Abstract
Objective
Exercise has been accepted and generally recommended for the management of type 1 diabetes mellitus (T1D) and for improving the overall quality of life in affected individuals. This meta-analysis was conducted to determine the overall effects of exercise (acute bouts of exercise and chronic exercise [or training]) on acute and chronic glycaemic control in patients with T1D, the effects of different types of exercise on glycaemic control and which conditions are required to obtain these positive effects.
Methods
PubMed, ISI Web of Knowledge and SPORTDiscus™ were consulted to identify studies on T1D and exercise. Cohen’s d statistics were used for calculating mean effect sizes (ES) as follows: small d = 0.3, medium d = 0.5 and large d = 0.8. Ninety-five percent confidence intervals (95% CIs) were used to establish the significance of our findings.
Results
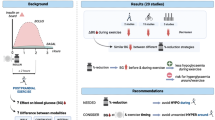
From a total of 937 studies, 33 that met the inclusion criteria were selected. Nine studies were used to calculate the ES of a single bout of aerobic exercise; 13 studies to calculate the ES of aerobic training; 2 studies to calculate the ES of strength training; 4 studies to calculate the ES of combined (aerobic and strength) training and 6 studies to calculate the ES of high-intensity exercise (HIE) and training. ES for exercise on acute glycaemic control were large, while they were small for chronic glycaemic control. Aerobic exercise, resistance exercise, mixed exercise (aerobic combined with resistance training) and HIE acutely decreased blood glucose levels. To prevent late-onset hypoglycaemic episodes, the use of single bouts of sprints into an aerobic exercise can be recommended. This meta-analysis also showed that a regular exercise training programme has a significant effect on acute and chronic glycaemic control, although not all exercise forms showed significant results. Specifically, aerobic training is a favourable tool for decreasing chronic glycaemic control, while resistance training, mixed and HIE did not significantly improve chronic glycaemic control. Although, this meta-analysis showed there was a tendency for improvement in glycaemic control due to resistance training or resistance training combined with endurance training, there were not enough studies and/or subjects to confirm this statistically.
Conclusions
Based on this meta-analysis, we can conclude that the addition of brief bouts of high-intensity, sprint-type exercise to aerobic exercise can minimize the risk of sustaining a hypoglycaemic episode. We can also conclude that only regular aerobic training will improve the glycated haemoglobin level of a patient with T1D.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. Background
Exercise has been accepted and generally recommended for the management of type 1 diabetes mellitus (T1D) and for improving the overall quality of life in affected individuals. In addition to increasing aerobic fitness, reducing cardiovascular risk factors, and reducing bodyweight and body fat, physical activity develops and maintains chronic glycaemic control by enhancing insulin sensitivity and stimulating muscle glucose uptake. The American College of Sports Medicine (ACSM) has published a guideline for exercise testing and prescription in T1D.[1] This guideline recommends that individuals with T1D need to work out for 20–45 minutes at an intensity of 40–60% of their maximal oxygen consumption (\({\rm{\dot V}}{{\rm{O}}_{{\rm{2max}}}}\)) for 5–7 days/week, or daily at low to moderate intensity. The ACSM guideline also advocates strength training as an integral part of the training programme. Both the American Diabetes Association and the ACSM recommend patients with T1D to keep blood glucose levels before, during and after exercise above 5.5 mmol/L and below 13.8–16.7 mmol/L. If these criteria are not met, it is recommended to delay exercise to determine as to whether or not ketones are present.
Unfortunately, due to the complexity of regulating exogenous insulin in a physiological manner during exercise, physical activity often results in episodes of hypoglycaemia or hyperglycaemia shortly following or even long after completing exercise.[2] Due to the persistent increase of insulin sensitivity and to the required repletion of muscle glycogen stores, in which hepatic glucose production is unable to match the peripheral uptake of glucose by muscle, exercise could affect blood glucose levels 24 hours following intense prolonged exercise and, therefore, late onset of hypoglycaemia can occur regardless of appropriate insulin reduction.[2,3] Besides this, previous exercise and the occurrence of previous hypoglycaemic episodes or poor glycaemic control can affect the hypoglycaemic counter-regulatory mechanisms, which may cause severe hypoglycaemia.[4] Moreover, T1D athletes with higher levels of physical activity tend to have an impaired glucose counter-regulatory hormone response to hypoglycaemia.[5] On the other hand, the opposite effect can occur in patients with poor glycaemic control. Patients with poor glycaemic control can easily develop hyperglycaemia (with or without ketosis) as a consequence of exercise. Even in well controlled T1D patients with adequate insulinization, acute high-intensity exercise (HIE) may cause hyperglycaemia due to an increase in catecholamines and sympathetic nervous system activation of hepatic glucose production, which exceeds the rate of glucose use.[4] Since circulating endogenous insulin levels cannot increase after exercise in T1D patients, even slight hyperglycaemic episodes should need small doses of supplemental insulin injection in order to prevent higher levels of blood glucose in the post-exercise phase. Chronic glycaemic control, expressed as glycated haemoglobin levels (HbA1c), represents a measurement to identify the average plasma glucose on haemoglobin. As red blood cells, which contain the haemoglobin, survive up to 120 days — in which a non-enzymatic glycation pathway is formed with plasma glucose — it is assumed that HbA1c levels are a good marker for average blood glucose levels over the previous months. HbA1c was traditionally expressed as percentage HbA1c (to the total amount of haemoglobin); however, recently, it has also been expressed as mmol/mol (HbA1c/total haemoglobin).
Although the current guidelines are well established, questions remain concerning the exact effect of training on glycaemic control in T1D. While a large body of literature exists, full comparison across individual studies are largely qualitative and hampered by a wide range of study characteristics, including subject population demographics and exercise modalities. For example, most existing exercise and T1D studies have focused on the effects of aerobic exercise (acute and training) on acute and chronic glycaemic control. In contrast, a relatively smaller subset of studies have been published determining the effects of an acute bout of strength exercise or strength training,[6,7] combined strength and aerobic exercise or training[8] and high-intensity (or sprint) exercise or training.[9–12] Therefore, many aspects concerning the optimal mode and amount of exercise per week remain unclear. Furthermore, studies vary widely in terms of insulin or dietary advice during exercise, or changes in \({\rm{\dot V}}{{\rm{O}}_{{\rm{2max}}}}\) levels through training. One approach to provide more precise and quantitative comparisons across such a large and heterogeneous body of literature is via a meta-analysis, which tends to be more objective and consistent than a narrative review because of its mathematical nature.[13] Meta-analyses have been successfully employed in a number of topics in exercise science, including the effects of thermal stress on cognition,[13] where wide heterogeneity existed in the degree of thermal stress and also the type of cognitive testing. To the best of our knowledge, this is the first meta-analysis on the effects of exercise and training on acute or chronic glycaemic control in T1D individuals. Therefore, our hypothesis is that exercise will have beneficial effects on acute and chronic glycaemic control. For the purpose of this article we defined acute exercise as ‘exercise’ while chronic exercise is defined as ‘training’. This meta-analysis consisted of the following three primary research areas:
-
1.
