Abstract
Aim
To investigate the effects of free oxygen radicals and various antioxidants on bone healing after experimental formation of fracture.
Materials and Methods
Fifty male rats were used and divided into five groups (ten rats in each). The right forelimbs of the rats were broken by bimanual compression method. One hour before this procedure, 5 ml/kg of intraperitoneal (i.p.) physiologic saline were given to the control Group 1. All 40 rats in the experimental Groups 2, 3, 4 and 5 were treated with i.p. zymosan at a dosage of 100 mg/kg to induce the production of free radicals by stimulating NADPH oxidase in polymorphonuclear leukocytes. Zymosan induction was stopped on the fifth post-fracture day. In addition to the zymosan, i.p. 1 g/kg/day of dimethyl sulfoxide were given to the animals in Group 3, 50 mg/kg/d of Ginko biloba Extract (EGb 761) in Group 4 and 500 mg/kg/day of vitamin C in Group 5. Radiographs of the fractures of all animals were obtained to assess callus formation, remodeling and bridging bone formation under ether anesthetics on postfracture day 7, 14 and 21. All rats were euthanized on day 22, and sections of the radius and ulna were examined both histologically with light and electron microscopy and ultrastructurally. Statistical analysis was made with Kruskal-Wallis variance analyze test and comparison between groups was performed by Dunn’s multiple comparision test.
Results
An impairment of bone healing was observed in Group 2 inducted with purely zymosan. Variable results were obtained for bone healing in the groups treated with various antioxidants. There was very significant difference of fracture healing between Groups 1 and 2 both histologically and radiologically (P < 0.001). There was significant difference between Groups 2 and 5 radiologically (P < 0.05).
Conclusion
Free oxygen radicals demonstrate a negative effect on fracture healing and vitamin C (an antioxidant) partially prevents the negative effect of zymosan on fracture healing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative stress defines the breakdown of the preoxidant-antioxidant balance toward the preoxidant side in the tissues and in the body. In the aerobic life, the continuously formed preoxidants should be regularly captured and be balanced with consuming by the antioxidants. Otherwise, oxidative damage will form and result in physiopathological events. Most of the preoxidants that create oxidative stress are free radicals. Being short surviving molecules, oxygen free radicals can produce cellular damage by causing various metabolic disarrangements in the cell and can eventually produce cell death [23, 27, 28].
Oxygen free radicals have been implicated as inducers of tissue injury in several pathologic conditions such as inflammation, circulatory shock injury, irradiation, respiratory distress syndrome and ischemic/reperfusion events in different tissues [39, 45–48].
Today, there are evidences for the roles of free radicals in appearance of many pathologic events including cancer and aging. Their roles in ischemia-reperfusion injury, in stress and in gastric mucosal damage by drugs have become precise. Besides these, their negative effects on wound healing, on granulation tissue, on collagen and cartilage tissue have been shown [9, 19, 20, 24].
Bone fractures are associated with inflammation and ischemia, stimulating free radical oxidation [47]. A free radical is defined as a molecular species capable of independent existence and contains one or more unpaired electrons [21]. Oxygen free radicals cause subcellular abnormalities in the tissues [38]. Oxygen-derived free radicals are believed to initiate a chain reaction leading to cell membrane damage via lipid peroxidation, thereby causing cell death [42, 50].
Although the mechanism of free radical formation is well known, there are a limited number of histopathological and ultrastructural studies regarding the effects of free radicals on fracture healing [16, 26, 27, 49]. This study aimed to investigate the effects of free oxygen radicals on fracture healing. In addition, the effects of antioxidants such as dimethyl sulfoxide (DMSO), Ginkgo biloba extract (EGb.761) and vitamin C were also evaluated on fracture healing under oxidative stress.
Materials and methods
The ethics committee of Erciyes University reviewed the study, and the experiments conformed to the Principles and Guidelines for the Use of Animals in Research, Testing and Education issued by the Committee on Educational Programmes in Laboratory Animal Science [11]. Rats were obtained from the Medical Sciences Experimental Research Unit of the University. All procedures were performed in the same unit.
