Abstract
Mosquito vector-borne diseases are a significant health problem in South and Southeast Asia. Some mosquito vectors in the region are well known to lay eggs and undergo pre-imaginal development in brackish water. However, a number of other important vectors, e.g. Anopheles culicifacies (malaria) and Aedes aegypti and Aedes albopictus (dengue and chikungunya), have previously been widely held to do so exclusively in freshwater. But recent evidence shows that these species can also lay eggs and undergo pre-imaginal development in brackish water collections in coastal areas of the region. This property produces a reservoir of vectors that are not targeted in larval control programmes. It can contribute to disease transmission in a previously unrecognised manner that can be compounded by environmental changes caused by expanding populations in coastal zones, climate change and rising sea levels due to global warming. Increased disease transmission in coastal areas will also lead to higher disease incidence in inland areas. Many countries in South and Southeast Asia have long coastlines, a high proportion of coastal zone relative to total land area and a large proportion of the population living in coastal areas. Hence, the region is particularly vulnerable to disease transmission by brackish water vectors. Appropriate policies and strategies need to be developed in a local, national and international context to counter this threat to human health in South and Southeast Asia.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Arboviral diseases
- Climate change
- Coastal zone
- Dengue
- Global warming
- Malaria
- Mosquito vectors
- Sea level rise
- South and Southeast Asia
1 Overview of Mosquito-Borne Human Diseases in Tropical South and Southeast Asia
Mosquito-borne human diseases are a cause of considerable mortality and morbidity worldwide. Diseases such as chikungunya, dengue and West Nile viral encephalitis are of great international concern because of their increasing incidence and global spread (Weaver and Reisen 2010). Mosquito vectors transmit many important human parasitic and arboviral diseases in tropical South and Southeast Asia. The World Health Organization (WHO) reported that there were approximately 130.6 million suspected cases of malaria caused by protozoan parasites of the genus Plasmodium, predominantly P. falciparum and P. vivax, in its Southeast Asian region in 2012 (WHO 2013a). This caused an estimated 1,226 deaths. The WHO Southeast Asian region includes countries with extensive coastal zones such as Bangladesh, India, Indonesia, Maldives, Myanmar, Sri Lanka, Thailand and Timor-Leste. In its Western Pacific region, which also contains many coastal countries regarded as part of Southeast Asia (Brunei, Cambodia, Malaysia, Papua New Guinea, the Philippines, Singapore and Vietnam), the WHO reported 10.8 million suspected cases and an estimated 458 malaria deaths in 2012 (WHO 2013a). The malaria situation in the greater South and Southeast Asian region is highly dynamic due to the increasing resistance of parasites to common antimalarial drugs and mosquito vectors to common insecticides, movement of human populations and changes in land use patterns, forest cover, health services and other pertinent factors. Several species of Anopheles mosquitoes are responsible for transmitting malaria in the region with some identified as dominant vectors (Sinka et al. 2011).
Lymphatic filariasis caused by the nematode parasites Wuchereria bancrofti, Brugia malayi and Brugia timori is widely prevalent in the region. It is responsible for considerable morbidity and disfigurement in infected persons. Recent WHO data suggest that approximately 60–65 million people in the two regions have lymphatic filariasis (WHO 2012). Culex quinquefasciatus in the Asian mainland and various species of Anopheles and Mansonia in the Southeast Asian archipelago are chiefly responsible for transmitting filariasis in the region (Walter Reed Biosystematics Unit 2013).
Dengue, the most common human arboviral disease, is widespread in South and Southeast Asia with 176,719 cases and 887 deaths reported in the WHO Southeast Asian region in 2010 and 242,424 cases with 784 deaths in the WHO Western Pacific region in 2009 (WHO 2012). Aedes aegypti and Aedes albopictus mosquitoes that are common throughout the region are predominantly responsible for transmitting dengue (Weaver and Reisen 2010; Walter Reed Biosystematics Unit 2013). Other mosquito-borne diseases that are endemic to the region include Japanese encephalitis and chikungunya. Yellow fever transmitted by Ae. aegypti and Ae. albopictus is endemic in parts of Africa and South America (WHO 2013b). An effective vaccine is available against yellow fever. The reasons for the absence of yellow fever from the South and Southeast Asian region, notwithstanding the presence of competent vectors, are not well understood. Increasing international travel and trade however poses the risk of its introduction to the region.
