Abstract
Purpose
To determine the influence of mild non-foveal involving epiretinal membrane (ERM) on visual outcome in eyes with multifocal intraocular lens (MIOL) implantation.
Methods
Patients with history of MIOL implantation were screened for the presence of ERM using spectral-domain optical coherence tomography (SD-OCT) at postoperative 6 months. Ninety-one eyes with mild non-foveal involving ERM and history of MIOL implantation were compared with 83 age-matched controls without ERM and history of MIOL implantation. The visual acuity (corrected and uncorrected) and visual quality (contrast sensitivity, Strehl ratio, area ratio, and higher-order aberrations; HOAs) of the eyes with mild non-foveal involving ERM were compared with the data of the age-matched control group.
Results
There was no difference in visual acuity between the groups at baseline and postoperative 6 months. The mild non-foveal involving ERM group showed significantly low contrast sensitivity at a visual angle of 4.0°, 2.5°, 1.0°, and 0.64° under scotopic conditions (P = .048, P = .025, P = .003, and P = .02, respectively) and 4.0°, 1.0°, and 0.64° under photopic conditions (P = .028, P = .002, and P = .001, respectively). The mean area ratio of the mild non-foveal involving ERM group was 45.13 ± 10.93, which was significantly lower than that of the control group, which measured 50.34 ± 12.66 (P = .044).
Conclusion
A mild non-foveal involving ERM has no effect on visual acuity, but it reduces visual quality in eyes with MIOL implantation. A thorough screening using SD-OCT is warranted for this condition when considering MIOL implantation.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epiretinal membrane (ERM) is defined as a cellular proliferation at the inner retinal surface [1, 2]. The reported incidence of ERM varies greatly depending on the study population and imaging devices that are used for ERM detection [3,4,5,6,7,8,9]. In the Alienor Study, spectral-domain optical coherence tomography (SD-OCT) detected ERMs with an incidence of 69.9% [9], whereas conventional retinal photographs detected ERMs with an incidence of 34.1% using the same dataset. These data suggest that there is significant discrepancy in ERM identification between the imaging methods and the interpreting clinicians.
Implantation of a multifocal intraocular lens (MIOL) increases a patient’s quality of life by reducing the dependency on eyeglasses after surgery. As the optical design has improved to minimize light loss, patient satisfaction levels have increased after MIOL implantation [10]. Current consensus on the indications for MIOL implantation depends on the patient’s lifestyle, personal character, and eye physiology [11]. Eyes with macular diseases that significantly reduce macular function are contraindicated for MIOL implantation [11]. On the other hand, the decision for eyes with macular pathology that does not affect vision is challenging. The most commonly encountered condition is ERM as a result of its high incidence as discussed previously.
The effect of ERMs on visual acuity varies significantly from no symptoms to decreased vision with metamorphopsia. This variation depends on ERM location, thickness, transparency, and contractility [12, 13]. Although eyes with deteriorating visual quality due to ERMs are not candidates for MIOL implantation, the question arises whether or not eyes with clinically insignificant ERMs that do not affect vision are candidates for MIOL surgery. Furthermore, it is not common practice to perform SD-OCT as a part of the preoperative evaluation for cataract surgery, which may result in an underestimation of the number of mild non-foveal involving ERMs preoperatively.
In the present study, we retrospectively reviewed the SD-OCT images of eyes that underwent cataract surgery, and we compared the visual prognosis of MIOL implantation in eyes with a mild non-foveal involving ERM and in eyes that did not have an ERM preoperatively.
