Abstract
Acetobacter xylinum BPR2001 produces water-insoluble bacterial cellulose (BC). Using a pH sensor for the accurate control of pH, which is one of the most critical factors for efficient BC production, is difficult especially in a baffled shake-flask and an airlift reactor. The buffering capacity of corn steep liquor (CSL) was estimated by measuring β (buffering capacity) values in advance and was used to maintain the pH within the optimal range during the production of BC. When CSL was added to either a shake-flask, a stirred-tank reactor or an airlift reactor, BC production was almost the same as that in cultivations where pH was controlled manually or by a pH sensor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Some bacteria secrete cellulose called bacterial cellulose (BC; Hestrin and Schramm 1954; Ross et al. 1991). BC production by the Gram-negative bacterium Acetobacter xylinum (Gluconacetobacter xylinus) has been widely studied, not only from the engineering point of view, but also from biochemical and genetic points of view (Nakai et al. 1998; Tanaka et al. 2000; Umeda et al. 1999; Volman et al. 1995; Wong et al. 1990). The primary structure of BC produced by A. xylinum is β-1,4-glucan, which is similar to that of plants, but the degree of polymerization, crystallinity and fineness of BC are significantly different from that produced by plants (Cannon and Anderson 1991; Yamanaka et al. 1989). Because of its unique properties, BC is expected to be a new biodegradable material and its application and massive production has been attracting attention recently (Yamanaka et al. 1989). For the efficient production of BC, cultivation methods are one of the key factors. The traditional BC production method is static cultivation, but this method demands a large area and a long cultivation time. Therefore, other methods are being investigated as an alternative to the static method. Stirred-tank reactors are widely used for BC production (Hwang et al. 1999; Kouda et al. 1996, 1997; Romano et al. 1989; Yang et al. 1998). The addition of ethanol to continuous cultivation in a stirred-tank reactor enhances the BC production rate 2-fold, compared with batch cultivation (Naritomi et al. 1998). In an airlift reactor, the BC productivity is equivalent to that in a stirred-tank reactor, with only one-fifth of the energy consumption of a stirred-tank reactor (Chao et al. 1997, 2000). In a modified airlift reactor, the BC concentration is 3-fold higher than that in a bubble column (Cheng et al. 2002). A rotation-disk reactor produced a BC pellicle in a significantly shorter time than a static culture (Serafica et al. 2002).
In those reactors, dissolved oxygen (DO) is an important control factor. If the DO is maintained above the critical DO value, the BC productivity is not influenced significantly (Chao et al. 2000). pH control is also critical to maintain a high BC productivity by A. xylinum. The optimum pH range for A. xylinum BPR2001 used in this study is pH 4.5–5.5. BC culture broth consists of solid (BC, cells), liquid (nutrients) and gas (oxygen) components. The viscous BC entraps cells and often attaches to the pH electrode, which provides inaccurate pH values. Furthermore, the increase in viscosity during cultivation, due to the accumulation of BC and the water-soluble by-product, acetan (Ishida et al. 2002), also delays the pH electrode response. This poses difficulty in accurate pH control. To overcome the problem of pH control during BC cultivation, one way is to use a buffer solution to maintain pH. In a static culture, the buffer effect of acetic acid results in the enhanced production of BC (Vandamme et al. 1998). However, the use of a buffer solution in a large-scale production is not practical. We focused on corn steep liquor (CSL), which is used as the nitrogen source in BC production (Chao et al. 1997; Kouda et al. 1997; Toyosaki et al. 1995; Yang et al. 1998). As CSL contains proteins, peptides and amino acids, the addition of an appropriate volume of CSL may strengthen the buffering capacity of the medium (Stanburg and Whitaker 1984).
In this study, the buffering capacity of CSL was evaluated by the addition of an acid or alkaline solution in advance and then an investigation was made into the possible use of CSL in maintaining the pH within the optimal range during the production of BC without the use of a pH electrode or pH controller in a shake-flask, a stirred-tank reactor and an airlift reactor.