The effect across all different forms of acute exercise on acute glycaemic control.
-
2.
The effects of different types of (chronic) training (endurance, strength, combined, or high-intensity training [HIT]) to determine whether different types of exercise have different effects on chronic glycaemic control.
-
3.
The nature of exercise training (e.g. frequency, duration) and modifying factors (e.g. dietary or insulin advice) required to provide a threshold for improved glycaemic control.
2. Materials and Methods
2.1 Data Sources
The overall goal of the current study was to examine whether the blood glucose values of subjects with T1D are influenced by physical activity. Three electronic databases were consulted: PubMed, ISI Web of Knowledge and SPORTDiscus™. Key terms (and synonyms searched by MeSH database) that were included and combined were: ‘type 1 diabetes mellitus’, ‘blood glucose’, ‘humans’, ‘HbA1c’, ‘metabolic control’, ‘glycaemic control’, ‘physical activity’ and ‘exercise’.
2.2 Study Selection
Studies in this meta-analysis needed to fulfill the following inclusion criteria: (i) subjects diagnosed with T1D; (ii) original data reported with sufficient information to allow calculation for effect sizes (ES) [group means, standard deviation (SD), or standard error of the mean (SEM), which were recalculated to SD)]; (iii) no severe methodological flaws; and (v) published before the end of 2011. Inclusion or exclusion of articles was performed by applying the above criteria on the title, abstract and/or full text. Case studies and reviews were excluded, although the latter’s bibliographies were consulted. The university’s library, hand searches, electronic databases and contact with the authors (by mail) were used for the extraction of more details of the manuscripts if necessary. Figure 1 shows the progress of the literature screening and the reasons for inclusion or exclusion.
2.3 Data Extraction, Synthesis and Report
Effects of exercise (i.e. single bout of exercise) and ‘training’ (i.e. chronic exercise) were distinguished in the analyses. Differences in exercise modes were classified into aerobic exercise, resistance exercise, combined studies (both aerobic and resistance training) and HIE. The dependent variables were HbA1c as a key marker of chronic glycaemic control and different values for capillary glucose levels or interstitial glucose levels or venous plasma glucose levels, as well as glucosuria as determinants of acute glycaemic control (depending on the displayed value in the studies). All glucose levels not displayed in mmol/L were recalculated in mmol/L to be able to compare all results. Study characteristics from the selected articles are shown in tables I to V. Cohen’s d statistics were used for calculating ES, weighted by the sample size of the study. Cohen[37] defined ESs (d) for means as small d = 0.3, medium d = 0.5 and large d = 0.8. Ninety-five percent confidence intervals (95% CI) were used to establish the significance of our findings. Positive effects indicate an increase in the dependent variable, while negative effects indicate a decrease. Both fixed and random effect models were included for calculating ESs. To display effects of exercise and training on (acute and chronic) glycaemic control in patients with T1D, the heterogeneity of the studies must be taken into account. The studies should be comparable in population, type of exercise, duration of exercise and age of subjects. Only if these conditions were met, a data pooling could be performed. Differences in exercise forms were classified into acute aerobic exercise, acute HIE, chronic aerobic training, chronic strength training, combined (aerobic + strength) training or chronic HIT. HIE (acute bout) or HIT (chronic) [also called ‘high-intensity intermittent exercise’, ‘high-intensity interval training’ or ‘sprint interval training’s] was defined as an exercise form including brief bouts of high-intensity, sprint-type exercise[38] and so have alternating periods of short intense anaerobic exercise near 100% peak \({\rm{\dot V}}{{\rm{O}}_{{\rm{2}}}}\) (\({\rm{\dot V}}{{\rm{O}}_{{\rm{2peak}}}}\)) and less-intense recovery periods. Nine studies were used to calculate the ES of a single bout of aerobic exercise; 13 studies to calculate the ES of aerobic training; 2 studies to calculate the ES of strength training; 4 studies to calculate the ES of combined (aerobic and strength) training; and 6 studies to calculate the ES of HIE and HIT.
2.4 Quality Assessment
Depending on the article, the methodological quality was assessed using different assessment tools of the Scottish Intercollegiate Guidelines Network (SIGN) checklists.[39] This checklist assesses the randomization, concealment method, blinding of subjects and/or investigators, drop-out, intention-to-treat analysis, eligibility criteria and follow-up. Two papers (research letters) were excluded because they did not provide enough methodological data for meta-analysing their results.[40,41]
3. Results
First, a meta-analysis looked at all different forms of exercise to provide an overall estimation of the effect of exercise on acute and chronic glycaemic control. Second, a separate meta-analysis for each of the different exercise forms was completed. This included an analysis of the effect of aerobic, resistance, mixed (aerobic + resistance) and acute HIE and HIT. The third level of the meta-analysis encompasses the changes in \({\rm{\dot V}}{{\rm{O}}_{{\rm{2max}}}}\), number of training sessions per week, duration of training protocol, dietary advice or insulin advice.
3.1 Study Characteristics
Figure 1 shows the development of the literature screening and the reasons for inclusion or exclusion. An initial raw screening using the listed search terms resulted in a selection of 947 articles. A more detailed screening of titles, abstracts and full-text articles resulted in a selection of 73 studies. Thirty-two studies ultimately met our inclusion and exclusion criteria for determining the effects of exercise on glycaemic control in T1D. Subject characteristics from these selected studies are shown in tables I–V. Sixteen additional studies were excluded because insufficient data were presented to perform a proper meta-analysis;[11,42–56] 4 studies were excluded because they did not define the level of physical activity;[55,57–59] 12 studies were excluded because no full-text data were found;[60–71] and 6 conference reports, reviews or brief reports and 1 case study, were excluded.[3,4,10,72–75]
All studies were subdivided into children, adolescents and adults. However, studies did not always mention the gender of the population; therefore, no meta-analysis could be performed for gender.
3.2 Effects of Acute Exercise on Glycaemia/Plasma Glucose in Patients with Type 1 Diabetes Mellitus (T1D)
Tables I and V show descriptive data of the studies included in the present meta-analysis for the effects of a single bout of exercise on acute glycaemic control (venous glucose levels, plasma venous glucose levels, interstitial glucose levels).
