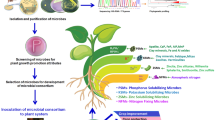
Abstract
Plant-associated microorganisms in the form of microbial consortia play an important role in agricultural production. The use of single strain or individual microorganism-based bioformulation has limitations. Thus, having a microbial consortium, where two or more interacting microorganisms have additive, synergistic, or mutual complementarity in nature, results in the desired effects on plants and soil. In this review, we have discussed the insights of interactions and mechanisms through which an effective microbial consortium promotes plant growth, improves nutrient utilization efficiency, enhances yield, induces tolerance to abiotic stresses, may contribute toward pest and phytopathogen management., etc. within the rhizosphere under their efficient root colonization and biofilm formation. In addition, the activity of microbial consortia has also been highlighted, mainly as a species of plant growth- and health-promoting bacteria. Furthermore, there is a huge impact of microbial consortia on the rhizosphere, which is enhanced by the concept of microbiome engineering and strain improvement. Augmentation of soil with synthetic microbial communities (SynComs), which are extended versions of traditional consortia, is recently being realized as a tool to modulate the complete rhizosphere microbiome for beneficial effects. This article is aimed to explain the wide horizon of the use of microbial consortia that facilitates the sustainable development of agriculture and its applications for human welfare.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The soil-plant ecosystem’s complexity often presents challenges for the single strain bioinoculants, when applied for plant growth promotion and disease control. By combining several microorganisms in the multi-species consortium, multiple beneficial activities are also added, and thus it is assumed that at least this group of microbes, i.e., the microbial consortium, will have more functional traits; hence, they perform better than respective individual microbial isolates (Woo and Pepe 2018; Nuti and Giovannetti 2015).
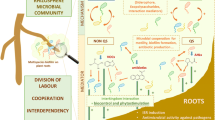
A microbial consortium is a group of different species of beneficial microorganisms, mainly plant growth-promoting bacteria, that act together as a microbial community. The co-culture of two or more microbial populations interacting synergistically forms a microbial consortium. It can perform diverse functions more efficiently which is difficult (not so efficient) or even impossible to carry out by a single organism. Since the division of labor is quite common in nature, it can be easily characterized in microorganisms established due to microbe-microbe interactions (Tshikantwa et al. 2018). Microbial consortia are “microbial cell factories” representing new synthetic biology approaches (Roell et al. 2019).
The microbial consortium proved effective in plant-microbe interactions, improvement of the soil profile, and soil nutrient status, which is supportive to induce plant growth, plant development in general, and enhancement of crop productivity. The microbial consortium helps in biofertilization, bioremediation, phytostimulation, and biological control of pests and pathogens (Sharma et al. 2018). They act in the solubilization of minerals in the soil, secreting phytohormones, producing enzymes (i.e., ACC deaminase) and chemical metabolites, and contributing to the bio-removal of soil pollutants and heavy metals (Arora et al. 2010; Pandey et al. 2012; Zhang et al. 2019a, b; Santoyo et al. 2021).
The synergism of microorganisms comprises the microbial consortium offering a new scope in agro-practices toward sustainable development. This may also avoid the agricultural requirements of microbial inoculants’ trade-off in individual microbial populations.
2 Microbe-Microbe Interactions
The interactions between/among the species or strains play a major role in the beneficial effects of bacterial consortia (Singh et al. 2019). The consortium (bearing bacteria) can be classified into three types. It is based upon the effect on each other, for example, (a) positive or stimulatory, (b) negative or inhibitory, and (c) neutral. The positive interactions comprise generating a network supporting individual members through cross-feeding wherein one bacterium utilizes the metabolic end products as nutrients for another member. Mutualism, protocooperation, and commensalism are some of the features. In mutualism, each or one of the members is benefitted in an obligatory association due to the exchange of required substances or mutual removal of toxins (Roell et al. 2019), while in the case of protocooperation, the interaction that occurs between species is beneficial to the growth rate of both populations but is not required either to persist. Similarly, commensalism is a positive one-way interaction in which one member benefits while the other remains unaffected (Dubey and Maheshwari 2022).
The suppression or inhibitory action of one another leads to the negative interactions that occur due to their growth inhibition of the structure and function. Such processes are (i) amensalism, (ii) predation, (iii) parasitism, and (iv) competition. When the growth of one of the members is affected due to the secretion of inhibitory substances (unidirectional), it is called amensalism, while predation and parasitism involved the growth of one species that depends upon the other species. Competition is mainly due to nutrition or space; therefore, the fast-growing organism dominated. In neutral interactions, members of the consortium do not influence each other. It occurs when two or more species consume different nutrients and neither produces any inhibitory compound to another consortium (Chaneton and Bonsall 2000).
