Abstract
Purpose
This systematic review and meta-analysis appraise the clinical evidence on efficacy and safety of dexmedetomidine (DEX), as a sedative and analgesic adjunct in adult patients undergoing spine surgery.
Methods
A database search was conducted to identify randomized clinical trials (RCTs) pertinent to the perioperative use of DEX in spine surgery. Sedative and analgesic efficacy of DEX constituted the primary outcomes, whilst the incidence of hemodynamic changes, quality of recovery and occurrence of adverse events served as secondary ones.
Results
Fifteen studies enrolling a total of 913 patients were selected for qualitative analysis, among which eight RCTs incorporating a placebo comparison group were included in the meta-analysis. Most of the retrieved studies were of moderate to good quality and demonstrated an acceptable risk of bias. DEX-treated patients showed a significant reduction of both propofol [mean difference (MD), −214.47 mg; 95%CI, −253.16 to −175.78; P < 0.001] and morphine equivalents consumption both intraoperatively and postoperatively (MD, −2.69; 95% CI, −3.05 to −2.33; P < 0.001 and MD, −4.36 mg; 95%CI, −6.93 to −1.79; P < 0.001, respectively) compared to those assigned to placebo. Postoperative nausea and vomiting incidence were comparable between DEX and placebo groups, whilst other adverse events were not consistently reported.
Conclusions
DEX emerges as an attractive alternative to standard sedative and analgesic modalities applied in spine surgery, by attaining a notable sedative and opioid-sparing effect, which goes with an enhanced safety profile. Yet, no definite conclusion can be drawn due to the considerable heterogeneity of available data.
Trial registration
PROSPERO CRD42015029537.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal surgery poses unique challenges concerning the provision of optimum perioperative management. Intraoperative hemodynamic changes, blood loss, the requirement of augmented doses of anesthetics or potent opioids to suppress the hemodynamic responses evoked by noxious stimulation, and rapid awakening for early neurological assessment, constitute the most prominent intraoperative concerns during spinal procedures [1,2,3,4]. Furthermore, spine surgeries are notorious for being painful and in high demand for adequate perioperative analgesia [3, 5]. As multiple pathways like nociceptive, inflammatory, and neuropathic ones seem to be implicated in the occurrence of pain following major spine surgery, the ideal analgesic strategy for these procedures remains an intriguing issue, yet. Opioids have long been considered as a first-line choice analgesics but their increased consumption carries the risk of opioid-induced hyperalgesia [6, 7]. Aiming to avoid any possible adverse effect associated with the use of systemic opioids, an analgesic approach targeting multiple antinociceptive and antihyperalgesic pathways is considered the best alternative choice [3, 5].
Dexmedetomidine (DEX) is a selective a-2 adrenergic receptor agonist exhibiting analgesic, sedative and sympatholytic actions without causing respiratory depression. On the basis of these properties, DEX can possibly reduce anesthetic requirements, as well as hemodynamic stress response (and consequently intraoperative blood loss), and improve quality of recovery [6, 8,9,10,11]. As DEX has an anesthetic-sparing effect, it can serve as an adjuvant to intravenous or inhalational anesthetics to reduce intraoperative requirements of these drugs, a practice which further minimizes any interference with neurophysiological monitoring and ameliorates recovery from anesthesia [8].
Moreover, intravenous DEX appears to potentiate the analgesic effects of opioids without increasing their hyperalgesic properties and side effects, as it exerts its analgesic effect by acting on different receptors [9, 10]. With its multiple beneficial effects, the systemic administration of DEX in the perioperative period is gaining acceptance as a beneficial sedative and analgesic agent in several types of surgical procedures, such as spinal surgery [12,13,14].
The aim of this systematic review is to evaluate the current evidence on efficacy and safety of DEX used as a sedative and analgesic adjunct in adult patients undergoing spine surgery, with a view to identifying any safe alternatives to standard anesthesia and perioperative practice.
Material and methods
Search strategy and study selection
This systematic review and meta-analysis were conducted according to the criteria of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement and the current recommendations of the Cochrane Collaboration [15, 16] Α dedicated study protocol was designed before the review started and registered with PROSPERO under the number CRD42015029537.
An electronic literature research of PubMed, EMBASE, Cochrane Central Register of Controlled Trials and International Web of Science databases from their inception to 2018 was performed to detect randomized controlled trials (RCTs) pertinent to the administration of DEX in patients undergoing surgery for all types of spine pathology (with the exception of scoliosis surgery). For literature search purposes the subject heading “dexmedetomidine” combined with free text words as “spine surgery”, “discectomy”, “laminectomy” or “fusion”, were applied. An ultimate check of the databases was performed on 10 March 2018. The search strategy is presented in Appendix 1.
