Abstract
Background
Aged patients are underrepresented in clinical trials on catheter ablation of atrial fibrillation (AF). In addition, results of outcomes after repeat ablation in the elderly are lacking. We report the results of first repeat AF ablation procedures of aged patients from a real-world multicenter prospective registry.
Methods
Patients undergoing second AF ablation included in the prospective, multicenter German Ablation Registry were divided in two groups (age > 70 years (group 1) and age ≤ 70 years (group 2)) and analyzed for procedural characteristics and clinical follow-up.
Results
738 patients were analyzed (108 patients in group 1, 630 patients in group 2). Significantly more aged patients had structural heart disease (56 patients (51.9%) vs. 203 patients (32.2%), p < 0.001). The majority of the patients underwent repeat pulmonary vein isolation (101 patients (93.5%) vs. 593 patients (94.1%), p = 0.98). More aged patients underwent ablation of left atrial linear lesions (78.1% vs. 57.3% of all linear lesions, p = 0.027). There was no difference in the occurrence of peri-procedural complications (7 patients (6.5%) vs. 24 patients (3.8%), p = 0.30). Recurrence of atrial arrhythmias was documented in 45/105 (42.9%) and 252/603 (41.8%) patients with available follow-up in groups 1 and 2 after a median of 447 (400; 532) and 473 (411; 544) days (p = 0.84). A comparable amount of patients were asymptomatic or reported symptom improvement after repeat ablation in both groups (80% (80/100) in group 1 and 77% (446/576) in group 2; p = 0.57).
Conclusion
Repeat ablation for AF in elderly patients can be performed with safety and efficacy comparable to younger patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Catheter ablation has emerged as an established approach to treat patients suffering from symptomatic atrial fibrillation (AF) [1]. The incidence of AF is increasing with age progression and the number of aged patients undergoing AF ablation is rising continuously [2]. AF ablation was demonstrated to be safe and effective with radiofrequency current (RF) and cryoballoon based (CB) ablation in elderly patients [4,5,6]. Albeit PVI-based ablation approaches have a high likelihood for the need of repeat ablation procedures to prevent recurrence of atrial tachyarrhythmias [6, 7], to our knowledge, there are no data in larger patient cohorts on the characteristics, safety and efficacy of repeat ablations in aged patients, yet.
This study sought to analyze safety and efficacy of first repeat AF ablation procedures in elderly patients which were followed in the large prospective, multicenter German Ablation Registry.
Methods
Registry structure and management
The German Ablation Registry (NCT01197638) is a prospective, multicenter non-profit registry under supervision of the “Institut für Herzinfarktforschung” (IHF, Ludwigshafen, Germany). The study was approved by local ethic boards of all participating centers. Project development and management, data acquisition and clinical monitoring were organized by the IHF. Fifty-five centers enrolled patients from January 2007 to January 2010. Patients gave written informed consent for procedure and registry participation. Data acquisition was conducted on a website-based platform, as previously published [8].
Patient selection
Patients undergoing first repeat ablation due to arrhythmia recurrence after previous AF ablation were enrolled. Patients undergoing AV node ablation were excluded from the analysis. Patients were divided into two groups according to their age (group 1 > 70 years and group 2 ≤ 70 years).
Ablation procedures and post-procedural care
Ablation procedures were performed according to institutional standards at the participating centers. Patients underwent transesophageal echocardiography (TEE) prior to the ablation procedure. Oral anticoagulation with vitamin-K antagonists was stopped before ablation procedure and replaced with low molecular-weight heparin. During procedures an activated clotting time (ACT) of 250–300 s was aimed. Procedures were performed under deep sedation using midazolam, sufentanil and/or continuous propofol infusion. The use of pre-interventional and intraprocedural imaging systems (cardiac computed tomography, cardiac magnetic resonance imaging, intracardiac echocardiography), the use of a 3D mapping system (CARTO or NavX) and the ablation system (radiofrequency (RF) or cryoballoon (CB) ablation) were at the discretion of the operators. The standard ablation protocol included assessment of PV isolation and re-ablation in case of PV reconnection to achieve persistent electrical disconnection of the PVs from the left atrium (LA). Additional ablation strategies including the creation of right atrial (RA) and LA linear lesions including block of the cavo-tricuspid isthmus (CTI), or ablation of complex fractionated atrial electrograms (CFAEs) were at the discretion of the operator. The post-procedural anticoagulation management and anti-arrhythmic drug therapy (AAD) were conducted according to local standards.