The results from the first and second stage of the statistical analysis on acute glycaemic control are presented in table VI. Exercise, including aerobic[9,14–18] and acute HIE[9–12,35–36] resulted in an overall ES of −4.17 (95% CI −4.57, −3.76). This indicates that the venous blood glucose values decreased significantly from performing exercise. This meta-analysis clearly shows that aerobic exercise contributes to a larger decrease in venous blood glucose values in adults (ES −6.0; 95% CI −6.86, −5.14) compared with acute HIE (ES −4.35; 95% CI −4.77, −3.92). Only one study explored the effects of an incremental exercise test on blood glucose concentrations in pre-pubertal children,[14] while no studies involving HIE in children or adolescents were found. West et al.[18] studied whether the ingestion of 75 g of carbohydrate 30 or 120 minutes before a 45-minute running exercise (70% of their \({\rm{\dot V}}{{\rm{O}}_{{\rm{2max}}}}\)) could assure that blood levels stayed within acceptable ranges. They found that venous blood glucose levels decreased more when carbohydrate was ingested 120 minutes before exercise (ES −10.6; 95% CI −14.4, −6.8) compared with 30 minutes before exercise (ES −7.26; 95% CI −9.97, −4.55).
3.3 Effects of Training on Fasting Plasma Glucose and Glycated Haemoglobin in T1D
Tables II–V show descriptive data from the studies included in the present meta-analysis for the effects of training on chronic glycaemic control. The results from the first and second stage of our statistical analysis on chronic glycaemic control are presented in table VII. Exercise training resulted in a small, although significant, decrease in levels of HbA1c (−0.27 [−0.47; −0.08]). Most of the individual studies on exercise training could not show significant results on glycaemic control (as shown in figure 2); however, since our calculations were weighted based on sample sizes and standard deviations, we found a significant overall decrease in HbA1c.
Twelve studies[7,21,22,26–32,59,76] were used for the estimation of the effect size of aerobic training on chronic glycaemic control in a total population of 171 T1D adults, adolescents and children. Overall ES of performing aerobic training is small, but significant (ES−0.23; 95% CI −0.44, −0.02). Chronic aerobic exercise had no significant effect (ES 0.23; 95% CI −0.28, 0.73) in a group of 30 poorly controlled T1D children (mean age 11.5 years).[22,76] When the effects of exercise training were compared with no training in T1D,[22,24] similar results were shown (ES 0.21; 95% CI −0.27, 0.32).[22,24] Chronic aerobic training significantly decreased (ES −0.66; 95% CI −0.99, 0.34) HbA1c levels in a group of 61 poorly controlled T1D adolescents (mean age of 13.8 years).[26–29,59] When comparing the effects of aerobic training with a no-exercise group, a significant decrease (ES −1.03; 95% CI −1.56, −0.49) in HbA1c levels was found.[25,26] No significant changes were observed for HbA1c levels in 66 poor and good controlled T1D adults (mean age 35.4 years) performing an aerobic training protocol (ES 0.02; 95% CI −0.32, 0.36).
Only two studies[6,7] reported data on strength training. Although there seems to be a trend for significant changes with a large ES, this was not statistically confirmed (ES −0.6; 95% CI −1.35, 0.16).
The effects of aerobic training combined with strength training were determined in four studies[8,25,33,34] using an adolescent population (10–18 years). The estimation of the size of decrease in HbA1c in the exercise group compared with the control T1D non-exercising group is −1.48 (95% CI −2.07, −0.89). HbA1c levels comparing pre- and post-training status in T1D adolescents showed a slightly decreasing effect −0.2 (95% CI −1.12, 0.73). We found no studies evaluating the effect of a combined exercise training programme on glycaemic control in T1D adults or children (<10 years). To determine the effects of sprint training on glycaemic control, Harmer et al.[11] performed a sprint-training study. They concluded that HbA1c levels were not influenced by long-term HIE training.
Fasting blood glucose levels were shown in four studies.[7,21,29,76] Fasting blood glucose levels decreased after an aerobic exercise programme in T1D adults (ES −0.16; 95% CI −0.52, 0.19).
3.4 Are There Specific Thresholds to Gain Significant Improvements in Chronic Glycaemic Control during Exercise Training?
The meta-analytic results from the third stage of analysis examining specific thresholds to gain significant improvements in glycaemic control are reported in table VIII. Chronic glycaemic control improved when training was performed for more than 3 months, training 1–3 times a week and having dietary or insulin advice. HbA1c did not change in subjects with adequate glycaemic control, while the remaining 11 studies (with poorly controlled [>8% HbA1c] T1D subjects had a significant decrease in HbA1c (ES −0.25; 95% CI −0.48, −0.02) by performing aerobic exercise.
4. Discussion
The major findings of this meta-analysis show that exercise has a significant effect on acute and chronic glycaemic control. Prolonged steady-state aerobic exercise has long been known to cause an acute decrease in blood glucose levels in individuals with T1D and may decrease even long after completion of the exercise. HIE gave a smaller decrease in blood glucose values compared with aerobic exercise. Aerobic training seems to be a favourable tool for improving chronic glycaemic control. There was a tendency for improvement in long-term glycaemic control due to resistance training or resistance combined with endurance training, but there were not enough studies and/or subjects to confirm this statistically.
4.1 Changes in Blood Glucose Values after a Single Bout of Exercise
Glycaemia during exercise can vary inter- as well as intra-individually given that it depends on various factors such as exercise modality and intensity,[10,77,78] nutritional status,[79] time of insulin injection,[80] or pre-exercise glycaemia level.[81] After performing moderate, aerobic exercise, all studies found a decrease in blood glucose values.[9,12,14–21] The blood glucose-lowering effect of moderate-intensity exercise can increase the risk of developing an episode of hypoglycaemia during and after exercising. MacDonald[2] followed 300 patients with T1D prospectively over 2 years. Sixteen percent developed late-onset (6–15 hours after vigorous exercise) hypoglycaemia. As previously described, through the persistent increase of insulin sensitivity and the required repletion of muscle glycogen stores, exercise could affect the blood glucose values the morning after exercise and so late onset of hypoglycaemia can occur regardless of appropriate insulin reduction.[2] However, from this meta-analysis, it seems that this risk can be minimized by appropriate insulin reduction and carbohydrate ingestion before and during exercise. Perrone et al.[40] studied whether ingestion of a drink containing sufficient carbohydrates (8% or 10% carbohydrate) can avoid exercise-induced hypoglycaemia in T1D adolescents. The authors concluded that supplementation of a carbohydrate drink before and during exercise was in most cases enough to maintain the blood glucose concentrations during moderate exercise. West et al.[18] concluded that the ingestion of carbohydrate 75 g in T1D patients 30 minutes before exercise resulted in less hypoglycaemic episodes during exercise and induced higher venous blood glucose levels after exercise compared with the ingestion of carbohydrate 75 g, 60, 90 or 120 minutes before exercise.