2 Vector Control Is Important for Reducing the Prevalence of Many Mosquito-Borne Diseases
A licensed vaccine is presently available against only Japanese encephalitis among all the major mosquito-borne diseases of the region. Vaccines for malaria, dengue and chikungunya are currently under development. The presence of four serotypes of the dengue virus hampers the production of a vaccine against dengue. In addition, a suboptimal immune response to any one of the serotypes can exacerbate disease caused by a subsequent infection with that serotype (Halstead 2003; Chun et al. 2007). Drugs for specific treatment of dengue are not presently available, and only symptomatic treatment is possible for the disease. On the other hand, specific and effective drugs are available to treat malaria and lymphatic filariasis. However, the Greater Mekong subregion in Southeast Asia is the origin of many strains of malaria parasites that have developed resistance to several antimalarial drugs and then spread worldwide. The recent emergence in this area of P. falciparum that is resistant to artemisinin combination therapies that are the first-line treatment for uncomplicated falciparum malaria is therefore of considerable international concern (Dondorp et al. 2010).
Minimising human-mosquito contact and reducing vector populations by the application of insecticides and through managing and eliminating pre-imaginal development sites therefore remain important components of mosquito-borne disease control programmes. The use of insecticide-impregnated bed nets and indoor residual spraying of insecticides is the mainstay of malaria vector control programmes throughout the world. These methods, together with early case detection and treatment as well as the management of larval habitats, have succeeded in reducing the incidence of malaria in many countries of the region over the past decade (WHO 2013b). However, the Aedes vectors of dengue can bite outdoors and during daytime making bed nets less effective against acquiring infections. Mosquito proofing houses, which has proved helpful in eliminating dengue from the southern United States, is not popular or widely affordable in the humid and densely populated countries of South and Southeast Asia. Therefore, reducing the Aedes vector populations through eliminating, minimising and larviciding pre-imaginal development habitats and the application of insecticide through fogging and residual spraying at infection foci are the principal methods used for controlling dengue in the region (WHO 2009). Such measures are additionally effective against chikungunya because Ae. aegypti and Ae. albopictus are also the principal vectors of chikungunya. Aedes aegypti is additionally capable of transmitting other Australasian arboviruses, such as Ross River and Murray Valley encephalitis viruses, as demonstrated in laboratory experiments (Ramasamy et al. 1990). It is also a potential vector of the filarial parasite Brugia malayi in Asia (Erickson et al. 2009).
3 Global Warming, Rising Sea Levels and Changes in Coastal Salinity
The United Nations Framework Convention on Climate Change described global climate change as long-term changes in commonly measured meteorological parameters, over and above natural variations, that are directly or indirectly attributable to human activity altering the composition of the atmosphere. Such parameters include temperature, rainfall and humidity. Global warming is the rise in average global temperatures due to the increasing emission of gases, principally carbon dioxide, causing a greenhouse effect in the atmosphere. Global warming leads to a rise in sea levels caused by the melting of polar ice and glaciers and the thermal expansion of seawater (Intergovernmental Panel on Climate Change 2013). The rise in sea levels, depending on the extent of the warming that is principally governed by the rate of burning of fossil fuels, is predicted to be about 82 cm by the end of the twenty-first century (Intergovernmental Panel on Climate Change 2013). Rising sea levels are anticipated to increase the extent of saline (>30 parts per thousand or ppt salt) or brackish (0.5–30 ppt salt) water bodies in coastal areas. These include coastal estuaries, lagoons, marshes and mangroves (Nicholls et al. 2007). The salinity of estuarine systems is projected to increase, and their boundaries move further inland with more pronounced tidal water flows into rivers (Nicholls et al. 2007). A proportion of coastal wetlands such as salt marshes and mangroves are expected to become inundated, but additional saline wetlands are anticipated to form further inland (Nicholls et al. 2007). Rising sea levels and higher water withdrawal rates from freshwater aquifers near the coast by expanding populations are also envisaged to increase saltwater intrusion into the aquifers (FAO 2007).
The tropical areas of South and Southeast Asia have extensive coastlines bordering the Indian and Pacific Oceans and various seas. Southeast Asia also has the largest archipelago in the tropics. Indonesia and the Philippines with 248 and 104 million persons, respectively, are the two most populous countries in this archipelago (CIA 2012). Table 8.1 presents data on the length of the coastline in relation to the land area for Indonesia and the Philippines and selected other countries in the region. A comparison is made with the Federated States of Micronesia, a collection of about 600 small islands in the Western Pacific tropics. India on the other hand has a long coastline but a low coast to land area ratio compared to countries composed of many islands.
Countries in South and Southeast Asia with extensive coastlines and high coast to land area ratios are particularly vulnerable to the consequences of rising sea levels on their biosphere and geosphere. One in ten persons worldwide lives in coastal areas that are less than 10 m above sea level (McGranahan et al. 2007). Such areas are prone to increasing salinisation caused by rising sea levels. The densely populated tropical South and Southeast Asian countries have a large proportion of their populations living in such vulnerable areas. For example, Vietnam, Bangladesh, Thailand and Indonesia, respectively, have 55, 46, 26 and 20 % of their population living on land that is less than 10 m above sea level (McGranahan et al. 2007).
