Abstract
Background
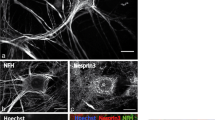
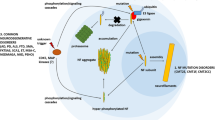
Massive neurofilament conglomeration in motor neurons has been described to occur in the early stages of both familial and sporadic amyotrophic lateral sclerosis (ALS). Previously, neurofilament conglomerates were immunolabeled for both superoxide dismutase (SOD1) and nitrotyrosine, suggesting the potential for oxidative nitration damage to neurofilament protein by peroxynitrite. Long-lived neurofilaments may also undergo modification by advanced glycation end-products (AGEs) with concomitant generation of free radicals, including superoxide. This radical species may then react with nitric oxide to form the potent oxidant, peroxynitrite, which in turn can nitrate neurofilament protein. Such a glycated and nitrated neurofilament protein may become resistant to proteolytic systems, forming high-molecular-weight protein complexes and cytotoxic, neuronal inclusions.
Materials and Methods
Paraffin sections containing both neurofilament conglomerates and neuronal inclusions were obtained from patients with sporadic (n = 5) and familial (n = 2) ALS and were probed with specific antibodies directed against the AGEs cypentodine/piper-idine-enolone, arginine-lysine imidazole, pentosidine, and pyrraline.
Results
Neurofilament conglomerates, but not neuronal inclusions, were intensely immunolabeled with each of the anti-AGE antibodies tested. The immunoreactivity was selective for neurofilament conglomerates and suggested that AGEs may form inter- or intramolecular cross-links in neurofilament proteins.
Conclusions
These data support the hypothesis that AGE formation affects neurofilament proteins in vivo and is associated with the concomitant induction of SOD1 and protein nitration in neurofilament conglomerates. AGE formation in neurofilament protein may not only cause covalent cross-linking but also generate superoxide and block nitric oxide-mediated responses, thereby perpetuating neuronal toxicity in patients with ALS.


Similar content being viewed by others
References
Chou SM. (1995) Pathology of motor system disorder. In: Leigh PN, Swash M (eds). Motor Neuron Disease: Biology and Management. Springer-Verlag, London, pp. 53–92.
Chou SM. (1997) Neuropathology of amyotrophic lateral sclerosis—new perspectives on an old disease. J. Formos. Med. Assoc. 96: 488–498.
Carpenter S. (1968) Proximal axonal enlargement in motor neuron disease. Neurology 8: 841–851.
Chou SM. (1979) Pathognomy of intraneuronal inclusions in ALS. In: Japanese Medical Research Foundation (ed). Amyotrophic Lateral Sclerosis. University of Tokyo Press, Tokyo, pp. 135–176.
Murayama SH, Mori, H, Ihara Y, Bouldin TW, Suzuki K, Tomonaga T. (1990) Immunocytochemical and ultrastructural studies of lower motor neurons in amyotrophic lateral sclerosis. Ann. Neurol 27: 137–148.
Leigh PN, Anderson BH, Dodison A, Gallo M, Swash M, Power D. (1992) Ubiquitin deposits in anterior horn cells in motor neuron disease. Neurosci. Lett. 93: 197–203.
Chou SM. (1994) Accumulation of disassembled neurofilament in upper motor neurons of ALS. In: Rose FC (ed). ALS—From Charcot to the Present and into the Future. Smith-Gordon & Nishimura Press, London, pp. 167–178.
Rosen DR, Siddique T, Patterson D. et al. (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362: 59–62.
Beckman JS, Ye YZ, Chen J, Conger KA. (1996) The interactions of nitric oxide with oxygen radicals and scavengers in cerebral ischemic injury. Adv. Neurobiol. 71: 339–351.
Beckman JS, Carson M, Smith CD, Koppenol WH. (1993) ALS, SOD, and peroxynitrite. Nature 364: 584–585.
Chou SM, Wang HS, Komai K. (1996) Co-localization of SOD-1 and NOS in neurofilament accumulation within motor neurons of amyotrophic lateral sclerosis: An immunohistochemical study. J. Chem. Neuroanat. 10: 249–258.
Chou SM, Wang HS, Taniguchi A. (1996) Role of SOD-1 and nitric oxide/cyclic GMP on neurofilament aggregation in ALS/MND. J. Neurol. Sci. 139(Suppl): 16–26.
Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. (1996) The advanced glycation end product, N-epsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J. Biol. Chem. 271: 9982–9986.
