Abstract
Seabird movements outside the breeding season are generally poorly known, but can cover thousands of square km and a multitude of habitats, feeding conditions and potential threats. During the last decades, many seabird species in the North Atlantic have experienced large reductions in population size and breeding success, probably caused by reduced prey abundance caused by climate alterations and overfishing. One of these seabird species is the common guillemot. We used global location sensors (geolocators) to identify inter-breeding movements of 10 individuals breeding at Sklinna, a colony off the coast of Central Norway during July 2009–July 2010. All individuals moved northwards after breeding, and eight of them (80 %) entered the Barents Sea where they probably completed their moult. Three individuals moved southwards before the winter, but in total, half of the individuals stayed in the Barents Sea during winter. The other half wintered off the coast of Central Norway–Lofoten. The fact that all individuals moved northwards to winter was surprising as ringing recoveries suggest they also moves southwards (to the Skagerrak area) to winter. This suggests variation (individual or annual) in wintering movements and calls for a multi-year geolocator study at a number of colonies. Much of the area in the Barents Sea–Lofoten area is classified as vulnerable with respect to specific environmental pressures such as oil pollution and other anthropogenic factors, and the importance of the Barents Sea as a major wintering area for common guillemots from central Norway certainly has implications for the management authorities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seabirds are important components of marine ecosystems, yet their ecology and spatial distribution during the non-breeding period is poorly known. Bird movements outside the breeding season can cover thousands of square km (e.g. Phillips et al. 2005; Egevang et al. 2010; Dunn et al. 2011; Mosbech et al. 2012), and the individuals experience a multitude of different habitats, feeding conditions and potential threats. The marine environment, in which the seabirds spend their whole life cycle, is changing due to over-harvesting, pollution, habitat modifications and global climate change (e.g. Halpern et al. 2008). Many seabird species in the North Atlantic have experienced large reductions in population size and breeding success during the last decades, probably caused by reduced prey abundance caused by climate alterations and overfishing (e.g. Frederiksen et al. 2004, 2007, 2008; Barrett et al. 2006).
One of the seabird species that have experienced the largest reductions in breeding numbers in Norwegian waters is the common guillemot (Uria aalge) whose numbers in most breeding colonies have declined with more than 95 % (e.g. Barrett et al. 2006), and the species is now classified as critically endangered (CR) on the Norwegian Red List (Kålås et al. 2010). The causes for this decline are partly unknown and probably related to both food availability and/or climate changes in the marine environment (e.g. Frederiksen 2010). However, the common guillemot is also one of the species that is most frequently affected by oil spills (e.g. Cadiou et al. 2004). Due to the status of the species, it is now critically important to have better knowledge on inter-breeding movements and wintering sites. This is particularly so because (1) while its breeding biology is well known, wintering ecology and wintering sites are poorly known, (2) climate changes are expected to be most pronounced in the Arctic (IPCC 2007), (3) mortality due to oil spills mostly occur during winter. Harris et al. (2007) found that most mortality of adult seabirds occurred outside the breeding season, and hence, knowing their wintering areas is essential in order to understand factors regulating the populations. This is particularly important for species that are declining and/or threatened.
Most information about inter-breeding movements and distribution of wintering seabirds are from ship-based surveys or from ringed birds. For seabirds, however, ring recoveries are biased by several factors, partly because many recoveries are from where carcasses have washed ashore and not necessarily where they died. In addition, only a small fraction of ringed birds are recovered. In Norway, only 2.4 % of the ringed common guillemots have been recovered (Bakken et al. 2003), indicating a huge effort, and potentially much disturbance in the breeding colonies, for a low return rate of data. During recent years, miniature global location sensors (GLS loggers or geolocators) that enable the movements of seabirds to be tracked over extended periods (up to several years) have been developed (e.g. Phillips et al. 2004; Bost et al. 2009; Dunn et al. 2011).
The main aim of this study was to map the inter-breeding movements of common guillemots breeding at a colony off the coast of Central Norway using GLS loggers. In particular, we aimed at identifying the key areas used during the non-breeding period when common guillemots may potentially be especially vulnerable to anthropogenic impacts. In order to evaluate possible seasonal differences in movement patterns, we also calculated the mean speed of the movements in 5-day periods.