Based on the search strategy applied, two investigators (G.T. and C.P.) independently screened and assessed titles and abstracts of all studies identified and discarded those that were obviously irrelevant or duplicates. If eligibility could not be ascertained from the title or the abstract, the full text of the study was retrieved and those deemed suitable were reviewed for eligibility according to the study characteristics and clinical relevance. Reference lists of the recovered articles were then scrutinized for any additional suitable articles in a further effort to ensure that relevant publications were not missed. Any disagreement over eligibility was resolved by consensus or by a third investigator (F.B.), as appropriate.
Inclusion and exclusion criteria
To be eligible for this systematic review, publications had to meet the following inclusion criteria: (1) adult patients (age ≥18 years) undergoing elective or emergency spine surgery; (2) RCTs involving the perioperative use of DEX either as a sedative and analgesic adjuvant (experimental group), compared to placebo or active comparators (control group); (3) provision of data with respect to at least one of the primary outcome measures up to 48 h postoperatively; and (4) availability of full text publication in English language.
Types of outcome measures
The primary outcome measures of this systematic review were the sedative and analgesic efficacy of DEX assessed by either perioperative consumption of supplementary anesthetic or analgesic modalities or pain evaluation scores between study groups. Perioperative hemodynamic performance, intraoperative blood loss, recovery from anesthesia (quality and time to awakening), and the occurrence of adverse events such as postoperative nausea or vomiting (PONV), somnolence, sedation, dizziness, respiratory depression, urine retention or other rare side effects constituted the secondary outcome end-points.
Data extraction and quality assessment
A dedicated data extraction form was developed for recording all relevant details. The extracted data were as follows: publication details (author, year of publication), study design, details of the study population (number and age range of patients); type of surgical procedure, interventions (anesthetic and analgesic protocol), dosage of tested drug dosage, results on primary or secondary outcomes of interest (anesthetic and analgesic drugs consumption, incidence and severity of postoperative pain assessed by a dedicated pain score, hemodynamic changes, quality of recovery and incidence of side effects in the postoperative period) and quality score assessment of each trial.
Selected full papers were critically appraised and quality-assessed, using the Jadad scale [17]. The bias risk in each study was judged by Cochrane Collaboration Risk of Bias Tool [18], which incorporates the following domains: sequence generation, allocation concealment, blinding (including participants and personnel, data collectors, outcome assessors), acquisition of data, selective outcome reporting and other sources of bias. Each item was classified as low, unclear, or at high risk of bias. An assessment of reporting biases (such as publication bias) by constructing a funnel plot and using tests for funnel plot asymmetry, was planned if there were at least ten studies included in the meta-analysis.
Statistical analysis
The results of RCTs being suitable for quantitative analysis were pooled and weighted separately and then together, using Review Manager (version 5.2.5; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). A P value of less than 0.05 was used to determine statistical significance. We computed risk ratios (RR) for and calculated the mean differences (MD) with 95% confidence interval (CI) for continuous data. Values presented as median and 25%–75% interquartile range (IQR) were transformed to mean and standard deviation (SD), while opioids consumption was expressed as morphine equivalents (mg). When data related to primary outcomes of this systematic review were provided as figures, we contacted the responsible authors to acquire the exact numerical values.
Between-study heterogeneity was assessed with the Cochrane Q test using a chi2 function (P values less than 0.10 were considered significant). Within-group heterogeneity was quantified using the I2 statistic. For substantial heterogeneity (I2 > 50%), a random-effect model was selected as appropriate for the analysis, otherwise, a fixed-effect model was applied. The Mantel–Haenszel or inverse variance methods were used to assess the effect of model assumptions on our conclusions, depending on study heterogeneity [19]. Due to the limited number of original publications included in this meta-analysis, further validation for possibly skewed data was not pursued.
Results
Studies selection
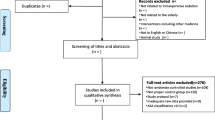
A total of 477 records relevant to DEX administration in patients subjected to elective spine surgery was retrieved from the database search. Among them, 182 records were screened and identified as eligible for inclusion after filtering, whilst 160 out of them were excluded as non-relevant, non-full-text clinical trials or duplicates, leaving 22 full-text papers available for this SR. Seven of them were considered unsuitable for inclusion in the final analysis, due to methodological issues. The articles deemed to be suitable for the final analysis consisted of 15 RCTs [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] enrolling a total of 913 adult patients of both sexes with age range 18 to 80 years, among which 415 were enrolled in DEX group and the remaining 498 in the placebo or active comparator group. All of these studies met the criteria to be included in the final qualitative appraisal, whilst only eight RCTs incorporating a comparison to the placebo group were included in the quantitative analysis. The literature review selection process is summarized in Appendix 2.
Quality assessment and risk of bias estimation of the included trials
Methodological quality assessment of the selected studies is summarized in Table 1. Only three RCTs were of poor quality due to the absence of data regarding randomization method or blinding [22, 24, 34]. The risk of bias estimation revealed that most of the studies enrolled are characterized by moderate to low risk of bias (Appendix 3). Publication bias analyses were not pursued due to the insufficient number of the studies included in the meta-analysis, as for less than ten studies the power of the tests is too low to distinguish chance from real asymmetry.