Fu MX, Wells-Knecht KJ, Blackledge JA, Lyons TJ, Thorpe SR, Baynes JW. (1994) Glycation, glycoxidation, and cross-linking of collagen by glucose. Kinetics, mechanisms, and inhibition of late stages of the Maillard reaction. Diabetes 43: 676–683.
Yim HS, Kang SO, Hah YC, Chok PB, Yim MB. (1995) Free-radicals generated during the glycation reaction of amino acids by methylglyoxal; a model study of protein-cross-linked free radicals. J. Biol. Chem. 270: 28228–28233.
Shibata N, Hirano A, Kobayashi M, et al. (1997) Advanced glycosylation end products (AGE) deposition in intraneuronal hyaline inclusions (IHIs) of spinal cords from familial amyotrophic lateral sclerosis (ALS) patients with superoxide dismutase-1 (SOD1) mutation and from transgenic mice expressing mutant human SOD1. Brain Pathol. 7: 1073A.
Shibata N, Hirano M, Klapporth K. (1993) Immunohistochemical demonstration of Cu/Zn superoxide dismutase in the spinal cord of patients with familial amyotrophic lateral sclerosis. Acta Histochem. Cytochem. 26: 619–621.
Shibata N, Hirano A, Kobayashi M, Sasaki S, Kato T, Matsumoto S, Shiozawa Z, Komori T, Ikemoto A, Umahara T, et al. (1994) Cu/Zn superoxide dismutase-like immunoreactivity in Lewy bodylike inclusions of sporadic amyotrophic lateral sclerosis. Neurosci. Lett. 179: 149–152.
Pardo CA, Xu Z, Borchet DR, Price DL, Sisodia SS, Cleveland DW. (1995) Superoxide dismutase is an abundant component in cell bodies, dendrites, and axons of motor neurons and in a subset of other neurons. Proc. Natl. Acad. Sci. U.S.A. 92: 954–958.
Nishiyama SH, Murayama S, Shimizu J, et al (1995b) Cu/Zu superoxide dismutase-like immunoreactivity is present in Lewy bodies from Parkinson’s disease: A light and electron microscopic immunocytochemical study. Acta Neuropathol. 89: 471–474.
Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. (1997) ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18: 327–338.
Wang HS, Taniguchi A, Chou SM. (1997) Role of SOD1 in nitration of neurofilamentous aggregation induced by IDPN in rats. Ann. Neurol. 43: 415–416A.
Abe K, Pan LH, Watanabe M, Konno H, Kato T, Itoyama Y. (1997) Upregulation of protein-tyrosine nitration in the anterior horn cells of amyotrophic lateral sclerosis. Neurol. Res. 19: 124–128.
Beal MF, Ferrante RJ, Browne SE, Matthews RT, Kowall NW, Brown RH Jr. (1997) Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann. Neurol. 42: 646–654.
Bruijn LI, Beal MF, Becher MW, Schulz JB, Wong PC, Price DL, Cleveland DW. (1997) Elevated free nitrotyrosine levels, but not protein-bound nitrotyrosine or hydroxyl radicals, throughout amyotrophic lateral sclerosis (ALS)-like disease implicated tyrosine nitration as an aberrant in vivo property of one familial ALS-linked superoxide dismutase 1 mutant. Proc. Natl. Acad. Sci. U.S.A. 94: 7606–7611.
Delanty N, Prezedborski S, Bandele AN, Lynch T, Rosario R. (1997) Elevated nitrotyrosine immunoreactivity in patients with amyotrophic lateral sclerosis. Ann. Neurol. 42: 427A.
Schiffer D, Autilio-Gambetti L, Chio A, Migheli A, Pezzulo T. (1991) Ubiquitin in motor neuron disease: A study at the light and electron microscopic level. J. Neuropathol. Exp. Neurol. 50: 463–473.
Migheli A, Attanasio A, Schiffer D. (1994) Ubiquitin and neurofilament expression in anterior horn cells in amyotrophic lateral sclerosis: Possible clues to the pathogenesis. Neuropathol. Appl. Neurobiol. 20: 282–289.
Zhang X, Mitsuhashi T, Pan S, Bucala R, Ulrich P. (1997) Two cyclic AGEs from dehydration of the Amadori product are recognized by polyclonal and monoclonal antibodies to AGEs. Diabetes 46(Suppl 1): A0598.