Materials and methods
Logger deployment and retrieval
Field work was conducted at Sklinna Nature Reserve (65°13′N 10°58′E), a small archipelago 20 km off the mainland coast of Central Norway. On 30 June 2009, 25 chick-rearing adults (9 males, 14 females and 2 individuals of unknown sex) were caught using a nose pole. A GLS logger (Lotek LAT 2500, 128 Kb memory, no pressure sensor, 8 × 35 mm, mass of 3.6 g in air, 0.4 % of the body mass of the equipped birds) attached to a plastic leg ring was attached the tarsus on each of the birds. Body mass (accuracy ±10 g when BM <1,000 g and ±20 g when BM >1,000 g) was measured using Pesola spring balances. In order to sex the birds, 25 μl of blood was collected in a capillary tube after puncturing one of the veins on the leg or the web and immediately suspended in 1 ml of “Queen’s lysis buffer” (Seutin et al. 1991). During 1–7 July 2010, 10 of the loggers were retrieved (4 males, 5 females and 1 of unknown sex).
Sexing
DNA was extracted from the blood using GeneMole® (Mole genetics, www.molegenetics.com). This instrument performs an automatic DNA extraction on a 1:10 solution of blood in a lysis buffer and PBS (pH = 7.4), see http://www.molecookbook.com//index.php?qlink=84. After DNA extraction, sexing was performed using the methods described by Bantock et al. (2008).
Data analysis
The GLS loggers basically record time, temperature and light intensity. Geographical positions are then estimated from changes in light intensity over time. Sunset and sunrise are estimated from thresholds in the light curves, and this enables estimations of latitude through daylength and longitude from the time of midday with respect to Greenwich Mean Time (e.g. Phillips et al. 2004).
Data from the loggers were processed in LAT Viewer Studio (Lotek wireless, Newmarket, Ontario) using the template fit option (Ekstrom 2004). Latitudes are unreliable around the vernal and autumnal equinox, and data for 18 September–4 October and 1–24 March were therefore removed. Latitudes were also unobtainable for periods when birds were in regions of constant darkness. Thus, in this study, all latitudinal data from 5 October–28 February were refined using the loggers’ records of sea surface temperature (SST), which were compared with SST data from NASA’s Terra and Aqua satellites and two polar-orbiting TIROS satellites using the algorithms integrated in LAT Viewer Studio. Satellite data were downloaded from http://whiteshark.stanford.edu/public/lotek_sst. The algorithm in LAT Viewer Studio was run with the start position manually set to 68°N, 10°E, as all equipped birds moved north the months following the breeding season. The loggers’ SST data were, additionally, compared (visually) with a map of sea surface temperatures from along the Norwegian coast and the Barents Sea during January 2010 (downloaded from http://modis.gsfc.nasa.gov/).
Positions obtained from GLS loggers have an inherent average error of approximately 186 km (Phillips et al. 2004). In order to reduce the influence of outliers when calculating distances and allocating positions to areas of interest, positions were smoothed using a 3-position moving average based on spherical trigonometry (e.g. Frederiksen et al. 2012). Distances between successive smoothed (and unsmoothed) positions were calculated. We estimated kernel utilization distributions over all 10 individuals for autumn (August–September), winter (October-February) and spring (March–April). From these utilization distributions, 25 and 50 % isopleths were derived. This removed any remaining outliers despite all previously mentioned filtering and calibrations. Kernel densities (Gaussian, bivariate normal) were estimated using the geospatial modelling environment (GME) extension (http://www.spatialecology.com/gme/index.htm). Mapping was done in ArcMap 10 (ESRI, Redlands, California).
Kernel utilization distributions are smoothed using the so-called bandwidth, which is represented as the radius of a circle within which points are counted around each cell. It is therefore important to choose the right bandwidth, to avoid over- or under-smoothing (GME manual downloaded from the address above). Although least-squares cross-validation (LSCV) is recommended as the bandwidth selection method (Seaman et al. 1999), newer methods such as the plug-in and solve-the-equation (STE) bandwidth methods are preferred in the statistical literature (Jones et al. 1996). Using simulated point patterns, Gitzen et al. (2006) found that although the relative differences were small, the plug-in and STE approaches provided good alternatives to LSCV. However, the choice of a bandwidth selection method may vary depending on the study goals, sample size and patterns of space use by the study species. Gitzen et al. (2006) recommended LSCV for clumped distributions, and plug-in and STE approaches for estimating smoother distributions. Given the extensive range use of our study species, the plug-in method was considered to be most appropriate.
