh (Medtronics, Minneapolis, MN USA) to bridge the fascia defect with 2-0 prolene sutures to secure the mesh.
After the posterior rectus sheath is closed, we place a large 30 × 30 cm Ultrapro mesh (Ethicon, LLC., Cincinnati, OH, USA ), a partially absorbable macro-pore lightweight mesh. This mesh is secured to the fascia using 2-0 prolene U-shaped sutures. Overlap past the hernia defect was always adequate (greater than 5 cm) using this 30 × 30 cm-sized mesh (Fig. 4). Also of importance was that the mesh expands beyond the transverse scar plications. If not adequate, we would have tied two Ultrapro meshes together with a prolene suture in order to guarantee 5 cm overlap of the mesh past the hernia defect. These sutures are placed through our midline incision and not transcutaneous. The anterior fascia is closed by a running Connell technique using a Polydioxanone (PDS) 0 suture. Routinely drains are not placed after the repair. Individual vicryl 3-0 sutures are placed subcutaneously to approximate the wound, and the skin is closed with running 3-0 monocryl sutures.
Results
Fourteen patients during the study time had an elective POVH repair secondary to a previous Mercedes incision by the lead surgeon on this study. There were eleven males and three females with a median age of 51 years. The average BMI at the time of the POVH repair was 28. Two of the patients (14.2%) had previous POVH repairs; one of these two had two previous repairs. The index operations utilizing a Mercedes incision were 10 (71.4%) liver transplants, 3 (21.4%) combined pancreas and kidney transplants, and 1 (7.1%) hepatectomy. The cohort’s demographics, comorbidities, and surgical history are summarized on Table 1.
The intraoperative finds were that six of the patients (43%) had multiple fascial defects and 8 (57%) had single fascial defects. Of those with single defects, 75% were under the midline incision and 25% were under a lateral aspect of the incision. In 12 of the patients (85.7%), the posterior rectus sheath was able to be reconstructed, and in all but one patient (92.8%), we were able to also close the anterior rectus sheath. In all 14 patients, a retrorectus mesh was placed (Ultrapro), and in two patients a bridging mesh (Symbotex composite mesh) was used between the posterior rectus sheaths and followed by a retrorectus mesh placed anterior. The average largest diameter was 8.6 cm with a range of 5 to 18 cm. All the surgeries were categorized as clean with no reported contamination or signs of local wound infection. The length of hospitalization was on average 5.6 days with a range of 2–12 days. No patients required the intensive care, and all were extubated after their surgery. Table 2 summarizes the operative details. Table 3 shows the measurements of each hernia defect.
The average follow-up time was 25.9 months with a range of 7–49. The short lower limit of our follow-up range was because that was when one of the recurrences occurred (after 7 months). Early postoperative complications included 3 (21.4%) hematomas that did not affect the patient’s hemodynamics or require blood transfusion, 1 (7.4%) superficial surgical site infection that was successfully treated with antibiotics, and 2 (14.2%) seromas. There was no dehiscence or other major or minor complications. Two patients (14.2%) were readmitted for observation after hematomas formed. During the follow-up period, 2 patients (14.2%) were found to have recurrence in which both were symptomatic. The recurrence on both patients was in the midline portion of the scar at site of previous hernia and not a lateral extension. The postoperative outcomes and follow-up course is summarized in Table 4.
The two patients who had recurrence are further described in Table 5. One was a transplant patient on steroids (along with other immunosuppressants), and the other after a hepatectomy for hepatocellular carcinoma. Both initial repairs did not require bridging of the posterior rectus sheath. Only the transplant patient had a significant postoperative course with a non-symptomatic seroma that did not require intervention. Neither recurrences required emergent surgery for the hernia.
Discussion
Here, we describe our experience and the technique of a single surgeon in repairing POVH secondary to a Mercedes abdominal incision. The combination of a complex anatomy and patients with multiple comorbidities (including immunosuppression) make the surgical technique critical in decreasing morbidity, complications, and recurrence. Despite exponential advances in surgery, there has not been a significant decrease in occurrence over the last decades [15], and for this cohort, incisional hernias continue to be a common cause of morbidity and hospital cost [16].