Description of included trials
Eleven RCTs claimed the use of a double-blind study design [20,21,22,23, 25, 26, 28, 29, 31,32,33]; among which two studies failed to delineate the method of blinding [20, 22]. Nevertheless, appropriate blinding of involved personnel was incorporated in nine of the included RCTs [21, 23, 25, 26, 28, 29, 31,32,33]. The majority of the included RCTs applied a two-arm study design [20,21,22,23, 26, 27,28,29,30,31, 33, 34], whilst three RCTs incorporated three comparison groups [24, 25, 32]. The DEX-treated group was compared either to placebo [20,21,22,23,24,25,26, 32, 33] and/or to an active comparator, namely propofol [34], midazolam [28], etomidate [30], ketamine [32], remifentanil [27], fentanyl [29], clonidine [31], and magnesium sulphate [25]. With the exception of Garg et al. [32] and Terao et al. [34] who applied DEX only for postoperative sedation, all the selected studies involved the administration of DEX in an intraoperative setting as an adjunct to general anesthesia [20,21,22,23,24,25,26,27, 29,30,31, 33] or for conscious sedation in local anesthesia cases [28]. Among them, Gandhi et al. [26] extended the administration of DEX up to 24 h postoperatively.
In most of the studies, DEX administration followed a standard pattern involving a combination of a bolus dose (0.3 μg/kg to 1 μg/kg) delivered over 10–15 min, with a subsequent maintenance infusion (0.2 μg/kg/h to 0.6 μg/kg/h). The single exception was the study conducted by Hwang et al. [27], which omitted the loading dose and applied DEX only as a continuous infusion. Most study designs incorporated an effect-oriented titration of DEX dose; however, dosage regimen varied considerably among the included RCTs. Of interest, two studies climaxed the infusion rate to 0.8 μg/kg/h [24] or even up to 1.2 μg/kg/h [27]. Characteristics of reviewed studies are shown in Table 1.
Regarding the invasiveness of the surgical procedure, multi-level (>2) spine surgeries were reported in three studies [21, 31, 34], while in four studies, the procedure complexity or the segments involved were not explicitly stated [20, 23, 24, 32].
Sedative efficacy
The sedative sparing effect of DEX in spine surgery was assessed for 11 RCTs [20, 21, 23,24,25,26,27, 29,30,31, 34], out of which five used this parameter as a primary outcome end-point [20, 25, 26, 29, 31].
A significant reduction of intraoperative propofol consumption - applied as the basic anesthetic regimen - was recorded in five RCTs [20, 24,25,26, 29], while in two other this positive effect was not documented [23, 27]. Similarly, intraoperative desflurane [22] and etomidate [30] needs were significantly reduced in the DEX-treated group. Nevertheless, besides the notable reduction of isoflurane requirements in an isoflurane-based anesthesia protocol, no considerable difference in supplementary propofol consumption was found between DEX and clonidine groups [31]. Intraoperative sedative needs were BIS-guided in a total of ten RCTs [20,21,22,23,24,25,26,27, 29, 31]. The difference in Ramsey Sedation Scale (RSS) score during awakening was used as an index of sedative efficacy of DEX in two studies; both documented an equal effect of DEX to midazolam [28] or propofol [34] administration, in terms of patients’ arousal level.
Quality of recovery after intraoperative DEX infusion was evaluated by a variety of indices. Time to achieve a BIS level of 80 [26] and time needed for the onset of spontaneous breathing, recovery time, response to verbal commands and safe extubation [20, 22, 25, 26, 29, 31] was either considerably shortened [20, 22, 25, 26] or unaffected [29, 31] in DEX-treated patients compared to placebo or control groups. Nevertheless, the time to eye opening and first verbal command response in PACU were significantly delayed in patients receiving DEX than in those assigned to remifentanil group [27].
Only four RCTs [21, 24,25,26], using a propofol-based anesthetic protocol, deemed as suitable to be included in a meta-analysis. Patients who underwent DEX administration presented significantly lower propofol consumption (mg) compared to those assigned to placebo group (mean difference (MD), −214.47; 95% CI, −253.16 to −175.78; P < 0.001; I2 = 58%) (Fig. 1a).
Analgesic efficacy
Consumption of intraoperative opioids was significantly reduced in the DEX-treated arm in six studies [20, 21, 24, 25, 28, 30], whilst analgesic needs were unaffected by the tested drugs in two studies [23, 31]. Α meta-analysis conducted on this parameter including data from three RCTs sharing a common propofol-based anesthetic protocol [21, 24, 25], detected a considerable reduction of opioid requirements - presented as morphine metabolic equivalents (mg) - between DEX and placebo groups (MD, −2.69; 95% CI, −3.05 to −2.33; P < 0.001; I2 = 0%) (Fig. 1b).
