Abstract
Object
Chiari malformation type II is almost exclusively found in patients with open spinal dysraphism. Etiology and pathophysiology are not yet completely understood, and management guidelines regarding the best follow-up and treatment of this pathological entity do not exist. In order to assess essential management aspects, literature and a series of secondary neurosurgical interventions in Chiari II patients have been reviewed.
Methods
A literature review regarding etiology, diagnostics, pathophysiology, and management of Chiari malformation type II (CMII) and a retrospective evaluation of a series (2009–2012) of secondary interventions in Chiari II patients have been performed. Inclusion criteria were ICD for myelomeningocele with or without hydrocephalus and ICD for Chiari malformation and neurosurgical OR procedure. Evaluated parameters were: patient demographics, primary management, secondary neurosurgical operations (cranio-cervical decompression, shunt revision, myelolysis) as well as specific findings pre- and postoperatively. Essential results from literature review and patients' series are compiled in order to define management recommendations.
Results
Fifty patients (28 f, 22 m; mean age, 7.1 years (range, 0.5–26 years)) with myelomeningocele-associated Chiari malformation type II were operated on between 2009 and 2012. Twenty-four patients had syringomyelia and scoliosis each, and 12 suffered from both. Orthopedic surgery for scoliosis or kyphosis had been performed in 13 cases. Shunt revision was performed in 38 cases, myelolysis in 17, and decompression of the foramen magnum in 14 (28 %). After a mean follow-up of 1.9 years, syringomyelia decreased from 24 to 16 cases. There was a postoperative reduction of neck pain (one third), sensorimotor (two fifths), and cranial nerve deficits (one half). CSF flow at the foramen magnum did not change visibly after surgery. Ventricular size improved in about half of the patients. Slit-like ventricles were found in nine (6 pre-surgical) and enlarged ventricles in nine (23 pre-surgical). Complication rate was 6 % (3/50) per cases, and no patient died or deteriorated neurologically after surgery.
Conclusion
CMII-related management guidelines are not well defined, since clinical constellations and presentations are varying. Often associated findings are syringomyelia, hydrocephalus, and scoliosis, and symptomatic CMII may be triggered by more than one underlying condition. According to literature and clinical experience, management recommendations can be defined. The most important finding is that hydrocephalus is often involved in symptomatic CMII and must always be considered first in any symptomatic patient. Intrinsic brain stem dysfunctions cannot be treated surgically, and monitoring of vital functions is sometimes the only clinical means that can be offered to the patient. Knowledge of the complex background has led to improved follow-up programs for the affected children and thus also improved longtime survival.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chiari malformation type II is almost exclusively found in patients with open spinal dysraphism. Etiology and pathophysiology are not yet completely understood, and management guidelines regarding the best follow-up and treatment of this pathological entity do not exist. Chiari malformation type II (CMII) originally was defined as a syndrome consisting of the downward displacement of the essential posterior fossa structures: brain stem with medulla oblongata, fourth ventricle, and lower cerebellar parts, also described as hindbrain herniation. With the increase of MRI imaging, more features of the syndrome, mainly in other parts of the brain, have been discovered. A variety of supratentorial and brain stem abnormalities are found in CMII such as dysplasia of the corpus callosum and commissural structures, an enlarged or duplicated massa intermedia, hemispheric white and gray matter abnormalities, hippocampus dysplasia as well as pons elongation and beaking of the midbrain tectum. Defects of the falx and tentorium are also present in numerous cases [17]. Hydrocephalus is associated in up to 80 % as well as abnormalities in different body systems, e.g., cardiovascular, gastrointestinal, or genitourinary. It is not clear which expressions of CMII are primarily malformative and which are secondary to in utero cranio-spinal fluid (CSF) outflow and developing hydrocephalus. Hemispheric and some callosal abnormalities as well as an accessory massa intermedia cannot be explained by altered CSF dynamics or other influences due to the underlying open spinal defect. On the other side, experimental and clinical experience from fetal myelomeningocele closure demonstrated that the presence and extent of hindbrain herniation is positively influenced by early myelomeningocele (MMC) repair [27, 28]. Typical clinical symptoms of CMII are alterations in breathing patterns, a depressed gag reflex, and oculomotor signs [22–24]. In some cases, e.g., when a cervical syringomyelia is present, upper limb symptoms like radiating pain and loss of sensorimotor function can be found. Neck pain and opisthotonic posturing are also reported as typical signs of symptomatic CMII [14]. Although CMII is well described as a single entity, it cannot be discussed without other pathologies closely associated like primary and secondary tethered cord, hydrocephalus, and syringomyelia [8].