Animal and tissue preparation
Fifty Wistar albino male rats weighting 150–250 g were used in this study. The rats were acclimated to caged laboratory conditions for 2 weeks. The rats were randomly divided into five equal groups (ten rats in each group), consisting of one control and four experimental groups. They were permitted to take water and standard laboratory diet. After rats were anesthetized intraperitoneally with ketamine (40 mg/kg Ketalar®, Eczacibasi, Istanbul, Turkey) and xylazine (5 mg/kg Rompum®, Bayer, Leverkusen, Germany), right forelimbs of the rats were broken by bimanual compression method using the standardized technique [17]. The fractures were detected to be simple transverse fractures in the radius and ulna by radiography taken just after production of fracture, while the rats were under anesthesia. All groups except control rats (Group 1) were inducted with intraperitoneal (i.p.) zymosan at 09:00 a.m. to produce free oxygen radicals (Zymosan A, MO, USA, Sigma chemical co.) at a dosage of 100 mg/kg [27]. The rats in Group 1 were treated with 5 mg/kg of i.p. saline 1 h before the fracture procedure. Injection of Zymosan and saline was continued once in a day until the fifth day of fracture.
In addition to zymosan, i.p. dimethyl sulfoxide (DMSO, Sigma chemical co.) was administered at a dosage of 1 g/kg/day for 21 days in Group 3 [25]. Ginkgo biloba extract (EGb.761) (Tebokan®, Abdi İbrahim Drug Co.) was administered at a dosage of 50 mg/kg/day in Group 4 for 21 days [43]. Group 5 rats were treated with 500 mg/kg/day of vitamin C (Redoxon®, Roche) for the same period [51].
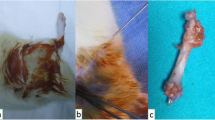
Radiographs of the fractures of all animals were obtained to assess callus formation, remodeling and bridging bone formation under ether anesthetics on postfracture day 7, 14 and 21. Modified Lane and Sandhu [34] method was used for radiological scoring system (Table 1). When mineralized bridging was radiographically detected in the fractures of control group, rats were euthanized by high dose ether anesthesia and evaluated under histological examination to perform the difference of complete and incomplete bone healing on day 22. The tissues were fixed in 10% neutral buffered formalin.
Forearm bones were decalcified in 10% ethylene diamine tetra acetic acid (EDTA). Paraffin blocks were prepared and sections of 5–6 mm around each callus were obtained [1]. Histological sections of 5 μm thick were stained with hematoxylin-eosin (HE) and were examined under light microscope [30]. All preparations were blindly examined by the same pathologist under light microscopy. The histological grading of fracture healing was performed according to the 5-grade system (Table 3) [1]. The least healed bone (radius or ulna) was considered in the grading of recovery. In case of technical irregularity in sections, grading was done by evaluating callus of both fractures [1]. For electron microscopic evaluation, fresh bone specimens were fixed by immersion in 2.5% gluteraldehyde and then post fixed in 1% osmium tetroxide in 0.1 M phosphate buffer at 4°C for 1 h. After washing in phosphate buffer, they were dehydrated in graded series of ethanols to absolute ethanol in preparation for embedding in araldite Cy 212 Agar-R1030 (epoxy resin kit). Thin sections were subsequently stained with uranyl acetate and lead citrate and evaluated with LEO 906 E transmission electron microscope.
Statistical analysis
Data are expressed as median (min–max). Comparison of histopathological and radiological scores between groups was made using the Kruskal–Wallis one way analysis of variance on Ranks test (KW). Post-hoc comparisons on parameters were performed using Dunn’s procedure. Statistical significance was set at P < 0.05. All analyses were performed with the statistical package for scientist (SİGMASTAT) Windows version 3.10.
Results
Radiographical evaluation
Simple transverse fracture was detected in all radius and ulna radiographically after production of fracture (Fig. 1).
At the end of the first week, there was significant difference between the mean scores (P < 0.05). The multiple comparison of the groups showed that there was a statistical difference between Group 2 and Group 5 (P < 0.001).
There was significant difference between the mean scores at the end of the second week (P < 0.001). The multiple comparison of the groups showed that there was significant difference between Group 2 and Group 5 (P < 0.01).
At the end of the third week, variable healing of fractures were observed between the groups. The most significant fracture healing was observed in Group 5 (Table 2) (Fig. 2) when compared with the second group, showing enhanced consolidation after 22 days. Six of the animals in Group 2 were found to have “no bridging” upon radiological examination. There was statistical difference between the mean scores of the groups (P < 0.001). The multiple comparison of the groups showed that there were significant differences between Group 1 and Group 2 (P < 0.01) and Groups 2 and 5 (P < 0.05).