Materials and methods
Microorganism
A. xylinum subsp. sucrofermentans BPR2001(abbreviated as BPR2001) was used. BPR2001 was isolated as a strain that showed 1.8-fold higher BC production in a stirred-tank reactor than A. xylinum ATCC23769 (Toyosaki et al. 1995). The cell suspension of BPR2001 was stored at −80 °C in a 30% glycerin solution.
Medium
For BC production, CSL with fructose (CSL-Fru) medium was used. CSL products were obtained from two different manufacturers: Nihon Denpun Co. [Kagoshima, Japan; denoted as CSL(N)] and Showa Sangyo Co. [Tokyo, Japan; denoted as CSL(S)]. Two different lots of the latter were used (the production dates of which were 6 months apart) and were denoted as CSL(S-lot1) and CSL(S-lot2). The added amount of CSL varied in each experiment. The original CSL solution was centrifuged at 6,000g for 20 min and the supernatant was used as the sample. Nutrients other than CSL, in 1 l medium, consist of 40 g fructose, 1 g KH2PO4, 0.25 g MgSO4·7H2O, 3.3 g (NH4)2SO4, 3.6 mg FeSO4·7H2O, 14.7 mg CaCl2·2H2O, 2.42 mg NaMoO4·2H2O, 1.73 mg ZnSO4·7H2O, 1.39 mg MnSO4·5H2O, 0.05 mg CuSO4·5H2O, 2.0 mg inositol, 0.4 mg nicotinic acid, 0.4 mg pyridoxine hydrochloride, 0.4 mg thiamine hydrochloride, 0.2 mg d-pantothenic acid calcium, 0.2 mg riboflavin, 0.2 mg p-aminobenzonic acid, 0.2 g folic acid and 0.2 μg d-biotin. The initial pH was adjusted to 5.0 and an anti-foam agent (Disfoam CB-442; Nihon Yushi Co., Tokyo, Japan) was added, when necessary.
Analysis of total nitrogen and lactate content in CSL
The total nitrogen content in CSL was analyzed by elementary analysis (MT-5; Yanaco Co., Japan). The lactic acid content in CSL was measured using Sigma Lactate kit 375, according to the manufacturer′s instructions.
Measurement of the buffering capacity of CSL
The buffering capacity of each CSL solution was measured as follows: 3 ml of either 0.2 N NaOH or 0.2 N H2SO4 was added to 500 ml of each CSL solution and the pH change after each addition was measured. The β value, a measure of buffering capacity, was calculated using the equation β=Δ[OH]/ΔpH (Perin and Dempsey 1974) and then plotted against pH.
In the equation, Δ[OH] indicates the change in molarity on addition of 0.2 N NaOH or 0.2 N H2SO4 and ΔpH indicates the change in pH.
Each CSL solution was autoclaved at 120 °C for 20 min and β was measured by the procedure described above, in order to check the pattern of β changes before and after the sterilization procedure.
Cultivation of BPR2001 in 500-ml baffled shake-flasks
One milliliter of the cell suspension stored at −80 °C was added to 100 ml of CSL-Fru medium in a 750-ml Roux flask and statically cultivated at 30 °C for 3 days. After shaking the flask vigorously by hand to detach the cells from the cellulose pellicle formed on the surface of the broth, the suspension was filtered through a sterile gauze to remove BC. The filtrate containing the cell suspension was used as an inoculum. Baffled shake-flasks (500 ml nominal volume) containing 112.5 ml of CSL-Fru medium were inoculated with 12.5 ml of cell suspension and shaken at 30 °C at 180 rpm for 5 days in a rotary shaker (Bio-Shaker BR-3000L; Taitec Co., Tokyo, Japan). On each day, one flask was taken and the BC concentration and pH were measured.