Whenever follow-up and treatment modalities are defined, these interacting relationships must be considered. In order to present the current state of evidence and practicability regarding CMII management, we reviewed the literature and evaluated our recent series of secondary operations in patients suffering from CMII and myelomeningocele.
Materials and methods
We reviewed the literature regarding etiology, diagnostics, pathophysiology, and management of CMII. In addition, we retrospectively evaluated our own series from 2009 to 2012 with all patients treated neurosurgically and having the diagnosis CMII and spina bifida with or without hydrocephalus. Inclusion criteria were the ICD for myelomeningocele with or without hydrocephalus (ICD-10-GM codes Q05.0-Q05.9) and ICD for Chiari malformation (ICD-10-GM code Q07.0) and neurosurgical OR procedure. These parameters were retrieved from our patient information system. Relevant clinical data from the electronic patient charts were evaluated. The parameters for evaluation were: patient demographics, primary management, secondary neurosurgical operations (cranio-cervical decompression, shunt revision, myelolysis) as well as specific findings pre- and postoperatively (scoliosis and related orthopedic surgery, syringomyelia, neurological findings, MRI findings, polysomnography results). Finally, considering these information and data, guidelines for the management of Chiari II in children with myelomeningocele are defined.
Results from CMII patient series with secondary neurosurgical interventions
Between January 2009 and December 2012, a consecutive series of 50 patients with myelomeningocele-associated Chiari malformation type II were treated neurosurgically in our department. All of these patients primarily received plastic repair of their myelomeningocele defect in the first days of life and were shunted due to associated hydrocephalus, 47 of them after birth. Three patients were treated fetoscopically in utero with patch coverage of the defect between gestational week 22 and 23. About half of the patients have not been treated primarily by our team. All of them were recruited from our interdisciplinary spina bifida center and underwent secondary surgery for neurological or orthopedic spina bifida syndrome sequelae in our children's hospital. The patients either underwent cranio-cervical decompression and/or shunt revision and/or myelolysis (or spinal cord adhesiolysis). The group consisted of 28 female and 22 male patients; the mean age was 7.1 years (range, 0.5–26 years). Syringomyelia and scoliosis were each present in 24 patients at time of secondary surgery. The mean age for patients with syringomyelia was 6.9 years and for scoliosis, 9.7 years. Twelve of 24 patients suffered from both scoliosis and syringomyelia. No patient demonstrated papilledema, and only three had typical neck pain. Five patients (10 %) underwent preoperative polysomnography (PSG) evaluation demonstrating pathological central and obstructive breathing disorders. Five suffered from new sensorimotor symptoms and two from additional cranial nerve deficits. Orthopedic surgery for neuromuscular scoliosis or kyphosis had been performed in 13 cases.
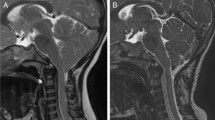
Shunt revision, performed in 38 patients, was the most frequent procedure in this patient group; myelolysis was performed in 17 patients, and decompression of the foramen magnum became necessary in 14 out of 50 (28 %) patients. In three cases, a bony decompression alone was sufficient; in 11, additional dural augmentation, with or without adhesiolysis of the neural structures, had been performed. Shrinking or removal of cerebellar tonsils was not performed (Table 1). In the decompression group, 8 additional procedures for myelolysis (three before, three during, and two after) and 13 for shunt revision (two before, ten during, and one after) became necessary (Table 3). The mean clinical follow-up interval was 1.9 years, and the mean MRI follow-up was 1.8 years (Table 2). Overall results after surgical treatment demonstrated a decreasing number of syringomyelia from 24 to 16. In three cases, the syrinx itself decreased in size. This was observed after decompression in 3, shunt revision in 14, and myelolysis in 7 cases. Postoperative neck pain was found in only one out of three patients. PSG was controlled in five patients demonstrating one early improvement of central breathing disorder. Two out of five new sensorimotor deficits disappeared after surgery as well as one cranial nerve deficit. CSF flow at the foramen magnum did not change after surgery: there was no visible flow in two out of three patients. In the remaining patients, one half showed only ventral CSF flow, the other half a ventro-dorsal flow. Ventricular size improved in about half of the patients. Slit-like ventricles were found in nine (6 pre-surgical) and enlarged ventricles in nine (23 pre-surgical) with three improved and two one-sided findings. Normalization was found in 18 (eight pre-surgical) cases (Table 1). Looking at the decompression group (14 cases, including 8 myelolysis and 13 shunt revisions), neck pain diminished in 2 out of 3 patients and syringomyelia in 3 (Fig. 1) out of 12 (two patients improved). There was no clear change in CSF dynamics around the foramen magnum. Ventricular size normalized in four cases, showed decreased width in five out of seven cases, and was slit like in three cases (formerly two) (Table 3).