Histopathological evaluation
Complete bony union was detected in nine rats in control group (Group 1). Pseudoarthrosis formation was observed in one rat.
In rats of zymosan administered group (Group 2), six were observed to have cartilaginous union, two were observed to have incomplete bony union and two were observed to have pseudoarthrosis formation. In animals which were administered DMSO with zymosan (Group 3), four were observed to have incomplete bony union, one was observed to have complete bony union, three were observed to have complete cartilaginous union and two were observed to have pseudoarthrosis formation. In animals that were administered EGb 761 with zymosan (Group 4), five were observed to have complete cartilaginous union, three were observed to have incomplete bony union, one was observed to have complete bony union and one was observed to have pseudoarthrosis formation. In animals that were given vitamin C (Redoxon) with zymosan (Group 5), seven were observed to have incomplete bony union, one was observed to have complete bony union, one was observed to have complete cartilaginous union and one was observed to have pseudoarthrosis formation.
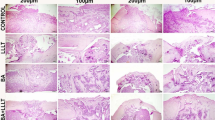
Histopathological evaluation showed that there was complete bony union in H.E stained preparations in control group with a healthy development of the cartilage, bone and the bone marrow (Fig. 3). Complete cartilaginous union was predominant in H.E stained sections of Group 2. Destruction was observed in bony tissue (bone resorption) (Fig. 4). Incomplete bony union and complete cartilaginous union were predominant in H.E stained sections of Group 3 (Fig. 5).
Complete cartilaginous union and incomplete bony union were predominant in H.E stained sections of Group 4 (Fig. 6). Incomplete bony union was predominant in H.E stained sections of animals in Group 5 (Fig. 7).
Statistical analysis showed that there was significant difference between mean histological scores of the groups at the end of the third week (P < 0.001) (Table 4). There was significant difference between Groups 1 and 2 (P < 0.001), and Groups 1 and 3 and Groups 1 and 4 (P < 0.05) with multiple comparisons. There were similar histological scores between Groups 1 and 5 (P > 0.05).
Electron microscopic evaluation
Findings of bone healing such as macrophages, fibroblasts, undifferentiated mesenchymal cells and collagen fibrils were observed in the control group (Fig. 8). In Group 2, bone destruction and undifferentiated migrating mesenchymal cells were observed (Fig. 9). On the other hand, collagen fibrils were observed in Group 5 treated with vitamin C (Fig. 10).
Discussion
The administration of zymosan to the rats in Group 2 significantly inhibited the fracture healing. The negative effects of zymosan on fracture healing were reduced by the addition of vitamin C in Group 5. Histological and radiological evaluation demonstrated that zymosan inhibited fracture healing in rats and vitamin C prevented this effect at least partially.
Inflammation is a step in fracture healing and involves just 5 days after fracture. Inflammation is an event occurring in all injured tissues. Blood, lymph and tissue exudate accumulates in between tissues as a result of destruction of endosteum, periosteum and surrounding soft tissue when bone is fractured. This accumulate named fracture hematoma involves the basic elements for fracture healing. After this first step, inflammatory changes like vasodilatation in soft tissues and leukocyte exudation from plasma appear. Soon, as a response, polymorphonuclear leukocytes (PNLs), histiocytes and macrophages increase in number. The early period of fracture healing is very important. Most of the biological insufficiencies appear at the first week after fracture [12, 22].
Since we obtained the autopsy materials on the 22nd day of production of fracture, we were unable to observe the inflammatory stage. We observed that there was a significant decrease in the callus formation in rats treated with zymosan. These changes were also partially prevented by administration of vitamin C in Group 5.
Oxygen free radicals are ubiquitous compounds that occur naturally in all biological tissues. They contain reactive unpaired electron which can attack susceptible chemical groups on all classes of macromolecules in the cell. Under aerobic conditions, the damage to macromolecules caused by free radicals is minimized by endogenous scavengers which convert the free radicals to less reactive and therefore less toxic forms [30]. Oxygen free radicals, the superoxide anion, the hydroxyl radical and their intermediary, hydrogen peroxide, are believed to be generated during ischemia and at the time of reperfusion. These reactive oxygen species can be cytotoxic by attacking fatty acids, which leads to lipid peroxidation of membranes, and reacting with proteins, including destruction of amino acids, oxidation of sulfhydryl groups and polypeptide chain [23, 30].