Cultivation of BPR2001 in 10-l stirred-tank reactors
BMS-10PI stirred-tank reactors (185 mm diameter, 390 mm height, 10 l nominal volume, 5 l working volume; Biott Co., Tokyo, Japan) were used for the cultivation of BPR2001. The reactors were equipped with two Rushton turbine impellers (Amanullah et al. 1998) with an impeller diameter/diameter of reactor (D/T) ratio of 0.5 and four baffle plates. DO was measured using a DO sensor. The DO concentration was adjusted to 6% O2, giving 21% O2 saturation by changing the agitation speed of the impellers. The air-flow rate was maintained at 0.4 vvm. In one reactor, the pH was maintained at 5.0 by a controller (DPC-2; Able Co., Tokyo, Japan) by adding 4 N NaOH or 4 N H2SO4. In another reactor, CSL(S-lot1) was added to the medium at an initial concentration of 40 ml/l and the cultivation was carried out without pH control. The preculture was prepared in baffled shake-flasks in CSL-Fru medium as described above and 500 ml of the culture was homogenized using a homogenizer (Nihon Seiki Co., Tokyo, Japan) at 10,000 rpm for 1 min and inoculated into 4.5 l of fresh CSL-Fru medium in each stirred tank reactor. Then, 5 ml of 1% anti-foam agent was added to the medium. Culture broths sampled periodically were subjected to an analysis of the amount of BC, fructose concentration, pH and viable cell number.
Cultivation of BPR2001 in a 50-l internal-loop airlift reactor
A 50-l airlift reactor (50 l nominal volume, 38 l working volume) was used. As the details of the airlift reactor were provided by Chao et al. (1997, 2000), only a brief outline of the airlift reactor is given. The stainless steel (SUS304) reactor is equipped with a draft tube inside it, consisting of a downcomer of 230 mm inner diameter and a riser of 134.2 mm inner diameter. The heights of the reactor and the draft tube are 1,200 mm and 800 mm, respectively. Four gas spargers with five holes of 5 mm diameter each are set in the bottom of the reactor. The jacket around the reactor is filled with water circulating from a water bath to maintain the temperature of the reactor at 30 °C during the cultivation.
Since accurate pH control was difficult due to the adhesion of BC and cells to the pH electrode, particularly after the logarithmic growth phase, 50 ml of culture broth was intermittently sampled and the pH was measured using a pH meter in the laboratory. Then, 4 N H2SO4 or 4 N NaOH solution was added to the reactor manually to maintain the pH at approximately 5.0. An appropriate volume of fructose-free CSL(N) medium was supplemented to the reactor to compensate for the decrease in the volume of the broth after each sampling. The inlet oxygen concentration and gas-flow rate in the reactor were adjusted by introducing a mixture of air and oxygen-fortified gas prepared using a pressure-swing adsorption oxygen generator (AS-45; Airsep Co., USA). The sampled culture broth was used to measure the amount of BC, fructose concentration and viable cell number. When CSL(S-lot2) was used, the BC production was carried out in the same way, as mentioned above, but without manual pH control.
Measurement of BC production
Five milliliters of each culture broth sample was centrifuged at 4,000g for 20 min (J6-HC; Beckman, USA) and the deposited BC was treated with 0.1 N NaOH solution at 80 °C for 20 min to dissolve the bacterial cells in the deposit. To remove other impurities, the BC was washed with deionized water more than three times. Finally, the purified BC was dried in a vacuum at 80 °C for 8 h and then weighed.
Measurement of viable cell number
The viable cell number was measured by the plate-dilution method. Eight milliliters of 0.1 M potassium acetate-acetate buffer (pH 5) and 1 ml of 20% cellulase (Celluclast; Novo Nordisk, Denmark) were added to 1 ml of culture broth and incubated at 30 °C with shaking at 100 strokes/min for 1 h to hydrolyze BC. Then, the suspension was diluted properly with the buffer and an aliquot of 100 µl was spread on plates containing CSL-Fru medium and 2 g agar/l. After 3 days of incubation at 30 °C, the number of colony-forming units was calculated. As the cell number in the culture liquid was approximately one-hundredth of that entrapped in the BC, the cell number measured from the BC sample was taken as the viable cell number.