Complication rate was 6 % (3/50) per cases. One girl developed cerebellar hemisphere bleeding after aspiration of painful CSF pseudomeningocele at the duraplasty site. Another girl suffered from repeated functional shunt complications due to gravitational unit and a deep venous thrombosis 1 week after discharge from hospital. One CSF pseudocele was not treated and resolved spontaneously. There was no mortality in this patient group.
In five cases, no MRI data were available retrospectively. In three patients, telesensor ICP monitoring revealed increased pressure values (Fig. 2) and indicated surgery (one decompression plus shunt revision, two shunt revisions). In three patients, preoperative functional deterioration (foot spasticity, foot deformity, radiating pain) was reversible after myelolysis.
A 17-year-old female with nocturnal and morning headache as well as intermittent episodes of severe headache. a Slit-like ventricles and mild unilateral papilledema were found. Telemetric ICP measurement recorded nocturnal B waves above 30 mmHg. Shunt revision revealed partial central occlusion. b Postoperative improved headache and less frequent B waves, but still elevated ICP during the night. Additional foramen magnum decompression was indicated. c and d After decompression, ICP normalized, but headache episodes persisted
Results from literature review
Regarding primary surgery for CMII and MMC, there is substantial evidence that early postnatal closure of the open spinal defect in between 48 h prevents major complications like infection and uncontrolled CSF outflow. The significance of spinal defect closure in respect of CMII has not been addressed in early publications. Morota et al. [18] have shown that postnatal MMC repair partially reverses or improves hindbrain herniation. They reviewed 20 cases with open spinal dysraphism and found that this series could be divided into two groups: 14 patients had a caudal tonsillar position ranging from C2 down to C7, and 11 out of them developed an ascent of the tonsillar tip of one to four segments (mean two segments). The ascent continued in most of them gradually after shunt placement. But 8 out of these 14 patients later on developed symptomatic CMII. The other group of six infants showed a tonsillar position at C2 down to C4/5. In four cases, the tonsils ascended 1 to 1.5 levels without signs of symptomatic CMII during follow-up. These results show that severe hindbrain herniation can partially be corrected after birth but does not prevent the occurrence of symptomatic CMII.
Stritzke et al. reported on a fatal case of vernix caseosa granulomatous meningitis, which appeared clinically like severe symptomatic CMII. The correlation of stridor at birth and poor prognosis and possible infectious complications already acquired in utero are discussed [25]. Since the 1980s, experimental and clinical studies on fetal MMC repair have been conducted. The two major hypotheses for this treatment approach were reduction of secondary toxic reactions caused by amniotic fluid as a second hit theory regarding spinal cord and neurological dysfunction on the one side. On the second side, a reduction of hindbrain herniation and consecutive hydrocephalus development were anticipated by stopping CSF outflow. Both have been proven to be the fact, although a series of technical problems and the so far inevitable prematurity do not allow fetal MMC repair being considered as a standard of care [1]. Probably the most striking effect of fetal defect closure before gestational week (GW) 20 is the reduction of hindbrain herniation in most of the affected fetuses. Due to the relatively accelerated cerebellar growth compared to the development of other brain structures after GW 20, early closure at midgestation would allow a normal cerebellar growth inside a normal posterior fossa [4]. Repeated fetal MRI videos in MMC fetuses have shown the relationship between CSF outflow from the spinal defect and development of hindbrain herniation (oral presentation by G. Kasprian, Vienna; during the 4th International Symposium Der kleine Patient in der Neurochirurgie, November 11th, 2012, Frankfurt/Main; Germany). Extent and level of the spinal defect also seem to be important factors regarding the size of the posterior fossa and cerebellar volume as well as of the downward position of hindbrain structures [26].