Although it was not possible to perform a meta-analysis for the effects of HIE due to the methodological differences in all studies (effects on venous, plasma and capillary glucose values before and after HIE+moderate exercise versus moderate exercise [table VI]), we can make some general observations. One has to be careful when interpreting table V because of the different protocols used. Therefore, more standardization of protocols is needed for the evaluation of the effects of HIE in T1D. While aerobic exercise elicits marked falls in glycaemia, which can often result in episodes of hypoglycaemia, this meta-analysis revealed that there was a smaller fall of blood glucose levels due to an acute bout of HIE compared with an acute bout of aerobic exercise. This reaction can be attributed to a greater increase in catecholamines and growth hormone and hence in glucose hepatic production observed during the repeated bouts of HIE during moderate exercise.[9,82] It is even demonstrated that glucose production was higher in HIE + moderate versus moderate exercise alone and that glucose utilization was greater and occurred faster in HIE + moderate compared with moderate exercise.[10] This hypothesis was confirmed by the studies of Iscoe and Riddell[36] and the studies of Bussau et al.[12,82] who found a more pronounced catecholamine response in HIE + moderate compared with the continuous moderate-intensity exercise trial. Harmer et al.[11] found that muscle free glucose values increased significantly after HIE. Most recently, Iscoe and Riddell[36] compared moderate exercise with an HIE form with equivalent mechanical load in T1D adults. They showed that HIE provided better protection against nocturnal hypoglycaemia. Rabasa-Lhoret et al.[77] observed that blood glucose levels decreased more in moderate continuous and/or longer exercise (periods ranging from 30 to 60 minutes and from 25% to 75% of \({\rm{\dot V}}{{\rm{O}}_{{\rm{2max}}}}\)) modes than in intense exercise forms. On the other hand, Iscoe and Riddell[36] did not find any difference in interstitial or plasma glucose levels during exercise between an intermittent high-intensity and a continuous moderate exercise in T1D patients. In this study, the effects of two different types of exercise with a total equivalent mechanical load were studied: a continuous moderate exercise of 45 minutes at 55% of \({\rm{\dot V}}{{\rm{O}}_{{\rm{2max}}}}\) and 45 minutes at 50% in which nine sequences of 15-second high-intensity sprints were incorporated. The bouts of intense exercise (100% \({\rm{\dot V}}{{\rm{O}}_{{\rm{2peak}}}}\)) represented only 5% of the total duration of the high-intensity intermittent exercise, which could contribute to the absence of differences in the change in blood glucose levels during exercise. We could thus hypothesize that the use of high-intensity bouts during a moderate form of exercise could successfully limit the risk of hypoglycaemia during and after exercise.
4.2 Changes in Glycaemic Control due to Training
The individual studies on aerobic training demonstrated no significant results on glycaemic control. However, when viewed ‘in total’, our meta-analysis of the grouped studies successfully demonstrated a reduction in HbA1c from aerobic training. Aerobic exercise is well known to enhance insulin action 24 hours following[83] both acute exercise and training. Therefore, it is recommended that exercise is performed frequently in order to maintain a constant increase in insulin sensitivity and thus improve HbA1c. Consequently, training once a week might not be enough to improve HbA1c levels. For example, Huttunen et al.[22] performed an exercise intervention of 45 minutes, once per week during 12 weeks and found that HbA1c levels were not affected by the intervention programme. The duration of the training period is also an important influencing factor for decreasing HbA1c. HbA1c levels decreased significantly only in training studies that lasted for more than 3 months. While HbA1c levels are inversely correlated with the duration (minutes) of the exercise training, the amount (times/week) of training per week can also influence the HbA1c levels. Besides this, baseline glycaemic control is also an important predictor of HbA1c improvement due to training. HbA1c decreases significantly more in T1D individuals with poor glycaemic control (>8% HbA1c) compared with individuals with good glycaemic control (<8% HbA1c). Lehman et al.[31] demonstrated only a slight decrease in HbA1c in well controlled subjects who performed exercise training. This might suggest that exercise can be beneficial in order to maintain a good glycaemic control in T1D subjects. In our meta-analysis, the ES of a decrease in HbA1c with training appeared more marked when training was not associated with an improvement in \({\rm{\dot V}}{{\rm{O}}_{{\rm{2max}}}}\). This strange result might in fact be explained by the fact that studies where \({\rm{\dot V}}{{\rm{O}}_{{\rm{2max}}}}\) was not changed probably included more poorly controlled patients. Furthermore, a study of Baldi et al.[83] demonstrated that despite similar training volumes, subjects with T1D with high HbA1c had lower peak workload, \({\rm{\dot V}}{{\rm{O}}_{{\rm{2peak}}}}\), and peak cardiac output than those with low HbA1c. Pulmonary function measures were also lower in the high HbA1c group during peak exercise. These data suggest that cardiopulmonary training adaptations are greater in patients with T1D who maintain good glycaemic control.[83] The mechanism through which poor glycaemic control influenced cardiac and pulmonary responses to exercise is an interesting area for further study. Several studies reported a blunted sympathoadrenal response to exercise in subjects with T1D.[16,84] The blunted sympathoadrenal response may be more obvious in those with poor glycaemic control.[84] The autonomic dysfunction can therefore influence the haemodynamic exercise response.
