Abstract
Background
While oligoclonality of circulating CD4− CD8− and of CD8+ T cells is not uncommon, clonal dominance within the CD4 compartment is not frequently found in healthy individuals. In contrast, the majority of patients with rheumatoid arthritis (RA) have clonally expanded CD4+ T cell populations. Previous studies have demonstrated that these clonogenic CD4+ T cells do not express the CD28 molecule. To examine the correlation between CD28 expression and clonal proliferation, we have analyzed the T cell receptor (TCR) diversity of CD4+ CD28− T cells in normal individuals and in RA patients.
Material and Methods
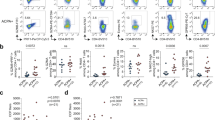
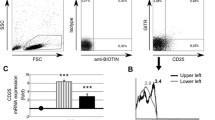
The size of the peripheral blood CD4+ CD28− compartment was determined in 30 healthy individuals and 30 RA patients by two-color FACS analysis. In 10 RA patients and five controls with more than 2.5% CD4+ CD28− T cells, TCR BV gene segment usage was analyzed with 19 BV-specific antibodies. Oligoclonality was assessed in sorted CD4+ CD28+ and CD28− T cells using TCR BV-BC-specific polymerase chain reaction and size fractionation. Clonal dominance was confirmed by direct sequencing.
Results
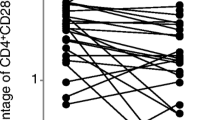
The CD4+ CD28− T cell compartment was expanded to more than 2.5% in 70% of the RA patients and 30% of the normal individuals. Compared with the CD4+ CD28+ T cells, the TCR BV gene segment usage among CD4+ CD28− cells was grossly skewed with the dominance of single BV elements. Molecular TCR analysis provided evidence for oligoclonality in 17 of 21 expanded BV elements. In two unrelated RA patients who shared both HLA-DRB1 alleles, the TCR β-chain sequences of dominant clonotypes were highly conserved.
Conclusions
Oligoclonality is a characteristic feature of CD4+ CD28− T cells which are expanded in some healthy individuals and in the majority of RA patients. The lack of CD28 expression is a common denominator of CD4+, CD8+, and CD4− CD8− T cells prone to develop clonal dominance. The limited TCR diversity of clonal CD4+ CD28− populations in RA patients suggests that these T cells recognize a limited spectrum of antigens. The fact that the majority of individuals with marked expansions and oligoclonality of CD4+ CD28− T cells are RA patients suggests a role for these unusual lymphocytes in the pathogenetic events leading to RA.




Similar content being viewed by others
References
Davis MM. (1990) T cell receptor gene diversity and selection. Annu. Rev. Biochem. 59: 475–496.
Blackman M, Kappler J, Marrack P. (1990) The role of the T cell receptor in positive and negative selection of developing T cells. Science 248: 1335–1341.
Matis LA. (1990) The molecular basis of T-cell specificity. Annu. Rev. Immunol. 8: 65–82.
Porcelli S, Yockey CE, Brenner MB, Balk SP. (1993) Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4−8−α/β T cells demonstrates preferential use of several Vβ genes and an invariant TCR α chain. J. Exp. Med. 178: 1–16.
Dellabona P, Casorati G, Friedli B, et al. (1993) In vivo persistence of expanded clones specific for bacterial antigens within the human T cell receptor α/β CD4−8− subset. J. Exp. Med. 177: 1763–1771.
Callahan JE, Kappler JW, Marrack P. (1993) Unexpected expansions of CD8-bearing cells in old mice. J. Immunol. 151: 6657–6669.
Hingorani R, Choi IH, Akolkar P, et al. (1993) Clonal predominance of T cell receptors within the CD8+ CD45RO+ subset in normal human subjects. J. Immunol. 151: 5762–5769.
Monteiro J, Hingorani R, Choi I-H, Silver J, Pergolizzi R, Gregersen PK. (1995) Oligoclonality in the human CD8+ T cell repertoire in normal subjects and monozygotic twins: Implications for studies of infectious and autoimmune diseases. Mol. Med. 1: 614–624.
Posnett DN, Sinha R, Kabak S, Russo C. (1994) Clonal populations of T cells in normal elderly humans: The T cell equivalent to “benign monoclonal gammapathy.” J. Exp. Med. 179: 609–618.
Brooks EG, Balk SP, Aupeix K, Colonna M, Strominger JL, Groh-Spies V. (1993) Human T-cell receptor (TCR) α/β+ CD4− CD8− T cells express oligoclonal TCRs, share junctional motifs across TCR Vβ-gene families, and phenotypically resemble memory T cells. Proc. Natl. Acad. Sci. U.S.A. 90: 11787–11791.
Bendelac A. (1995) Mouse NK1+ T cells. Curr. Opin. Immunol. 7: 367–374.
Makino Y, Yamagata N, Sasho T, et al. (1993) Extrathymic development of Vαl4+ T cells. J. Exp. Med. 177: 1399–1408.
Waase I, Kayser C, Carlson PJ, Goronzy JJ, Weyand CM. (1996) Oligoclonal T cell proliferation in patients with rheumatoid arthritis and their unaffected siblings. Arthritis Rheum. 39: 904–913.