In terms of postoperative analgesic efficacy, this was assessed by either various pain intensity scales, namely, Visual Analogue Scale (VAS), Numeric Rating Scale (NRS) or Verbal Rating Scales (VRS) or total rescue analgesics requirements. Nine RCTs recorded the impact of DEX administration on pain intensity scores from 60 min [22] up to 48 h [20, 26,27,28, 32,33,34], whilst in a single study, the observation period was extended up to 72 h postoperatively [21]. Approximately, half of these studies identified a positive effect of DEX on patients’ perception of pain [20, 22, 26, 27, 32]. Notably, a three-arm RCT using not only a placebo but an active comparator arm, as well, found that DEX was superior to placebo and inferior to ketamine, in terms of intraoperative analgesic control [32]. Furthermore, among the six studies evaluating the amount of postoperative morphine equivalents consumption [21, 22, 27, 28, 32, 33], only one study involving multilevel (>3 levels) thoracic and/or lumbar spine surgery failed to identify any considerable difference between DEX and placebo arms [21]. Two RCTs comparing the intraoperative use of DEX either to a potent analgesic drug as fentanyl [29] or to placebo [33] attributed a longer pain-free period to DEX arm.
However, an analysis regarding the comparable effect of DEX to placebo on postoperative analgesic requirements, including data from three RCTs [21, 32, 33] totalling 280 patients was performed. Patients who received DEX demonstrated a lower morphine equivalents consumption 12 and 48 h postoperatively to those assigned to placebo (MD, −1.56; 95% CI, −2.21 to −0.91; P < 0.001; I2 = 0% and MD, −7.74; 95% CI, −8.89 to −6.59; P < 0.001; I2 = 45%, respectively). A comparable effect was recorded only at 24 h after intervention (MD, −3.00; 95% CI, −9.19 to 3.19; P = 0.34; I2 = 81%) (Fig. 2).
On the basis of pain intensity assessment follow-up, three main subcategories of time-points were identified in the meta-analysis: 1 h [20, 22], 2 h [20, 21, 32] and 6 h [21, 32] after the end of the surgical procedure. A notable attenuation of pain intensity scores was recorded in the DEX group compared with placebo during the first 2 postoperative hours (MD, −3.39; 95% CI, −4.49 to −2.29; P < 0.001; I2 = 61% and MD, −2.11; 95% CI, −3.31 to −0.91; P = 0.005; I2 = 81%, respectively). This effect was eliminated at 6 h after surgery (Fig. 3).
Hemodynamic effects
Hemodynamic effects of DEX use were evaluated by all included studies, with a single exception [27]. Four studies showed that patients assigned to DEX were more prone to slower heart rate and lower blood pressure compared to placebo [20, 25, 26, 32] or magnesium [25], throughout the study period. A very transient hemodynamic deterioration - during the first minute after anesthesia induction – was documented in a study design comparing DEX to desflurane [22], while heart rate decline was the single hemodynamic effect in the remaining studies, using placebo [24], midazolam [28], or propofol [34] as a comparison group. Hypertensive response during intubation and awakening from anesthesia was more efficiently controlled in DEX-treated patients compared to placebo [20, 25, 26], magnesium [25] or desflurane [22]. On the contrary, four studies demonstrated equivalent hemodynamic changes between study groups; DEX being tested against placebo [21, 33], fentanyl [29] or clonidine [31] (Fig. 4).
Although Terao et al. [34] did not report any significant difference in terms of blood pressure between DEX and propofol groups, higher doses of dopamine were applied to maintain this parameter within clinically acceptable limits up to 2 h after DEX infusion was commenced. Surprisingly, Rozet et al. [23] recorded elevated blood pressure levels after DEX compared to placebo without any concomitant difference in heart rate values in either group.
Adverse effects
Incidence of PONV occurrence was reported in eight RCTs, with inconsistent findings [20, 21, 22, 27,28,29, 32, 33], showing either an improvement [27, 29, 33] or no effect [20, 22, 28, 32]. On the contrary, Naik et al. [21] demonstrated a notable augmentation of PONV incidence up to 3 h postoperatively, in DEX-treated patients compared to placebo. A further analysis of the findings regarding the comparable incidence of PONV between DEX and placebo groups revealed a non-significant effect (RR, 1.15; 95% CI, 0.80 to 1.66; P = 0.45; I2 = 44%). Other adverse effects were not consistently reported, as each study evaluated a different kind of adverse event in different cohorts of patients. In any case among the included RCTs, no adverse events of clinical importance were recorded.
Discussion
In this systematic review and meta-analysis, we originally report available clinical evidence on efficacy and safety of DEX used as a sedative and analgesic adjunct in adult patients subjected to elective spine surgery. Intraoperative DEX infusion promoted a sedative- and opioid-sparing effect, whilst a tendency towards to improved short-term perception of postoperative pain and de-escalation of rescue analgesia demands could also be identified. In terms of safety, no clear hemodynamic compromise or any other serious adverse effect could be attributed to DEX administration compared to placebo or active comparators. Moreover, the incidence of PONV seems to remain unaffected by the sedative or analgesic regimen applied.