4 Mosquito Vector Development in Brackish and Saline Waters
Global warming can alter the transmission of mosquito-borne diseases. This is due to the effects of changing temperature on mosquito survival and pre-imaginal development and the more rapid development of ingested pathogens in mosquitoes and shorter intervals between blood meals at moderately higher ambient temperatures (Ramasamy and Surendran 2012). However, global warming will also raise sea levels, leading to an increase in saline and brackish water bodies in coastal areas. We recently drew attention for the first time to the possible impacts of rising sea levels on the transmission of vector-borne diseases (Ramasamy and Surendran 2011).
There are about 3,500 species of mosquitoes that are present throughout the world, but only a few hundreds of them feed on humans. The pre-imaginal stages of several mosquito vectors of human disease are able to develop in brackish or saline water habitats. Larvae of such salinity-tolerant mosquitoes possess specific physiological mechanisms to overcome high osmolarity in their surrounding (Bradley 1987). A relatively thick cuticle that is impermeable to water and ions helps pupae to survive salinity (Bradley 1987). Details of the more important vector mosquitoes that possess salinity-tolerant pre-imaginal stages in South and Southeast Asia are summarised in Table 8.2.
We have postulated that an expansion of brackish/saline water bodies in coastal areas associated with rising sea levels can increase densities of salinity-tolerant vectors and lead to the adaptation of freshwater vectors to breed in brackish and saline waters (Ramasamy and Surendran 2011). Higher vector densities can increase transmission of diseases in coastal localities, which can then spread to inland areas through bridging vectors (Ramasamy and Surendran 2011, 2012). Because the tropical countries in South and Southeast Asia possess many lagoons, coastal marshes, mangroves and estuaries and many have extensive coastlines or a high ratio of coastline to land area, they are particularly susceptible to an increase in disease transmission caused by salinity-tolerant mosquito vectors.
Evidence that has been discussed in detail elsewhere (Ramasamy and Surendran 2011, 2012) supports this new perspective on coastal transmission of mosquito-borne diseases. In particular, the inland incursion of seawater caused by the 2004 Asian tsunami and the effect of draining coastal marshland in Western Europe provide specific supportive evidence (Ramasamy and Surendran 2011, 2012). Anopheles culicifacies, the predominant freshwater vector of malaria in South Asia, was recently shown to undergo pre-imaginal development in brackish waters in Sri Lanka (Jude et al. 2010). Anopheles sundaicus, a well-known salinity-tolerant malaria vector in Southeast Asia, is now reported to be widespread in coastal areas of Sri Lanka (Surendran et al. 2010, 2011). Many mosquitoes identified through morphology as Anopheles subpictus sibling species B, which is reported to be salinity tolerant in Sri Lanka and elsewhere in the region, have been shown to be members of the An. sundaicus complex by DNA sequencing (Surendran et al. 2010). An. sundaicus is able to develop in nearly saline water, but the physiological mechanisms responsible for this ability have not been determined. It is possible that a capacity to differentially localise Na+/K+ ATPase in rectal cells that permits osmoregulation through ion excretion as reported for Anopheles albimanus (Smith et al. 2008), a salinity-tolerant malaria vector in the Americas, may also be a feature of An. sundaicus. This requires experimental verification. Genetic selection for salinity tolerance over a period is possible in anopheline vectors because sibling species that differ in salinity tolerance are reportedly present in the Subpictus, Sundaicus and Farauti vector complexes of the region (Sinka et al. 2011). Maps of the recent distributions of the three vector complexes and the Culicifacies complex, whose members may also adapt to salinity, in South and Southeast Asia, are shown in Fig. 8.1. Mosquitoes of the Subpictus, Sundaicus and Farauti complexes are widely distributed in the region. A future expansion of brackish water habitats with rising sea levels has the potential to increase malaria transmission by the salinity-tolerant members of the three complexes as well as brackish water-adapted freshwater species like An. culicifacies. This can increase the incidence and geographical spread of malaria in coastal areas that can subsequently be extended by bridging vectors to inland areas of the region.
