Abstract
Twenty eight surface water samples were collected from fourteen sites of the West Bokaro coalfield, India. The concentration of Mn, Cu, Zn, Ni, As, Se, Al, Cr, Ba, and Fe were analyzed using inductively coupled plasma mass spectrometry (ICP-MS) for determination of seasonal fluctuations and a heavy metal pollution index (HPI). The HPI values were below the critical pollution index value of 100. Metal concentrations were higher in the pre-monsoon season as compared to the post-monsoon season. The Zn, Ni, Mn, As, Se, Al, Ba, Cu, and Cr concentrations did not exceed the desirable limits for drinking water in either season. However, at many sites, concentrations of Fe were above the desirable limit of the WHO (2006) and Indian drinking water standard (BIS 2003) in both seasons. The water that contained higher concentrations of Fe would require treatment before domestic use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Access to safe drinking water remains an urgent necessity, as 30 % of the urban and 90 % of the rural Indian population still depend completely on untreated surface or groundwater resources (Kumar et al. 2005). Scarcity of clean and potable drinking water has emerged in recent years as one of the most serious developmental issues in many parts of West Bengal, Jharkhand, Orissa, Western Uttar Pradesh, Andhra Pradesh, Rajasthan and Punjab (Tiwari and Singh 2014). The presence of heavy metals in both surface and groundwater supplies is a major environmental problem. The occurrence of toxic metals in pond, ditch and river water affect the lives of local people that depend upon these water sources for their daily requirements (Rai et al. 2002). Contamination of surface water may also degrade the groundwater quality, resulting in a very serious issue in many developing countries.
Water quality indices are tools, to determine conditions of water quality and, like any other tool, require knowledge about principles and basic concepts of water and related issues (Nikbakht 2004). Several researchers have used water quality indices methods for the assessing quality of waters (Zhang et al. 2009; Kikuchi et al. 2009; Pandey et al. 2009; Giri et al. 2010; Virha et al. 2011; Srivastava et al. 2011; Kumar et al. 2012; Prasanna et al. 2012; Díaz et al. 2013; Giri and Singh 2014; Mahato et al. 2014; Protano et al. 2014; Tiwari et al. 2014; Varghese and Jaya 2014; Panigrahy et al. 2015). However, in recent years much attention has been given towards the evaluation of heavy metal pollution in ground and surface water with the development of a heavy metal pollution index (HPI) (Reddy 1995; Mohan et al. 1996). The aim of this study was to assess the seasonal variations in heavy metals concentrations in river and pond water using the HPI approach for the determination of the suitability of the water for drinking.
Materials and Methods
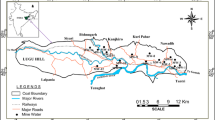
The West Bokaro coalfield lies between 23°41′ to 23°52′ N latitude and 85°24′ to 85°41′ E longitude (Fig. 1). The coalfield is drained by the Bokaro River passing through the central part of coalfield with easterly flows. Chutua River is the main tributary of the Bokaro River which drains the northern hilly terrain of the coalfield. Chotha River is a tributary of the Bokaro River which drains the southern region of the coalfield. The coalfield area experiences a tropical climate characterized by very hot pre-monsoon and cold post-monsoon season. The average annual rainfall of the district is 1418 mm and more than 85 % of annual rainfall occurs during the four monsoon months (June to September). The coalfield forms a broad syncline trending east to west. The complete sequence of lower Gondwana formation rests unconformably on basement rocks. The Barakar formation covers the major part of the coalfield and is comprised of coarse to fine grained sand stone, pebbly conglomerates, gritty sandstones, grey shales, carbonaceous shales, fire clays and coal seams.
Twenty eight of the surface water samples (fourteen samples in the post-monsoon season, and fourteen samples in the pre-monsoon season) were collected from fourteen sites of the West Bokaro coalfield area and placed into narrow-mouth pre-washed polyethylene bottles (capacity 100 mL) during month of November, 2012 and May, 2013 (Fig. 1). Samples were filtered with Millipore filtration unit, filter paper (pore size 0.45 µm) and preserved by adjusting the pH < 2 with 6 N ultrapure nitric acid (Radojevic and Bashkin 1999). Appropriate quality assurance procedures and precautions were carried out to ensure reliability, and samples were carefully handled to avoid contamination. Glassware was properly cleaned and analytical grade reagents were used. Milli Q water was used throughout the study. Concentrations of heavy metals were analyzed by ICP-MS (Perkin Elmer model ELAN DRCe, 710 Bridgeport Avenue Shelton, Connecticut 06484-4794, United States). Reagent blank determinations were used to correct the instrument readings. For the accuracy of the analysis, it was checked by analysing reference standard of water (NIST 1640a and NIST 1643b). The precision obtained in most cases was better than 5 % RSD with comparable accuracy.