The Mercedes incision remains a common incision for pancreatohepatobiliary surgeons, especially for transplant procedures. Nevertheless, in the recent years, studies have shown specific disadvantages to this incision when compared to alternative incision types. For example, a study from Memorial Sloan–Kettering found an increased POVH after Mercedes incision for hepatectomy when compared to an extended right subcostal incision [7]. They hypothesize that ischemia at the trifurcation point was a probable cause and risk factor for POVH occurrence. They sighted from ‘authors experience’ that this was the most common site of the hernia occurrence. With regard to orthotopic liver transplantations, studies have shown significantly less wound-related complications (surgical site infections and POVH) without compromising exposure when using a J-shape incision instead of the Mercedes incision [9, 10].
It has been shown that specific closure techniques are not a risk factor for POVH, but rather the patients’ underlying pathology and immunosuppressive regimen [17]. Therefore, an anatomic basis most probably also has influence on the formation of a POVH after a Mercedes incision. One study demonstrating an adverse anatomical effect showed clear atrophy of both rectus abdominis muscles after a Mercedes incision, which did not occur with midline incision. This was speculated to be because of denervation from the intercostal and subcostal branches innervating the rectus abdominis [18]. From the plastic surgery literature, Mercedes-like skin and subcutaneous incisions have been shown to have a better blood flow supply and less soft tissue breakdown when compared to inverted-T incisions with horizontal and vertical junctions further inferior on the abdominal midline (the Fleur-de-Lis incision) [19]. This may be a reason why we, nor the literature, have had no skin necrosis or deep wound infections.
There is a lack of data describing the technique and outcomes of POVH repair after a Mercedes incision. The studies that exist also mainly focus on only transplant patients, instead of the initial incision type, and there is also a wide variety of mesh types used without clear evidence-based recommendations. Examples of the heterogeneity of the research include a study by Perrakis et al. from Germany describing their technique of “inlay/onlay” after OLT in which only J-incisions were used [20], along with a group from Italy and France (Piardi et al.) describing the use of a polypropylene or intraperitoneal dual mesh after either a purely transverse incision or a transverse incision with a midline extension (unclear if this is a cranial or caudal extension) [21].
One of the strongest papers is by Tastaldi et al. and the group from Cleveland OH [14], who repaired all the hernias with a clear standard operative technique using TAR and a modified Rives–Stoppa, as in our study. Here, they used a variety of meshes, all of which were non-absorbable. Their recurrence rate was 25% and mainly involved those repaired with Versatex mesh. This group also suspected central mesh fractures (CMF) as the main stimulus for eight of these recurrences. In our series, we were unable to conclude if the recurrences were due to CMF. They concluded that further studies are needed using the same technique with different mesh selection.
In our practice, we used an Ultrapro mesh, which is a synthetic, partially absorbable lightweight mesh composed of the non-absorbable polypropylene and the absorbable polyglecaprone 25 (monocryl), which is absorbed within approximately 90 days [22]. Research has shown that this low-weight large (macro) pore mesh had good incorporation, adequate tensile strength, and the least shrinkage when compared to other meshes [23, 24]. Large pore and lightweight meshes have been shown not to compromise the strength of the repair or increase the chance of recurrence partially because of their low foreign body reaction and strong integration of local tissue [25, 26]. Smaller studies have shown that when compared to non-absorbable meshes there are no increased wound complications or seroma formation [27]. Over the years, we have found the Ultrapro mesh is easy to use, with low complications and recurrences. The unique flexibility of this soft mesh we believe adds to its strength and ability to incorporate well into surrounding tissue. Also, we have found an increase of patient satisfaction because they do not feel a ‘stiff’ material in the abdominal wall during movement. Biomechanical and technical questions remain regarding the objective retention force of a large (30×30 cm) Ultrapro mesh, its ability for strong scar tissue during the healing process, and the retention forces of our plication (compared to a non-plicated cohort). Further studies are indicated investigating these biomechanics and the incorporation process during healing of an Ultrapro mesh (and other meshes), especially taking into consideration immunosuppression and a difficult population such as transplant patients.