To obtain an estimate of the bandwidth, we projected the longitudinal data into UTM Zone 33 (WGS 1984) to render X/Y coordinates on a metric scale. We then increased the inaccuracy in 5 km steps from 0 to 200 km. For each step, the X and Y coordinates of the original data set were adjusted by adding randomly derived deviations from each coordinate using a normal distribution with accuracy as standard deviation around a mean of zero. From this adjusted data set, unique distances between all pairs of positions were calculated, which was used as input to the bandwidth calculations. This process was iterated 100 times to estimate mean and range for each (accuracy) step. In addition, the predicted trend was plotted based on regressing bandwidth against the square of accuracy.
Assuming absolute accuracy (i.e. 0 km), a mean bandwidth of 11.2 km (accuracy range, 11.2–21.4 km) was obtained. We therefore used the rounded value of 10 km as a measure of bandwidth.
Swimming speed during the autumn migration was calculated for every 5-day period (numbered consecutively from 1 January so that e.g. 5–9 June is 5-day period 32, 10–14 June is 5-day period 33) from when the birds left the colony until they arrived at the autumn staging site.
Results
There were no differences in the body mass of the guillemots at deployment and removal of the GLS loggers approximately 1 year after, indicating that the attachment of the loggers did not have any negative influence on the equipped individuals; Wilcoxon signed-ranks test, males Z = 1.07, p = 0.285, df = 3; females Z = 1.10, p = 0.273, df = 3 (Table 1). In 2010 and 2011, at least 18 of the 25 birds equipped with loggers were observed (8 individuals, no logger retrieval) or re-caught (10 individuals), giving a total return rate of 72 %. Although a small sample size, this is lower than the annual survival (return rate) of adults not equipped with geolocators at the same site (mean adult survival = 91.8 %, S.H. Lorentsen unpublished data).
Eight of 10 birds (80 %) moved directly to the Barents Sea after breeding (Table 1). The two remaining individuals also moved northwards, but did not enter the Barents Sea. All individuals started to move northwards shortly after the chicks had left for the sea, which at Sklinna normally occurs between 10 and 25 July. The mean swimming speed varied much between the individuals but increased slightly from 0.5 to 2 km/h during the first month (until the end of August, 5-day period 46 = 21–25 August, Fig. 1). The swimming speed was then quite low for a few weeks (<0.5 km/h) before it increased steeply during the first half of September (5–20 September, 5-day period 49–51).
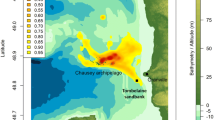
During the autumn (August–September), the area from central Norway to the Barents Sea was used (Fig. 2). However, all individuals that entered the Barents Sea did so in late August–early September. The individuals that did not enter the Barents Sea stayed in the area outside Central Norway and Lofoten, whereas the individuals entering the Barents Sea used the central parts as far east as 45°E.
GLS positions (yellow dots), kernel density surface plots (red and yellow) and isopleths (50 % = red lines, 25 % = green lines) for common guillemots during autumn (August–September, upper panel), winter (October–February, mid panel) and spring (March–April, lower panel). Geographical names used in the text are indicated in the lower panel
Three of the individuals that moved to the Barents Sea during autumn moved southward during late autumn/early winter and stayed outside Lofoten/Central Norway. Half of the individuals stayed in the Barents Sea during winter (mid-October–end of February). In the Barents Sea, the main wintering areas judged from the kernel isopleths were outside the coast of Finnmark and the eastern part of the Kola Peninsula. The other half of the individuals spent the winter in the area from off the coast of Lofoten in Northern Norway to Central Norway (Fig. 2). Most of the individuals had returned to the breeding colony in March. While the northwards movement in autumn was quite directed and fast, changes in latitude during the other time periods suggested much lower movements and also showed a greater variance in latitude (Fig. 3).