Material and methods
A retrograde chart review was performed on eyes with a history of MIOL implantation and SD-OCT data at postoperative 6 months from April 2018 to December 2019 at the KEYE Eye Center, Seoul, Korea. To eliminate confounding factors from MIOL type, the inclusion was limited to the eyes with Panoptix TFNT IOL (Alcon Laboratories, Inc., Fort Worth, TX) MIOL. The eyes with macular diseases, such as age-related macular degeneration, diabetic macular edema, retinal vascular occlusions, significant ERM, or a previous history of macular surgery, are not indicated for MIOL implantation in our institution. Eyes with intraoperative capsular damage or any kind of postoperative complication, such as cystoid macular edema, were also excluded. In cases where both eyes from a patient were eligible to be enrolled, only the right eye was included for analysis. To increase the efficiency of the study, we randomly selected an “age-matched control group” by “individual matching,” among patients with a history of phacoemulsification and MIOL implantation but no ERM. This study was approved by the Institutional Review Board (IRB)/Ethics Committee of KEYE EYE Center (IRB number P12361001-001). The study protocol adhered to the tenets of the Declaration of Helsinki.
Review of the preoperative ocular examinations was performed, including monocular uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), and uncorrected near visual acuity (UNVA); refraction; intraocular pressure measurement; biomicroscopic examination; indirect binocular ophthalmoscopy; optical biometry with a partial coherence interferometry device (IOL master, Carl Zeiss Meditec, Jena, Germany); corneal topography using the Pentacam Scheimpflug System (Oculus Inc., Berlin, Germany); ultra-widefield fundus photography (UWFP; Optos Optomap Panoramic 200A Imaging System; Optos plc, Dunfermline, Scotland); SD-OCT version 5 (Heidelberg Engineering, Heidelberg, Germany); and Cirrus HD-OCT (Carl Zeiss Meditec, Inc., Dublin, California). The ocular root mean square (RMS) of higher-order aberrations (HOAs) at 4 mm was measured with Pentacam Scheimpflug System. The presence of metamorphopsia was detected using an Amsler grid, and the presence of macropsia was detected using a double dot chart (DDC) as was developed by the author previously [10].
Contrast sensitivity using the CGT-2000 instrument (Takagi, Seiko, Japan) and OPD-Scan III (NIDEK Co. Ltd., Aichi, Japan) was examined at postoperative month 6 to assess the quality of vision. The ocular root mean square (RMS) of higher-order aberrations (HOAs), the Strehl ratio of the point spread function (PSF), and the modulation transfer function (MTF) from the postoperative RMS of the total ocular wave aberration Z (1 ≤ n ≤ 8) were assessed for a pupil diameter of 5.0 mm. The MTF was analyzed using the area ratio method.
The SD-OCT was routinely checked unless there was a severe media opacity. Both quantitative and qualitative analyses of SD-OCT were done to analyze regional retinal thickness, severity of ERM, and the presence of posterior vitreous detachment (PVD). The regional retinal thickness according to the 1-mm, 3-mm, and 6-mm Early Treatment Diabetic Retinopathy Study (ETDRS) map was obtained at baseline and postoperative month 6 by the built-in program on the SD-OCT instrument. The severity of the ERM was graded as previously defined by Delyfer et al. [9]: (1) stage 1 or continuous hyperreflectivity (Fig. 1a), presence of a continuous hyperreflective signal at the inner retinal surface on at least three consecutive sections of the macular cube; (2) stage 2 or mature ERM without foveal involvement (Fig. 1b), stage 1 associated with retinal folds but without alterations of the foveal depression.
Grading of epiretinal membrane (ERM) by spectral-domain optical coherence tomography (SD-OCT) scan. a Stage 1: presence of a continuous hyperreflective signal at the inner retinal surface on at least three consecutive sections of the macular cube. b Stage 2: stage 1 associated with retinal folds but without alterations of the foveal depression
Statistical analysis
SPSS, version 15.0 for Windows (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Descriptive data were recorded as mean ± standard deviation (SD) unless otherwise specified. Visual acuity was recorded as a Snellen value and then converted to logarithm of the minimum angle of resolution (logMAR) scale for statistical analysis. Student t test or Mann–Whitney U test was used for the comparison of continuous variables between groups. Chi-square test was used for the comparison of categorical variables between groups. Paired t test was used to compare reginal macular thickness, visual acuity, and contrast sensitivity before and after cataract surgery. Pearson correlation coefficient was determined to assess the association between continuous variables, according to the normality of distribution. Significant factors in the univariate analysis were entered into a multivariate logistic regression model to assess factors associated with decreasing in contrast sensitivity at night. A P value < 0.05 was considered statistically significant.