There are a few recent reports suggesting the successful use of the laparoscopic repair of a POVH after liver transplants [28], due to decreased infection rates and less morbidity in an already vulnerable patient population [29]. Currently, there remains an insufficiency of data and long-term follow-up needed to recommend this technique. Our department always considered a laparoscopic approach (or hybrid approach), yet specifically for this cohort, these hernias were determined too complex and large to adequately treat laparoscopically.
In conclusion, we have had success in this small group with repairing POVH after a Mercedes incision utilizing a modified Rives–Stoppa technique with TAR and the use of a macro-pore lightweight partially absorbable mesh. Our study is limited by the small patient size and its retrospective nature. Large randomly controlled prospective trials are needed to determine the optimal mesh type and anatomical placement. The patients’ factors (transplant patients versus non-transplant patients), mesh type, and surgical technique all need to be considered when attempting to determine evidence-based recommendations.
References
Dagradi A, Brearley R (1962) The surgery of hepatic tumours. Postgrad Med J 38(446):670
Calne RY (1978) Transplantation of the liver. Ann Surg 188(2):129
Sanders RL (1936) Transverse incision in the upper abdomen: its anatomic and physiologic advantages. Ann Surg 104(1):74
McGreevy PS, Miller FA (1969) Biography of Theodor Kocher. Surgery. 65(6):990–999
Lord RS, Crozier JA, Snell J, Meek AC (1994) From the Surgical Professional Unit SV. Transverse abdominal incisions compared with midline incisions for elective infrarenal aortic reconstruction: predisposition to incisional hernia in patients with increased intraoperative blood loss. J Vasc Surg 20(1):27–33
Grantcharov TP, Rosenberg J (2001) Vertical compared with transverse incisions in abdominal surgery. Eur J Surg 167(4):260–267
D’Angelica M, Maddineni S, Fong Y, Martin RC, Cohen MS, Ben-Porat L, Gonen M, DeMatteo RP, Blumgart LH, Jarnagin WR (2006) Optimal abdominal incision for partial hepatectomy: increased late complications with Mercedes-type incisions compared to extended right subcostal incisions. World J Surg 30(3):410–418
Davey S, Rajaretnem N, Harji D, Rees J, Messenger D, Smart NJ, Pathak S (2020) Incisional hernia formation in hepatobiliary surgery using transverse and hybrid incisions: a systematic review and meta-analysis. Ann R Coll Surg Engl (0):1–9
Wiederkehr JC, Igreja MR, Goncalves N, Nogara MS, Sequinel AP, Sampaio AL, Montemezzo GP, Wiederkehr HA, Wassen MP (2015) Comparison of J-shaped incision and Mercedes incision for liver transplantation. J Surg 3(1):3
Heisterkamp J, Marsman HA, Eker H, Metselaar HJ, Tilanus HW, Kazemier G (2008) AJ-shaped subcostal incision reduces the incidence of abdominal wall complications in liver transplantation. Liver Transpl 14(11):1655–1658
Maman D, Greenwald D, Kreniske J, Royston A, Powers S, Bauer J (2012) Modified Rives-Stoppa technique for repair of complex incisional hernias in 59 patients. Ann Plast Surg 68(2):190–193
Sakorafas G, Sarr MG (2002) Repair of ventral and incisional hernias using modifications of the Rives-Stoppa technique. Probl Gen Surg 19(4):51–58
Novitsky YW, Elliott HL, Orenstein SB, Rosen MJ (2012) Transversus abdominis muscle release: a novel approach to posterior component separation during complex abdominal wall reconstruction. Am J Surg 204(5):709–716
Tastaldi L, Blatnik JA, Krpata DM, Petro CC, Fafaj A, Alkhatib H, Svestka M, Rosenblatt S, Prabhu AS, Rosen MJ (2019) Posterior component separation with transversus abdominis release (TAR) for repair of complex incisional hernias after orthotopic liver transplantation. Hernia. 23(2):363–373