Latitudinal (mean ± SD) changes in average distribution between 5-day periods (40 = 20–24 July, 73 = 26–31 December) for common guillemots from Sklinna, Central Norway. The reference line indicates the latitude of the study colony. Time periods around vernal and autumnal equinoxes, and the breeding season, are filtered out
Discussion
Individuals equipped with geolocators had a lower return rate than birds without geolocators. There were however no differences in the body mass of the equipped individuals at deployment and retrieval of the loggers suggesting that the loggers did not have any negative influence on the equipped individuals. The cause of the lower return rate of the equipped individuals is not obvious, but might be related to inter-colonial differences in breeding sites of the individuals caught for logger deployment. The common guillemots at Sklinna nests among huge rocks, and most of the birds that were equipped were caught below a rock where they had few escape possibilities, whereas the rest were caught below rocks with more escape possibilities. Unfortunately, we did not take notes of where we caught the actual individuals within the colony as we wanted to make the catching events as quick as possible in order to reduce the disturbance. Thus, birds that were caught below rocks with several exits were much more difficult to re-trap, which might explain the differences in return rates between individuals with and without loggers. Hence, many of the birds with loggers that we were not able to catch were observed close to breeding sites with escape possibilities.
Most of the common guillemots (80 %) in this study moved directly from Sklinna to the Barents Sea after the breeding period. The remaining two individuals (20 %) also moved northwards but stopped in the Lofoten area. The movements were directional and with an increasing speed as the journey progressed, until late August when the daily movements dropped to <0.5 km/h before it increased from mid-late September. The drop in movements in late August–September coincides with the moult of their secondaries (Cramp 1985). Thus, the individuals that entered the Barents Sea in late August–early September probably were in, or had completed, their moult when they arrived.
Five of the eight individuals that entered the Barents Sea stayed there during the whole winter, whereas three moved out of the Barents Sea and wintered along the Norwegian coast from Lofoten to Central Norway. All individuals were back to the waters outside the breeding colony in March, about 2 months before egg laying (S.H. Lorentsen unpublished data).
The food of wintering common guillemots in the Barents Sea is not known. In the breeding season, the main food for common guillemots breeding at Hornøya in the eastern Barents Sea was capelin (Mallotus villosus), sandeel (Ammodytes sp.) and herring (Clupea harengus) (Barrett 2002; Bugge et al. 2011). It is strongly suggested that the food choice of common guillemots (and other seabirds) wintering in the Barents Sea are studied in order to understand its role in the marine ecosystem and to be able to access possible anthropogenic threats.
The GLS loggers record time, temperature and light intensity, and geographical positions are then estimated from changes in light intensity over time. However, when the birds carrying the loggers are in regions of constant darkness, latitudes are unobtainable. Light levels are low in the Barents Sea (Bear Island) from 7th November until 4th of February as the sun is below the horizon all the day. We therefore compared the logger’s records of sea surface temperature (SST) for the period 5 October–28 February with SST data from satellites. Due to cloud cover satellite, SST data were not available for all days in this period, but in general, most of the geographical positions were adjusted northwards to the Barents Sea after this procedure. Also, a visual comparison of the loggers’ SST data with a map of sea surface temperatures from along the Norwegian coast and the Barents Sea during January 2010 showed a close conformity of logger and satellite SST data.
Due to the inherited errors in the positions obtained from the GLS loggers (on average 186 km, Phillips et al. 2004), we used a 3-position moving average based on spherical trigonometry to reduce the influence of outliers when calculating distances and geographical positions. Still, we experienced some outliers, especially during spring and autumn (cf. Fig. 2). Although these outliers could be removed by manual inspection, we included them in the kernel analyses that, consequently, might be too wide especially outside the 50 % contour. Kernel density estimation does also not take into account topographic features such as the coast line. Thus, although the distributions may also to a certain extent encompass inland areas, we would like to stress that this study was performed, and gives insight into seasonal inter-breeding movements, on a rather large spatial scale. Also, the number of winter plots in the southern area might be misleading compared with the number of birds that wintered in the Barents Sea and outside the Barents Sea. This is probably related to the SST-based correction of plots for birds in the Barents Sea as satellite-based SST data could not be obtained for many days during winter due to extensive cloud cover. Thus, proportionally more of the plots from birds in the Barents Sea had to be removed as they could not be corrected than plots from birds wintering farther south.