Results
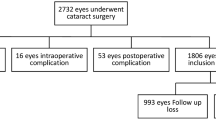
Among 2187 eyes with a history of TFNT implantation and a postoperative OCT data, 91 eyes (4.16%) with mild non-foveal involving ERM detected with SD-OCT. We randomly selected 83 age-matched control eyes from the eyes with a history of MIOL implantation. Table 1 shows the demographic and preoperative ocular characteristics of enrolled patients. There was no difference in the age, gender, presence of diabetes, hypertension, laterality, and other preoperative ocular parameters, such as pupil size, Chang-Waring (CW)-chord, SE, TCRP4, WTW, IOP, AXL, ACD, LT, and CST (P = 0.495, P = 0.104, P = 0.540, P = 0.386, P = 0.223, P = 0.600, P = 0.148, P = 0.803, P = 0.839, P = 0.522, P = 0.592, P = 0.941, P = 0.259, P = 0.469, and P = 0.455, respectively). Patients with mild non-foveal ERM group showed significantly higher TCIA than the patients without ERM (P = 0.019).
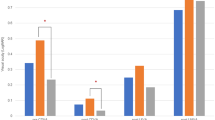
Figure 2 shows the changes in visual acuity in both groups. UDVA and UNVA improved significantly in both groups at postoperative months 1, 2, and 6 (P < 0.001, for all analyses; Fig. 2a and b). CDVA also improved significantly in both groups at postoperative months 1, 2, and 6 (P = 0.008, P = 0.006, and P = 0.004 for the control group and P = 0.001, P < . 001, and P < 0.001 for the mild non-foveal involving ERM group; Fig. 2c). There was no difference in visual acuity at 6 months (P = 0.081 for UDVA, P = 0.295 for UNVA, and P = 0.657 for CDVA). Eighty-two of 83 eyes (98.8%) in the control group showed UDVA equal to or better than 0.1, whereas 87 of 92 eyes (94.5%) in the mild non-foveal involving ERM group showed UDVA equal to or better than 0.1. Every eye (100.0%) in the control group showed CDVA equal to or better than 0.1, whereas 91 of 92 eyes (98.9%) in the mild non-foveal involving ERM group showed CDVA equal to or better than 0.1. Every eye (100.0%) in the control group showed UNVA equal to or better than 0.1, whereas 90 of 92 eyes (97.8%) in the mild non-foveal involving ERM group showed UNVA equal to or better than 0.1. Figure 3 shows the representative images from eyes with poor postoperative CDVA. There were no eyes in the mild non-foveal involving ERM group experiencing metamorphopsia or macropsia at baseline or the postoperative follow-up period. Table 2 shows the regional macular thickness from both groups at postoperative 6 months. There was no difference in the macular thickness at postoperative 6 months in both groups (P = 0.225, P = 0.884, P = 0.265, P = 0.637, P = 0.089, P = 0.457, P = 0.170, P = 0.959, and P = 0.681, for central 1 mm, superior 3 mm, superior 6 mm, nasal 3 mm, nasal 6 mm, inferior 3 mm, inferior 6 mm, temporal 3 mm, and temporal 6 mm, respectively).
