Abstract
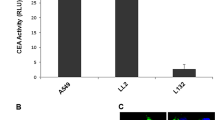
PURPOSE: This study was designed to characterize the mechanisms regulating the expression of the human carcinoembryonic antigen promoter (pCEA), in terms of tissue-specific targeting for gene therapy. The promoter was subcloned to a luciferase reporter gene (pCEA/Luc) in our laboratory and compared with a virally controlled luciferase vector (pSV40/Luc). METHODS: Four human cancer cell lines (HeLa, SW480, Caco2, and SW1116) were transfected with either pCEA/Luc or pSV40/Luc. Cells were treated with interferon-gamma and assayed at 72 hours after treatment. Carcinoembryonic antigen level was measured by enzyme immunoassay. Luciferase expression was measured at 48 hours and one week after transfection by luminometry. RESULTS: Luciferase activity after transfection with pCEA/Luc was higher in CEA-positive cells than in CEA-negative cells (P<0.0001). pCEA/Luc demonstrated higher activity than pSV40/Luc in CEA-positive cells (P<0.0001), but not in CEA-negative cells. In Caco2 cells, which before confluence are CEA-negative, luciferase expression increased on reaching confluence (P<0.0001). Well to moderately differentiated cells responded to the interferon-gamma treatment, but the increase in CEA secretion did not correspond to an increase in pCEA/Luc expression. CONCLUSIONS: The expression of pCEA correlates well with the CEA production by the specific cell line offering a potential tissue-specific targeting strategy for colon cancer gene therapy. Furthermore, the tissue-specific CEA promoter has a higher and more persistent activity in CEA-positive human cancer cells than a viral promoter. The lack of response to interferon-gamma treatment suggests a different mechanism of action for interferon-gamma other than directly interacting with the promoter.
Similar content being viewed by others
References
Gold P, Freeman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med 1965;122:467–81.
Ahnen DJ, Nakane PK, Brown WR. Ultrastructural localization of carcinoembryonic antigen in normal intestine and colon cancer. Cancer 1982;49:2077–90.
Beauchemin N, Benchimol S, Cournoyer D, Fuks A, Stanners CP. Isolation and characterization of full length functional cDNA clones for human carcinoembryonic antigen. Mol Cell Biol 1987;7:3221–30.
Zimmermann W, Ortlieb B, Friedrich R, von Kleist S. Isolation and characterization of cDNA clones encoding the human carcinoembryonic antigen reveal a highly conserved repeating structure. Proc Natl Acad Sci U S A 1987;84:2960–4.
Thompson JA, Pande H, Paxton RJ,et al. Molecular cloning of a gene belonging to the carcinoembryonic antigen gene family and discussion of a domain model. Proc Natl Acad Sci U S A 1987;84:2965–9.
Schrewe H, Thompson J, Bona M,et al. Cloning of the complete gene for the carcinoembryonic antigen: analysis of its promoter indicates a region conveying cell type-specific expression. Mol Cell Biol 1990;10:2738–48.
Di Maio JM, Clary BM, Via DF, Coveney E, Pappas TN, Lyerly HK. Directed enzyme pro-drug therapy for pancreatic cancerin vivo. Surgery 1994;116:205–13.
Lan KH, Kanai F, Shiratori Y,et al. Tumor specific gene expression in carcinoembryonic antigen-producing gastric cancer cells using adenovirus vectors. Gastroenterology 1996;111:1241–51.
Lyerly HK, DiMaio JM. Gene delivery systems in surgery. Arch Surg 1993;128:1197–206.
Fichera A, Guo Y, Romero L, Michelassi F, Arenas RB. Quantitation ofin vivo gene delivery by restriction enzyme PCR generated polymorphism. J Surg Res 1997;69:188–92.
Arenas RB, Fichera A, Mok P, Blanco M, Michelassi F. Introduction of human APC gene into Min mice using cationic liposomes. Surgery 1996;120:712–8.
Stewart MJ, Plautz GE, Del BL. Phosphatidylethanolamine liposomes: drug delivery, gene transfer and immunodiagnostic applications. Biochim Biophys Acta 1992;1113:201–27.
Tran R, Kashmiri SV, Kantor J,et al. Correlation of DNA hypomethylation with expression of carcinoembryonic antigen in human colon carcinoma cells. Cancer Res 1988;48:5674–9.
Toth CA, Thomas P. The effect of interferon treatment on 14 human colorectal cancer cell lines: growth and carcinoembryonic antigen secretionin vitro. J Interfer Res 1990;10:579–88.
Guadagni F, Witt PL, Robbins PF, Schlom J, Greiner JW. Regulation of carcinoembryonic antigen expression in different human colorectal tumor cell by interferon γ. Cancer Res 1990;50:6248–55.
Kolbeck JC, Padgett RA, Estevez EG, Harrel LJ. Bioluminescence screening for bacteriuria. J Clin Microbiol 1985;21:527–30.
Snedecor GW, Cochran WG. Statistical methods. Ames, IA: Iowa State University Press, 1980.
Kantor J, Tran R, Greiner J,et al. Modulation of carcinoembryonic antigen messenger RNA levels in human colon carcinoma cells by recombinant human γ-interferon. Cancer Res 1989;49:2651–5.
Hauck W, Stanners CP. Control of carcinoembryonic antigen gene family expression in a differentiating colon carcinoma cell line, Caco-2. Cancer Res 1991;51:3526–33.
Huber BE, Austin EA, Good SS, Knick VC, Tibbels S, Richards CA.In vivo antitumor activity of 5-fluorocytosin on human colorectal carcinoma cells genetically modified to express cytosin deaminase. Cancer Res 1993;53:4619–26.
Vachon PH, Beaulieu JF. Transient mosaic patterns of morphological and functional differentiation in the Caco-2 cell line. Gastroenterology 1992;103:414–23.
Greiner JW, Fisher PB, Pestka S, Schlom J. Differential effects of recombinant human leukocyte interferons on cell surface antigen expression. Cancer Res 1986;46:4984–90.
Wallach D, Fellous M, Revel M. Preferential effect of γ interferon on the synthesis of HLA antigens and their mRNAs in human cells. Nature 1982;299:833–6.
Levy D, Larner A, Chaudhuri A, Babiss LE, Darnell JE. Interferon-stimulated transcription: isolation of an inducible gene and identification of its regulatory region. Proc Natl Acad Sci U S A 1986;83:8929–33.
Reich N, Evans B, Levy D, Fahey D, Knight E, Darnell JE. Interferon-induced transcription of a gene encoding a 15 kDa protein depends on an upstream enhancer element. Proc Natl Acad Sci U S A 1987;84:6394–8.
Author information
Authors and Affiliations
Additional information
Supported in part by the University of Chicago Cancer Research Foundation, a National Institutes of Health Teaching Grant (T32 CA9576), and a 1995 American Society of Clinical Oncology Young Investigator's Award to Dr. Arenas.
About this article
Cite this article
Fichera, A., Michelassi, F. & Arenas, R.B. Selective expression of carcinoembryonic antigen promoter in cancer cell lines. Dis Colon Rectum 41, 747–754 (1998). https://doi.org/10.1007/BF02236263
Issue Date:
DOI: https://doi.org/10.1007/BF02236263