The Barents Sea is known to be a major wintering area for common guillemots of unknown origin (e.g. Barrett and Golovkin 2000 and references therein). This study shows that this includes birds from central Norway. Also puffins (Fratercula arctica) (Anker-Nilssen and Aarvak 2009) and black-legged kittiwakes (Rissa tridactyla) (B. Moe pers. comm.) winters in the Barents Sea. This certainly has implications for the management authorities. For instance, both Norwegian and Russian authorities now open the area for oil exploration, and an accident causing a major oil spill could have huge impacts on moulting and wintering common guillemots. Much of the area in the Barents Sea–Lofoten area is classified as vulnerable with respect to specific environmental pressures such as oil pollution and other anthropogenic factors (e.g. von Quillfeldt et al. 2009).
This study suggests that the Barents Sea and the Norwegian coast from Central Norway and northwards are important staging and wintering grounds for common guillemots breeding in Central Norway. However, analyses of ring recoveries show that common guillemots from Central and Northern Norway also migrates south to the Skagerrak area during winter (Bakken et al. 2003). This might suggest variation (individual and/or annual) in inter-breeding movements and, thus, call for multi-year geolocator studies at a number of breeding colonies. We strongly hope that such studies are initiated in the near future, and that they also incorporate studies of the food choice of the common guillemot during the inter-breeding period.
References
Anker-Nilssen T, Aarvak T (2009) Satellite telemetry reveals post-breeding movements of Atlantic puffins Fratercula arctica from Røst, North Norway. Polar Biol 32:1657–1664
Bakken V, Runde O, Tjørve E (2003) Norwegian bird ringing Atlas vol 1. Stavanger Museum, Stavanger (In Norwegian)
Bantock TM, Prys-Jones RP, Lee PLM (2008) New and improved molecular sexing methods for museum bird specimens. Mol Ecol Res 8:519–528
Barrett RT (2002) Atlantic puffin (Fratercula arctica) and common guillemot (Uria aalge) chick diet and growth as indicators of fish stocks in the Barents Sea. Mar Ecol Prog Ser 230:275–287
Barrett RT, Golovkin AN (2000) Common guillemot Uria aalge In: Anker-Nilssen T, Bakken V, Strøm H, Golovkin AN, Bianki VV, Tatarinkova IP (eds) The status of the marine birds breeding in the Barents Sea region. Norsk Polarinstitutt, Tromsø, pp 114–118
Barrett RT, Lorentsen SH, Anker-Nilssen T (2006) The status of breeding seabirds in mainland Norway. Atl Seabirds 8:97–126
Bost CA, Thiebot JB, Pinaud D, Cherel Y, Trathan PN (2009) Where do penguins go during the inter-breeding period? Using geolocation to track the winter dispersion of the macaroni penguin. Biol Lett 5:473–476
Bugge J, Barrett RT, Pedersen T (2011) Optimal foraging in chick-raising common guillemots (Uria aalge). J Ornithol 152:253–259
Cadiou B, Riffaut L, McCoy KD, Cabelguen J, Fortin M, Gélinaud G, Le Roch A, Tirard C, Boulinier T (2004) Ecological impact of the “Erika” oil spill: determination of the geographic origin of the affected common guillemots. Aquat Living Resour 17:369–377
Cramp S (ed) (1985) The birds of the western Palearctic, vol 4. Oxford University Press, Oxford
Dunn MJ, Silk JRD, Trathan PN (2011) Post-breeding dispersal of Adélie penguins (Pygoscelis adeliae) nesting at Signy Island, South Orkney Islands. Polar Biol 34:205–214
Egevang C, Stenhouse IJ, Phillips RA, Petersen A, Fox JW, Silk JRD (2010) Tracking of Arctic terns Sterna paradisea reveals longest animal migration. Proc Nat Acad Sci USA 107:2078–2081
Ekstrom PA (2004) An advance in geolocation by light. Mem Natl Inst Polar Res 58:210–226
Frederiksen M (2010) Seabirds in the North East Atlantic. A review of status, trends and anthropogenic impact. Appendix 1 TemaNord 587:47–122
Frederiksen M, Wanless S, Harris MP, Rothery P, Wilson LJ (2004) The role of industrial fisheries and oceanographic change in the decline of North Sea black-legged kittiwakes. J Appl Ecol 41:1129–1139
Frederiksen M, Edwards M, Mavor RA, Wanless S (2007) Regional and annual variation in black-legged kittiwake breeding productivity is related to sea surface temperature. Mar Ecol Prog Ser 350:137–143