Complications were categorized in severe, moderate and minor complications (see Supplement Table 1 for further details).
Clinical follow-up
Follow-up was conducted according to local standards and included patient visits, ECG and Holter-ECG recordings. Additionally, a centralized telephonic follow-up was conducted after 12 months using a standardized protocol questioning the incidence of adverse events, arrhythmia recurrence, repeat ablations, symptoms and patients quality of life. Patients subjective perception of the ablation therapy was questioned and defined as successful, partly successful, or unsuccessful.
In case of arrhythmia recurrence documentation of the ECG or medical treatment was obtained. Adverse events (AE) were categorized in serious, moderate and related to the repeat ablation procedure (see Supplement Table 2).
Statistics
Continuous data are summarized as mean and standard deviation or median plus interquartile range (IQR; first and third quartile) in case of skewed data. Categorical data are presented as absolute and relative frequencies. Differences between the patient groups were compared with a Chi-square test or Mann–Whitney–Wilcoxon test. The Kaplan–Meier method was used to calculate 12-month event-rate of MACE (composite endpoint of death and myocardial infarction), MACCE (composite endpoint of death, myocardial infarction and stroke) and quality endpoints (composite endpoint of death, myocardial infarction, stroke and major bleeding). A log rank test was used to compare incidences of MACE, MACCE and quality endpoints. Statistical calculations were based on available data and cases at the timepoint of follow-up.
Results
Patient population and baseline characteristics
A total of 738 patients undergoing first repeat ablation were analyzed (Group 1 = 108 patients > 70 years, group 2 = 630 patients ≤ 70 years). Median ages were 73 (71; 75) and 61 (53; 66) years (p < 0.001) and group 1 consisted of significantly more females (41 (38.0%) vs. 174 (27.6%) patients, p < 0.001). The underlying arrhythmia leading to repeat ablation was paroxysmal AF in 60 (55.6%) and 397 (63.0%) patients (p = 0.14) and persistent AF in 48 (44.4%) and 233 (37.0%) patients (p = 0.14) in groups 1 and 2, respectively. Significantly more patients in group 1 suffered from coronary artery disease and valvular heart disease was significantly more common in the elderly patients (31 (28.7%) and 17 (15.7%) patients vs. 88 (14.0%) and 50 (7.9%) patients, p < 0.001 for each parameter). There were more patients with dilated or hypertrophic cardiomyopathy in group 2 (0 (0.0%) vs. 28 (4.4%) patients, p < 0.001). Group 1 patients had did significantly more often a pacemaker, implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy (CRT) device (15 (13.9%) vs. 31 (4.9%) patients, p < 0.001). Table 1 gives an overview of patients baseline data.
Procedural parameters
Most patients underwent repeat ablation using RF ablation (698/738 patients, 94.6%). There was a statistical trend for a more frequent use of cryoballoon ablation in group 2 (2/108 (1.9%) patients in group 1 and 38/630 (6.0%) patients in group 2, p = 0.076). There were significantly less patients in group 1 undergoing manual catheter navigation (77/83 (92.8%) vs. 424/430 (98.6%) patients, p = 0.001) due to a significantly more frequent use of magnetic navigation in group 1 (5/83 (6.0%) vs. 5/430 (1.2%) patients, p = 0.003). In 1 patient of group 1 the method of catheter navigation was unknown and in an additional patient of group 2 ablation was performed using remote robotic navigation. Repeat ablation was performed under deep sedation in a comparable amount of the patients in group 1 and 2, respectively (97.6% (81/83) and 96.6% (402/416); p = 0.65). The remaining patients underwent repeat ablation without sedation in 1.2% (1/83) and 2.4% (1/416) (p = 0.50), with invasive ventilation in 0% and 0.7% (3/416) (p = 0.44) and an undetailed sedation method in 1 patient of each group (p = 0.20).