Late deterioration of neurological and skeletomuscular functions are typical in patients with open spinal dysraphism [5], and the risk of secondary complications will not become smaller in adulthood. Therefore, interdisciplinary follow-up throughout the lifetime is essential to ensure a good long-term prognosis, e.g., in times of improved overall survival in this patient group. Piatt et al. reviewed outcome studies and found that the most critical and prognostically determinant factor is shunt failure instead of CMII [21]. Patients with CMII and MMC are best followed in an interdisciplinary group of specialized physicians, and whenever patients are seen by neurosurgeons or orthopedic surgeons for possible secondary worsening of their preexisting conditions, MRI of the cranio-spinal neuroaxis is strongly recommended, since CMII as well as hydrocephalus and tethered cord with or without syringomyelia may contribute to the actual symptomatology [7]. Besides regular clinical follow-up, neurophysiological tests like somatosensory evoked potentials (SEPs) [20] and/or brain stem auditory evoked potentials combined with blink reflex measurements [19] can be useful as well. Breathing disorders and sleep apnea are possible signs of brain stem dysfunction in CMII [2, 9, 12, 29]. But also obstructive apnea is found in dysraphic patients due to tracheomalacia or other MMC-related conditions [9]. This can be of significant risk for the patient and also affect all day life by causing attention deficit disorders due to inadequate sleep during the night [13]. Regarding functional testing in CMII patients, literature does not provide much information and evidence. There is one cross-sectional single-institution study investigating the correlation of PSG findings with clinical data of CMII patients. Eighty-three children could be included (out of 109). Thirty one (37 %) had a normal sleep pattern, 35 (42 %) showed a slightly abnormal pattern, and a moderately to severely abnormal breathing function was seen in 17 (20 %) cases. In this group of 17 children, 12 showed predominantly central apneas and 5 more obstructive apneas. Children with thoracic or thoracolumbar MMCs (relative risk (RR), 9.2), previous foramen magnum decompression (RR, 3.5), significant brain stem malformation (RR, 3.0), and pulmonary function abnormalities (RR, 11.6) had a relatively high risk of moderate to severe sleep apnea. Nocturnal pulse oximetry was able to detect sleep apnea in these patients (sensitivity, 100 %; specificity, 67 %) and is recommended in high-risk patients [29].
Various publications regarding surgical management of secondary complications can be found, but regarding surgical indications and strategies, no well-defined guidelines are available. In order to offer the most adequate treatment to a secondarily symptomatic patient, all interrelating factors of the pathomechanisms must be considered. Most of the patients with open spinal dysraphism present with associated CMII (more than 90 %) and hydrocephalus (up to 80 %) as well as with kyphoscoliosis (overall 80 %, depending on MMC level 5–100 %). The etiopathological relationship between these entities is not clear. An extensive literature review according to the guidelines of the Oxford Centre for Evidence-Based Medicine Levels of Evidence was undertaken by M. S. Dias [10] regarding the major underlying cause of scoliosis in MMC patients. He found that there was some evidence that only spinal cord tethering influenced scoliosis development. Little evidence was found to support a positive correlation of CMII and/or syringomyelia and scoliosis.
Surgical indication for cranio-cervical decompression needs to be discussed in typical symptomatic CMII with sleep apnea, lower cranial nerve dysfunction, and upper limb symptoms or progressive tetraparesis, but also in cases of progressive holocord hydrosyringomyelia and before planned orthopedic surgery for scoliosis correction. The goal of decompression is pressure relief of the brain stem and cranial nerve structures as well as reestablishing CSF flow around the foramen magnum, addressing syringomyelia. A holocord syrinx involving cervical segments as well is also considered being related to CMII and not only to the tethering of the spinal cord due to dysraphism. Additional postinflammatory arachnoiditis can also promote holocord syrinx in these patients.
La Marca et al. [14] published their management strategies in children with CMII and hydromyelia. In 231 MMC cases with MRI studies, they found hydromyelia in 48.5 % of patients; 45 of these patients had significant hydromyelia and required treatment, and one group had holocord hydromyelia, the other segmental syringomyelia; 15 patients had typical CMII symptoms, 18 typical tethered cord symptoms with deteriorating scoliosis and bladder/bowel-dysfunction and lower limb symptoms, and 12 children presented with a mixed symptomatology. In this latter group, decompression and detethering or syringo-pleural shunting was performed. In the other groups, only decompression or detethering or syrinx shunting was indicated depending on the predominant cause. In two other publications by Caldarelli et al. [5, 6], the impact of inappropriate hydrocephalus drainage on symptomatic CMII and hydromyelia was highlighted. In one series of 26 children with secondary neurological deterioration, they performed 22 interventions. In half of them, spinal cord detethering was sufficient; in the other half, five shunt revisions, four syrinx shunts, and two cranio-cervical decompressions improved the symptoms [5]. In the other series, 142 MRI studies of children with spina bifida were evaluated; 32 had hydromyelia and 18 of them were not symptomatic. The remaining 14 (10 %) were symptomatic. In this group, a correlation between symptoms and extent of the hydromyelia as well as adequate hydrocephalus drainage was found. Fifteen interventions became necessary: seven syrinx shunts, five decompressions, two ventriculoperitoenal shunt revisions, and one untethering procedure. Mean age of the symptomatic children was 4 years [6]. Symptomatic CMII with sleep apnea can be caused by hydrocephalic complications alone and may resolve only by appropriate CSF drainage [15]. Endoscopic treatment of the associated hydrocephalus is also an option in selected cases [11].