Frederiksen M, Jensen H, Daunt F, Mavor RA, Wanless S (2008) Differential effects of a local industrial sand lance fishery on seabird breeding performance. Ecol Appl 18:701–710
Frederiksen M, Moe B, Daunt F, Phillips RA, Barrett RT, Bogdanova MI, Boulinier T, Chardine JW, Chastel O, Chivers LS, Christensen-Dalsgaard S, Clément-Chastel C, Colhoun K, Freeman R, Gaston AJ, González-Solís J, Goutte A, Grémillet D, Guilford T, Jensen GH, Krasnov Y, Lorentsen SH, Mallory ML, Newell M, Olsen B, Shaw D, Steen H, Strøm H, Systad GH, Thórarinsson TL, Anker-Nilssen T (2012) Multi-colony tracking reveals the winter distribution of a pelagic seabird on an ocean basin scale. Divers Distrib 18:530–542
Gitzen RA, Millspaugh JJ, Kernohan BJ (2006) Bandwidth selection for fixed-kernel analysis of animal utilization distributions. J Wildl Manag 70:1334–1344
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D’Agrosa C, Bruno JF, Casey KS, Ebert C, Fox HE, Fujita R, Heinemann D, Lenihan HS, Madin EMP, Perry MT, Selig ER, Spalding M, Steneck R, Watson R (2008) A global map of human impact on marine ecosystems. Science 319:948–952
Harris MP, Frederiksen M, Wanless S (2007) Within and between-year variation in the juvenile survival of common guillemots Uria aalge. Ibis 149:472–481
IPCC, Core Writing Team (2007) Climate change 2007: synthesis report. In: Pachauri RK, Reisinger A (eds) Contribution of working groups I, II and III to the fourth assessment. Report of the intergovernmental panel on climate change. IPCC, Geneva
Jones MC, Marron JS, Sheather SJ (1996) A brief survey of bandwidth selection for density estimation. J Am Stat Assoc 91:401–407
Kålås JA, Viken Å, Henriksen S, Skjelseth S (eds) (2010) The 2010 Norwegian red list for species. Artsdatabanken, Norway
Mosbech A, Johansen KL, Bech NI, Lyngs P, Harding AMA, Egevang C, Phillips RA, Fort J (2012) Inter-breeding movements of little auks Alle alle reveal a key post-breeding staging area in the Greenland sea. Polar Biol 35:305–311
Phillips RA, Silk JRD, Croxall JP, Afanasyev V, Briggs DR (2004) Accuracy of geolocation estimates for flying seabirds. Mar Ecol Prog Ser 266:265–272
Phillips RA, Silk JRD, Croxall JP, Afanasyev V, Bennett VJ (2005) Summer distribution and migration of nonbreeding albatrosses: individual consistencies and implications for conservation. Ecology 86:2386–2396
Seaman DE, Millspaugh JJ, Kernohan BJ, Brundige GC, Raedeke KJ, Gitzen RA (1999) Effects of sample size on kernel home range estimates. J Wildl Manag 63:739–747
Seutin GB, White N, Boag PT (1991) Preservation of avian blood and tissue samples for DNA analyses. Can J Zool 69:82–90
Von Quillfeldt C, Olsen E, Dommasnes A, Vongraven D (2009) Integrated ecosystem-based management of the Barents Sea-Lofoten area. In: Sakshaug E, Johnsen G, Kovacs K (eds) Ecosystem Barents Sea. Tapir Academic Press, Trondheim, pp 545–562
Acknowledgments
We are indebted to the field assistants during the 2009 and 2010 field seasons at Sklinna, especially Emma Bengtsson, Torunn Moe and Eike Stüebner, and to Vidar Bakken for help with some of the data smooting. We are also indebted to Børge Moe, the editor, and the referees for valuable comments on the manuscript, and to Lotek Inc. for their kind support on Lat Viewer Studio. Permissions to catch, handle and attach geolocators to the guillemots were obtained from the Directorate for Nature Management and the Animal Research Authorities. All handling of the birds was in accordance with the animal welfare act and other legal requirements in Norway. Permission to work in the Sklinna Nature Reserve was obtained from the County Governor of Nord-Trøndelag. Financial support was granted by the Directorate for nature Management.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lorentsen, SH., May, R. Inter-breeding movements of common guillemots (Uria aalge) suggest the Barents Sea is an important autumn staging and wintering area. Polar Biol 35, 1713–1719 (2012). https://doi.org/10.1007/s00300-012-1215-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-012-1215-2