3 Microbial Consortia
3.1 Definition and Design
A microbial consortium constitutes two or more compatible microorganisms of diverse/similar genera of different species in synergistic or additive interactions (Stockwell et al. 2011; Sarma et al. 2015). Long back, Higa and Parr (1994) advocated the use of effective microorganisms (EM) in the growth promotion of crops. The EM may also contain non-microbial biostimulants and stress-mediated/stress-protective nutrients. Even less-defined microbial populations originating from the fermentation of various natural substrates, farmyard manure, or composting processes are recommended as inoculants. The microbial consortia contain a network of microorganisms and represent an elegant way to identify specific microbes that have a more central position in the network, often defined as “keystone” species or “hub.” Such microorganisms generally co-occur with other taxa and likely exert a strong influence on the structure of microbial communities. The identified “hub” species may act on microbial communities and/or indirectly through (a) cascade modifications in the interconnected microbial network, (b) competition for space and nutrients, (c) alteration of the plant immunity, and (d) modification of the host physiology as identified (Kang et al. 2020). Microbial consortia consists either (i) a synthetic assembly by combining several isolated strains (Puentes-Téllez and Salles 2018) or (ii) complex microbial communities from nature (Skariyachan et al. 2017). In this scenario, the enrichment process is often used to get the desired microbial consortia.
3.2 Types, Process, and Development
Bashan and Prabhu (2020) highlighted the formation of advanced consortia with microbe-based products. Two types of consortia, i.e., simple and complex, are based on their differences in fermentation strategy (production of a large population of bacteria to be later formulated into an inoculant). For this, strains are grown individually or in combination including staggering into other species/strains in a suitable medium for all the plant growth-promoting (PGP) organisms. The consistency in results under field conditions is a benchmark of the success of bacterial consortia application which not only depends upon the type and function of strains but also includes their adaptation to adverse climate conditions, survival, and persistence in the soil after application (Verbruggen et al. 2013; Gosal and Kaur 2017).
4 Formulations: Difficulties and Success
Consortia formulation can be carried out by using selected PGP bacteria by combining a uniform bacterial cell concentration of all the participating strains. Later, after mixing, inoculant suspensions are prepared to achieve a final bacterial concentration of approximately 108 CFU/ml (⁓OD 600) as described by Gomez et al. (2021). To ensure different genera of PGP bacteria, for consortia formulations, the strain must be evaluated for some traits such as N fixation, P solubilization, siderophore production, IAA production, biofilm formation, ACC deaminase activity, etc. Thus, PGPR selected are recommended to design and construct microbial-based bioformulation for their application in a wide range of agro-ecosystem (Pandey et al. 2005, 2010). Santoyo et al. (2021) described plant growth stimulation by microbial consortia. Although many publications are appearing on plant-microbe interaction, it is significant to note that comparatively few appeared on the use of microbial consortium to perform plant growth and development enhancement to perform a variety of tasks in an ecosystem.
Consortium communication is governed by molecular signals. In this, quorum sensing plays a major role in the compatibility of bacterial communities comprising consortium formulations. Quorum sensing (QS) allows bacteria to switch between two gene expression programs: (i) at low density for individual and social behavior and (ii) at high cell density for social and group behaviors which are preferential for consortia (Ng and Bassler 2009). The QS enables bacterial cells in a formulation to function in unison, and they carry out as a collective, not allowing the desired effect of compatible consortia (Schikora et al. 2016).
A proper description of the consortium, the taxonomic affiliation of the strains and identification protocols, the process of formulation, the effect of edaphic and other related parameters, and the population of consortia formulations can be carried out by using selected PGP bacteria. The PGP strain is to be evaluated for their nature not to inhibit the growth of each other by the “cross-streaking” method of Pierson and Weller (1994). This was further confirmed by the filter paper disk method as given by Sindhu et al. (1999). The strains are further listed for their consortium-forming abilities following the spectrophotometric method of Shanmugam et al. (2002). In one of the reports from our research group, we have designed different combinations of bacteria, viz., (i) Pseudomonas aeruginosa KRP1 + B. licheniformis KRB1, (ii) B. licheniformis KRB1 + Sinorhizobium meliloti RMP1, (iii) S. meliloti RMP1 + P. aeruginosa KRP1, and (iv) KRP1 + RMP1, a multi-species bacterial consortium of all the above strains (Maheshwari et al. 2010). The healthy seeds of Brassica campestris (Indian mustard) were bacterized with KRP1, KRB1, or RMP1 and by consortia as given above, and the maximum enhancement of vegetative growth parameters was observed in the consortium, in comparison to those that emerged due to individual treatment with KRP1, KRB1, or RMP1. This application of bacterial consortium proved to be most desirable for plant growth and development of B. campestris (Maheshwari et al. 2010).