A similar amount of patients underwent repeat PVI due to reconduction to the PVs in groups 1 and 2, respectively (101 (93.5%) vs. 593 (94.1%) patients, p = 0.98). Circumferential repeat PVI was conducted in 85 (78.7%) and 491 (77.9%) and segmental repeat PVI in 16 (14.8%) and 102 (16.2%) patients. The patients underwent additional RA and LA linear lesion ablation in 32 (29.6%) and 164 (26.0%) and CFAE ablation in 30/99 (30.3%) and 131/542 (24.2%) of the cases in groups 1 and 2 (p = 0.43 and p = 0.20). There was a significantly higher amount of patients undergoing ablation of linear lesions in the LA in the older patient group (25/32 (78.1%) vs. 94/164 (57.3%), p = 0.027). The procedural data are depicted in Table 2.
Acute procedure related complications during hospital stay
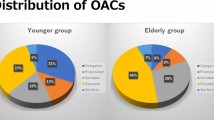
There was no significant difference in the incidence of severe, moderate and minor complications between the two groups (Table 2; Fig. 1). The total incidence of complications was 6.5% (7 patients with complications) and 3.8% (24 patients with complications) in groups 1 and 2 (p = 0.30). Details of the incident complications are shown in Table 2 and Fig. 1. No patient died during the procedure or the hospital stay.
Arrhythmia recurrence and patients satisfaction after repeat ablation
Clinical follow-up was available for 107 and 611 patients in groups 1 and 2 (99.1% and 97.0% of the total patient population, p = 0.36). Median follow-up duration was 447 (400; 532) days in group 1 and 473 (411; 544) days in group 2 (p = 0.10). Rhythm follow-up with ECG documentation was available in 105 patients of group 1 (97.2%) and 603 patients of group 2 (95.7%). Recurrence of atrial tachyarrhythmias was documented in 45/105 (42.9%) and 252/603 (41.8%) of the patients in each group, which was statistically not significant (p = 0.84). A similar amount of patients in each group underwent another repeat ablation procedure during the follow-up period (19/105 (18.1%) and 104/603 (17.2%) in groups 1 and 2, p = 0.83) after a median of 285 (144; 376) and 208 (103; 331) days after the first repeat procedure (p = 0.050). At the end of the follow-up 40.8% (42/103) and 33.2% (191/576) of the patients were still on AAD therapy (class I, III or IV). In group 1 significantly less patients were on class I AAD, but significantly more patients on class III AAD (10/103 (9.7%) vs. 104/576 (18.1%) patients on class I, p = 0.037; 28/103 (27.2%) vs. 86/576 (14.9%) patients on class III AAD, p = 0.002). In total, a comparable 35.0% (36/103) and 42.0% (333/603) of the patients in groups 1 and 2 were without documented arrhythmia recurrence and off AAD therapy at the end of the follow-up (p = 0.17).
At the end of the follow-up a comparable amount of patients stated to be free of arrhythmia-related symptoms or to have symptom improvement in both groups (80/100 patients (80%) and 446/576 patients (77%) in groups 1 and 2; p = 0.57). Additionally, a comparable amount of patients with available data stated the ablation therapy to be successful and partly successful (13/21 (61.9%) and 96/157 (61.1%) patients in groups 1 and 2, p = 0.95 and 6/21 (28.6%) and 38/157 (24.2%) patients in groups 1 and 2, p = 0.66 stated to have successful and partly successful ablation therapy, respectively). Table 3 and Fig. 2 give an overview about the follow-up data.
Patient survival and long-term safety
Kaplan–Meier estimates of 366-day incidence for MACE (death and myocardial infarction) was 0.9% and 0.2% (95% CI 0.36–91.65, log-rank p = 0.16, HR 5.73), for MACCE (death, myocardial infarction and stroke) was 0.9% and 0.5% (95% CI 0.20–18.41, log-rank p = 0.57, HR 1.92) and for quality endpoints (death, myocardial infarction, stroke and major bleeding) was 1.9% and 1.3% (95% CI 0.31–6.79, log-rank p = 0.64, HR 1.44).