Bar graphs showing the comparison between the control group and mild non-foveal involving ERM group for visual acuities, including uncorrected distance visual acuity (UDVA; a), uncorrected near visual acuity (UNVA; b), and corrected distance visual acuity (CDVA; c) at baseline and postoperative months 1, 2, and 6. *Represents P value < .05
Images from one of the enrolled patients. A 58-year-old woman with no previous medical or surgical history was implanted with an MIOL. a-c At baseline, an epiretinal membrane (ERM) was detected in the superotemporal area which shows retinal folds but without alteration of the foveal depression on spectral-domain optical coherence tomography (SD-OCT); this indicates a stage-2 ERM. d-f At postoperative month 3, the reflectivity of the ERM increased on fundus photography, and the macular thickness increased on OCT scan. A thin, tractional membrane at the fovea was detected (arrows) which altered the inner and outer retinal structure
To evaluate the objective quality of vision, we further analyzed the contrast sensitivity, Strehl ratio, area ratio, and HOAs. Figure 4 shows the contrast sensitivity detected under photopic and mesopic conditions. The mild non-foveal involving ERM group showed significantly low contrast sensitivity at a visual angle of 4.0°, 2.5°, 1.0°, and 0.64° under scotopic conditions (P = 0.048, P = 0.025, P = 0.003, and P = 0.020, respectively) and 4.0°, 1.0°, and 0.64° under photopic conditions (P = 0.028, P = 0.002, and P = 0.001, respectively; Fig. 4). Table 3 shows the comparison of data from the OPD-Scan between the two groups. The mean area ratio ± SD of the mild non-foveal involving ERM group was 45.13 ± 10.93, which was significantly lower than that of the control group, which was 50.34 ± 12.66 (P = 0.044). The mild non-foveal involving ERM group showed a lower Strehl ratio than the control group, but lacked statistical significance (P = 0.078). There was no difference in the total HOA (P = 0.316).
Since the contrast sensitivity can be affected by multiple factors, we performed multivariate regression analysis to see whether the presence of mild non-foveal involving ERM is an independent factor for the decreased contrast sensitivity (Table 4). Univariate analysis revealed that the age, presence of mild non-foveal involving ERM, TCIA, ACD, and presence of PVD were associated with the contrast sensitivity at 0.64 cpd (P < 0.001, P = 0.008, P < 0.001, P = 0.044, and P < 0.001, respectively). Multivariate analysis showed that the age, presence of mild non-foveal involving ERM, and TCIA were independently associated with contrast sensitivity at night (P < 0.001, P = 0.008, and P = 0.002, respectively).
Because the visual prognosis of eyes with a grade 1 or grade 2 ERM can differ, we subanalyzed the visual prognosis according to the grade of ERM. Figure 5 shows the visual prognosis of eyes with grade 1 and grade 2 ERM. There was no difference in UDVA and UNVA during 6 months of follow-up (P = 0.836, P = 0.174, P = 0.590, and P = 0.318 for UDVA; and P = 0.064, P = 0.740, P = 0.982, and P = 0.214 for UNVA). There was no difference in CDVA during the preoperative and postoperative 1-month and 2-month examinations (P = 0.692, 0.492, and 0.471, respectively). At postoperative month 6, CDVA was significantly worse in the eyes with grade 2 ERM when compared with those diagnosed with grade 1 ERM (P = 0.025).
Bar graphs showing the comparison between the eyes with grade 1 ERM and eyes with grade 2 ERM for visual acuities, including uncorrected distance visual acuity (UDVA; a), uncorrected near visual acuity (UNVA; b), and corrected distance visual acuity (CDVA; c) at baseline and postoperative months 1, 2, and 6. *Represents P value < .05
Discussion
As imaging devices used in ophthalmology have become more advanced, their sensitivity to disease detection has tremendously increased. One of these advancements, the SD-OCT, has enabled an in-depth understanding of the retinal structure, especially the macular area. In terms of ERMs, there have been many OCT-related studies regarding structural–functional correlation [12, 14] and surgical prognosis [15,16,17,18]. However, there have been only a limited number of studies regarding mild non-foveal involving ERMs because conventional imaging studies could not detect these lesions. The condition was not clinically relevant as the lesion does not deteriorate visual quality in the phakic or monofocal IOL-implanted state [9, 19]. However, these mild non-foveal involving ERMs may become clinically relevant when the eye is implanted with an MIOL, which has an optical design that divides light into multiple planes and results in some loss of light. In the present study, the mild non-foveal involving ERM, which did not distort the foveal structure, did not affect the postoperative visual acuity at 6 months. However, the objective quality of vision in the mild non-foveal involving ERM group, including the contrast sensitivity under both scotopic and photopic conditions and the MTF represented by the area ratio, was significantly worse than that of the control group. Also, the CDVA was markedly worse in the eyes with grade 2 ERM than those diagnosed with grade 1 ERM at postoperative 6 months. The typical symptoms of ERM, such as metamorphopsia and macropsia, were not reported as the lesion did not cause asymmetric traction of the outer retinal structures [12].