According to Nuti and Giovannetti (2015), microbial consortia are based on multiple PGP microbial strains with complementary properties. Sometimes non-microbial biostimulants and stress-protective nutrients are added to reduce the product cost. Molina-Romero et al. (2021) observed the potential of a second-generation consortium formulated with Azospirillum brasilense SP7, Pseudomonas putida KT2440, Acinetobacter sp. EMM02, and Sphingomonas sp. OF-178A. The bacterial strains present in the consortium proved compatible and efficient for field applications and resistant to desiccation.
5 Root Colonization and Biofilm Formation
PGPR-plant interaction is an intricate and interdependent relationship that involved not only the microorganisms but also other abiotic and biotic factors of the rhizosphere region that also play a role in their successful partnership (Kshetri et al. 2015). Root colonization and biofilm formation by the microbial community and the underlying principles are also behind the success of these organisms to tide over unfavorable conditions as suggested (Dutta and Podile 2010). The nature of bacterial genera and their relationship with host plants are exhibited by aggressive root colonization due to adequate adhesiveness to its surface. The adhesion improved when the strains of Azotobacter brasilence, Acinetobacter spp., and Sphingomonas spp. were applied to Zea mays together in a consortium. The inoculation of the bacterial consortium also improves the root colonization capacity in comparison to that of individual treatments. De Oliveira et al. (2006) observed the root colonization of a consortium formulated with Gluconacetobacter diazotrophicus, Herbaspirillum seropedicae, H. rubrisubalbicans, Azospirillum amazonense, and Burkholderia tropica. Even the different isolates of Burkholderia sp. RHT8 and RHT12 led to synergism and root colonization in fenugreek’s rhizosphere (Kumar et al. 2017). The combined effects of rhizo-competitive rhizosphere and non-rhizosphere Bacillus species enhanced the growth and yield in Eleusine coracana (Dheeman et al. 2020).
Root zone or “rhizosphere effect” is pronounced due to the successful establishment of bacterial consortia. This phenomenon is a crucial step to obtaining the beneficial effect of consortia on the host plant, which is further improved due to adequate adhesion and colonization (Shahzad et al. 2013). A significant difference was seen in maize when inoculated with A. brasilense, P. putida, Acinetobacter, and Sphingomonas spp. together. The plant taxa, variety, and other morphological features are also supportive of bacterial colonization formulated with Gluconacetobacter diazotrophicus, Herbaspirillum seropedicae, H. rubrisubalbicans, Azotobacter amazonense, and Burkholderia tropica in sugarcane (De Oliveira et al. 2006). The colonization capability of consortia of P. striata and Piriformospora indica is also dependent on corn varieties as observed by Singh et al. (2009). Earlier, Gusain and Bhandari (2019) studied the root colonization of Sinorhizobium meliloti, A. chroococcum, Serratia marcescens, and P. aeruginosa in different combinations of consortia which showed quite effective colonization in comparison to their counterparts. Santoyo et al. (2021) described plant growth stimulation and root colonization by microbial consortia.
The bacterial biofilm formation occurs quite commonly on the root surface and represents a hotspot for microbial interactions assisting them to form a consortium. It plays a significant role in the ecological network for shaping microbial communities for playing their role in sustainable agrobiological practices. The desired role of microbe-microbe interaction or mixed consortium involved in stimulating ecosystem functioning as well as in the enhancement of plant productivity (Pandit et al. 2020).
Currently, bioinformatics tools have been devised and used to investigate inter-microbial co-occurrence networks from community profiling or metagenomic data (Faust and Raes 2012); Layeghifard et al. 2017) study of the microbial networks. Plant interaction tends to indicate that positive correlation dominates among microbes from the same kingdom, whereas negative interaction primarily occurs through inter-kingdom microbe-microbe interaction (Agler et al. 2016). Thus, the role of microbial consortia is complex, and a more holistic understanding of microbial networks for holobiont fitness, is required (Hassani et al. 2018).
Aggressive bacterial genera in the root rhizosphere must have adequate adhesion and root colonization. Molina-Romero et al. (2021) highlighted that the adhesion improved when strains of A. brasilense, Acinetobacter sp., and Sphingomonas sp. were applied to maize together in a consortium. The inoculation of the bacterial consortium improved the bacterial colonization capacity in comparison to that of individual treatments. In another study, De Oliveira et al. (2006) observed the colonization of a consortium formulated with Gluconacetobacter diazotrophicus, Herbaspirillum seropedicae, H. rubrisubalbicans, Azospirillum amazonense, and Burkholderia tropica.