The distributions of (a) Anopheles (Cellia) culicifacies species complex, (b) Anopheles (Cellia) farauti species complex, (c) Anopheles (Cellia) subpictus species complex in South and Southeast Asia (Reproduced with permission from Sinka et al. 2011, under the Creative Commons licence)
We also recently showed that Aedes aegypti and Aedes albopictus, well known to be the vectors of dengue and chikungunya in the region, undergo pre-imaginal development in brackish water collections in coastal urban and peri-urban environments in the island of Sri Lanka (Ramasamy et al. 2011). Larvae and pupae were found in brackish water in discarded plastic and glass containers and disused boats and wells in beach areas. Figure 8.2 illustrates examples of such brackish water larval habitats. In the field surveys carried out in the northern and eastern provinces of Sri Lanka (Fig. 8.3), 17 % of brackish water containers in coastal beaches of Thannamunai in the eastern Batticaloa district were found to have Ae. aegypti and Ae. albopictus larvae, while 6 % of brackish water containers along the coast of Jaffna City in the north had Ae. aegypti larvae (Ramasamy et al. 2011). In Kurunagar, a coastal division of the city of Jaffna, 25 % of brackish water wells were found to have Ae. aegypti larvae in a salinity range of 2–9 ppt (Surendran et al. 2012). Such Aedes larval positivity rates in brackish water of up to 15 ppt salt in Jaffna (Ramasamy et al. 2011) are higher than the House Index (percentage of houses positive for Aedes larvae) or Breteau Index (number of containers with larvae per 100 houses) for freshwater habitats that have been typically associated with dengue epidemics in other countries (Sanchez et al. 2006). Pre-imaginal development in brackish water can play an unappreciated role in the transmission of dengue as dengue control efforts worldwide are presently directed only towards freshwater habitats of the two Aedes vectors because of the long and widely held view that they only develop naturally in freshwater (Barraud 1934; Chan et al. 1971; Kulatilaka and Jayakuru 1998; Ooi et al. 2006; WHO 2009, 2013c). The adaptation of Ae. aegypti and Ae. albopictus to coastal brackish water habitats could have major consequences to human health since vaccines are presently not available against dengue and chikungunya that are increasing in incidence globally (Weaver and Reisen 2010). Small island countries like Singapore and Sri Lanka within the region have not been able to eliminate dengue despite well-resourced and long-established control programmes (Chan et al. 1971; Kulatilaka and Jayakuru 1998; Ooi et al. 2006). We hypothesise that the exclusive targeting of freshwater pre-imaginal habitats for vector source reduction and management until now has contributed to this failure. Our observations in Sri Lanka also suggest that Ae. aegypti and Ae. albopictus from different locations in the country may vary in their ability to tolerate salinity (Ramasamy et al. 2011). We observed that Ae. albopictus and Ae. aegypti first and third instar larvae from the Jaffna peninsula, where there is more extensive groundwater salinisation (Rajasooriyar et al. 2002), had significantly higher LC50 for salinity in developing into adults (11.9 and 13.0 ppt for first instar larvae of Ae. aegypti and Ae. albopictus, respectively) than the corresponding larvae from Batticaloa in mainland Sri Lanka (9.8 and 10.2 ppt for first instar larvae of Ae. aegypti and Ae. albopictus, respectively) (Ramasamy et al. 2011).
Brackish water development habitats of Aedes aegypti and Aedes albopictus larvae in Sri Lanka. The photographs show the brackish water collections containing larvae in (a) and (b), disused boats; (c) and (e), abandoned wells; (d) and (f), discarded food and beverage containers (Reproduced with permission from Surendran et al. 2012)
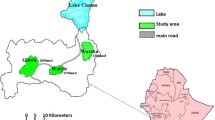
Map of Sri Lanka showing the different provinces and the Aedes larvae collection sites in the Jaffna and Batticaloa districts. Sri Lanka is an island in the Indian Ocean with an area of 65,525 km2 located between latitudes 5′55 and 9′50 North of the equator. The central hills of the island divide the surrounding plains into two distinct rainfall zones: the wet and dry zones. The wet zone receives an annual rainfall exceeding 2,500 mm in two main rainy seasons: the north-east monsoon in October–December and the south-west monsoon in May–July. Inter-monsoonal rains also occur in the wet zone. The dry zone, with an annual rainfall below 2,000 mm, receives maximal rainfall during the north-east monsoon and little or no rain during the rest of the year. An intermediate zone, with mixed characteristics, lies between the dry and wet zone. The beige, green and pink shaded areas show the dry, intermediate and wet rainfall zones, respectively (Reproduced with permission from Surendran et al. 2012)
In Brunei Darussalam, which is 3,775 km away from Sri Lanka, we observed larvae of Ae. albopictus in discarded plastic food and beverage containers in beach areas in water with salinity of up to 8 ppt (Idris et al. 2013). The laboratory determined LC50 for the development of first instar Ae. albopictus larvae to adults in Brunei Darussalam in brackish water was approximately 9 ppt salinity (Idris et al. 2013). Aedes aegypti was however found in brackish water in a house from a water village on the Brunei river estuary (Ramasamy and Surendran 2013). The failure to detect Ae. aegypti pre-imaginal stages in brackish water containers along beaches in Brunei Darussalam may be due to the peri-urban nature of the beaches and the greater endophilicity of Ae. aegypti in Brunei Darussalam, a country that is less densely populated than the surveyed coastal areas of Sri Lanka. The observations in Brunei Darussalam and Sri Lanka suggest that the development of Ae. aegypti and Ae. albopictus in brackish water may be a widespread phenomenon in tropical countries that can contribute to dengue transmission.