Measurement of fructose concentration
The culture broth was centrifuged at 4,000 rpm for 20 min and the supernatant was filtered through a 0.45-μm filter (GL chromato disk; GL Science, Tokyo, Japan). The fructose concentration of the filtrate was measured by high-performance liquid chromatography, using an ODS Shodex NH2P-50 4E column (Showa Denko Co., Tokyo, Japan) eluted with a mixture of acetonitrile and H2O at 3:1 (v/v), using a flow rate of 1 ml/min at a column temperature of 40 °C.
Results
Characterization of CSL
Figure 1 shows the changes in β values for five different CSL solutions in the pH range from 4.0 to 6.0 before (Fig. 1A) and after (Fig. 1B) sterilization. CSL(N) at 20 ml/l, CSL(S-lot1) at 20 ml/l and CSL(S-lot2) at 20 ml/l showed a smooth change in β (Fig. 1A), indicating a weak buffering capacity. However, CSL(S-lot1) at 40 ml/l and CSL(S-lot2) at 54 ml/l showed several significant peaks with β values of more than 0.02, implying that CSL(S) at about 40 ml/l will function as a significant buffering agent during cultivation. These patterns of β changes are more complicated than those in typical buffer solutions of chemicals (Perin and Dempsey 1974). When CSL(S-lot1) at 40 ml/l and CSL(S-lot2) at 54 ml/l were autoclaved at 121 °C for 20 min, the patterns of β changes were simplified, although some peaks remained (Fig. 1B).
Buffering capacities (β) of different corn steep liquors (CSLs) before sterilization (A) and after sterilization (B). Trace 1 CSL(N) at 20 ml/l, trace 2 CSL(S-lot1) at 20 ml/l, trace 3 CSL(S-lot1) at 40 ml/l, trace 4 CSL(S-lot2) at 20 ml/l, trace 5 CSL(S-lot2) at 54 ml/l. See Materials and methods for CSL codes
As shown in Table 1, the total nitrogen and lactate contents were different between the CSL solutions used, mainly due to differences between their sources and preparation processes. Thus, CSL(S-lot1) at 40 ml/l and CSL(S-lot2) at 54 ml/l were prepared such that the total nitrogen and lactic acid contents were almost the same as those in CSL(N) at 20 ml/l. Therefore, the buffering effects of CSL(S-lot1) at 40 ml/l and CSL(S-lot2) at 54 ml/l were judged to be much stronger than that of CSL(N) at 20 ml/l, using the patterns of β changes shown in Fig. 1A.
Cultivation of BPR2001 with baffled shake flasks with added CSLs
BPR2001 was cultivated in baffled shake-flasks containing CSL(N) or CSL(S-lot1) where pH was not controlled. As CSL(S-lot1) at 20 ml/l contains less lactate than CSL(N) at 20 ml/l, as shown in Table 1, 1.1 g lactate/l was added to the CSL(S-lot1) at 20 ml/l and the lactate concentrations in the CSL(N) and CSL(S-lot1) media were adjusted to be almost equal. Changes in BC production and pH are shown in Fig. 2. The pH of the broth containing CSL(N) at 20 ml/l increased to 6.4 within 48 h (which is not appropriate for efficient BC production) and then declined towards the end of the cultivation. The pH of CSL(S-lot1) at 40 ml/l was maintained at approximately 4.5 during the cultivation, which is optimal for BC production, as reflected by the high buffering capacity of CSL(S-lot1) at 40 ml/l between 4.0 and 4.5 (Fig. 1B). The pH of CSL(S-lot1) at 20 ml/l supplemented with lactate was maintained within the optimal range for 48 h, but later declined to 4.0.