When free oxygen radicals were generated in the bone environment, osteoclasts were formed and bone resorption occurred. It is possible that the generation of oxygen-derived free radicals may be particularly important in the bone resorption that occurs in association with inflammatory diseases [10, 54]. Oxygen-derived species are produced by activated phagocytes including monocytes, macrophages and neutrophils. Since these cells accumulate adjacent to bone surfaces in chronic inflammatory diseases, radical production by these cells could be responsible for stimulating osteoclast formation or activation to resorb bone [23, 54].
Antioxidants are essential molecules in preventing the cellular damage caused by free radicals. Oxygen-related free radicals are produced in inflamed and ischemic tissues. These free radicals are responsible for tissue destruction by lipid peroxidation of biologic membranes. In normal metabolism, there is a balance between the generation of free radicals and antioxidant defense mechanism [20, 52]. These findings have led us to speculate if free radicals play an important role in inflammation and induction of reperfusion-induced muscle and tendon damage as well as fracture healing.
Several compounds are able to prevent the damage induced by oxygen free-radicals [20, 23, 27, 32, 43, 53]. We have used DMSO, Ginkgo biloba extract and vitamin C to prevent the negative effects of zymosan on fracture healing process.
It is known that free radicals formed by activated polymorphonuclear cells (PNL) disrupt wound healing and granulation tissue. In the studies of Foschi et al. [19, 20] on the effects of free oxygen radicals on wound healing and granulation tissue, they used zymosan to produce free oxygen radicals in PNL by stimulation of NADPH oxidase. They used zymosan with a dose of 100 mg/kg for 5 days and showed that this dosage produced enough free oxygen radicals to produce a pathological condition. We also observed such findings of destruction in bone tissue in light and electron microscopy in Group 2 (Figs. 4, 9).
Zymosan, being a 3–5 μm cell wall fragment of Saccharomyces cerevisiae, is formed by polysaccharide, protein and lipid [14, 18]. Glucan, a polysaccharide compound, forms 60% of the dry weight of zymosan. A second polysaccharide mannan forms 20% of zymosan. Although glucan being the main component in the activation of PNL, mannan also plays an important role in the transmembrane activation of respiratory burst in these cells [31, 55]. Zymosan is a xenobiotic known to stimulate oxygen free radicals production by stimulation of NADPH oxidase of the polymorphonuclear cells. It increases the anion superoxide radical (with formation of such secondary toxic products as H2O2, the singlet oxygen, OH• and hypochloric acid) and by this way necrotic effects are induced in the target tissues [14, 44].
Dimethyl sulfoxide (C2H6OS), first found in 1866, has the ability of using H• atoms in unsaturated sulfur atom in methyl group, and is a strong scavenger of hydroxyl radical [27, 29]. It traps the hydroxyl radical (OH•), dimethyl sulfide (DMS) and the oxygen radical [7]. In addition, it inhibits thrombocyte aggregation, protects cell membrane components and mitochondrial oxidative phosphorylation, decreasing inflammatory response. DMSO possesses many in vivo properties that mediate its effect on musculoskeletal trauma. Besides these, DMSO has prostaglandin and fibroblast inhibiting, immune modulating and pain relieving effects [28, 40].
There was a significant difference histopathologically when Group 3 animals were compared to the control group. The difference was not significant when it was compared to the other groups. Therefore, DMSO has not preventive effect on reversing the detrimental effects of zymosan on fracture healing.
Both classic drug–receptor interactions and certain enzyme inhibition must be considered to explain the therapeutic effects of EGb 761 [13]. It may prolong the half-life of endothelium-derived relaxing factor by scavenging superoxide anions (O2•–), and thus stimulate the relaxation of contracted blood vessels. In addition, the ginkgolide constituents of the extract, especially ginkgolide B, may inhibit platelet aggregation and O2 generation induced by platelet-activating factor [6].
In vitro and in vivo experimental studies in humans and animals have indicated that EGb 761 has significant free radical scavenging activity [2]. It leads to the inhibition of the formation of lipid peroxides in the brain and liver microsomes, and could protect the retina against lipoperoxidation, reperfusion-induced injury and argon laser (photocoagulation) damage [25, 32, 53]. Indeed, we found a significant difference histologically between Group 4 animals and the control group. The difference was not significant when it was compared to the other groups. Therefore, EGb 761 has not preventive effect on reversing the detrimental effects of zymosan on fracture healing. When Group 2 and Group 4 have been compared for litic lesions of cartilage and bone tissue histopathologically, Group 4 showed significant improvement. There were also histopathological findings of callus tissue formation to bone tissue in Group 4.