The ideal perioperative sedation strategy for patients undergoing spine surgery should minimize intraoperative sympathetic response to a surgical stimulus, facilitate neurophysiologic monitoring, be easily titrated and monitored, have predictable arousal, ensure stable hemodynamics, address postoperative pain, and have a tolerable side effect profile [35, 36]. None of the commonly used sedative agents fulfills all these criteria or has a distinct superiority to the others.
DEX emerges as an attractive alternative to standard anesthetic approaches, as it holds unique hypnotic and analgesic properties through the stimulation of a2 - receptors located in the locus coeruleus, and spinal dorsal horn, respectively [36, 37]. Having both central and peripheral sympatholytic action, DEX can be applied as an adjuvant in spine surgical procedures with a view to attenuate perioperative stress, in addition to minimizing sedative and opioids requirements.
Our review clearly confirms that DEX reduces intraoperative propofol and opioids consumption in spine surgery; the available data are insufficient for conclusions to be drawn for inhalational [22, 31] and other sedative agents [22, 30]. Apparently this propofol-sparing effect is attained with relatively low infusion rates of DEX ranging from 0.2 μg/kg/h [22, 29] up to 0.5 μg/kg/h [21, 26, 30]. A plausible reasoning for the failure of Rozet et al. [23] and Hwang et al. [27] to confirm this positive effect could be the likelihood of their studies being underpowered to detect consumption of anesthetics, as the sample size was calculated with respect to VAS and evoked potentials changes, respectively, which served as primary outcomes for these studies.
An issue of concern regarding the assessment of sedation effectiveness is the accuracy of the applied instruments. Albeit, most of the RCTs included in this SR incorporated a BIS-guided anesthesia protocol, it is widely known that the BIS is not an ideal monitoring of anesthesia depth and incurs high inter-subject variability [38, 39]. Considering that, DEX induces a sedation state that mimics natural sleep, caution is required when interpreting the output of currently available EEG-based monitors in patients sedated with DEX over to GABA-acting sedatives. As the plasma concentrations of propofol and DEX are not routinely measured in clinical studies, the lower threshold of the doses of both medications is established intuitively rather than scientifically to prevent accidental awareness with an inherent risk of interpretation bias and inconsistency. However, it seems possible that the lower dose of propofol could be used safely if the anesthesia protocol involves the concomitant infusion of DEX [23, 32]. Moreover, any delay in quality of recovery attributed to DEX could be explained under the light of the aforementioned issues of concern.
Spine procedures - complex ones in particular- could be implicated in severe perioperative pain [40, 41]. Opioids have long been a mainstay for perioperative analgesia in major spine surgery, however, their use is challenged by numerous side effects and thus current analgesic approach aims to the implementation of other analgesic alternatives [42]. Our data shows that DEX yielded a positive impact on intraoperative opioids consumption when being tested against placebo [20, 21, 24, 30], midazolam [28] or magnesium [25], but its effect in reducing pain perception and rescue analgesics requirements postoperatively was less clear, as the relevant trials were of moderate to high heterogeneity. The latter could be addressed to the diversity of intraoperative and postoperative analgesic modalities, time of the assigned drug regimen commencement, duration of administration, cumulative dose of DEX, pain assessment follow-up period since the tested drug was concluded, subjective nature of tools used for pain scoring and the possible impact of opioid-induced hyperalgesia. Consequently, the short-term (up to 6 h postoperatively) pain intensity attenuation and reduction of total rescue analgesic requirements recorded in DEX-treated individuals, should be interpreted with extreme caution.
Nevertheless, the appealing performance of DEX is tempered by the reported unfavorable hemodynamic sequelae, consisting of bradycardia, hypotension, and hypertension; an effect being more apparent with rapid infusion [43]. These features are attributed to complex vasodilative and vasoconstrictive hemodynamic effects specific to its activation of pre- and post-synaptic α2-receptors, with the net hemodynamic effect depending on the balance between central and peripheral mechanisms [44]. By central mechanisms, DEX reduces sympathetic outflow and causes hypotension, whereas the peripheral direct action of vasoconstriction may lead to hypertension [44, 45]. Thus, a loading dose of DEX usually causes systemic hypertension, followed by hypotension [43, 45].
In spine surgery, hemodynamic stability is of paramount importance as an abrupt elevation of arterial blood pressure can cause intraoperative bleeding, which impairs quality of vision of the surgical field leading to an increased rate of complications [42]. On the contrary, critical arterial hypotension incurs the risk of spinal ischemia further aggravating patients’ neurological outcome [43]. Considering that acute hemodynamic fluctuations due to autonomic dysfunction of central cord origin constitute a rather ordinary implication, especially in spinal procedures involving cervical and thoracic segments, any anesthetics-related disturbances might act synergistically to further hemodynamic compromise [46, 47].