Time-courses of pH (A) and bacterial cellulose (BC) concentration (B) when Acetobacter xylinum was cultivated in baffled shake-flasks containing CSL(N) at 20 ml/l (circles), CSL(S-lot1) at 40 ml/l (upright triangles), CSL(S-lot1) at 20 ml/l supplemented with 1.1 g lactate/l (squares) and CSL(S-lot2) at 54 ml/l (inverted triangles)
BC production in CSL(S-lot1) at 40 ml/l reached 7.2 g/l, which is 2-fold higher than that in CSL(N) at 20 ml/l. BC production in CSL(S-lot1) at 20 ml/l supplemented with lactate was between that in CSL(N) at 20 ml/l and CSL(S-lot1) at 40 ml/l. As the total nitrogen, lactate and fructose contents of the three culture broths were almost the same initially, the differences in BC production were mainly due to the pH changes derived from the differences in CSL buffering capacity in the three media.
Cultivation of BPR2001 in baffled shake-flasks containing different volumes of CSL(S-lot1)
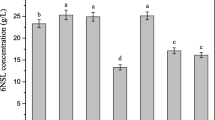
Different volumes of CSL added may affect the BC productivity. The volume of CSL(S-lot1) was varied from 20 ml/l to 80 ml/l and the BC productivity after each addition was compared. Fig. 3 shows the result. The addition of CSL(S-lot1) at 40, 60, or 80 ml/l maintained the pH range at 4.6–4.8, while the addition of CSL(S-lot1) at 20 ml/l caused a constant decline in pH, as shown in Fig. 3A. These changes in pH were well reflected in BC production, namely 2.9 g/l at 120 h for CSL(S-lot1) at 20 ml/l and 7.2 g/l for CSL(S-lot1) at 40–80 ml/l. This indicates that the addition of CSL(S-lot1) at more than 40 ml/l is sufficient for BC production, both from the nutritional point of view and for the buffering effect.
Cultivation of BPR2001 in a stirred-tank reactor
The effect of the buffering capacity of CSL was tested by the cultivation of BC in stirred-tank reactors, as shown in Fig. 4. As a stirred-tank reactor vigorously agitates the culture broth, pH control by a pH sensor and a controller is possible because adhesion of cells or BC to the pH sensor is minimized. In the cultivation of BPR2001 in CSL(N) at 20 ml/l, pH was maintained at 5.0 by a pH controller. The significant increase in BC (Fig. 4B) in the pH-controlled culture is obvious compared with no pH control, reflecting a fluctuation of pH when pH was not controlled. In CSL(S-lot1) at 40 ml/l, a pH controller was not used, but the pH was maintained between 5.0 and 5.2 (as shown in Fig. 4A) and BC production was much faster and reached a maximum earlier than that in the pH-controlled CSL(N), as shown in Fig. 4B. The maximum BC concentrations were 7.95 g/l at 48 h for CSL(N) and 9.22 g/l at 40 h for CSL(S-lot1). Changes in fructose concentration and cell number are shown in Fig. 4B and C, respectively. As the initial cell number in CSL (S-lot1) at 40 ml/l was higher, the fructose consumption of CSL(S-lot1) at 40 ml/l was much faster and cell numbers in CSL(S-lot1) at 40 ml/l were higher, reflecting higher BC production.
Time-courses of: A pH, B BC concentration (black symbols) and fructose concentration (white symbols) and C cell number, when stirred-tank reactors were used in the cultivation of A. xylinum in media containing the following CSLs: CSL(N) at 20 ml/l with pH control at 5.0 using a sensor (circles),CSL(S-lot1) at 40 ml/l without pH control (triangles), CSL(N) at 20 ml/l without pH control (squares)
Cultivation of BPR2001 in an airlift reactor
As the agitation power of an airlift reactor is weaker than that of a stirred-tank reactor, pH control using a pH sensor is impossible, especially with any significant accumulation of BC. Therefore, the buffering effect of CSL can be anticipated if the CSL is added at the initial stage of cultivation. In an airlift reactor, CSL(N) at 20 ml/l and CSL(S-lot2) at 54 ml/l were compared, because the lactate concentrations in these two CSLs were almost the same (Table 1). Furthermore, CSL(S-lot1) was completely consumed and there was none left for the 50-l airlift reactor experiment. As CSL(N) at 20 ml/l was weak in buffering capacity, the pH was controlled manually at 5.0.