We speculate that Ae. aegypti and Ae. albopictus may be evolving strains that are adapting to brackish water habitats in the urban or peri-urban environment by a process that may be driven by anthropogenic changes that increase such habitats, the exclusive application of larval control methods to freshwater habitats and the use of insecticides in mainly inland locations for agriculture and vector control. Aedes albopictus has spread from Asia to Africa, America and Europe since the 1980s (Rezza 2012). It recently caused a chikungunya epidemic in northern Italy and dengue transmission in southern France (Rezza et al. 2007; Cavrini et al. 2009; La Ruche et al. 2010). We hypothesise that salinity-tolerant and diapausing Ae. albopictus will further increase the potential for arboviral disease transmission in coastal areas of the temperate zone in Asia and elsewhere.
The physiological mechanisms that permit Ae. aegypti and Ae. albopictus to oviposit and for the eggs to hatch into larvae that then develop into adults are not known. Larvae of the American brackish water mosquito Aedes taeniorhynchus, a vector of Eastern equine encephalitis virus, osmoregulate by drinking the surrounding fluid and excreting Na + and Cl− from the posterior rectum to produce a hyperosmotic urine (Bradley 1987). Early laboratory studies show that Ae. aegypti larvae are able to tolerate a limited increase in salinity in their surroundings by increasing the concentrations of free amino acids and ions in their haemolymph in order to osmoconform to their environment (Edwards 1982). Recent evidence suggests that the pre-imaginal development of Ae. aegypti in brackish water of up to 15 ppt salinity in Sri Lanka is accomplished by a combination of reversible and irreversible physiological changes (Ramasamy et al. 2014).
Many tropical South and Southeast Asian countries are experiencing a rapid growth of populations living in coastal areas (McGranahan et al. 2007; UNEP 2007). Discarded containers along coasts that can accumulate brackish water and serve as habitats for the pre-imaginal development of mosquito vectors will become more common if refuse collection becomes increasingly inadequate because the relevant government authorities fail to cope with the rising need. Such a scenario, coupled with the application of mosquito control methods only to inland, urban and freshwater habitats, can favour the adaptation of vector mosquitoes to undergo pre-imaginal development in coastal brackish water habitats. This can additionally increase the prevalence of mosquito-borne diseases in the region.
The Jaffna peninsula is located at the apex of northern Sri Lanka (Fig. 8.3). Jaffna is traditionally an agricultural area with an extensive coastline. It is largely composed of sedimentary limestone of the Miocene period (Rajasooriyar et al. 2002), has a maximum altitude of 10.4 m and contains many lagoons and other seawater inlets. Almost all locations in the peninsula are <10 km from the sea, lagoon or other seawater inlets. Therefore, the entire peninsula may be considered to be a coastal zone. Dengue is endemic (Ramasamy and Surendran 2012, 2013), and there have been recent epidemics of malaria (Ramasamy and Surendran 2012, 2013) and chikungunya (Surendran et al. 2007) in the Jaffna peninsula. There are no reports of the local transmission of Japanese encephalitis and filariasis in the Jaffna peninsula in recent times although their respective primary vectors Culex tritaeniorhynchus and Culex quinquefasciatus are present in the peninsula (Rajendram and Antony 1991; Jude et al. 2012). Furthermore, the larvae of Culex sitiens, a known vector of arboviruses including the Japanese encephalitis virus, are present in domestic wells with salinity ranging from 10 to 20 ppt in the islands off the peninsula (Jude et al. 2012). We propose that the Jaffna peninsula is therefore a model location for studying the future impact of global climate change and rising sea levels on mosquito vector populations and disease transmission in tropical coastal zones of Asia. The availability of relevant expertise and resources in the University of Jaffna makes a systems-based approach for studying the primary and secondary effects of different climate change parameters, changing salinity and other ecological and socio-economic factors, on mosquito populations and disease transmission possible in the Jaffna peninsula.
In summary, several mosquito vector species that have traditionally been regarded as developing exclusively in freshwater habitats are now known to be able to undergo pre-imaginal development in brackish water in the peri-urban and urban environments. The data are summarised in Table 8.3. The relative contribution of brackish water-developing vectors, compared to vectors developing in freshwater habitats, towards disease transmission in coastal areas remains to be elucidated in detailed epidemiological studies. An analysis of dengue incidence in relation to rainfall in Jaffna and the rest of Sri Lanka is compatible with the hypothesis that brackish water vectors are a perennial source of dengue transmission and act as a virus source to help generate the increase in dengue transmission that follows soon after the onset of monsoonal rains (Ramasamy and Surendran 2013).