The phenomenon of a crowded foramen magnum may be overestimated. Milhorat et al. [16] investigated the size of the posterior fossa and the foramen magnum in Chiari type I (CMI) and type II. They could demonstrate that the size of the foramen magnum was significantly larger in CMII cases and CMI with associated tethered cord.
Aronson et al. [3] investigated the cervical spine of children with CMII after decompression and found a ratio of 19/20 instabilities after suboccipital decompression and cervical laminectomy.
Discussion
Primary management of CMII
Regarding primary management of CMII in MMC patients, early defect closure of the open spinal dysraphism is essential. It has been shown by Morota et al. [18] that even in mature newborns, closure of the spinal defect leads to upward movement of the herniated hindbrain which continues after ventriculoperitoneal shunting of the related hydrocephalus in some patients. Prevention of secondary infection which may deteriorate CMII-related symptoms is another aspect of the initial management [25]. Theoretically, the best management is prevention of hindbrain herniation, which can only be achieved by in utero defect closure before onset of disproportionate growth of the cerebellum after GW 20 [1, 4, 27, 28]. But up to now, fetal MMC surgery is not considered as standard of care due to a high fetal and maternal risk, e.g., inevitable prematurity, and uterus complications. Results from several series, and also from our own, could demonstrate that adequate treatment of hydrocephalus is more important in order to prevent symptomatic CMII compared to direct decompression of brain stem structures in the early postnatal period. This essential statement can be applied to primary and secondary treatment [5, 6, 14, 15].
It is important to identify newborns and infants with CMII who are at risk regarding their brain stem functions. Initial stridor and swallowing difficulties as well as a hoarse and low voice together with opisthotonic posture are ominous signs [18, 25] of symptomatic CMII. Brain stem compression is not necessarily the only underlying cause. Also, a primary dysplastic brain stem, postinfectious complications after in utero contamination of CSF, and increased intracranial pressure due to hydrocephalus must be ruled out before any indication for treatment is set.
In these cases, 24-h monitoring and pulse oximetry surveillance are strongly recommended. Daily ultrasound for ventricular size assessment and foramen magnum visualization as well as head circumference measurements are useful. Early MRI of the whole cranio-spinal axis together with venous MR angiograph is necessary before indicating decompression surgery. In rare cases, tube feeding and continuous positive airway pressure ventilation becomes necessary. Tracheostomy should be reserved for very rare cases with resolvable secondary complications. In most of these symptomatic cases, early CSF shunting will prevent severe symptomatic courses. There is some evidence that children especially with thoracic and large spinal defects tend to develop symptomatic CMII. Since prenatal counseling is a standard in many Western countries, this constellation became rare. The incidence of early foramen magnum decompression is substantially low, and in these cases, the most appropriate approach is probably simple extradural bony decompression. But care must be taken in order to avoid secondary cervical instability be detaching muscles from C2 and lower segments [3].
Secondary management of CMII
It is important to monitor patients with CMII and spina bifida syndrome throughout their lives. Since hydrocephalus shunting became a standard in the 1950s, life expectancy of spina bifida babies increased significantly, and permanent vital and functional risks, e.g. from inadequate hydrocephalus drainage, have to be addressed in follow-up programs [21]. MMC patients followed by an experienced interdisciplinary spina bifida group will have regular tests of all involved organ systems. Regarding CMII attention, focus is to be put on the following aspects:
-
1.
Specific pain sensations in the suboccipital and neck region and pain radiation into the upper limbs: the patient should be interviewed regarding these specific pain patterns.
-
2.
Direct or indirect signs of sleep disorders: snoring and frequent sleepless episodes during the night as well as sleepiness and attention deficits during daytime. Polysomnography and ENT counseling to exclude obstructive causes are indicated in suspicious cases.
-
3.
Lower cranial nerve dysfunction: A hoarse and low voice as well as swallowing problems are typical symptoms. Laryngoscopy and swallowing studies are recommended.
-
4.
Upper and lower limb symptoms: testing of sensorimotor functions and muscle reflexes as well as SEP and MEP help to detect long tract dysfunction (motor weakness and fine motor skills in the upper limbs and increased spasticity in the lower limbs).
-
5.