Before the airlift reactor experiment, two lots of CSL(S-lot1) at 40 ml/l and CSL(S-lot2) at 54 ml/l (which showed significant buffering capacity at 4.0–6.0, as shown in Fig. 1B) were compared in terms of BC production in 500-ml baffled shake-flasks. As shown in Fig. 2, the final BC concentration was 7.8 g/l in both CSLs, although the pH was stably maintained at 4.5 for CSL(S-lot1) and 5.0 for CSL(S-lo2), indicating that BC productivities are not significantly different in these two CSLs.
In an airlift reactor, the pH of CSL(S-lot2) at 54 ml/l increased to 5.7 and then stabilized at 5.6, while the pH of CSL(N) at 20 ml/l was maintained at 5.0–5.4 by manual control, as shown in Fig. 5A. Changes in fructose concentration (Fig. 5B) and cell number (Fig. 5C) were slightly different between the two CSLs but the BC productions were similar (Fig. 5B). The cultivation of BPR2001 in the two CSLs was stopped mainly because the broth became stagnant in the reactor, due to accumulated BC and a high cell concentration.
Time-courses of: A pH, B BC concentration (black symbols) and fructose concentration (white symbols) and C cell number, when an airlift reactor was used in the cultivation of A. xylinum in media containing the following CSLs: CSL(S-lot2) at 54 ml/l without pH control (triangles), CSL(N) at 20 ml/l with pH controlled manually at approximately 5.0 (circles)
Discussion
The unreliable pH control by a controller and a pH sensor inserted in a reactor sometimes occurs, especially in the microbial production of viscous products such as exopolysaccharides. This is due to either the lack of steam-sterilizable and reliable pH sensors or an unstable response caused either by solids attaching to the sensor or an inhomogeneous culture broth with a non-Newtonian character (Lapasin et al. 1992; Kouda et al. 1996). Furthermore, pH control induces contamination from alkali or acid supply pipe lines. Therefore, if proper pH control is possible by selecting the correct medium or buffering agent, these problems can be minimized. In BC cultivation, we experienced a difficulty in pH control, mainly due to the production of solid BC and viscous acetan by the bacterium. When the natural resources of wastes such as CSL are used as a nutrient, various substances contained in them may contribute to the buffering effect, but the difference in quality between the materials may cause a deviation in the productivity of BC and the controllability of pH. We focused on the buffering capacity of CSL used as a medium. Then, we introduced β as a quantitative measure of the buffering capacity of a CSL solution.
The patterns of β changes typical of the buffering effect, with distinct peaks observed in different CSLs, are shown in Fig. 1. However, β varies with changes in CSL concentration or with sterilization. Lactic acid in CSLs plays an important role in the growth of A. xylinum, because lactic acid activates the tricarboxylic acid cycle and contributes to the supply of ATP for the initial growth of the bacterium (Matsuoka et al. 1996). Consequently, any increase in pH at the initial stage of growth is mainly due to the consumption of lactic acid. Therefore, in this experiment, the adjustment of lactic acid concentration was taken into consideration, based on the data shown in Table 1.
It was not easy to accurately predict a stable pH from only the pattern of β, as shown in Fig. 1B. However, a controllable pH range could be estimated. The quantity of CSL added to the medium was considered, in order to determine a stable buffering effect, as shown in Fig. 3. The fact that pH remained stable until the end of the experiment indicated that the components contributing to the buffering capacity were not completely degraded by the cells.
In shake-flasks, the role of CSL(S-lot1) and CSL(S-lot2) on the buffering capacity was clearly observed (Fig. 2). In correspondence to the changes in β, as shown in Fig. 1B, the addition of CSL(S-lot1) at 40 ml/l maintained the pH at 4.4 and the addition of CSL(S-lot2) at 54 ml/l gave pH 5.2.