Aerobic and strength training have different actions in the body and can therefore influence glycaemic control through different pathways, for example, fat mass decrease after a period of aerobic training.[85] A prospective study of Svensson and Eriksson[86] indicates that the change in the amount of body fat contributes to the change in insulin resistance over time in T1D patients. On the other hand, strength training has enhanced insulin sensitivity and improved glucose tolerance.[87] A meta-analysis of nine randomized controlled trials evaluated 372 subjects with type 2 diabetes.[88] When compared with not exercising, progressive resistance training led to a small but statistically significant absolute reduction of 0.3% in HbA1c, indicating that resistance training is a reasonable option in the management of glycaemic control in diabetic subjects.[89] This could be the result of obtaining greater muscle mass. At rest, skeletal muscle consumes 54.4 kJ/kg (13.0 kcal/kg) per day, which is larger than adipose tissue at 18.8 kJ/kg (4.5 kcal/kg).[90] A greater muscle mass would thus consume more glucose and therefore could affect glycaemic control. Out of our meta-analysis, strength training[6,7] seems to have a decreasing effect on long-term HbA1c. However, this effect is only shown in a small sample size and may, for this reason, not be applied generally. Combined training (a combination of strength and aerobic training) showed, compared with a no-exercise group, a significant improvement in chronic glycaemic control. A possible explanation for this is the combined effect of a greater use of glucose, caused by an increased muscle mass and the decreased insulin resistance.[86,90] We have to mention that these results are processed from only two studies.[8,25] The study of Heyman et al.[8] did not show a significant decrease of HbA1c levels, while the study of Bernardini et al.[25] did show a large, significant decrease in HbA1c. This might depend on the type of intervention: Bernardini et al.[25] defined his ‘combined training’ as ‘soccer, volleyball, tennis, basketball’. Thus they did not improve their glucose levels due to specific aerobic or strength training programmes, but due to the combined effect in different sports. On the other hand, children who were very active during this study, were often children who were active during their lifetime (during several precedent years). In the study of Heyman et al.[8] children only benefited from the training during 6 months. Moreover, in a cross-sectional study, subjects with poor glycaemic control could be less motivated to be involved in physical activity.
The relative difficulty of improving HbA1c with exercise training (especially when patients do not benefit from specific advice about diet and insulin adaptations) might be partly caused by the difficulty for the patients to manage important and various glycaemic variations depending on a large number of factors (e.g. time since the last meal or insulin dose, insulin absorption, initial glycaemia, hour of the day). Therefore, it could be difficult to adapt insulin and diet to these important day-to-day glycaemic variations, resulting in more hypoglycaemic episodes. In response, T1D individuals can consume more carbohydrate or reduce too much of their insulin dose, which in turn can induce slight hyperglycaemia and prevent improvement in HbA1c.
4.3 Limitations of the Literature
Pooling of data on the effects of acute exercise on glycaemia in T1D populations is difficult. A great variability of glycaemic responses exist according to factors such as pre-exercise glycaemia and pre-exercise insulinaemia[91] that depend on numerous factors such as time since the last insulin dose or meal, insulin dose and factors modifying insulin absorption (e.g. location of injection, ambient temperature). There are only few studies providing information on the use of the insulin pump, insulin injections or a continuous glucose measurement system. Small sample studies were included, meaning that the power of these studies might not be satisfactory, but those studies present the advantage of often testing novel interventions. In analysing our cohort of studies, few data on glycaemia or insulin regimens were presented, along with the presence of potential long-term micro- or macrovascular complications as well as acute diabetic complications, reflecting markers such as ketonuria or glucosuria. To enable better cross-comparison, future studies should include a standardized set of T1D subject characteristics.
4.4 Literature Weaknesses
Not all studies met our inclusion and exclusion criteria. Three cross-sectional studies were excluded because not enough data were presented to be included in this meta-analysis, or because physical activity level was not defined.[27,55,58] The study of Herbst et al.[55] (a study performed in a population of 23 251 T1D subjects) concluded that physically active T1D subjects have significantly lower HbA1c levels compared with sedentary T1D subjects. The cross-sectional study of Ligtenberg et al.[58] found no correlations between long-term physical activity and HbA1c. However, we have to be careful when interpreting cross-sectional studies because some biases may appear. For example, subjects can ‘over report’ their level of physical activity. Therefore, the level of physical activity must be verified (e.g. in a small sample) with more objective measurement systems (e.g. accelerometers). In addition, positive correlations in cross-sectional studies do not give the sense of the cause-effect relationships and thus they cannot conclude whether this indicates that poorly controlled patients might be less motivated to practice[92] and/or physical activity may have positive effects on glycaemic control.
Mostly, only studies where a significant difference is found are published, this implies that some completed studies are not published and therefore cannot be considered in the meta-analysis. It should be mentioned that it is possible that a publication bias occurred. Funnel plots (plots of effect estimates against sample size) are effective and relatively powerful tests for evaluating the existence of publication bias in a meta-analysis.[93,94] They assume that the largest studies will be near the average, and small studies will be spread on both sides of the average (because of their larger/smaller sample sizes and standard errors). Variation from this assumption can indicate publication bias. A symmetric inverted funnel shape arises from a ‘well behaved’ data set, in which publication bias is unlikely, as detected in our funnel plot.
A major issue is that there are no data available on the exact (or minimum) amount of studies needed to perform a meta-analysis. In this meta-analysis some analyses were made on two or three studies, which is probably not enough to make uniform conclusions. Furthermore, a common criticism of the meta-analysis technique is that it focuses on the summary effect and ignores the fact that the treatment effect may vary from study to study. The goal of a meta-analysis should be to synthesize the ESs (random model effect), and not simply (or necessarily) to report a summary effect (fixed model effect). If the effects are consistent, then the analysis shows that the effect is robust across the range of included studies. If there is modest dispersion, then this dispersion should serve to place the mean effect in context. If there is substantial dispersion, then the focus should shift from the summary effect to the dispersion itself.[58] By ignoring the heterogeneity of the studies, one also misses the point of the synthesis. Overall, meta-analysing two to three studies might be enough to be able to give directions for further research.
5. Conclusion and Directions for Future Research
Some limitations were found in the existing literature concerning the effects of exercise on glycaemic control in T1D. While aspects such as micro- and macrovascular complications, insulin advice and dietary advice are very important in interpreting results emanating from exercise, these aspects are not systematically displayed in the literature. Also, little research is done concerning the effects of resistance training, combined training, or the implementation of HIT in a moderate exercise training programme. Due to the few data in these topics, we had to calculate ESs on only two or three papers. Therefore, we have to be careful interpreting these results.
We can conclude that exercise has an overall beneficial lowering effect on acute and chronic glycaemic control in T1D. Exercise can clearly help subjects with poor glycaemic control to decrease their HbA1c and can help to sustain good glycaemic control in T1D subjects. Therefore, T1D subjects should integrate exercise into their lifestyle and should try to exercise every second day. To avoid excessive fluctuation in blood glucose levels during and after exercising, subjects with T1D might need to adjust their insulin doses. Depending on the form of exercise, T1D subjects should ingest some carbohydrate for preventing hypoglycaemic episodes.
References
Medicine ACoS. ACSM’s guidelines for exercise testing and prescription. 6th ed. Philadeplhia (PA): Lippincott Williams, Wilkins; 2000.
MacDonald MJ. Postexercise late-onset hypoglycemia in insulin-dependent diabetic patients. Diabetes Care. 1987 Sep–Oct; 10(5): 584–8.
Graveling AJ, Frier BM. Risks of marathon running and hypoglycaemia in type 1 diabetes. Diabet Med 2010 May; 27(5): 585–8.