In addition, the bacterial consortium offered an alternative allowing the efficient use of half of the recommended dose of nitrogen fertilizer. The use of the consortium allowed the lowering of a 50% mineral N application and generated beneficial agronomic practices along with the lower cost to the cultivars (Molina-Romero et al. 2021). Recently, a new approach is devised wherein the effect of microbial consortia is applied as fertilizer coating. For this, the use of illumine high-throughput sequencing (HTS) is involved to influence the bulk soil and rhizosphere microbial community applied to potato fields (Overbeek et al. 2021). On the other hand, bacterial consortium acts as a substitute to chemical fertilizers such as urea, DAP, etc. because decreased application of chemical fertilization along with bacterial consortium exhibited a similar effect on plant growth and yield as revealed while applying the recommended doses of chemical fertilizers (Kumar et al. 2010; Da Costa et al. 2013).
Other characteristics such as strain evaluation to salinity stress under drought resistance cannot be ruled out, to stimulate crop growth and improve tolerance to abiotic stresses, and prove more effective in extreme climate change conditions. Microbial inoculants may improve salt tolerance by altering hormonal root-shoot signaling that manages IAA production in plants by bacterial action, thus having the potential in enhancing salt tolerance (Etesami and Maheshwari 2018). Such an approach is beneficial for a realistic assessment of the potential of microbial consortia in a climate change world.
6 Abiotic Stress: Action and Mechanism
The application of microbial consortia can reduce the negative effects that arise due to abiotic stress conditions on crops. But for their effective application in the crops, novel approaches are required to explore bacteria-bacteria and plant-bacteria interactions or bacteria-fungi interactions. Isolating and identifying the stress-tolerant or stress-resistant microbes to recalcitrant agrochemicals and heavy metals is important (Xia et al. 2020; Katiyar et al. 2021).
Abiotic stresses inhibit plant growth and development due to oxidative damage attacking DNA and cellular membranes. The antioxidant enzymes neutralize the reactive molecules; thus, cells are protected. PGPB having catalase and peroxidase properties are proven more protective. The beneficial bacteria also produce trehalose which also benefits the plants to abiotic stress (Glick 2015; Kumar and Verma 2018). Microbial production of phytohormones also protects plants by the involvement of various physiological actions. PGPB induces the level of proline in plants. Proline scavenges reactive oxygen molecules and acts to stabilize proteins through molecular chaperons in stress conditions (Meena et al. 2019). The effect of the consortium of Bacillus cereus AR156, B. subtilis SM21, and Serratia sp. XY21 was reported to develop healthy cucumber plants, with much darker green leaves containing increased proline and chlorophyll contents, and induce superoxide dismutase activity (Wang et al. 2012). An increase in ethylene level is injurious to plants causing senescence and other deleterious effects which occur due to the accumulation of a consortium of ACC deaminase-producing bacteria (Ochrobactrum pseudogrignonense, Pseudomonas sp., and B. subtilis) that significantly increased early vegetative growth plant parameters in Vigna mungo and P. sativum.
As human populations continue to increase, the disturbance of the soil ecosystem to enhance productivity may place greater demand on supplying soil essential nutrients. Therefore, it is essential to increase the understanding of the biological, physical, and chemical properties of soil along with the soil-microbe-plant relationship to enhance productivity with available nutrient pools (Millard and Singh 2010). The soil native ability to supply sufficient nutrients continuously decreases and emerges as a greater challenge for enhancing the productivity of crops and the quality of water, air, and fragile soil ecosystems. The relationship of soil-plant-microbes especially soil interaction influences plant compounds accurately, identifies the yield-limiting potential factors and growth and development, and minimizes the influence of those to manage the enhancement productivity (Metcalfe et al. 2011). Most of the research during the last decades was focused on the use of fertilizers and manures. Thus, information on the integrated approach of plant nutrition on the sustainability of soil fertility and crop productivity is necessary.
Soil fertility is the status or the inherent capacity of the soil to supply nutrients to plants in adequate amounts in suitable proportions. On the other hand, soil productivity is the capacity of the soil to produce crops with a specific system of management and is expressed in terms of yields (Van Ittersum et al. 2013). All productive soils are fertile, but all fertile soils are not necessarily productive. To produce crops of economic value and to maintain the health of the soil without deterioration is most important. Modern farming, driven by economic constraints, is forced to use artificial fertilizers, often to the detriment of the soil’s natural fertility (Rana and Rana 2011).