Geographic information system (GIS) is widely used for collecting diverse spatial data and for overlay analysis in spatial register domain to represent spatially variable phenomena (Bonham-Carter 1996; Babiker et al. 2004; Gupta and Srivastava 2010). The water quality indices and GIS which synthesize different available water quality data into an easily understood format, provide a way to summarize overall water quality conditions in a manner that can be clearly communicated to policy makers (Singh et al. 2013a). The spatial distribution maps were prepared using ARC GIS- 10.2 software (Environmental Systems Research Institute, Redlands 380 New York Street 92373, California, United States).
The heavy metal pollution index (HPI) is a rating technique that provides the composite influence of individual heavy metal on the overall quality of water. The rating system is an arbitrary value between zero to one and its selection depends upon the importance of individual quality considerations in a comparative way or it can be assessed by making values inversely proportional to the recommended standard for the corresponding parameter (Horton 1965; Mohan et al. 1996). In computing the HPI, Prasad and Bose (2001) considered unit weightage (Wi) as a value inversely proportional to the recommended standard (Si) of the corresponding parameter as proposed by Reddy (1995). The critical pollution index of HPI value for drinking water as given by Prasad and Bose (2001) is 100. However, a modified scale using three classes has been used in the present study after Edet and Offiong (2002). The classes have been demarcated as low, medium and high for HPI values <15, 15–30 and >30, respectively.
For this study, the concentration limits [i.e. highest permissive value for drinking water (Si) and maximum desirable value (Ii) for each parameter] were taken from the Indian drinking water specifications (BIS 2003). The highest permissive value for drinking water (Si) refers to the maximum allowable concentration in drinking water in the absence of any alternate water source. The desirable maximum value (Ii) indicates the standard limits for the same parameters in drinking water.
The HPI model (Mohan et al. 1996) is given by Eq. (1)
where, Q i is the sub-index of the ith parameter. W i is the unit weightage of ith parameter, and n is the number of parameters considered.
The sub index (Qi) of the parameter is calculated by Eq. (2)
where M i is the monitored value of heavy metal of ith parameter, I i is the ideal value (maximum desirable value for drinking water) of the ith parameter and S i is the standard value (highest permissive value for drinking water) of the ith parameter. The sign (−) indicates numerical difference of the two values, ignoring the algebraic sign.
Results and Discussion
The results of the metal analysis for the two seasons viz for the post- and pre-monsoon seasons are provided in Table 1. The Zn, Ni, Mn, As, Se, Al, Ba, Cu, and Cr concentrations did not exceed the desirable limits for drinking water in either season. However, at many sites, concentrations of Fe were above the desirable limit of the WHO (2006) and Indian drinking water standard (BIS 2003) in both seasons. The concentrations of Fe ranged from 198.5 to 905 µg L−1 in the post-monsoon and 267 to 1621 µg L−1 in the pre-monsoon season, exceeding the desirable limit of 300 µg L−1 in 57 % of the surface water samples in the post-monsoon season and 86 % of the surface water samples in the pre-monsoon season, respectively. The spatial variation showed higher Fe values at sites 1, 4, 7, 8, 9 in the post-monsoon season (Fig. 2). However, in the pre-monsoon season the higher Fe values were measured at sites 1, 4, 7, 8, 9, 10, 11 and 12 (Fig. 3). Excess Fe in water is thought to result from industrial effluents. Except for agricultural based activity and coal mining and washing, there are no other major anthropogenic or industrial activities in this region. Excess Fe is an endemic water quality problem in many part of India (Singh et al. 2013b).
The concentrations of variables displayed great seasonality. The total concentrations of all the studied elements in surface water had an average of 532 and 921 µg L−1 in the post- and pre-monsoon seasons, respectively. Metal concentrations were higher in the pre-monsoon season as compared to the post-monsoon season irrespective of the locations. This may be attributed to the high evaporation and intense anthropogenic activities (high degree of mining activities and agriculture) in summer (Vega et al. 1998; Olias et al. 2004). The dilution effect due to heavy rainfall may also results in the consequent reduction in the total concentration of the metals in the post-monsoon season. Eighty-five percent of the annual precipitation falls in the rainy season and subsequently dilutes pollutants in surface waters.