This meta-analysis failed to attribute a clear impact of DEX on hemodynamics compared to placebo or active comparators; only a tendency towards to higher risk for bradycardia and relatively lower blood pressure was demonstrated. In general terms, these hemodynamic alterations were maintained within clinically acceptable limits, whilst the use of rescue drugs for maintaining stable hemodynamics was hardly reported [26, 34].
In line with the known pharmacodynamics of DEX, half of the studies included in this systematic review demonstrated that DEX administration was implicated with bradycardia incidences [20, 24,25,26, 28, 32, 34] and this could be per se a contraindication in patients undergoing high-risk surgeries for hypotension development, such as complex spine procedures. An intriguing remark was that all studies failing to ascribe any considerable hemodynamic effect to DEX infusion [21, 23, 29, 31, 33], used a propofol-based anesthesia protocol. Presumably, this finding further supports what has already been documented in a neurocritical care setting, namely that propofol and DEX might share equal blood-lowering properties [46, 48].
Furthermore, the degree of hemodynamic effects has been related to the dosage of DEX and hydration status of the patient [45, 46, 49]. However, in this systematic review, no clear association between loading or maintenance dose of DEX and hemodynamic adverse events could be identified.
Different authors evaluated various adverse events related to DEX administration. The impact of DEX administration on the incidence of PONV was assessed by half of the studies. The meta-analysis conducted on this parameter failed to identify any superiority of DEX over placebo. Interestingly, the two RCTs using opioids as controls suggested a clear benefit of DEX use compared to remifentanil [27] and fentanyl [29] for PONV prevention. Other adverse events were not consistently reported and thus could not be thoroughly assessed.
In conclusion, DEX emerges as an attractive alternative to standard sedative and analgesic modalities applied in spine surgery, by attaining a notable reduction of intraoperative consumption of both anesthetics and opioids. Moreover, DEX seems to offer satisfactory control of pain and reduce rescue analgesic requirements in the postoperative period. These properties are coming along with an enhanced safety profile as from the currently available evidence no clear hemodynamic compromise or any other adverse event could be documented.
Implication for research
Taking into consideration the observed heterogeneity among included trials regarding patients’ characteristics, surgical invasiveness, dosing, and type of tested sedative and analgesic agents, outcome parameters and length of follow-up, our results need to be interpreted with caution. Furthermore, our findings could not be easily generalizable, as only adult populations were included. Much of the available data are in minor spine procedures while those supporting the use of DEX in major spine surgery are limited. These two cohorts of patients have different analgesic needs, thus well-designed RCTs are warranted to address the efficacy of DEX as an adjunct to other sedatives and analgesic in major spine surgeries, as well. Finally, the use of DEX in clinical settings involving volatile-based anesthesia protocols need to be elucidated.
Implication for practice
The use of DEX infusion as a sedative adjunct intraoperatively in patients subjected to spine procedures should be carefully titrated to avoid the risk for clinically significant bradycardia or systemic hypotension requiring vasopressors.
References
Edgcombe H, Carter K, Yarrow S (2008) Anesthesia in the prone position. Br J Anaesth 100:165–183
Willner D, Spennati V, Stohl S, Tosti G, Aloisio S, Bilotta F (2016) Sa pine surgery and blood loss: systematic review of clinical evidence. Anesth Analg 123:1307–1315
Sharma S, Balireddy RK, Vorenkamp KE, Durieux ME (2012) Beyond opioid patient-controlled analgesia: a systematic review of analgesia after major spine surgery. Reg Anesth Pain Med 37:79–98
Sekimoto K, Nishikawa K, Ishizeki J, Kubo K, Saito S, Goto F (2006) The effects of volatile anesthetics on intraoperative monitoring of myogenic motor-evoked potentials to transcranial electrical stimulation and on partial neuromuscular blockade during propofol/fentanyl/nitrous oxide anesthesia in humans. J Neurosurg Anesthesiol 18:106–111
Mathiesen O, Dahl B, Thomsen BA, Kitter B, Sonne N, Dahl JB, Kehlet H (2013) A comprehensive multimodal pain treatment reduces opioid consumption after multilevel spine surgery. Eur Spine J 22:2089–2096
Zheng Y, Cui S, Liu Y, Zhang J, Zhang W, Zhang J, Gu X, Ma Z (2012) Dexmedetomidine prevents remifentanil-induced postoperative hyperalgesia and decreases spinal tyrosine phosphorylation of N-methyl-d-aspartate receptor 2B subunit. Brain Res Bull 87:427–431