Makita Z, Bucala R, Rayfield EJ, Friedman EA, Kaufman AM, Korbert SM, Barth RH, Winston JA, Fuh H, Manogue KR, et al. (1994) Reactive glycosylation endproducts in diabetic uremia & treatment of renal failure. Lancet 343: 1519–1522.
Al-Abed Y, Bucala, R. (1997) A novel AGE crosslink that exhibits immunological crossreactivity with in vivo-formed AGEs. Diabetes 46:(Suppl 1): A0271.
Monnier VM, Sell DR, Ramanakoppa H, et al. (1992) Maillard reaction-mediated molecular damage to extracellular matrix and other tissue proteins in diabetes, aging, and uremia. Diabetes 41(Suppl 20: 36–41.
Nakamura Y, Horii Y, Nishino T, et al. (1993) Immunohistochemical localization of advanced glycosylation endproducts (AGEs) in coronary atheroma and cardiac tissue in diabetes mellitus. Am. J. Pathol. 143: 1649–1656.
Stitt A, Li YM, Gardiner TA, Bucala R, Archer D, Vlassara H. (1997) Advanced glycation end products (AGEs) co-localized with AGE receptors in the retinal vasculature of diabetic and of AGE-infused rats. Am. J. Pathol. 150: 523–531.
Smith MA, Taneda S, Richey PL, Miyata S, Yan SD, Stern D, Sayre LM, Monnier VM, Perry G. (1994) Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc. Natl. Acad. Sci. U.S.A. 91: 5710–5714.
Xu Z, Cork LC, Griffin JW, Cleveland DW. (1993) Increased expression of neurofilament subunit NF-L produces morphological alterations that resemble the pathology of human motor neuron disease. Cell 73: 23–33.
Lee MK, Marszalek JR, Cleveland DW. (1994) A mutant neurofilament subunit causes massive, selective motor neuron death: Implication for the pathogenesis of human motor neuron disease. Neuron 13: 975–988.
Ma D, Descarries L, Julien JP, Doucet G. (1995) Abnormal perikaryal accumulation of neurofilament light protein in the brain of mice transgenic for the human protein sequence of postnatal development. Neuroscience 68: 135–149.
Collard JF, Côté F, Julien JP. (1995) Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis Nature 375: 61–64.
Côté F, Collard JF, Houle D, Julien JP. (1994) Copy-dependent and correct developmental expression of the human neurofilament heavy gene in transgenic mice. Brain Res. Mol. Brain Res. 26: 99–105.
Del Canto MC, Gurney ME. (1994) Development of central nervous system pathology in a mouse transgenic model of human amyotrophic lateral sclerosis. Am. J. Pathol. 145: 1271–1280.
Julien JP, Côté F, Collard JF. (1995) Mice over-expressing the human neurofilament heavy gene as a model of ALS. Neurobiol. Aging 16: 487–490.
Tu PH, Raju P, Robinson KA, Gurney ME, Trojanowski JQ, Lee VM. (1996) Transgenic mice carrying a human mutant superoxide dismutase transgene develop neuronal cytoskeletal pathology resembling human amyotrophic lateral sclerosis lesions. Proc. Natl. Acad. Sci. U.S.A. 93: 3155–3160.
Rouleau GA, Clark AW, Rooke K, Pramatarova A, Krizus A, Suchowersky O, Julen JP, Figlewicz D. (1996) SOD1 mutation is associated with accumulation of neurofilaments in amyotrophic lateral sclerosis. Ann. Neurol. 39: 128–131.
McLean WG, Pekiner C, Cullum NA, Casson IF. (1992) Post-translational modifications of nerve cytoskeletal proteins in experimental diabetes. Mol. Neurobiol. 6: 225–237.
Yagihashi S. (1993) Axonal cytoskeleton and diabetic neuropathy. Diabet. Med. Suppl. 2: 107S–109S.
Ryle C, Donaghy M. (1995) Non-enzymatic glycation of peripheral, nerve proteins in human diabetics. J. Neurol. Sci. 129: 62–68.
Ryle C, Leow CK, Donaghy M. (1997) Nonenzymatic glycation of peripheral and central nervous system proteins in experimental diabetes mellitus. Muscle Nerve 20: 577–584.
Vlassara H, Bucala R, Striker L. (1994) Pathogenic effects of advanced glycosylation: Biochemical, biologic, and clinical implications for diabetes and aging. J. Lab. Invest. 70: 138–151.