A paired sample t test was run on the metals data, comparing the post- versus pre-monsoon seasons, for each of the ten parameters. Table 2 depicts the mean differences of the post- versus pre-monsoon seasons metals data for all the ten variables along with the corresponding t values, degrees of freedoms (df) and p values for two-tailed paired sample t tests. Metal concentrations of the pre-monsoon season were statistically higher than the post-monsoon season and shows significant variations in their concentration (Table 2).
Mean concentrations of the analyzed metals were used to calculate the HPI values. These values ranged from 2.1 to 6.4 (mean 3.3 ± 1.3) in the post-monsoon season and from 2.3 to 26 (mean 9 ± 8.0) in the pre-monsoon season. The highest HPI values were calculated in water from sites 4, 7, 8 and 9. The higher values of HPI may be attributed due to the natural and anthropogenic activities. Lower HPI values in the post-monsoon season again indicate a dilution affect. The HPI values of the samples within the study area were less than the critical HPI value of 100 (Prasad and Bose 2001). However, considering the classes of HPI, all the locations for both the seasons fall under the low class (HPI < 15) to medium class (HPI 15–30) (Figs. 4, 5). In the post-monsoon season among all of the surface water samples, the percentage (%) of HPI categories, low class (100 %) were observed. However, in the pre-monsoon season the percentage (%) of HPI categories, low class (86 %) and medium class (14 %) were observed. This indicates the water is not critically polluted with respect to heavy metals.
Heavy pollution index class map of the West Bokaro coalfield area in the post-monsoon season (according to Edet and Offiong 2002)
Heavy pollution index class map of the West Bokaro coalfield area in the pre-monsoon season (according to Edet and Offiong 2002)
In conclusion, the HPI values within the study area were below the critical pollution index value of 100. The concentrations of most metals in the surface water in the study area were well below the limits for the desirable/permissible levels recommended for drinking water by the Bureau of Indian Standard (BIS 2003) and World Health Organisation (WHO 2006). The water that contained higher concentrations of Fe would require treatment before domestic use.
References
Babiker IS, Mohamed MAA et al (2004) Assessment of groundwater contamination by nitrate leaching from intensive vegetable cultivation using geographical information system. Environ Int 29(8):1009–1017
BIS (2003) Indian standard drinking water specifications IS 10500:1991, edition 2.2 (2003–2009). Bureau of Indian Standards, New Delhi
Bonham-Carter GF (1996) Geographic information systems for geoscientists: modeling with GIS. Comput Methods Geosci 13:1–50
Díaz RO, Fonticiella MD, Arado LJ, Borrell MJ, D'Alessandro RK, López PN (2013) Spatial distribution and contamination assessment of heavy metals in urban topsoils from Las Tunas City, Cuba. Bull Environ Contam Toxicol 91(1):29–35
Edet AE, Offiong OE (2002) Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower Cross River Basin (southeastern Nigeria). GeoJournal 57:295–304
Giri S, Singh AK (2014) Assessment of surface water quality using heavy metal pollution index in Subarnarekha River, India. Water Qual Expo Health 5(4):173–182
Giri S, Singh G, Gupta SK, Jha VN, Tripathi RM (2010) An evaluation of metal contamination in surface and groundwater around a proposed Uranium mining site, Jharkhand, India. Mine Water Environ 29(3):225–234
Gupta M, Srivastava PK (2010) Integrating GIS and remote sensing for identification of groundwater potential zones in the hilly terrain of Pavagarh, Gujarat, India. Water Int 35:233–245
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37(3):300–306
Kikuchi T, Furuichi T, Hai HT, Tanaka S (2009) Assessment of heavy metal pollution in river water of Hanoi, Vietnam using multivariate analyses. Bull Environ Contam Toxicol 83(4):575–582
Kumar R, Singh RD, Sharma KD (2005) Water resources of India. Curr Sci 89:794–811
Kumar PS, Delson PD, Babu PT (2012) Appraisal of heavy metals in groundwater in Chennai city using a HPI model. Bull Environ Contam Toxicol 89(4):793–798
Mahato MK, Singh PK, Tiwari AK (2014) Evaluation of metals in mine water and assessment of heavy metal pollution index of East Bokaro Coalfield area, Jharkhand, India. Int J Earth Sci Eng 7(04):1611–1618