Schmidt D, Goronzy JJ, Weyand CM. (1996) CD4+ CD7− CD28− T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J. Clin. Invest. 97: 2027–2037.
Arnett FC, Edworthy SM, Bloch DA, et al. (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31: 315–324.
Choi YW, Kotzin B, Herron L, Callahan J, Marrack P, Kappler J. (1989) Interaction of Staphylococcus aureus toxin “super antigens” with human T cells. Proc. Natl. Acad. Sci. U.S.A. 86: 8941–8945.
Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P. (1993) The sizes of the CDR3 hypervariable regions of the murine T-cell receptor β chains vary as a function of the recombined germ-line segments. Proc. Natl. Acad. Sci. U.S.A. 90: 4319–4323.
Gorski J, Yassai M, Zhu X, Kissella B, Keever C, Flomenberg N. (1994) Circulating T cell repertoire complexity in normal individuals and bone marrow receptors analyzed by CDR3 size spectratyping. Correlation with immune status. J. Immunol. 152: 5109–5119.
Stoflet ES, Koeberl DD, Sarkar G, Sommer SS. (1988) Genomic amplification with transcript sequencing. Science 239: 491–494.
Goronzy JJ, Bartz-Bazzanella P, Hu W, Jendro MC, Walser-Kuntz DR, Weyand CM. (1994) Dominant clonotypes in the repertoire of peripheral CD4+ T cells in rheumatoid arthritis. J. Clin. Invest. 94: 2068–2076.
Morishita Y, Sao H, Hansen JA, Martin PJ. (1989) A distinct subset of human CD4+ cells with a limited alloreactive T cell receptor repertoire. J. Immunol 143: 2783–2789.
Azuma M, Phillips JH, Lanier LL. (1993) CD28− T lymphocytes. Antigenic and functional properties. J. Immunol. 150: 1147–1159.
Jenkins MK, Johnson JG. (1993) Molecules involved in T-cell costimulation. Curr. Opin. Immunol. 5: 361–367.
Linsley P, Ledbetter J. (1993) The role of the CD28 receptor during T cell responses to antigen. Annu. Rev. Immunol. 11: 191–212.
DerSimonian H, Sugita M, Glass DN, et al. (1993) Clonal Vαl2.1 T cell expansion in the peripheral blood of rheumatoid arthritis patients. J. Exp. Med. 177: 1623–1631.
Fitzgerald JE, Ricalton NS, Meyer A-C, et al. (1995) Analysis of clonal CD8+ T cell expansions in normal individuals and patients with rheumatoid arthritis. J. Immunol. 154: 3538–3547.
Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. (1994) An invariant Vα 24-Jα Q/Vβ 11 T cell receptor is expressed in all individuals by clonally expanded CD4−8− T cells. J. Exp. Med. 180: 1171–1176.
Casanova J-L, Cerottini J-C, Matthes M, et al. (1992) H-2 restricted cytolytic T lymphocytes specific for HLA display T cell receptors of limited diversity. J. Exp. Med. 176: 439–447.
Moss PA, Moots RJ, Rosenberg WM, et al. (1991) Extensive conservation of α and β chains of the human T-cell antigen receptor recognizing HLA-A2 and influenza A matrix peptide. Proc. Natl. Acad. Sci. U.S.A. 88: 8987–8990.
Jorgenson JL, Reay PA, Ehrich EW, Davis MM. (1992) Molecular components of T-cell recognition. Annu. Rev. Immunol. 10: 835–873.
Porcelli S, Morita CT, Brenner MB. (1992) CD lβ restricts the response of human CD4(−) 8(−) T lymphocytes to a microbial antigen. Nature 360: 593–597.
Boise LH, Minn AJ, Noel PJ, et al. (1995) CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity 3: 87–98.
Walunas TL, Sperling AI, Khattri R, Thompson CB, Bluestone JA. (1996) CD28 expression is not essential for positive and negative selection of thymocytes or peripheral T cell tolerance. J. Immunol. 156: 1006–1013.
Gribben JG, Freeman GJ, Boussiotis VA, et al. (1995) CTLA4 mediates antigen-specific apoptosis of human T cells. Proc. Natl. Acad. Sci. U.S.A. 92: 811–815.
Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3: 541–547.
Waterhouse P, Penninger JM, Timms E, et al. (1995) Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science 270: 985–988.
Behar SM, Porcelli SA, Beckman EM, Brenner MB. (1995) A pathway of costimulation that prevents anergy in CD28− T cells: B7-independent costimulation of CD 1-restricted T cells. J. Exp. Med. 182: 2007–2018.
Acknowledgments
Supported in part by National Institutes of Health grants (ROI AR41974 and AR42527) and by the Mayo Clinic and Foundation. The authors thank Toni L. Higgins for secretarial support and James W. Fulbright for technical assistance.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schmidt, D., Martens, P.B., Weyand, C.M. et al. The Repertoire of CD4+ CD28− T Cells in Rheumatoid Arthritis. Mol Med 2, 608–618 (1996). https://doi.org/10.1007/BF03401644
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03401644