5 Impact of Increased Disease Transmission on Coastal Communities in South and Southeast Asia
Rising sea levels can therefore act synergistically with climate change and then interact in a complex manner with other environmental and socio-economic factors near coasts to generate a greater potential for the transmission of malaria, dengue and other mosquito-borne diseases. More than half the world’s population lives within 60 km of a shoreline, and population densities in coastal areas will increase markedly in disease-endemic countries of the region (McGranahan et al. 2007; UNEP 2007). Growing numbers of people will therefore be at risk of acquiring infections from increasing vector populations in coastal areas. Greater host density, by increasing vector-host contact, will also contribute to an increase in disease transmission rates.
Factors such as climate, agricultural practice, forest cover, animal reservoirs of disease, efficiency of garbage collection in coastal locations and human population changes can influence the greater mosquito-borne disease transmission risk in coastal areas (Ramasamy and Surendran 2012). Higher incidence of such disease in coastal areas can serve as a focus to spread the disease inland through efficient freshwater vectors. The resulting health impacts may be particularly significant in the more resource-poor countries of the South and Southeast Asian region.
6 Mitigating Measures
Controlling the transmission of malaria, dengue and other mosquito-borne diseases and providing medical care to the corresponding patients consume a significant part of the health and national budgets of several South and Southeast Asian countries. This diverts scarce resources from improving medical services in other areas. Illness due to mosquito-borne diseases also reduces economic productivity. Therefore, improved control of dengue and other mosquito-borne diseases, through targeting brackish water larval habitats, is an important consideration for health authorities and governments in the region.
There is a clear need for supporting more research at local, national and international levels into the impact of rising sea levels and climate change on mosquito vector populations in coastal areas of the region. The impact of rising sea levels on the expansion of brackish and saline water bodies along coasts is subject to local geosphere factors, occurs over a timescale of decades and is therefore best studied at local levels. The bionomics of salinity-tolerant mosquito vector populations needs to be investigated in more detail. Present methods of identifying sibling species in malaria vector species complexes based on morphology or karyotyping have proved unreliable and unsuitable for field studies (Surendran et al. 2010). Robust and readily usable methods for differentiating sibling species that differ in salinity tolerance in the field need to be developed for different localities of the region. Mathematical models of disease transmission that take into consideration vector development in brackish water habitats have to be developed. The physiological mechanisms employed by mosquitoes such as An. culicifacies and Ae. aegypti that were considered to be freshwater species in adapting to brackish water need to be elucidated. Besides furthering knowledge on mosquito biology, this research can lead to the development of specific larvicides that can be used in brackish water habitats.
Therefore, greater attention needs to be devoted to monitoring disease incidence and mosquito vector development in coastal brackish and saline water habitats. Countermeasures to prevent an expansion of such habitats and reduce vector development therein are required. The extension of vector source reduction and management programmes to the brackish water habitats of the Aedes vectors of dengue and chikungunya is likely to immediately improve disease control in coastal areas. Pilot studies are needed in dengue-endemic areas to confirm this. Similarly, greater attention to brackish and saline water development of malaria vectors may also be helpful in further reducing malaria transmission in the region. Larval source reduction in defined habitats is effective in controlling dengue (WHO 2009) and malaria in many situations (Fillinger and Lindsay 2011). Managing and eliminating brackish water mosquito vector habitats may assume even greater importance in a future context of global warming, as sea levels rise and increase the extent of such habitats (Ramasamy and Surendran 2011, 2012). In this context, the stability and effectiveness of larvicides that are developed for freshwater use need to be examined in brackish and saline waters (Jude et al. 2012).
Community understanding and action have contributed to minimising freshwater larval development sites of dengue vectors in many disease-endemic countries. It is expected that this can be usefully extended to the brackish water habitats of the vectors. Governments can publicise the importance of brackish water mosquito vectors for transmitting disease through newspapers, radio, television and appropriate websites. This can include, for example, highlighting the additional importance of brackish water habitats of dengue vectors in national dengue awareness campaigns.
It is also important that the many local and national authorities responsible for different concerned sectors, e.g. health, agriculture, coastal planning, environment, irrigation, livestock development, etc., are made aware of the increased risk of mosquito-borne diseases in coastal areas due to rising sea levels, in order to incorporate it into their strategic development plans. Application of appropriate countermeasures can greatly reduce the potential for increased transmission of mosquito-borne diseases in coastal areas of South and Southeast Asia in the context of climate change and a rise in sea levels.