7 Metagenomics and Biotechnological Approach to Increase Efficiency of Microbial Consortium for Plant Growth Promotion
When compared to single microorganisms, consortia are superior throughout many situations. The selection of consortium members in a way that maximizes performance is a significant obstacle. Microbial consortia have the advantage of being more adaptive to environmental changes due to their high stability, resilience, and multifunction. Human health, bioremediation and biodegradation, chemical and bioenergy generation, and food manufacturing are just a few of the areas where microbial consortia are playing crucial roles in the developing sector (Lee et al. 2013). Recent breakthroughs in synthetic biology have significantly enhanced both the synthesis of microbial consortia and the comprehension of microbial communication mechanisms (Song et al. 2014). Cell-cell interactions in relatively small synthetic microbial consortia have recently been studied. Synthetic microbial consortia are typically less complex and easier to genetically modify than real microbial consortia, making the interaction and control processes easier to explore (Sanchez-Gorostiaga et al. 2019).
7.1 Microbiome Engineering
Microorganisms found on or within a plant have been shown to have beneficial effects, such as promoting growth or inhibiting pathogens (Ab Rahman et al. 2018). Altering the microbiome with plant growth-promoting rhizobacteria (PGPR) can improve plant development and reduce infections and abiotic stress (Kumar et al. 2018). Microbiome engineering can enhance agricultural yields and resilience by manipulating the plant holobiome. The plant’s genotype is also very important for the formation and function of rhizospheric microbiomes and for getting the most out of PGPR (Arif et al. 2020). Beneficial interactions between plants and microbes have been studied to learn how to change plant genomes to attract and keep beneficial microbiomes. Different plant genotypes attract helpful and disease-suppressing microorganisms to varying degrees, reorganizing the microbiome assembly (Gao et al. 2021). The endophytic microbiome of plants also influences functional genes related to plant growth promotion (Singha et al. 2021).
The study of plant functional genomics during mutually beneficial plant-microbe interactions has allowed the manipulation of plant genomes to entice and sustain such microbiomes (Rosier et al. 2018; Vandana et al. 2021). This led to the idea of “designer plants.” These genetically modified plants can release hormones or other substances that attract and keep good microbiomes (Stringlis et al. 2018). The targeted crop’s yield can be dramatically increased through the application of a consortium that is compatible with the chosen plant and able to repair the rhizospheric microbiome (Tabacchioni et al. 2021). Several studies have also pointed out that wild-type relatives of domesticated crops can help us learn more about the role of genes in wild plants that are linked to microbiome assembly (Pérez-Jaramillo et al. 2018). The microbiome is often commonly referred to as the brain of a given environment because of the significant impact it has on the general health and well-being of that environment (Lavazza and Sironi 2019). Inoculated groups of microorganisms can rebuild the structure and function of the microbiome in plants and soil. Microbes create functional consortia in the rhizosphere; soil conditioning and important microbial strains can modify the rhizosphere microbiome’s structure (Voges 2019). It is feasible to create artificial consortia with several functions for promoting plant development. This could fix some of the problems with traditional microbial biofertilizers, like not getting along with the host, not being able to compete well with native microbes, and not being able to adapt to the local environment (Hart et al. 2018). The development of the optimum artificial microbial consortium involves studying the microbes’ origin, getting and cultivating the microorganisms, optimizing microbial interactions as per compatibility, and finally investigating the consortia’s performance. Microbiome breeding is another technique by which the microbiome can be altered for betterment. It requires allowing the host to filter which populations of bacteria are permitted to interact with it and will be passed straight to their progeny, thus indirectly affecting the microbiome (Mueller and Linksvayer 2022). This strategy involves spreading a microbiome-influenced phenotype of the host. For example, to study the microbial influence on the flowering pattern of Arabidopsis thaliana, the early and late flowering microcosms are studied over generations, and it was found that more phenotypic inflorescence was observed in the plants inoculated with microbiome from late flowering plants. The repeatability of flowering phenotypes shows that microbiomes can be regulated to influence plant characteristics and coordinate soil resource pools (Panke-Buisse et al. 2015, 2017). In the same study, an increase in total biomass and increased enzyme activity for the mineralization of nitrogen were observed in Brassica rapa when inoculated with the same (Panke-Buisse et al. 2015). Likewise, microbiome transformation is another technique where the beneficial microbiota from one species was inoculated in other species to promote plant growth (Arif et al. 2020). For example, Leptospermum scoparium is reported to release antibacterial agents to counter the growth of Pseudomonas pathogens. A similar biocontrol activity was observed in the kiwi plant when PGP bacterial microbiome from this species was inoculated in it (Wicaksono et al. 2018).