In a stirred-tank reactor, BC production increased in CSL(S-lot1)-supplemented medium; but this difference was mainly due to the difference in the initial cell number, because BC production is said to be strongly sensitive to the initial cell number. Therefore, if the initial cell concentration was adjusted to be the same, the productivities of BC in the media used should be almost the same. This indicates that, even in a stirred-tank reactor, the addition of CSL makes culture possible without pH control.
In an airlift reactor, where the fluidity of the medium was significantly low because it was not equipped with a mechanical agitation system, the accumulation of BC hindered the fluidity of the culture broth. However, BC production was similar between CSL(N) with manual pH control and CSL(S-lot2) without pH control. The pH after the addition of CSL(S-lot2) at 54 ml/l without pH control was stabilized at 5.5, although the pH in the cultivation in a baffled shake-flask was stabilized at 5.2, as shown in Fig. 2. In spite of these differences, laborious manual pH control in an airlift reactor can be avoided when CSL(S-lot2) at 54 ml/l is added. This also gives the advantage of CSL addition in pH control.
The optimal pH range of 4.5–5.5 is critical for BC production and growth of BPR2001, because the growth of A. xylinum declines when the pH is more than 6.0 and the increase in cellulase activity when the pH is less than 4.0 causes a degradation of BC (Tahara et al. 1997; Yang et al. 1998). When CSL(S-lot1) and CSL(S-lot2) were compared, the stable pH values maintained were different between the two (Fig. 2). However, their BC production was almost the same. The details of the differences between the two CSL lots were not clear, but the buffering capacity of the two CSLs was similar. When the pH is maintained within the optimal range for BC production, the pattern of BC production will be the same, irrespective of the use of different CSL lots.
The advantage of CSL addition and lack of pH control by a sensor minimizes contamination and maintains a homogeneous fluid flow during the cultivation period, resulting in a BC production that is almost the same as that in a shake-flask or an airlift reactor. When the lactate concentration varies in each CSL, it should be adjusted. The difference in the buffering capacity between different CSLs can be compensated by measuring β in advance and calculating the change in the volume of CSL to be added.
References
Amanullah A, Serrano-Carreon L, Castro B, Galindo E, Nienow AW (1998) The influence of impeller type in pilot scale xanthan fermentations. Biotechnol Bioeng 57:95–108
Cannon RE, Anderson SM (1991) Biogenesis of bacterial cellulose. Crit Rev Microbiol 17:435–447
Chao Y, Sugano Y, Kouda T, Yoshinaga F, Shoda M (1997) Production of bacterial cellulose by Acetobacter xylinum with an air-lift reactor. Biotechnol Tech 11:829–832
Chao Y, Ishida T, Sugano Y, Shoda M (2000) Bacterial cellulose production by Acetobacter xylinum in a 50-l internal-loop air-lift reactor. Biotechnol Bioeng 68:345–352
Cheng HP, Wang PM, Chen JW, Wu WT (2002) Cultivation of Acetobacter xylinum for bacterial cellulose production in a modified airlift reactor. Biotechnol Appl Biochem 35:125–132
Hestrin S, Schramm M (1954) Synthesis of cellulose by Acetobacter xylinum: preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem J 58:345–352
Hwang JW, Yang YK, Hwang JK, Pyun YR, Kim YS (1999) Effects of pH and dissolved oxygen on cellulose production by Acetobacter xylinum BRC5 in agitated culture. J Biosci Bioeng 88:183–188
Ishida T, Sugano Y, Shoda M (2002) Novel glycosyltransferase genes involved in the acetan biosynthesis of Acetobacter xylinum. Biochem Biophys Res Commun 295:230–235