One death with unknown reason occurred during the follow-up period in each patient group (0.9% and 0.2% of all patients, p = 0.69; Table 4). A total of 16 severe AE occurred in both groups during follow-up with an overall incidence of severe AE of 1.0% vs. 2.6% in groups 1 and 2 (1 major bleeding in group 1, 2 myocardial infarctions in group 2, 6 strokes in group 2 and 7 major bleedings in group 2, p = 0.32). In detail, there occurred numerically more strokes in group 2 but the difference was not significantly different (0/103 (0%) vs. 6/577 (1%) of the patients, p = 0.30). Moderate and late ablation procedure related AEs occurred in 5.4% (5/103)/0.0% (0/103) and 8.1% (38/472)/0.5% (3/579) of the patients in group 1 and 2, which was statistically not significantly different (p = 0.39 and 0.46).
More patients in group 1 were still on oral anticoagulants at the end of the follow-up (55/103 (53%) vs. 226/576 (39%) patients, p = 0.007), which did not result in a significantly different amount of major or minor bleedings in the patient cohorts with 0.9% (1/108) and 0.5% (3/630) major bleedings (p = 0.56) and 1.9% (2/108) and 1.6% (10/629) minor bleedings (p = 0.84) in groups 1 and 2, respectively.
The total incidence of re-hospitalisation during follow-up was not different between the two groups (51.5% and 43.1%, p = 0.11). The median time from discharge after repeat ablation to re-hospitalisation was 214 (126; 357) and 202 (94; 346) days (p = 0.74) and the reason for hospitalisation was stated as cardiovascular cause in 73.1% and 71.9% in groups 1 and 2 (p = 0.86). Table 4 gives an overview on follow-up safety data.
Comparison of patients > 75 and ≤ 75 years of age
Subgroup analysis of patients > 75 years of age (n = 23) as compared to patients ≤ 75 years of age (n = 715) revealed a significantly higher incidence of CAD (9/23 (39.1%) and 110/715 (15.4%) patients, p = 0.002), history of myocardial infarction (4/23 (17.4%) and 42/715 (5.9%) patients, p = 0.025) and of cardiac device carriers (9/23 (39.1%) and 37/715 (5.2%) patients, p < 0.001). There was no significant difference between procedural parameters, occurrence of adverse events and follow-up parameters in patients > 75 and ≤ 75 years of age except shorter duration of fluoroscopy during procedures in patients > 75 years (19 (15; 32) vs. 27 (28; 44) min, p = 0.049) and a higher amount of patients being on oral anticoagulation during clinical follow-up in the older patient group (16/22 (72.2%) and 265/657 (40.3%) patients with available follow-up, p = 0.002).
Discussion
This is the first report on real-world outcomes of first repeat AF ablation procedures in elderly patients, assessed in a large, prospectively enrolled multicenter patient cohort. Our main finding is that repeat ablation can be conducted safely in the elderly. Furthermore, repeat ablation results in a comparable procedural success, patients satisfaction and symptom improvement in aged patients as compared to younger patients.
Catheter ablation in the elderly: current knowledge
The majority of patients investigated in large trials of AF ablation are of younger age [6, 7] and data on AF ablation in older patients are sparse. There is no unique definition of an elderly patient but most studies used a cut-off value between 65 and 75 [4,5,6, 9,10,11,12].
The majority of available studies reported on patient cohorts with a limited number of individuals [4, 9, 11, 13]. A larger number of elderly patients was reported by the German Ablation Registry which analysed the first attempt of AF ablation in patients older than 75 years [5]. Two studies reported on the feasibility of more complex ablation strategies for the treatment of AF in elderly including extensive ablation of CFAEs [14, 15].
Repeat ablations in the elderly patient
To achieve long-lasting clinical success rates after AF catheter ablation repeat ablation procedures are often necessary, in particular, in patients with persistent AF [6, 7]. In contrast to the positive results of the above mentioned studies on AF ablation in elderly patients, Bunch et al. found an association between higher age and the likelihood of AF recurrence after catheter ablation [16]. Therefore, data addressing the procedural outcome and long-term data of repeat ablation for AF in the elderly are needed.
PV-reconnection was seen in a comparable number of patients in both groups and was the main driver of arrhythmia recurrence after the initial ablation procedure [6, 7]. However, aged patients in our study underwent significantly more often ablation of linear lesions within the LA in addition to repeat PVI as compared to the younger patients. Since advanced age is associated with a higher amount of atrial fibrosis in AF patients [17] this might explain why left atrial substrate modification was performed more often in the older patients.