7.2 Molecular Tools to Increase Efficiency of Microbiome Engineering
Understanding the physiological and biochemical functionality of the consortium can be greatly aided by genetic engineering or the use of molecular tools in the microorganism involved with plant growth promotion. The extensive collection of genes that are engaged in the processes will be taken into consideration as potential targets to achieve an accurate comprehension of the function that each gene is carrying out (Kumar et al. 2020). The discovery of RNA interference (RNAi) and CRISPR is the most recent and commonly used biotechnological development in genetic tools in this regard (Boettcher and McManus 2015; Schultenkämper et al. 2020). RNAi relies on an endogenous process that regulates gene expression with short RNAs. Synthetic tiny RNAs (siRNAs or short hairpin RNAs) can be used to seize the indigenous RNAi mechanism. Either way, the inserted RNA is put into the RNA-induced silencing complex (RISC), which promotes target mRNA destruction (Carthew and Sontheimer 2009; Mohr et al. 2014). Reduced amounts of the target protein are the result achieved post-translationally by targeting the expression of the corresponding mRNA (Boettcher and McManus 2015).
CRISPR is a revolutionary way to change the genes of plants to improve specific traits, and thus it has become one of the most useful tools in the field of functional genomics (Pérez-Jaramillo et al. 2018). One important use of CRISPR-based genetic engineering tools is to alter the genes of plants or microbes to study how the genes work. One of the best things about the CRISPR tool is that it can completely shut down the target gene. To do this, designer plants could be genetically engineered using the CRISPR tool to make and release mass hormones or exudates that attract and keep beneficial microbial populations in the rhizosphere microbiome ecosystem (Bisht et al. 2019) (Fig. 1.1).
7.3 Next-Generation Microbial Synthetic Communities (SynComs) for Plant Yield Promotion
From basic natural or synthetic consortia to a more complex applied consortium of microbes, omics-based techniques can paint a comprehensive picture of a consortium’s operation. Based on single omics approaches, metabolomics, metagenomics, transcriptomics, and metaproteomics have been developed to make it easier to study groups of microbes (Chandran et al. 2020). Quantifying the meta-proteome in a group of microorganisms is important for understanding how different protein functions work together and how they change over time which makes it the best of the different meta-omics approaches at showing how a microbial consortium’s system works (Franzosa et al. 2015). Rapid developments in mass spectrometry have led to the creation of many quantification strategies. Among these techniques, the isobaric leveling method and isobaric tags for relative and absolute quantitation (iTRAQ) are widely utilized for comparative proteomic research because of their high sensitivity and accuracy. The metabolomic analysis of the consortium dedicated to any process gives a prediction of how the combined effects the production of intermediate metabolites so that the best can be grouped to obtain the best results (Ma et al. 2019).
SynComs are microbial consortia designed to imitate the natural microbiome. The goal is to minimize the sophistication of the microbiome while keeping some of the natural interactions between bacteria and hosts, offering a spectrum of capabilities unattainable by a single bacterium. Additionally, synergistic interactions between members of SynComs may improve community stability (Kaminsky et al. 2019; McCarty and Ledesma-Amaro 2019). To unlock the potential of soil microbes and boost agricultural yields, microbial synthetic communities (SynComs) have been proposed as a useful technique that incorporates both microbial ecology and genetics in the construction of inoculants. The goal of this strategy is to identify and then recruit a group of microbes that can stimulate plant development in a variety of climates and the face of harsh events (York 2018). In recent times, the focus has been given to the development of microbially based goods due to the worldwide potential of these SynComs to boost agricultural production and sustainability (Singh et al. 2020).
Computational approaches, such as machine learning algorithms, will improve the screening and identification of beneficial bacteria, as well as the process of establishing the optimal microbe combination for a particular plant phenotype (Harfouche et al. 2019). The growing number of reference genomes and metagenomes in public databases helps to find bacteria with desirable features, and by using these genomic information and gene expression patterns, one can choose microorganisms with plant-beneficial functional features or metabolic capabilities (Vorholt et al. 2017). Genome surveys for several gene markers will be critical to finding relevant microorganisms because important properties like colonization efficiency and frequency of other attributes are likely to relate to multiple genes, and to solve this problem, genomics-based datasets filter microbiological candidates on a genomic markers’ basis (Finkel et al. 2017). Thus, genome and metagenome sequencing, together with microbial characterization, could assist in building SynComs that bestow stable plant phenotypes and increase plant colonization and permanence (De Souza et al. 2020). A systematic flow to develop a successful SynCom is presented in Fig. 1.2.