References
Barraud PJ (1934) Diptera Vol V Family Culicidae. Tribes Megarhinini and Culicini. In: Sewell RBS, Edwards PW (eds) The fauna of British India, including Ceylon and Burma. Taylor and Francis, London
Bradley TJ (1987) Physiology of osmoregulation in mosquitoes. Annu Rev Entomol 32:439–462
Cavrini F, Gaiban P, Pierro AM et al (2009) Chikungunya: an emerging and spreading arthropod-borne viral disease. J Infect Dev Ctries 3:744–752
Central Intelligence Agency (2012) The world factbook. Washington, DC, USA
Chan KL, Ho BC, Chan YC (1971) Aedes aegypti (L.) and Aedes albopictus (Skuse) in Singapore City. Bull World Health Organ 4:629–633
Chun L, Telisinghe PU, Hossain MM et al (2007) Vaccine development against dengue and shigellosis and implications for control of the two diseases in Brunei Darussalam. Brunei Darussalam J Health 2:60–71
Dondorp AM, Yeung S, White L et al (2010) Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol 8:272–280
Edwards HA (1982) Free amino acids as regulators of osmotic pressure in aquatic insect larvae. J Exp Biol 101:153–160
Erickson SM, Xi Z, Mayhew GF et al (2009) Mosquito infection responses to developing filarial worms. PLoS Negl Trop Dis 3:e529
Fillinger U, Lindsay SW (2011) Larval source management for malaria control in Africa: myths and reality. Malar J 10:353
Food and Agricultural Organisation (2007) Seawater intrusion in coastal aquifers – guidelines for study, monitoring and control. FAO, Rome
Gunasekaran K, Jambulingam P, Srinivasan R et al (2005) Malaria receptivity in the tsunami-hit coastal villages of southern India. Lancet Infect Dis 5:531–532
Halstead SB (2003) Neutralisation and antibody-dependent enhancement of dengue viruses. Adv Virus Res 60:421–467
Idris FH, Usman A, Surendran SN et al (2013) Detection of Aedes albopictus pre-imaginal stages in brackish water habitats in Brunei Darussalam. J Vect Ecol 38:197–199
Intergovernmental Panel on Climate Change (2013) Working group I contribution to the IPCC Fifth Assessment Report Climate Change 2013: the physical science basis. Summary for policymakers. http://www.climatechange2013.org/images/uploads/WGIAR5-PM_Approved27Sep2013.pdf. Accessed 17 Oct 2013
Jude PJ, Dharshini S, Vinobaba M et al (2010) Anopheles culicifacies breeding in brackish waters in Sri Lanka and implications for malaria control. Malar J 9:106
Jude PJ, Tharmasegaram T, Sivasubramaniyam G et al (2012) Salinity-tolerant larvae of mosquito vectors in the tropical coast of Jaffna, Sri Lanka and the effect of salinity on the toxicity of Bacillus thuringiensis to Aedes aegypti larvae. Parasit Vectors 5:269
Kulatilaka TA, Jayakuru WS (1998) Control of dengue/dengue haemorrhagic fever in Sri Lanka. Dengue Bull 22:53–61
La Ruche G, Souares Y, Armengaud A et al (2010) First two autochthonous dengue virus infections in metropolitan France. Euro Surveill 15:19676
McGranahan G, Balk D, Anderson B (2007) The rising tide: assessing the risks of climate change and human settlements in low elevation coastal zones. Environ Urban 19:17
Nicholls RJ, Wong PP, Burkett VR (2007) Coastal systems and low-lying areas. Climate change 2007: impacts, adaptation and vulnerability. In: Parry ML, Canziani OF, Palutikof JP et al (eds) Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 315–356
Ooi EE, Goh KT, Gubler DJ (2006) Dengue prevention and 35 years of vector control in Singapore. Emerg Infect Dis 12:887–893
Rajasooriyar LD, Mathavan V, Dharmagunewardene HA et al (2002) Groundwater quality in the Valigamam region of the Jaffna Peninsula, Sri Lanka. In: Hiscock KM, Rivett MO, Davison RM (eds) Sustainable groundwater development. Geological Society, London, pp 181–197, Special publications 193
Rajendram G, Antony NR (1991) Survey of peri-domestic mosquito species of Jaffna peninsula in Sri Lanka. Southeast Asian J Trop Med Public Health 22:637–642
Ramasamy R, Surendran SN (2011) Possible impact of rising sea levels on vector-borne infectious diseases. BMC Infect Dis 11:18. doi:10.1186/1471-2334-11-18
Ramasamy R, Surendran SN (2012) Global climate change and its potential impact on disease transmission by salinity-tolerant mosquito vectors in coastal zones. Front Physiol 3:198