Toni S, Reali MF, Barni F, et al. Managing insulin therapy during exercise in type 1 diabetes mellitus. Acta Biomed 2006; 77 Suppl. 1: 34–40.
Aussedat B, Dupire-Angel M, Gifford R, et al. Interstitial glucose concentration and glycemia: implications for continuous subcutaneous glucose monitoring. Am J Physiol Endocrinol Metab 2000 Apr; 278(4): E716–28.
Durak EP, Jovanovic-Peterson L, Peterson CM. Randomized crossover study of effect of resistance training on glycemic control, muscular strength, and cholesterol in type I diabetic men. Diabetes Care 1990 Oct; 13(10): 1039–43.
Ramalho AC, de Lourdes Lima M, Nunes F, et al. The effect of resistance versus aerobic training on metabolic control in patients with type-1 diabetes mellitus. Diabetes Res Clin Pract 2006 Jun; 72(3): 271–6.
Heyman E, Toutain C, Delamarche P, et al. Exercise training and cardiovascular risk factors in type 1 diabetic adolescent girls. Pediatr Exerc Sci 2007 Nov; 19(4): 408–19.
Guelfi KJ, Jones TW, Fournier PA. The decline in blood glucose levels is less with intermittent high-intensity compared with moderate exercise in individuals with type 1 diabetes. Diabetes Care 2005 Jun; 28(6): 1289–94.
Guelfi KJ, Ratnam N, Smythe GA, et al. Effect of intermittent high-intensity compared with continuous moderate exercise on glucose production and utilization in individuals with type 1 diabetes. Am J Physiol Endocrinol Metab 2007 Mar; 292(3): E865–70.
Harmer AR, Chisholm DJ, McKenna MJ, et al. Sprint training increases muscle oxidative metabolism during high-intensity exercise in patients with type 1 diabetes. Diabetes Care 2008; 31(11): 2097–102.
Bussau VA, Ferreira LD, Jones TW, et al. The 10-s maximal sprint: a novel approach to counter an exercise-mediated fall in glycemia in individuals with type 1 diabetes. Diabetes Care 2006 Mar; 29(3): 601–6.
Pilcher JJ, Nadler E, Busch C. Effects of hot and cold temperature exposure on performance: a meta-analytic review. Ergonomics 2002 Aug 15; 45(10): 682–98.
Heyman E, Briard D, Gratas-Delamarche A, et al. Normal physical working capacity in prepubertal children with type 1 diabetes compared with healthy controls. Acta Paediatr 2005 Oct; 94(10): 1389–94.
Tansey MJ, Tsalikian E, Beck RW, et al. The effects of aerobic exercise on glucose and counterregulatory hormone concentrations in children with type 1 diabetes. Diabetes Care 2006 Jan; 29(1): 20–5.
Heyman E, Delamarche P, Berthon P, et al. Alteration in sympathoadrenergic activity at rest and during intense exercise despite normal aerobic fitness in late pubertal adolescent girls with type 1 diabetes. Diabetes Metab 2007 Dec; 33(6): 422–9.
Poortmans JR, Saerens P, Edelman R, et al. Influence of the degree of metabolic control on physical fitness in type I diabetic adolescents. Int J Sports Med 1986 Aug; 7(4): 232–5.
West DJ, Stephens JW, Bain SC, et al. A combined insulin reduction and carbohydrate feeding strategy 30 min before running best preserves blood glucose concentration after exercise through improved fuel oxidation in type 1 diabetes mellitus. J Sports Sci 2011 Feb; 29(3): 279–89.
Yamanouchi K, Abe R, Takeda A, et al. The effect of walking before and after breakfast on blood glucose levels in patients with type 1 diabetes treated with intensive insulin therapy. Diabetes Res Clin Pract 2002 Oct; 58(1): 11–8.
Zinman B, Murray FT, Vranic M, et al. Glucoregulation during moderate exercise in insulin treated diabetics. J Clin Endocrinol Metab 1977 Oct; 45(4): 641–52.
Zinman B, Zuniga-Guajardo S, Kelly D. Comparison of the acute and long-term effects of exercise on glucose control in type I diabetes. Diabetes Care 1984 Nov–Dec; 7(6): 515–9.
Huttunen NP, Lankela SL, Knip M, et al. Effect of once-a-week training program on physical fitness and metabolic control in children with IDDM. Diabetes Care 1989 Nov–Dec; 12(10): 737–40.
Rowland TW, Swadba LA, Biggs DE, et al. Glycemic control with physical training in insulin-dependent diabetes mellitus. Am J Dis Child 1985 Mar; 139(3): 307–10.
Wong CH, Chiang YC, Wai JP, et al. Effects of a homebased aerobic exercise programme in children with type 1 diabetes mellitus. J Clin Nurs 2010 Mar; 20(5–6): 681–91.
Bernardini AL, Vanelli M, Chiari G, et al. Adherence to physical activity in young people with type 1 diabetes. Acta Biomed 2004 Dec; 75(3): 153–7.
Marrero DG, Fremion AS, Golden MP. Improving compliance with exercise in adolescents with insulin-dependent diabetes mellitus: results of a self-motivated home exercise program. Pediatrics 1988 Apr; 81(4): 519–25.
Michaliszyn SF, Faulkner MS. Physical activity and sedentary behavior in adolescents with type 1 diabetes. Res Nurs Health 2011 Oct; 33(5): 441–9.
Ruzic L, Sporis G, Matkovic BR. High volume-low intensity exercise camp and glycemic control in diabetic children. J Paediatr Child Health 2008 Mar; 44(3): 122–8.
Sideraviciute S, Gailiuniene A, Visagurskiene K, et al. The effect of long-term swimming program on glycemia control in 14–19-year aged healthy girls and girls with type 1 diabetes mellitus. Medicina (Kaunas) 2006; 42(6): 513–8.
Laaksonen DE, Atalay M, Niskanen LK, et al. Aerobic exercise and the lipid profile in type 1 diabetic men: a randomized controlled trial. Med Sci Sports Exerc 2000 Sep; 32(9): 1541–8.
Lehmann R, Kaplan V, Bingisser R, et al. Impact of physical acitivity on cardiovascular risk factors in IDDM. Diabetes Care 1997; 20(10): 1603–11.
Wallberg-Henriksson H, Gunnarsson R, Rossner S, et al. Long-term physical training in female type 1 (insulin-dependent) diabetic patients: absence of significant effect on glycaemic control and lipoprotein levels. Diabetologia 1986; 29: 53–7.