Hydrocephalus: signs of raised ICP should be ruled out by asking about headache during the night and early morning, as well as vomiting. Fundoscopy is essential for papilledema assessment. Imaging will give information regarding the ventricular size. Raised ICP in CMII is possible without enlarged ventricles. In these cases, invasive ICP monitoring and venous outflow studies may be additionally helpful. Full shunt assessment is essential.
-
6.
MRI: in symptomatic cases and during regular follow-up, entire cranio-spinal axis imaging is necessary to identify all possible causes of symptomatic CMII, such as hydrocephalus, brain stem compression, vascular problems, hydrosyringomyelia or cystic lesions, and tethering of the spinal cord.
Not all symptoms caused by CMII can be addressed by subsequent therapies. Typical malformative features of CMII in the supratentorial and brain stem structures are inherent, and their impact on neurocognitive and behavioral functions, as well as oculomotor dysfunctions, are not well understood [17]. Neuropsychological testing and regular ophthalmological and orthoptic controls are recommended as well as EEG controls.
Patients who are elected for orthopedic correction of neuromuscular scoliosis and kyphosis deserve special attention. Straightening of the sometimes severely curved spinal column in patients with marked hindbrain herniation and crowed foramen magnum together with a tethered spinal cord can lead to deterioration of spinal cord and brain stem functions. The significance of associated syringomyelia remains unclear. The incidence of neurological complications in scoliosis surgery is not known. Accordingly an interdisciplinary team will carefully discuss the necessity of neurosurgical interventions in such a patient. It is recommended to check each spina bifida patient prior to scoliosis surgery regarding all possibly affected body systems including MRI of the whole neuroaxis. Surgery itself should be performed under intraoperative neuromonitoring, which also applies to any neurosurgical intervention involving the cranio-spinal region except ventricular shunting and endoscopy.
The results from our series of patients with secondary interventions reflect the management proposed by our interdisciplinary spina bifida group consisting of neurosurgeons, orthopedics, pediatric neurologists, pediatric urologist, and the neurophysiology team. Additionally involved are orthopedic technicians, physiotherapists, and health care managers who organize home-based family support.
In 4 years, we performed secondary neurosurgical operations in 50 patients with spina bifida-related CMII. The majority of them was female (28 girls), and the mean age was 7.1 years (0.5–26 years). Thirteen of them underwent surgery for spinal kyphoscoliotic deformity.
Twenty four of them had scoliosis (mean age, 9.7 years) and also 24 showed a hydrosyringomyelia in the preoperative MRI. Twelve of them presented with scoliosis and syringomyelia. The preoperative symptoms were neck pain in three patients, which resolved in two cases after the intervention. Five children demonstrated new sensorimotor deficits and two new lower cranial nerve deficits. After surgery, two sensorimotor deficits and one cranial nerve deficit disappeared. Due to clinical signs of sleep disorder, we performed PSG in five patients before and after surgery and found combined central and obstructive sleep disorder in all of them. In one case, the pathological episodes improved markedly after decompression.
Treatment was divided into shunt revision and/or myelosysis and/or decompression. The type of decompression was differentiated into bony decompression alone and additional dural enlargement. Syrinx shunting and spinal cyst fenestration were not investigated but had been performed only in very rare cases.
The most frequent procedure was shunt revision in 38 patients, myelolysis in 17, and decompression in 14 (three bony decompressions alone). In some patients, more than one technique was performed, either in one session or subsequently. In three patients, complications occurred; no patient developed new neurological deficits postoperatively. There was no mortality in this series. After scoliosis surgery, no additional postoperative deficits have been observed as well.
Primary postnatal or in utero (fetoscopic patch application in three children) treatment consisted of closure of the defect and ventriculoperitoneal shunting. None of these newborns needed primary foramen magnum decompression and/or tracheostomy. After fetoscopy, preterm delivery resulted in all cases, and breathing disorders were observed, mainly because of bronchopulmonary dysplasia. The other children had, despite marked hindbrain herniation, no pathological breathing and swallowing patterns. All of them were monitored with electrocardiogram and pulse oximetry for 3 weeks. Indication for primary CSF shunting was set as soon as enlargement of the inner and/or outer CSF spaces was observed in transfontanellar ultrasound. The head circumference curve was less decisive.
All children were put on a follow-up protocol, and parents were informed about risks of shunt dysfunction and signs of brain stem compression. First, MRI imaging of the neuroaxis in patients not suspicious for brain stem dysfunction was performed around the third month of age.