Smith MA, Richey PL, Harris R, Sayre LM, Beckman JS, Perry G. (1997) Widespread peroxynitrite-mediate damage in Alzheimer’s disease. J. Neurosci. 17: 2653–2657.
Dong D, Xu Z, Chevrier M, Cotter R, Cleveland D, Hart G. (1993) Glycosylation of mammalian neurofilaments. Localization of mutiple O-linked N-acetylglucosamine moieties on neurofilament polypeptides L and M. J. Biol. Chem. 268: 16679–16687.
Dong D, Xu Z, Hart G, Cleveland D. (1996) Cytoplasmic O-GlcNAc modification of the head domain and the KSP repeat motif of the neurofilament protein neurofilament-H. J. Biol. Chem. 271: 20845–20852.
Bucala R. (1996) Laboratory evaluation of advanced glycosylation end products: Relevance to diabetes, aging and renal failure. Diagn. Endocrinol. Metab. 14: 99–106.
Zhang X, Ulrich P. (1996) Directed approaches to reactive Maillard intermediates: Formation of a novel 3-alkylamineo-2-hydroxy-4-hydroxymeth-yl-2-cyclopenten-l-one (“Cypentodine”). Tetrahedron Lett. 37: 4667–4670.
Hart GW, Greis KD, Dong LY, Blomberg MA, Chou TY, Jiang MS, Roquemore EP, Snow DM, Kreppel LK, Cole RN. (1995) O-linked N-acetylglucosamine: The “yin-yang” of Ser/Thr phosphorylation? Nuclear and cytoplasmic glycosylation. Adv. Exp. Med. Biol. 376: 115–123.
Vlassara H. (1997) Recent progress in advanced glycation end products and diabetic complications. Diabetes 46(Suppl 2): S19–25.
Bucala R. (1996) Lipid and lipoprotein oxidation: Basic mechanisms and unsolved questions in vivo. Redox Rep. 2: 291–307.
Yim H-S, Kang S-O, Hah Y-C, Chock PB, Yim, MB. (1995) Free radicals generated during the glycation reaction of amino acids and methylglyoxal J. Biol. Chem. 270: 28228–28233.
Yu WH. (1994) Nitric oxide synthase in motor neurons after axotomy. J. Histochem. Cytochem. 42: 451–457.
Wu W. (1993) Expression of nitric-oxide synthase (NOS) in injured CNS neurons as shown by NADPH diaphorase histochemistry. Exp. Neurol. 120: 153–159.
Wu Y, Li Y, Liu H, Wu W. (1995) Induction of nitric oxide synthase and motoneuron death in newborn and early postnatal rats following spinal root avulsion. Neurosci. Lett. 194: 109–112.
Gou JP, Eyer J, Leterrier JF. (1995) Progressive hyperphosphorylation of neurofilament heavy subunits with aging: Possible involvement in the mechanism of neurofilament accumulation. Biochem. Biophys. Res. Commun. 215: 368–376.
Zhu M, Spink D, Yan B, Bank S, DeCaprio A. (1994) Formation and structure of cross-linking and monomeric pyrrole autoxidation products in 2,5-hexanedione-treated amino acids, peptides, and protein. Chem. Res. Toxicol. 7(Suppl 4): 551–558.
Chou, SM, Wang HS, Taniguchi A. (1996) Does nonenzymatic glycation influence neurofilamentous aggregation in motor neurons? An experimental study. Presented at the 9th AOCN (Asian Oceanic Congress of Neurology) September 5, Seoul, Korea. Abstract pp97.
Brett J, Schmidt AM, Yan SD, et al. (1993) Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am. J. Pathol. 143: 1699–1712.
Schmidt AM, Hori O, Brett J, Yan S-D, Wautier J-L, Stern D. (1994) Cellular receptors for advanced glycation endproducts. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler. Thromb. 14: 1521–1528.
Acknowledgements
The authors gratefully acknowledge the generous contribution of antibodies against pyrraline and pentosidine by Drs. V. Monnier, G. Perry, and M. Smith of the Case Western Reserve University, Cleveland, Ohio.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Bucala.
Rights and permissions
About this article
Cite this article
Chou, S.M., Wang, H.S., Taniguchi, A. et al. Advanced Glycation Endproducts in Neurofilament Conglomeration of Motoneurons in Familial and Sporadic Amyotrophic Lateral Sclerosis. Mol Med 4, 324–332 (1998). https://doi.org/10.1007/BF03401739
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401739