D’Hooge R, Hellinckx T, Van Laethem C, et al. Influence of combined aerobic and resistance training on metabolic control, cardiovascular fitness and quality of life in adolescents with type 1 diabetes: a randomized controlled trial. Clin Rehabil 2011 Apr; 25(4): 349–59.
Mosher PE, Nash MS, Perry AC, et al. Aerobic circuit exercise training: effect on adolescents with well-controlled insulin-dependent diabetes mellitus. Arch Phys Med Rehabil 1998 Jun; 79(6): 652–7.
Iscoe KE, Campbell JE, Jamnik V, et al. Efficacy of continuous real-time blood glucose monitoring during and after prolonged high-intensity cycling exercise: spinning with a continuous glucose monitoring system. Diabetes Technol Ther 2006 Dec; 8(6): 627–35.
Iscoe KE, Riddell MC. Continuous moderate-intensity exercise with or without intermittent high-intensity work: effects on acute and late glycaemia in athletes with type 1 diabetes mellitus. Diabet Med 2011 Jul; 28(7): 824–32.
Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale (NJ): Lawrence Erlbaum Associates, 1988.
Gibala MJ. High-intensity interval training: a time-efficient strategy for health promotion? Curr Sports Med Rep 2007 Jul; 6(4):211–3.
Harbour R. A new system for grading recommendations in evidence based guidelines. BMJ 2001; 1(1): 334–6.
Perrone C, Laitano O, Meyer F. Effects of carbohydrate ingestion on the glycemic response of type 1 diabetic adolescents during exercise. Diabetes Care 2005 Oct; 28(10): 2537–8.
Raile K, Kapellen T, Schweiger A, et al. Physical activity and competitive sports in children and adolescents with type 1 diabetes. Diabetes Care 1999 Nov; 22(11): 1904–5.
Aman J, Skinner TC, de Beaufort CE, et al. Associations between physical activity, sedentary behavior, and glycemic control in a large cohort of adolescents with type 1 diabetes: the Hvidoere Study Group on Childhood Diabetes. Pediatric Diabetes 2009; 10: 234–9.
Michaliszyn SF, Shaibi GQ, Quinn L, et al. Physical fitness, dietary intake, and metabolic control in adolescents with type 1 diabetes. Pediatr Diabetes 2009 Sep; 10(6): 389–94.
Costill DL, Fink WJ, Getchell LH, et al. Lipid metabolism in skeletal muscle of endurance-trained males and females. J Appl Physiol 1979 Oct; 47(4): 787–91.
Wallymahmed ME, Morgan C, Gill GV, et al. Aerobic fitness and hand grip strength in type 1 diabetes: relationship to glycaemic control and body composition. Diabet Med 2007 Nov; 24(11): 1296–9.
Kelly RB. Diet and exercise in the management of hyperlipidemia. Am Fam Physician 2010 May 1; 81(9): 1097–102.
Ludvigsson J. Physical exercise in relation to degree of metabolic control in juvenile diabetics. Acta Paediatr Scand Suppl 1980; 283: 45–9.
Nermoen I, Jorde R, Sager G, et al. Effects of exercise on hypoglycaemic responses in insulin-dependent diabetes mellitus. Diabetes Metab 1998 Apr; 24(2): 131–6.
Roberts L, Jones TW, Fournier PA. Exercise training and glycemic control in adolescents with poorly controlled type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2002 May; 15(5): 621–7.
Sonnenberg GE, Kemmer FW, Berger M. Exercise in type 1 (insulin-dependent) diabetic patients treated with continuous subcutaneous insulin infusion: prevention of exercise induced hypoglycaemia. Diabetologia 1990 Nov; 33(11): 696–703.
Valerio G, Spagnuolo MI, Lombardi F, et al. Physical activity and sports participation in children and adolescents with type 1 diabetes mellitus. Nutr Metab Cardiovasc Dis 2007 Jun; 17(5): 376–82.
Vanelli M, Corchia M, Iovane B, et al. Self-monitoring adherence to physical activity in children and adolescents with type 1 diabetes. Acta Biomed 2006; 77 Suppl. 1: 47–50.
Fayolle C, Brun JF, Bringer J, et al. Accuracy of continuous subcutaneous glucose monitoring with the GlucoDay in type 1 diabetic patients treated by subcutaneous insulin infusion during exercise of low versus high intensity. Diabetes Metab 2006 Sep; 32(4): 313–20.
Meinders AE, Willekens FL, Heere LP. Metabolic and hormonal changes in IDDM during long-distance run. Diabetes Care 1988 Jan; 11(1): 1–7.
Herbst A, Kordonouri O, Schwab K, et al. Impact of physical activity on cardiovascular risk factors in children with type 1 diabetes. Diabetes Care 2007; 30(8): 2098–100.
Sackey AH, Jefferson IG. Physical activity and glycaemic control in children with diabetes mellitus. Diabet Med 1996 Sep; 13(9): 789–93.
Faulkner MS, Michaliszyn SF, Hepworth JT. A personalized approach to exercise promotion in adolescents with type 1 diabetes. Pediatr Diabetes 2010 May; 11(3): 166–74.
Ligtenberg PC, Blans M, Hoekstra JB, et al. No effect of long-term physical activity on the glycemic control in type 1 diabetes patients: a cross-sectional study. Neth J Med 1999 Aug; 55(2): 59–63.
Dahl-Jorgensen K, Meen HD, Hanssen KF, et al. The effect of exercise on diabetic control and hemoglobin A1 (HbA1) in children. Acta Paediatr Scand Suppl 1980; 283: 53–6.
Nugent AM, Steele IC, al-Modaris F, et al. Exercise responses in patients with IDDM. Diabetes Care 1997 Dec; 20(12): 1814–21.
Clarke WL, Cox DJ, Gonder-Frederick LA, et al. The relationship between nonroutine use of insulin, food, and exercise and the occurrence of hypoglycemia in adults with IDDM and varying degrees of hypoglycemic awareness and metabolic control. Diabetes Educ 1997 Jan–Feb; 23(1): 55–8.
Szmigiel C, Dziadkowiak H, Jesionek D, et al. The influence of physical effort of variable intensity on glycemia in children with diabetes. Pediatr Pol 1996 May; 71(5): 423–30.
McCargar LJ, Taunton J, Pare S. Benefits of exercise training for men with insulin-dependent diabetes mellitus. Diabetes Educ 1991 May–Jun; 17(3): 179–84.
Stratton R, Wilson DP, Endres RK, et al. Improved glycemic control after supervised 8-wk exercise program in insulin-dependent diabetic adolescents. Diabetes Care 1987 Sep–Oct; 10(5): 589–93.