In another way, SynComs help in understanding the physiology and function of microorganisms and the parameters regulating community assembly by manipulating a SynCom formulation by adding, removing, or replacing microorganisms (Vorholt et al. 2017). For example, removing a single strain of Enterobacter cloacae reduced the activity of microbial consortium which was related to reducing the severity of maize blight disease (Niu et al. 2017). Similarly, a SynCom with more microorganisms from the indica strain had a bigger effect on rice growth than a SynCom with more microorganisms from the japonica strain (Zhang et al. 2019a, b).
Few obstacles or problems exist with the employment of SynComs. Most microbial species are likely to be uncultivable, making it difficult to assemble in a microbial consortium. Further, the cost of sequencing hundreds of thousands of samples is extremely expensive (Lewis et al. 2021). In addition, soil microbiomes are complicated, and the relationships between soil single taxa and environmental factors are inadequately documented, limiting our understanding of microbial candidates that might be employed to increase plant growth and productivity in the wild (Jayaraj et al. 2016). Therefore, most of the recently developed SynComs are comprised of bacteria only and a group of culturable microbes equipped with good plant growth-promoting dexterities.
8 Application: Microbial Inoculation and Soil Community
Microbial inoculation directly impacts the soil microbial community to increase the relative abundance of inoculated microbial genera. The rhizospheric microbial community composition differed substantially from the bulk soil microbial community composition (Overbeek et al. 2021). For example, in the case of potato roots, enrichment of the rhizosphere community over bulk soil was observed for Proteobacteria and Eurotiomycetes. A similar difference in the microbial community was also observed by several workers (Berendsen et al. 2012; Xue et al. 2018). The external input of microorganisms closely associated with the rhizosphere contributed as core microorganisms and the alteration in the rhizospheric microbiome help in designing microbial inoculants beneficial to the plants growing under a variety of soil conditions (Sathya et al. 2017).
The biological management for the growth and development of plants is still at an early stage of development, while the approach appears to have tremendous potential, and many of the basic concepts necessary for the implementation are in place, and apparent obstacles such as information on biomass, formation of a product, site of application, and registration difficulties exist (Kumar et al. 2017). For increasing crop productivity and the maintenance and improvement of soil fertility for sustainable crop production, the multifunctional formulation may be promoted that involves microbial consortium utilizing the PGPR, which has been proven better and eco-friendly in comparison to that of formulation alone (Kshetri et al. 2017).
The microbial consortia are also used to control and optimize various industrial processes. Puentes-Téllez and Salles (2018) described the construction of effective minimal active microbial consortia for lignocellulose degradation. The simplification of the microbial community makes it easier to help and understand the individual roles of the strains in the consortia.
Skariyachan et al. (2018) worked on polymer degradation by novel thermophilic consortia of Brevibacillus spp. and Aneurinibacillus sp. associated with waste management landfills and sewage treatment plants. Earlier, the authors formulated bacterial consortia from plastic-contaminated cow dung. It is interesting to note that Subhashchandrabose et al. (2011) studied the biotechnology potential of consortia of cyanobacteria/microalgae and bacteria.
The PGP strain was evaluated for their nature to inhibit the growth of each other by the “cross-streaking” method of Pierson and Weller (1994). This was further confirmed by the filter paper disk method as given by Sindhu et al. (1999). The strains are further listed for their consortium-forming abilities following the spectrophotometric method of Shanmugam et al. (2002). Recently, Baliyan et al. (2022) reviewed the bacteriophage cocktails and antibacterial agents in crop protection.
9 Conclusions
The development of artificial consortiums developed with multifarious characteristics is a growing interest in using similar or diverse genera of beneficial bacteria in agriculture applications. The microbial consortia offer consistency and higher reproducibility of data under various environmental conditions and provide a broader array of the mechanism of action in comparison to that of individual beneficial bacteria applied alone for friendly crop production and protection system in agriculture. However, some critical challenges are yet to be resolved. Certain issues with the registration and marketing of formulations comprising mixed cultures limit their potential use in modern agriculture. The difficulties in understanding the specific role of each component of microbial consortium and their desirable effects may limit the predicted effect on the growth and development of crops. The molecular tools and biotechnological approach involving plant-microbe interaction, soil-microbe engineering, metagenomic soil profile, and next-generation synthetic microbial consortia are some of the most useful tools to make it easier to study microbe involved in the design and construction of microbial consortium systems. Further, bioinformatics and computational tools may improve the understanding of function of microbial consortia and their products for sustainable agriculture.