Vitamin C is a water-soluble free radical scavenger and is found in many fruits and vegetables, acting as an antioxidant. It is required for the optimal function of a number of enzymes. Its deficiency causes scurvy and poor wound repair [3, 33, 43]. Matsuda et al. extensively investigated the effects of high-dose vitamin C therapy on dermal burns [35, 36] and found that it stops the progression of vascular permeability after burns and, therefore, reduces the microvascular leakage of fluid and protein. It also reduces lipid peroxidation after burns [37]. Lipid peroxidation damages the microvascular endothelial cells, thereby increasing capillary permeability [15]. The lipid peroxide in the cell membrane can only be scavenged by vitamin E, the primary lipid-soluble small-molecule antioxidant, [8] producing a vitamin E free-radical complex [4, 41]. In the extracellular fluid vitamin C, the terminal water-soluble small-molecule antioxidant, acts on this complex and removes the free radical moiety, regenerating vitamin E. Vitamin C is a natural antioxidant that can scavenge hydroxyl radicals [5] and superoxide radicals that produce hydroxyl radicals [56]. By scavenging these radicals, vitamin C stops free-radical reactions and prevents the propagation of chain reactions, protecting the capillary endothelium and circulating cells such as erythrocytes and leukocytes [4, 41].
When the animals in Group 5 were compared to the animals with control group, the difference was not found both histologically and radiologically. When Group 5 was compared to the second group, there was significant difference radiologically. The difference was not significant when it was compared to the other groups. Even though histopathological results showed that there was significant difference among the second, third, fourth groups and the control group, the fifth group’s and control group’s histopathological scores were similar. This result demonstrates that vitamin C administered group prevented the detrimental effects of zymosan and showed similar histopathological results as control group.
We conclude that free oxygen radicals have a role in the disruption of fracture healing, and vitamin C can partially prevent the negative effects of zymosan on fracture healing. Further investigations are needed to clarify the mechanism of the prevention of negative effects of oxidants.
References
Allen HL, Wase A, Bear WT (1980) Indomethacin and aspirin: effect of nonsteroidal anti-inflammatory agents on the rate of fracture repair in the rat. Acta Orthop Scand 51:595–600
Bekerecioglu M, Tercan M, Ozyazgan I (1998) The effect of Gingko biloba extract (Egb 761) as a free radical scavenger on the survival of skin flaps in rats. A comparative study. Scand J Plast Reconstr Surg Hand Surg 32(2):135–139
Benito E, Bosch MA (1997) Impaired phosphatidylcholine biosynthesis and ascorbic acid depletion in lung during lipopolysaccharide-induced endotoxemia in guinea pigs. Mol Cell Biochem 175:117–123
Beyer RE (1994) The role of ascorbate in antioxidant protection of biomembranes: interaction with vitamin E and coenzyme Q. J Bioenerg Biomembr 26:349–358
Bielski BHJ, Richter HW, Chan PC (1975) Some properties of ascorbate free radical. Ann NY Acad Sci 258:231–237
Braquet P, Hosford D (1991) Ethnopharmacology and the development of natural PAF antagonists as therapeutic agents. J Ethnopharmacol 32:135–139
Brown JM (1967) Clinical experiences with DMSO in acute musculoskeletal conditions, comparing a non-controlled series with a double blind study. Ann N Y Acad Sci 141:496
Buettner GH (1993) The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys 300:535–543
Bulkley GB (1983) The role of oxygen free radicals in human disease processes. Surgery 94:407–411
Cetinus E, Kilinc M, Uzel M, Inanc F, Kurutas EB, Bilgic E, Karaoguz A (2005) Does long-term ischemia affect the oxidant status during fracture healing? Arch Orthop Trauma Surg 125:376–380