We found a comparable long-term effectiveness in older and younger patients, which is in line with the majority of studies on first ablation procedures for atrial fibrillation [4, 9, 12,13,14, 18]. Younger patients were more often on class I and older on class III AAD therapy. This difference can be attributed to the higher amount of structural heart disease in the older patients of this study population. Our registry assessed the patient’s satisfaction after repeat ablation and found a comparable amount of patients that termed repeat ablation as successful or at least partly successful and a comparable amount of patients reporting on improvement in subjective symptoms in both groups. Our data show that catheter ablation even with repetitive ablation procedures is a feasible treatment for symptom control in AF also for elderly patients. This finding is of major importance since there is a high likelihood to undergo a repeat procedure after arrhythmia recurrence after catheter ablation.
Beneath these favorable results of repeat ablations in older patients our data show that both, young and aged patients had a high incidence of rehospitalisation after first repeat ablation. Nearly half of the patients had hospital admission during the first 12 months after repeat ablation and nearly 20% of the patients underwent a second repeat ablation procedure. This demonstrates the ongoing need to improve the long-term efficacy of AF ablation and the need for development of new approaches in AF treatment [19].
Safety of repeat ablation
Although in most studies AF ablation in older patients was not associated with a higher complication rate, data about the safety of AF ablation in the elderly are still conflicting [3, 9,10,11,12, 20]. Studies found a higher incidence of periprocedural thromboembolic events in aged patients [5, 18]. Data from the US Nationwide Inpatient Sample demonstrated a higher incidence of periprocedural complications in patients older than 80 years [20].
We did not find a statistically significant incidence of overall periprocedural complications, in particular of major complications, in the elderly as compared to younger patients. Contrary to a previous report, which associated extensive linear ablation with an increased risk of cardiac tamponade [21], the incidence of cardiac tamponade was not higher in older patients in our study, although older patients had more often extensive ablations including left atrial linear lesions. In summary, our study shows, that repeat ablation, even when more extensive ablation is performed, can be performed with a comparable complication rate in the elderly.
Limitations
Data acquisition was conducted in a registry based on voluntary reports of procedural results and adverse events and in a non-randomized fashion. Additionally, follow-up was conducted according to local standards and we cannot exclude an influence of heterogenous methods and quality of data acquisition on study results. Nevertheless, the German Ablation Registry represents real-world data in a large patient cohort and we report the so far largest patient population in which repeat ablation and the influence of ageing on safety and procedural efficacy was studied. Since recent technological innovations like contact-force catheters [22], the use of the second-generation CB [23], laser balloon [24] or other advanced ablation techniques like rotor-mapping [25] were not available during the enrolment period of the registry, the influence of these technological advances has to be addressed in further studies. Nevertheless, we show that AF ablation in the elderly is effective and safe even without implementation of these novel technologies.
Conclusions
Repeat catheter ablation for AF can be safely performed in elderly patients with comparable results to patients in younger ages regarding periprocedural complications, long-term efficacy and patient satisfaction.