We speculate at least two critical issues that need to be discussed with the patients who want MIOL implantation and have a clinically insignificant ERM. First, the postoperative visual quality might be worse than the patients without ERM. Even a clinically insignificant ERM that does not involve the fovea was shown to affect the postoperative visual quality in the present study. Second, the ERM may progress to a clinically relevant stage as they age. Although ERMs can be removed surgically, the surgical outcome is not always predictable. The removal of an advanced ERM frequently results in poor postoperative visual quality, whereas the surgical excision of an early ERM runs a high risk–benefit ratio from unexpected events during the surgical procedure. Also, the operative field during vitrectomy and ERM peeling is greatly influenced by the implanted IOL’s optical design, resulting in iatrogenic retinal damage [20, 21].
The number of cases of patients who have had presbyopic correction using an MIOL increases as the optical design improves, and the need for a glass-free life increases. Whereas mature ERMs can be detected by biomicroscope or fundus photographs, mild non-foveal involving ERMs can be underdiagnosed or misdiagnosed by conventional methods, including a previous version OCTs. Especially, grade 2 ERM showed statistically significant worse CDVA at postoperative 6 months, stressing the importance of a detailed preoperative macular screening using SD-OCT for a proper indication of MIOL implantation.
The present study has the limitation of cross-sectional data. Although most eyes with an ERM show a stable clinical course, some of the eyes with an ERM could progress in the long term [19, 22, 23]. Subclinical ERM can affect the postoperative visual quality as irregular corneal astigmatism does. A consensus for the proper indication of subclinical macular changes for MIOL implantation is mandatory.
References
Wise GN (1975) Clinical features of idiopathic preretinal macular fibrosis. Schoenberg Lecture. Am J Ophthalmol 79:349–347
Bu SC, Kuijer R, Li XR, Hooymans JM, Los LI (2014) Idiopathic epiretinal membrane. Retina 34:2317–2335
Kim JM, Lee H, Shin JP, Ahn J, Yoo JM, Song SJ, Kim SJ, Kang SW (2017) Epidemiologic Survey Committee of the Korean Ophthalmologic Society. Epiretinal membrane: prevalence and risk factors from the Korea National Health and Nutrition Examination Survey, 2008 through 2012. Korean J Ophthalmol 31:514–523
Ng CH, Cheung N, Wang JJ, Islam AF, Kawasaki R, Meuer SM, Cotch MF, Klein BE, Klein R, Wong TY (2011) Prevalence and risk factors for epiretinal membranes in a multi-ethnic United States population. Ophthalmology 118:694–699
Zhu XF, Peng JJ, Zou HD, Fu J, Wang WW, Xu X, Zhang X (2012) Prevalence and risk factors of idiopathic epiretinal membranes in Beixinjing blocks, Shanghai, China. PLoS One 7:e51445
Ko TH, Fujimoto JG, Schuman JS, Paunescu LA, Kowalevicz AM, Hartl I, Drexler W, Wollstein G, Ishikawa H, Duker JS (2005) Comparison of ultrahigh- and standard-resolution optical coherence tomography for imaging macular pathology. Ophthalmology 112:1922.e1921–1915
Duan XR, Liang YB, Friedman DS, Sun LP, Wei WB, Wang JJ, Wang GL, Liu W, Tao QS, Wang NL, Wong TY (2009) Prevalence and associations of epiretinal membranes in a rural Chinese adult population: the Handan Eye Study. Invest Ophthalmol Vis Sci 50:2018–2023
Meuer SM, Myers CE, Klein BE, Swift MK, Huang Y, Gangaputra S, Pak JW, Danis RP, Klein R (2015) The epidemiology of vitreoretinal interface abnormalities as detected by spectral-domain optical coherence tomography: the beaver dam eye study. Ophthalmology 122:787–795