In the presented series, secondary surgery was discussed when a patient became symptomatic with signs of raised intracranial pressure and brain stem dysfunction, e.g., sleep abnormalities or when MRI demonstrated clear worsening of hydrosyringomyelia or brain stem compression. Additionally, all children scheduled for scoliosis underwent MRI of the neuroaxis and were examined by a neurosurgeon. In cases of symptoms or a crowded foramen magnum with significant holocord hydrosyringomyelia and multisegmental tethering of the spinal cord, decompression and/or myelolysis was performed a few weeks before the orthopedic surgery.
All three patients with neck pain had small ventricles without papilledema and a crowded foramen magnum without visible CSF spaces around the hindbrain structures. Shunt revision could solve the problems in two of them. Telemetric ICP measurement demonstrated nocturnal B waves. Venous congestion as a possible cause had been excluded before.
Depending on the position of a progressive syringomyelia, either foramen magnum decompression and/or myelolysis had been performed: in cases with a syrinx prominence in the cervical cord, decompression or shunt revision was chosen. A more caudal site of the syringomyelia led to spinal cord adhesiolysis.
It is remarkable that adequate shunting after shunt revision was able to reduce CMII-related symptoms and decreased the size of the hydrosyringomyelia in some cases. This could be shown in other series as well [5, 6, 14].
Phase-contrasted CSF flow studies seemed to be less useful in CMII cases compared to CMI regarding the assessment of surgical indication. In most of the CMII patients, CSF flow is compromised by several factors, not only by a disturbed flow around the foramen magnum [16]. In our series, there were cases with no visible CSF space at the level of the foramen but also no associated syringomyelia or signs of symptomatic CMII.
Primary dysplastic brain stem dysfunction, e.g., pathological sleep patterns, could necessarily be resolved by any surgical procedure.
Conclusion
CMII is a syndrome nearly exclusively occurring in association with open spinal dysraphism. It is characterized by multiple dysplastic changes of the central nervous system, the most prominent one being the herniation of the hindbrain into the upper spinal canal. Some of these changes are considered as true malformations, and others are interpreted as secondary to the underlying MMC. Only the latter ones are amenable to surgical interventions.
CMII-related management guidelines are not well defined, probably due to the fact that the clinical presentations are broadly varying, as well as its constellations. Often associated findings are syringomyelia, hydrocephalus, and scoliosis, and symptomatic CMII may be triggered by more than one underlying condition.
The experience reflected by clinical series and additional sparse evidence from literature allow the definition of management recommendations. The most important fact is that hydrocephalus is often involved in symptomatic CMII and is addressed surgically in most of the cases. Therefore, hydrocephalus must always be considered first in any symptomatic patient.
Intrinsic brain stem dysfunctions cannot be treated surgically, and monitoring of vital functions is sometimes the only clinical measure that can be offered to the patient. Knowledge of the complex background has led to interdisciplinary follow-up programs for the affected children and thus also improved longtime survival.
References
Adzick NS, Thom EA, Spong CY, Brock JW III, Burrows PK, Johnson MP, Howell LJ, Farrell JA, Dabrowiak ME, Sutton LN, Gupta N, Tulipan NB, D’Alton ME, Farmer DL (2011) A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 364:993–1004
Alsaadi MM, Iqbal SM, Elgamal EA, Gozal D (2012) Sleep-disordered breathing in children with Chiari malformation type II and myelomeningocele. Pediatr Int 54:623–626
Aronson DD, Kahn RH, Canady A, Bollinger RO, Towbin R (1991) Instability of the cervical spine after decompression in patients who have Arnold-Chiari malformation. J Bone Joint Surg Am 73:898–906
Beuls E, Vanormelingen L, Van AJ, Vandersteen M, Adriaensen P, Cornips E, Vles H, Temel Y, Gelan J (2003) The Arnold-Chiari type II malformation at midgestation. Pediatr Neurosurg 39:149–158
Caldarelli M, Di Rocco C, Colosimo C Jr, Fariello G, Di GM (1995) Surgical treatment of late neurological deterioration in children with myelodysplasia. Acta Neurochir (Wien) 137:199–206