References
Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B et al (2016) 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 18(11):1609–1678
Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J et al (2010) Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 3(1):32–38. https://doi.org/10.1161/CIRCEP.109.859116(Epub 2009 Dec 7)
Metzner I, Wissner E, Tilz RR, Rillig A, Mathew S, Schmidt B et al (2016) Ablation of atrial fibrillation in patients ≥ 75 years: long-term clinical outcome and safety. Europace. 18(4):543–549. https://doi.org/10.1093/europace/euv229(Epub 2016 Jan 29)
Tscholl V, Lin T, Abdullah K, Lsharaf AKA, Bellmann B, Nagel P et al (2018) Cryoballoon ablation in the elderly: one year outcome and safety of the second-generation 28 mm cryoballoon in patients over 75 years old. Europace 20(5):772–777. https://doi.org/10.1093/europace/eux128
Moser JM, Willems S, Andresen D, Brachmann J, Eckardt L, Hoffmann E et al (2017) Complication rates of catheter ablation of atrial fibrillation in patients aged ≥ 75 years versus < 75 years-results from the German Ablation Registry. J Cardiovasc Electrophysiol 28(3):258–265. https://doi.org/10.1111/jce.13142(Epub 2017 Jan 14)
Ouyang F, Tilz R, Chun J, Schmidt B, Wissner E, Zerm T et al (2010) Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation 122(23):2368–2377. https://doi.org/10.1161/CIRCULATIONAHA.110.946806
Tilz RR, Rillig A, Thum AM, Arya A, Wohlmuth P, Metzner A et al (2012) Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol 60(19):1921–1929. https://doi.org/10.1016/j.jacc.2012.04.060
Eitel C, Ince H, Brachmann J, Kuck KH, Willems S, Gerds-Li JH, Tebbenjohanns J, Richardt G, Hochadel M, Senges J, Tilz RR (2019) Atrial fibrillation ablation strategies and outcome in patients with heart failure: insights from the German ablation registry. Clin Res Cardiol. https://doi.org/10.1007/s00392-019-01411-3(Epub ahead of print)
Zado E, Callans DJ, Riley M, Hutchinson M, Garcia F, Bala R et al (2008) Long-term clinical efficacy and risk of catheter ablation for atrial fibrillation in the elderly. J Cardiovasc Electrophysiol 19(6):621–626. https://doi.org/10.1111/j.1540-8167.2008.01183.x(Epub 2008 May 5)
Corrado A, Patel D, Riedlbauchova L, Fahmy TS, Themistoclakis S, Bonso A et al (2008) Efficacy, safety, and outcome of atrial fibrillation ablation in septuagenarians. J Cardiovasc Electrophysiol 19(8):807–811. https://doi.org/10.1111/j.1540-8167.2008.01124.x(Epub 2008 Mar 21)
Leong-Sit P, Zado E, Callans DJ, Garcia F, Lin D, Dixit S et al (2010) Efficacy and risk of atrial fibrillation ablation before 45 years of age. Circ Arrhythm Electrophysiol 3(5):452–457. https://doi.org/10.1161/CIRCEP.110.938860(Epub 2010 Sep 21)
Kautzner J, Peichl P, Sramko M, Cihak R, Aldhoon B, Wichterle D (2017) Catheter ablation of atrial fibrillation in elderly population. J Geriatr Cardiol 14(9):563–568. https://doi.org/10.11909/j.issn.1671-5411.2017.09.008
Abugattas JP, Iacopino S, Moran D, De Regibus V, Takarada K, Mugnai G et al (2017) Efficacy and safety of the second generation cryoballoon ablation for the treatment of paroxysmal atrial fibrillation in patients over 75 years: a comparison with a younger cohort. Europace 19(11):1798–1803. https://doi.org/10.1093/europace/eux023
Santangeli P, Di Biase L, Mohanty P, Burkhardt JD, Horton R, Bai R et al (2012) Catheter ablation of atrial fibrillation in octogenarians: safety and outcomes. J Cardiovasc Electrophysiol 23(7):687–693. https://doi.org/10.1111/j.1540-8167.2012.02293.x(Epub 2012 Apr 11)
Nademanee K, Amnueypol M, Lee F, Drew CM, Suwannasri W, Schwab MC et al (2015) Benefits and risks of catheter ablation in elderly patients with atrial fibrillation. Heart Rhythm 12:44–51
Bunch TJ, May HT, Bair TL, Jacobs V, Crandall BG, Cutler M et al (2016) The impact of age on 5-year outcomes after atrial fibrillation catheter ablation. J Cardiovasc Electrophysiol 27(2):141–146. https://doi.org/10.1111/jce.12849
Akoum N, Mahnkopf C, Kholmovski EG, Brachmann J, Marrouche NF (2017) Age and sex differences in atrial fibrosis among patients with atrial fibrillation. Europace. https://doi.org/10.1093/europace/eux260(Epub ahead of print)