Mohan SV, Nithila P, Reddy SJ (1996) Estimation of heavy metal in drinking water and development of heavy metal pollution index. J Environ Sci Health 31(2):283–289
Nikbakht M (2004) The effect assessment of ahvaz No. 1, 2 water treatment plant on Karoon water quality. Environ Pollut B6:51–67
Olias M, Nieto JM, Sarmiento AM, Ceron JC, Canovas CR (2004) Seasonal water quality variations in a river affected by acid mine drainage: the Odiel River (South West Spain). Sci Total Environ 333:267–281
Pandey J, Shubhashish K, Pandey R (2009) Metal contamination of Ganga River (India) as influenced by atmospheric deposition. Bull Environ Contam Toxicol 83(2):204–209
Panigrahy BP, Singh PK, Tiwari AK, Kumar B, Kumar A (2015) Assessment of heavy metal pollution index for groundwater around Jharia coalfield region, India. J Bio Env Sci 6(3):33–39
Prasad B, Bose JM (2001) Evaluation of heavy metal pollution index for surface and spring water near a limestone mining area of the lower Himalayas. Environ Geol 41:183–188
Prasanna MV, Nagarajan R, Chidambaram S, Elayaraja A (2012) Assessment of metals distribution and microbial contamination at selected Lake waters in and around Miri city, East Malaysia. Bull Environ Contam Toxicol 89(3):507–511
Protano C, Zinnà L, Giampaoli S, Spica VR, Chiavarini S, Vitali M (2014) Heavy metal pollution and potential ecological risks in rivers: a case study from Southern Italy. Bull Environ Contam Toxicol 92(1):75–80
Radojevic M, Bashkin VN (1999) Practical environmental analysis. Royal Society of Chemistry, London
Rai UN, Tripathi RD, Vajpayee P, Jha V, Ali MB (2002) Bioaccumulation of toxic metals (Cr, Cd, Pb and Cu) by seeds of Euryale ferox Salisb (Makhana). Chemosphere 46:267–272
Reddy SJ (1995) Encyclopaedia of environmental pollution and control, vol 1. India. Environmental Media Karlia, p 342
Singh AK, Raj B, Tiwari AK, Mahato MK (2013a) Evaluation of hydrogeochemical processes and groundwater quality in the Jhansi district of Bundelkhand region, India. Environ Earth Sci 70(3):1225–1247
Singh PK, Tiwari AK, Panigarhy BP, Mahato MK (2013b) Water quality indices used for water resources vulnerability assessment using GIS technique: a review. Int J Earth Sci Eng 6(6–1):1594–1600
Srivastava PK, Mukherjee S, Gupta M, Singh SK (2011) Characterizing monsoonal variation on water quality index of River Mahi in India using geographical information system. Water Qual Expo Health 2(3–4):193–203
Tiwari AK, Singh AK (2014) Hydrogeochemical investigation and groundwater quality assessment of Pratapgarh district, Uttar Pradesh. J Geol Soc India 83(3):329–343
Tiwari AK, Singh PK, Mahato MK (2014) GIS-based evaluation of water quality index of groundwater resources in West Bokaro coalfield, India. Curr World Environ 9(3):843–850
Varghese J, Jaya DS (2014) Metal pollution of groundwater in the vicinity of Valiathura sewage farm in Kerala, South India. Bull Environ Contam Toxicol 93(6):694–698
Vega M, Pardo R, Barrado E, Deban L (1998) Assessment of seasonal and polluting effects on the quality of river water by exploratory data analysis. Water Res 32:3581–3592
Virha R, Biswas AK, Kakaria VK, Qureshi TA, Borana K, Malik N (2011) Seasonal variation in physicochemical parameters and heavy metals in water of upper lake of Bhopal. Bull Environ Contam Toxicol 86(2):168–174
WHO (2006) Guidelines for drinking-water quality, 3rd edn. World Health Organization, Geneva
Zhang XY, Tang LS, Zhang G, Wu HD (2009) Heavy metal contamination in a typical mining town of a minority and mountain area, South China. Bull Environ Contam Toxicol 82(1):31–38
Acknowledgments
The authors are grateful to the Director, Central Institute of Mining and Fuel Research, Dhanbad to providing research facilities and also thankful to Professor D. C. Panigarhi, Director, Indian School of Mines for his kind support. One of the authors (Ashwani Kumar Tiwari) is grateful to ISM/MHRD/Government of India and European Commission (Erasmus Mundus AREaS+ Program) for Grant support. Our hearty thanks to the Editor-in-Chief and anonymous reviewer for his valuable suggestions to improve the study in the present form.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tiwari, A.K., De Maio, M., Singh, P.K. et al. Evaluation of Surface Water Quality by Using GIS and a Heavy Metal Pollution Index (HPI) Model in a Coal Mining Area, India. Bull Environ Contam Toxicol 95, 304–310 (2015). https://doi.org/10.1007/s00128-015-1558-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-015-1558-9