Jenkins TP (1980) Incisional hernia repair: a mechanical approach. Br J Surg 67(5):335–336
Gillion JF, Sanders D, Miserez M, Muysoms F (2016) The economic burden of incisional ventral hernia repair: a multicentric cost analysis. Hernia. 20(6):819–830
Perkins JD (2007) Incisional hernia following liver transplantation: today’s incidence and causes of this pesky problem. Liver Transpl 13(9):1339–1342
Matsumoto K, Noda T, Eguchi H, Iwagami Y, Akita H, Asaoka T, Gotoh K, Kobayashi S, Marubashi S, Umeshita K, Mori M (2019) Atrophy of the rectus abdominis after left-side donor hepatectomy: comparison of upper abdominal midline vs mercedes incision. Transplant Proc 51(5):1496–1501 Elsevier
Khansa I, Janis JE (2018) Complex open abdominal wall reconstruction: management of the skin and subcutaneous tissue. Plast Reconstr Surg 142(3S):125S–132S
Perrakis A, Knüttel D, Rahimli M, Andric M, Croner RS, Vassos N (2020) Incisional hernia after liver transplantation: mesh-based repair and what else? Surg Today:1–5
Piardi T, Audet M, Panaro F, Gheza F, Cag M, Portolani N, Cinqualbre J, Wolf P (2010) Incisional hernia repair after liver transplantation: role of the mesh. Transplant Proc 42(4):1244–1247 Elsevier
Biondo-Simões MD, Sichciopi AA, Ioshii SO, Robes RR, Biondo-Simões R (2018) Comparative study of fibrosis induced by Marlex®, Parietex Composite®, Vicryl® and Ultrapro® meshes1. Acta Cir Bras 33(9):792–798
Burger JW, Halm JA, Wijsmuller AR, ten Raa S, Jeekel J (2006) Evaluation of new prosthetic meshes for ventral hernia repair. Surg Endosc Other Interv Tech 20(8):1320
Schug-Pass C, Tamme C, Sommerer F, Tannapfel A, Lippert H (2008) Köckerling F. A lightweight, partially absorbable mesh (Ultrapro) for endoscopic hernia repair: experimental biocompatibility results obtained with a porcine model. Surg Endosc 22(4):1100–1106
Schumpelick V, Junge K, Rosch R, Klinge U, Stumpf M (2002) Retromuscular mesh repair for ventral incision hernia in Germany. Der Chirurg Z Alle Gebiete Oper Medizen 73(9):888–894
Schumpelick V, Klinge U, Rosch R, Junge K (2006) Light weight meshes in incisional hernia repair. J Minim Access Surg 2(3):117
Seiler C, Baumann P, Kienle P, Kuthe A, Kuhlgatz J, Engemann R, v Frankenberg M, Knaebel HP (2010) A randomised, multi-centre, prospective, double blind pilot-study to evaluate safety and efficacy of the non-absorbable Optilene® Mesh Elastic versus the partly absorbable Ultrapro® Mesh for incisional hernia repair. BMC Surg 10(1):21
Garmpis N, Spartalis E, Schizas D, Patsouras D, Damaskos C, Spartalis M, Garmpi A, Nikiteas NI, Dimitroulis D (2019) Incisional hernias post liver transplantation: current evidence of epidemiology, risk factors and laparoscopic versus open repair. A review of the literature. In Vivo 33(4):1059–1066
Goodney PP, Birkmeyer CM, Birkmeyer JD (2002) Short-term outcomes of laparoscopic and open ventral hernia repair: a meta-analysis. Arch Surg 137(10):1161–1165
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Disclaimer
No animals were used in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Goldstein AL is an equal contributor to the first author
Rights and permissions
About this article
Cite this article
Nevo, N., Goldstein, A.L., Yakubovsky, O. et al. How-we-do-it: the repair of postoperative ventral hernias after a Mercedes abdominal incision. Langenbecks Arch Surg 406, 2117–2123 (2021). https://doi.org/10.1007/s00423-021-02087-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-021-02087-y