Committee on Educational Programmes in Laboratory Animal Science (1991) Education and Training in the Care and Use of Laboratory Animals: a guide for developing Institutional Programmes. Institute of Laboratory Animal Resources, National Research Council 1991
Cornell CN, Lane JM (1992) Newest factors in fracture healing. Clin Orthop 277:297–311
Defeudis FV (1991) Pharmacological activities and clinical applications. In: Ginkgo Biloba Extract EGb 761. Scientifiques Elsevier, Paris, pp 1–8, 25–94
DiCarlo FC, Fiore JV (1964) On the composition of zymosan. Science 127:756–757
Doly M, Braquet P, Bonhomme B, Meyniel G (1984) Effects of lipid peroxidation on the isolated rat retina. Ophthalmic Res 16:292–296
Durak K, Sönmez G, Sarısözen B, Özkan S, Kaya M, Öztürk C (2003) Histological assessment of the effect of alpha-tocopherol on fracture healing in rabbits. J Int Med Res 31:26–30
Ekeland A, Engesaeter LB, Langeland N (1981) Mechanical properties of fractured and intact rat femora evaluated by bending, torsional and tensile tests. Acta Orthop Scand 52:605–613
Fitzpatrick FW, DiCarlo JW (1964) Zymosan. Ann NY Acad Sci 118:233–262
Foschi D, Trabucchi E, Musazzi M, Castoldi L, Di Mattia D, Radaelli E, et al (1988) The effects of oxygen free radicals on wound healing. Int J Tissue React 10:373–379
Foschi D, Castoldi L, Radaelli E, Abelli P, Calderini G, Rastrelli A, et al (1990) Hyaluronic acid prevents oxygen-free radical damage to granulation tissue: a study in rats. Int J Tissue React 12:333–339
Freeman BA, Crapo JD (1982) Biology of disease: free radicals and tissue injury. Lab Invest 47:412–426
Frost HM (1989) The biology of fracture healing. An overview for clinicians. Part II. Clin Orthop 248:294–308
Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR (1990) Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest 85:632–639
Greenwald RA, Moy WW (1979) Inhibition of collagen gelation by action of the superoxide radical. Arthritis Rheum 22:251–259
Göktürk E, Seber S, Özakçe H (1989) Protective effect of dimethyl sulfoxide on skeletal muscle ischemia/reperfusion injury. Anatolian Med J 2:173–189
Göktürk E, Turgut A, Baycu C, Gunal I, Seber S, Gulbas Z (1995) Oxygen-free radicals impair fracture healing in rats. Acta Orthop Scand 66:473–475
Göktürk E (1997) The effect of free oxygen radicals on fracture healing in rats. Acta Orthop Traumatol Turc 31:353–356
Haigler HJ, Spring DD (1983) Comparison of the analgesic effects of dimethyl sulfoxide and morphine. Ann N Y Acad Sci 411:19–27
Kharasch N, Thyagarajan BS (1983) Structural basis for biological activities of dimethyl sulfoxide. Ann N Y Acad Sci 411:391–402
Kiernan JA (1990) Histological–histochemical methods. In: Theory and practice, 2nd edn. Pergamon Press, Oxford, pp 96–97
Korthuis RJ, Granger DN, Townsley MI (1985) The role of oxygen-derived free radicals in ischemia-induced increases in canine skeletal muscle vascular permeability. Circ Res 57:599–609
Kose K, Dogan P (1995) Lipoperoxidation induced by hydrogen peroxide in human erythrocyte membranes. 1. Protective effect of Ginkgo Biloba Extract (EGb 761). J Int Med Res 23:1–8
LaLonde C, Nayak U, Hennigan J, Demling RH (1997) Excessive liver oxidant stress causes mortality in response to burn injury combined with endotoxin and is prevented with antioxidants. J Burn Care Rehabil 18:187–192
Lane JM, Sandhu HS (1987) Current approaches to experimental bone grafting. Orthop Clin North Am 18:213–225
Matsuda T, Tanaka H, Hanumadass M, Gayle R, Yuasa H, Abcarian H, et al (1992) Effects of high-dose vitamin C administration on postburn microvascular fluid and protein flux. J Burn Care Rehabil 13:560–566
Matsuda T, Tanaka H, Shimazaki S, Matsuda H, Abcarian H, Reyes H, et al (1992) High-dose vitamin C therapy for extensive deep dermal burns. Burns 18:127–131
Matsuda T, Tanaka H, Yuasa H, Forrest R, Matsuda H, Hanumadass M, et al (1993) The effects of high-dose vitamin C therapy on postburn lipid peroxidation. J Burn Care Rehabil 14:624–629