Kouda T, Yano H, Yoshinaga F, Kamanoyama M, Kamiwano M (1996) Characterization of non-Newtonian behavior during mixing of bacterial cellulose in a bioreactor. J Ferment Bioeng 82:382–386
Kouda T, Yano H, Yoshinaga F (1997) Effect of agitator configuration on the productivity of bacterial cellulose production. J Ferment Bioeng 83:371–376
Lapasin R, Pricl S, Bertocchi C, Navarini L, Cesaro A, Philippis R (1992) Rheology of culture broths and exopolysaccharide of Cyanospira capsulate at different stages of growth. Carbohydr Polym 17:1–10
Matsuoka M, Tsuchida T, Matsushita K, Adachi O, Yoshinaga F (1996) A synthetic medium for bacterial cellulose production by Acetobacter xylinum subsp. sucrofermentans. Biosci Biotech Biochem 60:575–579
Nakai T, Moriya A, Tonouchi N, Tsuchida T, Yoshinaga F, Horinouchi S, Sone Y, Mori H, Sakai F, Hayashi T (1998) Control of expression by the cellulose synthase (bcsA) promoter region from Acetobacter xylinum BPR2001. Gene 213:93–100
Naritomi T, Kouda T, Yano H, Yoshinaga F (1998) Effect of ethanol on bacteria cellulose production from fructose in continuous culture. J Ferment Bioeng 85:598–603
Perin DD, Dempsey B (1974) Buffers for pH and metal ion control. Chapman and Hall, London, pp 10–12
Romano R, Franzosi G, Seves A, Sora S (1989) Study of the production of cellulose gel and cellulose by Acetobacter xylinum. Cellul Chem Technol 23:217–223
Ross P, Mayer R, Benzimann M (1991) Cellulose biosynthesis and function in bacteria. Microbiol Rev 55:35–38
Serafica G, Mormino R, Bungay H (2002) Inclusion of solid particles in bacterial cellulose. Appl Microbiol Biotechnol 58:756–760
Stanburg P F, Whitaker A (1984) Principles of fermentation technology. Pergamon Press, Oxford, pp 78–82
Tahara N, Yano H, Yoshinaga F (1997) Two types of cellulase activity produced by a cellulose-producing Acetobacter strain. J Ferment Bioeng 83:389–392
Tanaka M, Murakami S, Shinke R, Aoki K (2000) Genetic characteristics of cellulose-forming acetic acid bacteria identified phenotypically as Gluconacetobacter xylinus. Biosci Biotechnol Biochem 64:757–760
Toyosaki H, Naritomi T, Seto A, Matsuoka M, Tsuchida T, Yoshinaga F (1995) Screening of bacterial cellulose-producing Acetobacter strains suitable for agitated culture. Biosci Biotechnol Biochem 59:1498–1502
Umeda Y, Hirano A, Ishibashi M. Akiyama H, Onizuka T, Ikeuchi M, Inoue Y (1999) Cloning of cellulose synthase genes from Acetobacter xylinum JCM 7664: implication of a novel set of cellulose synthase genes. DNA Res 6:109–115
Vandamme EJ, De Baets S, Vanbaelen A, Joris K, De Wulf P (1998) Improved production of bacterial cellulose and its application potential. Polym Degrad Stabil 59:93–99
Volman G, Ohana P, Benziman M (1995) Biochemistry and molecular biology of cellulose biosynthesis. Carbohydr Eur 12:20–27
Wong HC, Fear AL, Calhoon RD, Eichinger GH, Mayer R, Amikam D, Benziman M, Gelfand DH, Measde JH, Emerick AW, Bruner R, Ben-Bassat A, Tal R (1990) Genetic organization of the cellulose synthase operon in Acetobacter xylinum. Proc Natl Acad Sci USA 87:8130–8134
Yamanaka S, Watanabe K, Kitamura N, Iguchi M, Mitsuhashi S, Nishi Y, Uryu M (1989) The structure and mechanical properties of sheets prepared from bacterial cellulose. J Mater Sci 24:3141–3145
Yang YK, Park SH, Hwang JW, Pyun YR, Kim YS (1998) Cellulose production by Acetobacter xylinum BRC5 under agitated condition. J Ferment Bioeng 85:312–317
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noro, N., Sugano, Y. & Shoda, M. Utilization of the buffering capacity of corn steep liquor in bacterial cellulose production by Acetobacter xylinum . Appl Microbiol Biotechnol 64, 199–205 (2004). https://doi.org/10.1007/s00253-003-1457-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1457-6