Lee C, Kim YD, Kim JN (2013) Antihyperalgesic effects of dexmedetomidine on high-dose remifentanil-induced hyperalgesia. Korean J Anesthesiol 64:301–307
Kang WS, Kim SY, Son JC, Kim JD, Muhammad HB, Kim SH, Yoon TG, Kim TY (2012) The effect of dexmedetomidine on the adjuvant propofol requirement and intraoperative hemodynamics during remifentanil-based anesthesia. Korean J Anesthesiol 62:113–118
Grewal A (2011) Dexmedetomidine: new avenues. J Anaesthesiol Clin Pharmacol 27:297–302
Blaudszun G, Lysakowski C, Elia N, Tramèr MR (2012) Effect of perioperative systemic alpha2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology 116:1312–1322
Tsaousi GG, Lamperti M, Bilotta F (2016) Role of Dexmedetomidine for sedation in neurocritical care patients: a qualitative systematic review and meta-analysis of current evidence. Clin Neuropharmacol 39:144–151
Schnabel A, Meyer-Friessem CH, Reichl SU, Zahn PK, Pogatzki-Zahn EM (2013) Is intraoperative dexmedetomidine a new option for postoperative pain treatment? A meta-analysis of randomized controlled trials. Pain 154:1140–1149
Peng K, Wu S, Liu H, Ji F (2014) Dexmedetomidine as an anesthetic adjuvant for intracranial procedures: meta-analysis of randomized controlled trials. J Clin Neurosci 21:1951–1958
McQueen-Shadfar LA, Megalla SA, White WD, Olufolabi AJ, Jones CA, Habib AS (2011) Impact of intraoperative dexmedetomidine on postoperative analgesia following gynecologic surgery. Curr Med Res Opin 27:2091–2097
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339. https://doi.org/10.1136/bmj.b2700
Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration, http://handbook.cochrane.org
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Higgins JP, Altman DG, Gotzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group; Cochrane Statistical Methods Group (2011) The Cochrane Collaboration’s tool for assessing the risk of bias in randomized trials. BMJ 343:d5928
Kontopantelis E, Springate DA, Reeves D (2013) A re-analysis of the Cochrane library data: the dangers of unobserved heterogeneity in meta-analysis. PLoS One 8:e69930
Bojaraaj DRK, Senthilkumar S, Vijayaragavan S, Gnanavelrajan A (2016) Effect of intravenous use of dexmedetomidine on anesthetic requirements in patients undergoing elective spine surgery: A double-blinded randomized controlled trial. International J Scientific Study 4:251–255
Naik BI, Nemergut EC, Kazemi A, Fernández L, Cederholm SK, McMurry TL, Durieux ME (2016) The effect of dexmedetomidine on postoperative opioid consumption and pain after major spine surgery. Anesth Analg 122:1646–1653
Ozkose Z, Demir FS, Pampal K, Yardim S (2006) Hemodynamic and anesthetic advantages of dexmedetomidine, an alpha 2-agonist, for surgery in prone position. Tohoku J Exp Med 210:153–160
Rozet I, Metzner J, Brown M, Treggiari MM, Slimp JC, Kinney G, Sharma D, Lee LA, Vavilala MS (2015) Dexmedetomidine does not affect evoked potentials during spine surgery. Anesth Analg 121:492–501
Chen Z, Lin S, Shao W (2015) Effects on somatosensory and motor evoked potentials of senile patients using different doses of dexmedetomidine during spine surgery. Ir J Med Sci 184:813–818
Srivastava VK, Mishra A, Agrawal S, Kumar S, Sharma S, Kumar R (2016) Comparative evaluation of dexmedetomidine and magnesium sulphate on propofol consumption, hemodynamics and postoperative recovery in spine surgery: a prospective, randomized, placebo-controlled, double-blind study. Adv Pharm Bull 6:75–81
Gandhi KA, Panda NB, Vellaichamy A, Mathew PJ, Sahni N, Batra YK (2017) Intraoperative and postoperative administration of dexmedetomidine reduces anesthetic and postoperative analgesic requirements in patients undergoing cervical spine surgeries. J Neurosurg Anesthesiol 29:258–263
Hwang W, Lee J, Park J, Joo J (2015) Dexmedetomidine versus remifentanil in postoperative pain control after spinal surgery: a randomized controlled study. BMC Anesthesiol 15:21
Peng K, Liu HY, Liu SL, Ji FH (2016) Dexmedetomidine-fentanyl compared with midazolam-fentanyl for conscious sedation in patients undergoing lumbar disc surgery. Clin Ther 38:192–201
Turgut N, Turkmen A, Gökkaya S, Altan A, Hatiboglu MA (2008) Dexmedetomidine-based versus fentanyl-based total intravenous anesthesia for lumbar laminectomy. Minerva Anestesiol 74:469–474
Lin S, Dai N, Cheng Z, Shao W, Fu Z (2014) Effect of dexmedetomidine-etomidate-fentanyl combined anesthesia on somatosensory- and motor-evoked potentials in patients undergoing spinal surgery. Exp Ther Med 7:1383–1387
Mariappan R, Ashokkumar H, Kuppuswamy B (2014) Comparing the effects of oral clonidine premedication with intraoperative dexmedetomidine infusion on anesthetic requirement and recovery from anesthesia in patients undergoing major spine surgery. J Neurosurg Anesthesiol 26:192–197