McCord JM (1985) Oxygen derived free radicals in postischemic tissue injury. N Engl J Med 312:159–163
McKechnie K, Furman BL, Parratt JR (1986) Modification by oxygen free radical scavengers of the metabolic and cardiovascular effects of endotoxin infusion in conscious rats. Circ Shock 19:429–439
Nylander G, Otamiri T, Lewis DH, Larsson J (1989) Lipid peroxidation in postischemic skeletal muscle and after treatment with hyperbaric oxygen. Scand J Plast Reconstr Surg Hand Surg 23:97–103
More RC, Kabo JM, Dorey FJ, Meals RA (1988) The effects of dimethyl sulfoxide on posttraumatic limb swelling and joint stiffness: a review and experimental study in rabbits. Clin Orthop Relat Res 233:304–310
Niki E (1987) Interaction of ascorbate and alpha-tocopherol. Ann NY Acad Sci 498:186–198
Ozenirler S, Dincer S, Akyol G, Ozogul C, Oz E (1998) The protective effect of ginkgo biloba extract on CCl4-induced hepatic damage. Acta Physiol Hung 85:277–285
Parks DA, Bulkley GB, Granger DN, Hamilton SR, McCord JM (1982) Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology 82:9–15
Perkowski SZ, Havill AN, Flynn JT, Gee MH (1983) Role of intrapulmonary release of eicosanoids and superoxide anion as mediators of pulmonary dysfunction of endothelial injury in sheep with intermittent complement activation. Circ Res 53:574–583
Petkau A, Chelack WS, Pleskach SD (1978) Protection by superoxide dismutase of white blood cells in x-irradiated mice. Life Sci 22:867–882
Petrovich YA, Podorozhnaya RP, Kichenko SM, Kozlova MV (2004) Effects of selenium-containing compounds and their metabolism in intact rats and in animals with bone fractures. Bull Exp Biol Med 137:74–77
Rowley D, Gutteridge JMC, Blake D, Farr W, Halliwell B (1984) Lipid peroxidation in rheumatoid arthritis: thiobarbituric acid-reactive material and catalytic iron salts in synovial fluid from rheumatoid patients. Clin Sci 66:691–695
Sarısözen B, Durak K, Dinçer G, Bilgen OF (2002) The effects of vitamin E and C on fracture healing in rats. J Int Med Res 30:309–313
Sugino K, Dohi K, Yamada K, Kawasaki T (1987) The role of lipid peroxidation in endotoxin induced hepatic damage and the protective effect of antioxidants. Surgery 101:746–752
Suresh MV, Sreeranjit Kumar CV, Lal JJ, Indira M (1999) Impact of massive ascorbic acid supplementation on alcohol induced oxidative stress in guinea pigs. Toxicol Lett 104:221–229
Suzuki H, Hayakawa M, Kobayashi K, Takiguchi H, Abiko Y (1997) H2O2-derived free radicals treated fibronectin substratum reduces the bone nodule formation of rat calvarial osteoblast. Mech Ageing Dev 98:113–125
Szabo ME, Droy-Lefaix MT, Doly M, Braquet P (1991) Free radical-mediated effects in reperfusion injury: a histologic study with superoxide dismutase and EGb 761 in rat retina. Ophthalmic Res 23:225–234
Weiss SJ, LoBoglio AF (1982) Biology of disease phagocyte-generated oxygen metabolites and cellular injury. Lab Invest 47:5–17
Williams JD, Topley N, Alobaidi HM, Harber MJ (1986) Activation of human polymorphonuclear leukocytes by particulate zymosan is related to both its major carbohydrate components: glucan and mannan. Immunology 58:117–124
Yilmaz C, Erdemli E, Selek H, Kinik H, Arikan M, Erdemli B (2001) The contribution of vitamin C to healing of experimental fractures. Arch Orthop Trauma Surg 121:426–428
Acknowledgment
Authors have no financial or proprietary interest in any instrument or products used in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript has been presented in 7th Congress of the European Federation of National Associations of Orthopaedics and Traumatology (EFORT), Lizboa, P1-78, Portugal, 2005.
Rights and permissions
About this article
Cite this article
Duygulu, F., Yakan, B., Karaoglu, S. et al. The effect of zymosan and the protective effect of various antioxidants on fracture healing in rats. Arch Orthop Trauma Surg 127, 493–501 (2007). https://doi.org/10.1007/s00402-007-0395-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-007-0395-7