Ramasamy R, Surendran SN (2013) Global environment changes and salinity adaptation in mosquito vectors. Lambert Academic Publishing, Saarbrucken
Ramasamy MS, Sands M, Kay BH et al (1990) Anti-mosquito antibodies reduce the susceptibility of Aedes aegypti to arbovirus infection. Med Vet Entomol 4:49–55
Ramasamy R, Surendran SN, Jude PJ et al (2011) Larval development of Aedes aegypti and Aedes albopictus in peri-urban brackish water and its implications for transmission of arboviral diseases. PLoS Negl Trop Dis 5:e1369
Ramasamy R, Jude PJ, Veluppillai T et al (2014) Biological differences between brackish and fresh water-derived Aedes aegypti from two locations in the Jaffna peninsula of Sri Lanka and the implications for arboviral disease transmission. PLoS One 9:e104977
Rezza G (2012) Aedes albopictus and the re-emergence of dengue. BMC Public Health 12:72
Rezza G, Nicoletti L, Angelini R et al (2007) Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 370:1840–1846
Sanchez L, Vanlerberghe V, Alfonso L et al (2006) Aedes aegypti larval indices and risk of dengue epidemics. Emerg Infect Dis 12:800–806
Sinka ME, Bangs MJ, Manguin S et al (2011) The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit Vectors 4:89
Smith KE, Van Ekeris LA, Okech BA et al (2008) Larval anopheline mosquito recta exhibit a dramatic change in localization patterns of ion transport proteins in response to shifting salinity: a comparison between anopheline and culicine larvae. J Exp Biol 211:3067–3076
Surendran SN, Kannathasan S, Kajatheepan A et al (2007) Chikungunya type fever outbreak: some aspects related to this new epidemic in Jaffna district, northern Sri Lanka. Trop Med Health 35:249–252
Surendran SN, Singh OP, Jude PJ et al (2010) Genetic evidence for malaria vectors of the Anopheles sundaicus complex in Sri Lanka with morphological characteristics attributed to Anopheles subpictus species B. Malar J 9:343
Surendran SN, Jude PJ, Ramasamy R (2011) Variations in salinity tolerance of malaria vectors of the Anopheles subpictus complex in Sri Lanka and the implications for malaria transmission. Parasit Vectors 4:117
Surendran SN, Jude PJ, Thabothiny V et al (2012) Pre-imaginal development of Aedes aegypti in brackish and fresh water urban domestic wells in Sri Lanka. J Vector Ecol 37:471–473
United Nations Environment Programme (2007) Global programme of action for the protection of the marine environment from land-based activities: physical alteration and destruction of habitats. UNEP, Nairobi. http://gpa.unep.org/content.html?id=199&ln=6. Accessed 15 Jun 2010
Walter Reed Biosystematics Unit (2013) Keys to medically important mosquito species. Smithsonian Institution, Silver Spring, http://wrbu.org/command_aors_MQ.html. Accessed 26 Dec 2013
Weaver SC, Reisen WK (2010) Present and future arboviral threats. Antiviral Res 85:328–345
World Health Organization (2009) Dengue guidelines for diagnosis, treatment, prevention and control. http://www.whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf. Accessed 13 Jun 2013
World Health Organisation (2012) http://www.searo.who.int and http://www.wpro.who.int. Accessed 15 May 2012
World Health Organisation (2013a) Fact sheet No 100. Yellow fever. WHO, Geneva, http://www.who.int/mediacentre/factsheets/fs100/en/. Accessed 26 Dec 2013
World Health Organisation (2013b) World malaria report 2012. http://www.who.int/malaria/publications/world_malaria_report_2013/report/en/. Accessed 27 Feb 2014
World Health Organization (2013c) Fact sheet No. 117 – Dengue and severe dengue. http://www.who.int/mediacentre/factsheets/fs117/en/. Accessed 26 Jan 2014
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Ramasamy, R., Surendran, S.N., Jude, P.J., Dharshini, S., Vinobaba, M. (2015). Adaptation of Mosquito Vectors to Salinity and Its Impact on Mosquito-Borne Disease Transmission in the South and Southeast Asian Tropics. In: Morand, S., Dujardin, JP., Lefait-Robin, R., Apiwathnasorn, C. (eds) Socio-Ecological Dimensions of Infectious Diseases in Southeast Asia. Springer, Singapore. https://doi.org/10.1007/978-981-287-527-3_8
Download citation
DOI: https://doi.org/10.1007/978-981-287-527-3_8
Publisher Name: Springer, Singapore
Print ISBN: 978-981-287-526-6
Online ISBN: 978-981-287-527-3
eBook Packages: MedicineMedicine (R0)