Blandino A, Toso E, Scaglione M, Anselmino M, Ferraris F, Sardi D et al (2013) Long-term efficacy and safety of two different rhythm control strategies in elderly patients with symptomatic persistent atrial fibrillation. J Cardiovasc Electrophysiol 24(7):731–738. https://doi.org/10.1111/jce.12126(Epub 2013 Apr 1)
Rillig A, Schmidt B, Di Biase L, Lin T, Scholz L, Heeger CH et al (2017) Manual versus robotic catheter ablation for the treatment of atrial fibrillation the man and machine trial. JACC: Clin Electrophysiol 3(8):875–883. https://doi.org/10.1016/j.jacep.2017.01.024
Deshmukh A, Patel NJ, Pant S, Shah N, Chothani A, Mehta K et al (2013) In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: analysis of 93,801 procedures. Circulation 128:2104–2112
Chun KR, Perrotta L, Bordignon S, Khalil J, Dugo D, Konstantinou A et al (2017) Complications in catheter ablation of atrial fibrillation in 3,000 consecutive procedures balloon versus radiofrequency current ablation. JACC Clin Electrophysiol 3(2):154–161. https://doi.org/10.1016/j.jacep.2016.07.002
Maurer T, Rottner L, Makimoto H, Reissmann B, Heeger CH, Lemes C, Fink T, Riedl J, Santoro F, Wohlmuth P, Volkmer M, Mathew S, Metzner A, Ouyang F, Kuck KH, Sohns C (2018) The best of two worlds? Pulmonary vein isolation using a novel radiofrequency ablation catheter incorporating contact force sensing technology and 56-hole porous tip irrigation. Clin Res Cardiol. https://doi.org/10.1007/s00392-018-1270-y(Epub ahead of print)
Heeger CH, Bellmann B, Fink T, Bohnen JE, Wissner E, Wohlmuth P, Rottner L, Sohns C, Tilz RR, Mathew S, Reissmann B, Lemeš C, Maurer T, Lüker J, Sultan A, Plenge T, Goldmann B, Ouyang F, Kuck KH, Metzner I, Metzner A, Steven D, Rillig A (2019) Efficacy and safety of cryoballoon ablation in the elderly: a multicenter study. Int J Cardiol 1(278):108–113. https://doi.org/10.1016/j.ijcard.2018.09.090(Epub 2018 Sep 27)
Reissmann B, Budelmann T, Wissner E, Schlüter M, Heeger CH, Mathew S, Maurer T, Lemes C, Fink T, Rillig A, Santoro F, Riedl J, Ouyang F, Kuck KH, Metzner A (2018) Five-year clinical outcomes of visually guided laser balloon pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation. Clin Res Cardiol 107(5):405–412. https://doi.org/10.1007/s00392-017-1199-6
Bellmann B, Lin T, Ruppersberg P, Zettwitz M, Guttmann S, Tscholl V, Nagel P, Roser M, Landmesser U, Rillig A (2018) Identification of active atrial fibrillation sources and their discrimination from passive rotors using electrographical flow mapping. Clin Res Cardiol. https://doi.org/10.1007/s00392-018-1274-7(Epub ahead of print)
Funding
This work was funded by an unrestricted grant from foundation ‘Stiftung Institut für Herzinfarktforschung Ludwigshafen’ (Ludwigshafen, Germany).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Metzner received speaker’s honoraria from Medtronic. Dr. Willems received speaker honoraria from Abott, Boston Scientific, Boehringer Ingelheim, Bristol Myers Squibb, Bayer Vital, Acutus and study funding from Abott, Boston Scientific and Acutus. Dr Eckardt consulting fees/speaker honoraria from Bayer Health Care, Daiichi Sankyo, Pfizer, Bristol-Myers, Squibb, Boehringer Ingelheim, Johnson&Johnson, Medtronic, Boston Scientific, Abbott, Novartis and research support by the DFG the German Heart Foundation. Dr Brachmann received consulting fees/honoraria from Biotronik, Biosense Webster, Medtronic, Boston Scientific, and St. Jude Medical. Dr Rillig received travel grants/lecture fees from Biosense, Hansen Medical, Medtronic, EP Solutions and St. Jude Medical and participated at the Boston scientific EP-fellowship. The other authors report no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fink, T., Metzner, A., Willems, S. et al. Procedural success, safety and patients satisfaction after second ablation of atrial fibrillation in the elderly: results from the German Ablation Registry. Clin Res Cardiol 108, 1354–1363 (2019). https://doi.org/10.1007/s00392-019-01471-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-019-01471-5