Jensen MD, Miles JM. The roles of diet and exercise in the management of patients with insulin-dependent diabetes mellitus. Mayo Clin Proc 1986 Oct; 61(10): 813–9.
Hubinger A, Ridderskamp I, Lehmann E, et al. Metabolic response to different forms of physical exercise in type I diabetics and the duration of the glucose lowering effect. Eur J Clin Invest 1985 Aug; 15(4): 197–203.
Schiffrin A, Parikh S, Marliss EB, et al. Metabolic response to fasting exercise in adolescent insulin-dependent diabetic subjects treated with continuous subcutaneous insulin infusion and intensive conventional therapy. Diabetes Care 1984 May–Jun; 7(3): 255–60.
Sills IN, Cerny FJ. Responses to continuous and intermittent exercise in healthy and insulin-dependent diabetic children. Med Sci Sports Exerc 1983; 15(6): 450–4.
Guler HP, Walter H, Morell B, et al. Effect of prolonged optimal blood sugar control by i.v. insulin administration on plasma free fatty acid and glycerol concentrations of insulin-dependent diabetics during exercise. Acta Diabetol Lat 1982 Jul–Sep; 19(3): 261–71.
Zander E, Schulz B, Chlup R, et al. Muscular exercise in type I-diabetics, II: hormonal and metabolic responses to moderate exercise. Exp Clin Endocrinol 1985 Feb; 85(1): 95–104.
Baharlouei k. The effect of aerobic training on selected variables in patients with type 1 diabetes mellitus. Br J Sports Med 2010; 44 Suppl. 1:121.
Jette DU. Physiological effects of exercise in the diabetic. Phys Ther 1984 Mar; 64(3): 339–42.
Riddell MC, Iscoe KE. Physical activity, sport, and pediatric diabetes. Pediatr Diabetes 2006 Feb; 7(1): 60–70.
Riddell MC, Milliken J. Preventing exercise-induced hypoglycemia in type 1 diabetes using real-time continuous glucose monitoring and a new carbohydrate intake algorithm: an observational field study. Diabetes Technol Ther 2011 Aug; 13(8): 819–25.
Steppel JH, Horton ES. Exercise in the management of type 1 diabetes mellitus. Rev Endocr Metab Disord 2003 Dec; 4(4): 355–60.
Smith NJ, Stanitski CL, Dyment CL, et al. Glycemic control with physical trainin in insulin-dependent diabetes mellitus. Sports Med 1985; 139: 307–10.
Rabasa-Lhoret R, Bourque J, Ducros F, et al. Guidelines for premeal insulin dose reduction for postprandial exercise of different intensities and durations in type 1 diabetic subjects treated intensively with a basal-bolus insulin regimen (ultralente-lispro). Diabetes Care 2001 Apr; 24(4): 625–30.
Riddell M, Perkins BA. Exercise and glucose metabolism in persons with diabetes mellitus: perspectives on the role for continuous glucose monitoring. J Diabetes Sci Technol 2009; 3(4): 914–23.
Jimenez C, Santiago M, Sitler M, et al. Insulin-sensitivity response to a single bout of resistive exercise in type 1 diabetes mellitus. J Sport Rehabil 2009 Nov; 18(4): 564–71.
Iafusco D. Diet and physical activity in patients with type 1 diabetes. Acta Biomed 2006; 77 Suppl 1: 41–6.
Zander E, Bruns W, Wulfert P, et al. Muscular exercise in type I-diabetics, I: different metabolic reactions during heavy muscular work in dependence on actual insulin availability. Exp Clin Endocrinol 1983 Jul; 82(1): 78–90.
Bussau VA, Ferreira LD, Jones TW, et al. A 10-s sprint performed prior to moderate-intensity exercise prevents early post-exercise fall in glycaemia in individuals with type 1 diabetes. Diabetologia 2007 Sep; 50(9): 1815–8.
Baldi JC, Cassuto NA, Foxx-Lupo WT, et al. Glycemic status affects cardiopulmonary exercise response in athletes with type I diabetes. Med Sci Sports Exerc 2011 Aug; 42(8): 1454–9.
Khoharo HK, Ansari S, Ali Shaikh I, et al. Cardiac autonomic neuropathy (CAN) in type-1 diabetes mellitus patients and its association with the duration of disease and glycemic control. J Coll Physicians Surg Pak 2009 Apr; 19(4): 232–5.
Ismail I, Keating SE, Baker MK, et al. A systematic review and meta-analysis of the effect of aerobic vs. resistance exercise training on visceral fat. Obes Rev 2012 Jan; 13(1): 68–91.
Svensson MK, Eriksson JW. Change in the amount of body fat and IL-6 levels is related to altered insulin sensitivity in type 1 diabetes patients with or without diabetic nephropathy. Horm Metab Res 2011 Mar; 43(3): 209–15.
Tresierras MA, Balady GJ. Resistance training in the treatment of diabetes and obesity: mechanisms and outcomes. J Cardiopulm Rehabil Prev 2009 Mar–Apr; 29(2): 67–75.
Irvine C, Taylor NF. Progressive resistance exercise improves glycaemic control in people with type 2 diabetes mellitus: a systematic review. Aust J Physiother 2009; 55(4): 237–46.
Sundell J. Resistance training is an effective tool against metabolic and frailty syndromes. Adv Prev Med 2011; 984683–7.
Heymsfield SB, Gallagher D, Kotler DP, et al. Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am J Physiol Endocrinol Metab 2002 Jan; 282(1): E132–8.
Brun J-F, Marti B, Fédou C, et al. Two parameters statistically explain blood glucose decrease during exercise at steady state in type 1 diabetics: pre-exercise blood glucose and insulinemia. Sci Sports. In press.
Brazeau AS, Rabasa-Lhoret R, Strychar I, et al. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care 2008 Nov; 31(11): 2108–9.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000 Jun; 56(2): 455–63.
Song F, Gilbody S. Bias in meta-analysis detected by a simple, graphical test. Increase in studies of publication bias coincided with increasing use of meta-analysis. BMJ 1998 Feb 7; 316(7129): 469–71.
Acknowledgements
The authors wish to acknowledge funding through the Vrije Universiteit Brussel (OZR2096BOF). Bart Roelands is a postdoctoral fellow of the Fund for Scientific Research Flanders (FWO). S.S. Cheung is supported by a Canada Research Chair. The authors have no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tonoli, C., Heyman, E., Roelands, B. et al. Effects of Different Types of Acute and Chronic (Training) Exercise on Glycaemic Control in Type 1 Diabetes Mellitus. Sports Med 42, 1059–1080 (2012). https://doi.org/10.1007/BF03262312
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03262312