Delyfer MN, Legout P, Le Goff M, Blaizeau M, Rougier MB, Schweitzer C, Korobelnik JF, Delcourt C (2020) Prevalence of epiretinal membranes in the ageing population using retinal colour images and SD-OCT: the Alienor Study. Acta Ophthalmol. https://doi.org/10.1111/aos.14422
Wolffsohn JS, Davies LN (2019) Presbyopia: effectiveness of correction strategies. Prog Retin Eye Res 68:124–143
Braga-Mele R, Chang D, Dewey S, Foster G, Henderson BA, Hill W, Hoffman R, Little B, Mamalis N, Oetting T, Serafano D, Talley-Rostov A, Vasavada A, Yoo S, Cataract Clinical Committee ASCRS (2014) Multifocal intraocular lenses: relative indications and contraindications for implantation. J Cataract Refract Surg 40:313–322
Jeon S, Jung B, Lee WK (2018) Spectral-domain optical coherence tomography findings in patients with macropsia. Ophthalmic Surg Lasers Imaging Retina 49:656–663
Jeon S, Baek J, Lee WK (2017) Gli1 expression in human epiretinal membranes. Invest Ophthalmol Vis Sci 58:651–659
Alkabes M, Fogagnolo P, Vujosevic S, Rossetti L, Casini G, De Cillà S (2020) Correlation between new OCT parameters and metamorphopsia in advanced stages of epiretinal membranes. Acta Ophthalmol. https://doi.org/10.1111/aos.14336
Yeo JH, Kim JG (2020) Bare retinal nerve fiber layer and internal limiting membrane tear associated with epiretinal membrane: OCT findings and visual outcomes. Graefes Arch Clin Exp Ophthalmol 258:1379–1387
Bae K, Choi JH, Kim KT, Kang SW (2020) EN-FACE optical coherence tomography in patients with epiretinal membrane: intuitive method for predicting functional outcomes. Retina 40:1972–1979
Hirata A, Nakada H, Mine K, Masumoto M, Sato T, Hayashi K (2019) Relationship between the morphology of the foveal avascular zone and the degree of aniseikonia before and after vitrectomy in patients with unilateral epiretinal membrane. Graefes Arch Clin Exp Ophthalmol 257:507–515
Jeon S, Jung B, Lee WK (2019) Long-term prognostic factors for visual improvement after epiretinal membrane removal. Retina 39:1786–1793
Byon IS, Pak GY, Kwon HJ, Kim KH, Park SW, Lee JE (2015) Natural history of idiopathic epiretinal membrane in eyes with good vision assessed by spectral-domain optical coherence tomography. Ophthalmologica 234:91–100
Yoshino M, Inoue M, Kitamura N, Bissen-Miyajima H (2010) Diffractive multifocal intraocular lens interferes with intraoperative view. Clin Ophthalmol 4:467–469
Hadayer A, Jusufbegovic D, Schaal S (2017) Retinal detachment repair through multifocal intraocular lens- overcoming visualization challenge of the peripheral retina. Int J Ophthalmol 10:1008–1010
Wiznia RA (1982) Natural history of idiopathic preretinal macular fibrosis. Ann Ophthalmol 14:876–878
Fraser-Bell S, Guzowski M, Rochtchina E, Wang JJ, Mitchell P (2003) Five-year cumulative incidence and progression of epiretinal membranes: the Blue Mountains Eye Study. Ophthalmology 110:34–40
Funding
This work was supported by the grant from National Research Foundation of Korea (NRF) grant (grant number 2020R1G1A1006948) funded by the Korea government (MSIT) and Investigator-Initiated Trials grant (grant number: 61580831) funded by Alcon, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The research was conducted in accordance with the tenets of the Declaration of Helsinki and approved by the Institutional Review Board (IRB)/Ethics Committee of KEYE EYE Center (IRB number P12361001-001).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, B., Kwon, S., Choi, A. et al. Influence of mild non-foveal involving epiretinal membrane on visual quality in eyes with multifocal intraocular lens implantation. Graefes Arch Clin Exp Ophthalmol 259, 2723–2730 (2021). https://doi.org/10.1007/s00417-021-05225-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-021-05225-w