Caldarelli M, Di Rocco C, La Marca F (1998) Treatment of hydromyelia in spina bifida. Surg Neurol 50:411–420
Cardoso M, Keating RF (2009) Neurosurgical management of spinal dysraphism and neurogenic scoliosis. Spine (Phila Pa 1976) 34:1775–1782
Christensen B, Rand-Hendriksen S (1998) The significance of associated malformations of the central nervous system in myelomeningocele. Tidsskr Nor Laegeforen 118:4232–4234
Cochrane DD, Adderley R, White CP, Norman M, Steinbok P (1990) Apnea in patients with myelomeningocele. Pediatr Neurosurg 16:232–239
Dias MS (2005) Neurosurgical causes of scoliosis in patients with myelomeningocele: an evidence-based literature review. J Neurosurg 103:24–35
Gorayeb RP, Cavalheiro S, Zymberg ST (2004) Endoscopic third ventriculostomy in children younger than 1 year of age. J Neurosurg 100:427–429
Gozal D, Arens R, Omlin KJ, Jacobs RA, Keens TG (1995) Peripheral chemoreceptor function in children with myelomeningocele and Arnold-Chiari malformation type 2. Chest 108:425–431
Henriques Filho PS, Pratesi R (2009) Sleep disorder: a possible cause of attention deficit in children and adolescents with Chiari malformation type II. Arq Neuropsiquiatr 67:29–34
Marca L, Herman M, Grant JA, McLone DG (1997) Presentation and management of hydromyelia in children with Chiari type-II malformation. Pediatr Neurosurg 26:57–67
Luigetti M, Losurdo A, Dittoni S, Testani E, Colicchio S, Gnoni V, Farina B, Scarano E, Zampino G, Mariotti P, Rendeli C, Di RC, Massimi L, Della MG (2010) Improvement of obstructive sleep apneas caused by hydrocephalus associated with Chiari malformation type II following surgery. J Neurosurg Pediatr 6:336–339
Milhorat TH, Nishikawa M, Kula RW, Dlugacz YD (2010) Mechanisms of cerebellar tonsil herniation in patients with Chiari malformations as guide to clinical management. Acta Neurochir (Wien) 152:1117–1127
Miller E, Widjaja E, Blaser S, Dennis M, Raybaud C (2008) The old and the new: supratentorial MR findings in Chiari II malformation. Childs Nerv Syst 24:563–575
Morota N, Ihara S (2008) Postnatal ascent of the cerebellar tonsils in Chiari malformation type II following surgical repair of myelomeningocele. J Neurosurg Pediatr 2:188–193
Nishimura T, Mori K (1996) Blink reflex in meningomyelocele, with special reference to its usefulness in the evaluation of brainstem dysfunction. Childs Nerv Syst 12:2–12
Nishimura T, Mori K (1996) Somatosensory evoked potentials to median nerve stimulation in meningomyelocele: what is occurring in the hindbrain and its connections during growth? Childs Nerv Syst 12:13–26
Piatt JH Jr (2010) Treatment of myelomeningocele: a review of outcomes and continuing neurosurgical considerations among adults. J Neurosurg Pediatr 6:515–525
Salman MS, Dennis M, Sharpe JA (2009) The cerebellar dysplasia of Chiari II malformation as revealed by eye movements. Can J Neurol Sci 36:713–724
Salman MS, Sharpe JA, Lillakas L, Dennis M, Steinbach MJ (2008) The vestibulo-ocular reflex during active head motion in Chiari II malformation. Can J Neurol Sci 35:495–500
Salman MS, Sharpe JA, Lillakas L, Dennis M, Steinbach MJ (2009) Visual fixation in Chiari type II malformation. J Child Neurol 24:161–165
Stritzke AI, Dunham CP, Smyth JA, Steinbok P (2011) Congenital stridor in the context of Chiari malformation type II: the etiological role of vernix caseosa granulomatous meningitis. J Neurosurg Pediatr 8:372–376
Sweeney KJ, Caird J, Sattar MT, Allcutt D, Crimmins D (2013) Spinal level of myelomeningocele lesion as a contributing factor in posterior fossa volume, intracranial cerebellar volume, and cerebellar ectopia. J Neurosurg Pediatr 11:154–159
Tulipan N, Hernanz-Schulman M, Bruner JP (1998) Reduced hindbrain herniation after intrauterine myelomeningocele repair: a report of four cases. Pediatr Neurosurg 29:274–278
Tulipan N, Hernanz-Schulman M, Lowe LH, Bruner JP (1999) Intrauterine myelomeningocele repair reverses preexisting hindbrain herniation. Pediatr Neurosurg 31:137–142
Waters KA, Forbes P, Morielli A, Hum C, O’Gorman AM, Vernet O, Davis GM, Tewfik TL, Ducharme FM, Brouillette RT (1998) Sleep-disordered breathing in children with myelomeningocele. J Pediatr 132:672–681
Conflict of interests
The authors declare that they have no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Messing-Jünger, M., Röhrig, A. Primary and secondary management of the Chiari II malformation in children with myelomeningocele. Childs Nerv Syst 29, 1553–1562 (2013). https://doi.org/10.1007/s00381-013-2134-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-013-2134-4