Garg N, Panda NB, Gandhi KA, Bhagat H, Batra YK, Grover VK, Chhabra R (2016) Comparison of small dose ketamine and dexmedetomidine infusion for postoperative analgesia in spine surgery - a prospective randomized double-blind placebo controlled study. J Neurosurg Anesthesiol 28:27–31
Song Y, Shim JK, Song JW, Kim EK, Kwak YL (2016) Dexmedetomidine added to an opioid-based analgesic regimen for the prevention of postoperative nausea and vomiting in highly susceptible patients: a randomised controlled trial. Eur J Anaesthesiol 33:75–83
Terao Y, Ichinomiya T, Higashijima U, Tanise T, Miura K, Fukusaki M, Sumikawa K (2012) Comparison between propofol and dexmedetomidine in postoperative sedation after extensive cervical spine surgery. J Anesth 26:179–186
Bekker A, Haile M, Kline R, Didehvar S, Babu R, Martiniuk F, Urban M (2013) The effect of intraoperative infusion of dexmedetomidine on the quality of recovery after major spinal surgery. J Neurosurg Anesthesiol 25:16–24
Jamaliya RH, Chinnachamy R, Maliwad J, Deshmukh VP, Shah BJ, Chadha IA (2014) The efficacy and hemodynamic response to Dexmedetomidine as a hypotensive agent in posterior fixation surgery following traumatic spine injury. J Anaesthesiol Clin Pharmacol 30:203–207
Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M (2003) The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology 98:428–436
Kaskinoro K, Maksimow A, Långsjӧ J, Aantaa R, Jääskeläinen S, Kaisti K, Särkelä M, Scheinin H (2011) Wide inter-individual variability of bispectral index and spectral entropy at loss of consciousness during increasing concentrations of dexmedetomidine, propofol, and sevoflurane. Br J Anaesth 107:573–580
Lobo FA, Schraag S (2011) Limitations of anaesthesia depth monitoring. Curr Opin Anaesthesiol 24:657–664
Yu L, Ran B, Li M, Shi Z (2013) Gabapentin and pregabalin in the management of postoperative pain after lumbar spinal surgery: a systematic review and meta-analysis. Spine (Phila Pa 1976) 38:1947–1952
Boezaart AP, Davis G, Le-Wendling L (2012) Recovery after orthopedic surgery: techniques to increase duration of pain control. Curr Opin Anaesthesiol 25:665–672
Dunn LK, Durieux ME, Nemergut EC (2016) Non-opioid analgesics: novel approaches to perioperative analgesia for major spine surgery. Best Pract Res Clin Anaesthesiol 30:79–89
Constantin JM, Momon A, Mantz J, Payen JF, De Jonghe B, Perbet S, Cayot S, Chanques G, Perreira B (2016) Efficacy and safety of sedation with dexmedetomidine in critical care patients: a meta-analysis of randomized controlled trials. Anaesth Crit Care Pain Med 35:7–15
Bloor BC, Ward DS, Belleville JP, Maze M (1992) Effects of intravenous dexmedetomidine in humans: II. Hemodynamic changes. Anesthesiology 77:1134–1142
Ice CJ, Personett HA, Frazee EN, Dierkhising RA, Kashyap R, Oeckler RA (2016) Risk factors for Dexmedetomidine-associated hemodynamic instability in noncardiac intensive care unit patients. Anesth Analg 122:462–469
Erdman MJ, Doepker BA, Gerlach AT, Phillips GS, Elijovich L, Jones GM (2014) A comparison of severe hemodynamic disturbances between dexmedetomidine and propofol for sedation in neurocritical care patients. Crit Care Med 42:1696–1702
Karlsson AK (1999) Autonomic dysreflexia. Spinal Cord 37:383–391
Jakob SM, Ruokonen E, Grounds RM, Sarapohja T, Garratt C, Pocock SJ, Bratty JR, Takala J, Dexmedetomidine for Long-Term Sedation Investigators (2012) Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA 307:1151–1160
Jones GM, Murphy CV, Gerlach AT, Goodman EM, Pell LJ (2011) High-dose dexmedetomidine for sedation in the intensive care unit: an evaluation of clinical efficacy and safety. Ann Pharmacother 45:740–747
Author information
Authors and Affiliations
Contributions
1. Georgia Tsaousi: conception and design of the work; acquisition, analysis, and interpretation of data; wrote the paper; drafted the work or revised it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work.
2. Chryssa Pourzitaki: acquisition, analysis, and interpretation of data; wrote the paper; Drafted the work or revised it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work.
3. Simone Aloisio: acquisition and interpretation of data; Drafted the work or revised it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work.
4. Federico Bilotta: conception and design of the work; interpretation of data; drafted the work or revised it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work.
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 739 kb)
Rights and permissions
About this article
Cite this article
Tsaousi, G.G., Pourzitaki, C., Aloisio, S. et al. Dexmedetomidine as a sedative and analgesic adjuvant in spine surgery: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 74, 1377–1389 (2018). https://doi.org/10.1007/s00228-018-2520-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-2520-7