References
Ab Rahman SFS, Singh E, Pieterse CM, Schenk PM (2018) Emerging microbial biocontrol strategies for plant pathogens. Plant Sci 267:102–111
Agler MT, Ruhe J, Kroll S, Morhenn C, Kim ST, Wiegel D, Kemen EM (2016) Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol 14(1):1–9
Arif I, Batool M, Schenk PM (2020) Plant microbiome engineering: expected benefits for improved crop growth and resilience. Trend Biotechnol 38(12):1385–1396
Arora NK, Khare E, Maheshwari DK (2010) Plant growth promoting rhizobacteria: constraints in bioformulation, commercialization, and future strategies. In Plant growth and health promoting bacteria, Springer, Berlin, Heidelberg, pp 97–116
Baliyan N, Dhiman S, Dheeman S, Vishnoi VK, Kumar S, Maheshwari DK (2022) Bacteriophage cocktails as antibacterial agents in crop protection. Environ Sustain:1–7
Bashan Y, Prabhu SR, de-Bashan LE, Kloepper JW (2020) Disclosure of exact protocols of fermentation, identity of microorganisms within consortia, formation of advanced consortia with microbe-based products. Biol Fertil Soils 56:443-445
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trend Plant Sci 17:478–486
Bisht DS, Bhatia V, Bhattacharya R (2019) Improving plant-resistance to insect-pests and pathogens: the new opportunities through targeted genome editing. Semin Cell Dev Biol 99:65–78
Boettcher M, McManus MT (2015) Choosing the right tool for the job: RNAi, TALEN, or CRISPR. Mol Cell 58(4):575–585
Carthew RW, Sontheimer EJ (2009) Origins and mechanisms of miRNAs and siRNAs. Cell 136(4):642–655
Chandran H, Meena M, Sharma K (2020) Microbial biodiversity and bioremediation assessment through omics approaches. Front Environ Chem 1(570326):1–22
Chaneton EJ, Bonsall MB (2000) Enemy-mediated apparent competition: empirical patterns and the evidence. Oikos 88(2):380–394
Da Costa PB, Beneduzi A, De Souza R, Schoenfeld R, Vargas LK, Passaglia LMP (2013) The effects of different fertilization conditions on bacterial plant growth promoting traits: guidelines for directed bacterial prospection and testing. Plant Soil 368:267–280
De Oliveira ALM, De Canuto EL, Urquiaga S, Reis VM, Baldani JI (2006) Yield of micropropagated sugarcane varieties in different soil types following inoculation with diazotrophic bacteria. Plant Soil 284:23–32
De Souza RSC, Armanhi JSL, Arruda P (2020) From microbiome to traits: designing synthetic microbial communities for improved crop resiliency. Front Plant Sci 11(1179):1–7
Dheeman S, Baliyan N, Dubey RC, Maheshwari DK, Kumar S, Chen L (2020) Combined effects of rhizo-competitive rhizosphere and non-rhizosphere Bacillus in plant growth promotion and yield improvement of Eleusine coracana (ragi). Can J Microbiol 66(2):111–124
Dubey RC, Maheshwari DK (2022) Textbook of microbiology. S. Chand and Company Limited, New Delhi
Dutta S, Podile AR (2010) Plant growth promoting rhizobacteria (PGPR): the bugs to debug the root zone. Critic Rev Microbial 36(3):232–244
Etesami H, Maheshwari DK (2018) Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: action mechanisms and future prospects. Ecotoxicol Environ Safe 156:225–246
Faust K, Raes J (2012) Microbial interactions: from networks to models. Nat Rev Microbiol 10:538–550
Finkel OM, Castrillo G, Paredes SH, González IS, Dangl JL (2017) Understanding and exploiting plant beneficial microbes. Curr Opin Plant Biol 38:155–163
Franzosa EA, Hsu T, Sirota-Madi A, Shafquat A, Abu-Ali G, Morgan XC, Huttenhower C (2015) Sequencing and beyond: integrating molecular ‘omics’ for microbial community profiling. Nat Rev Microbiol 13(6):360–372
Gao M, Xiong C, Gao C, Tsui CK, Wang MM, Zhou X, Cai L (2021) Disease-induced changes in plant microbiome assembly and functional adaptation. Microbiome 9(1):1–18
Glick BR (2015) Stress control and ACC deaminase. In: Principles of plant-microbe interactions. Springer, Cham, pp 257–264
Gomez JA, Höffner K, Barton PI (2021) Production of biofuels from sunlight and lignocellulosic sugars using microbial consortia. Chem Eng Sci 239(116615):1–14
Gosal SK, Kaur J (2017) Microbial inoculants: a novel approach for better plant microbiome interactions. In: Probiotics in agroecosystem. Springer, Singapore, pp 269–289
Gusain P, Bhandari BS (2019) Rhizosphere associated PGPR functioning. J Pharmacog Phytochem 8(5):1181–1191
Harfouche AL, Jacobson DA, Kainer D, Romero JC, Harfouche AH, Mugnozza GS, Altman A (2019) Accelerating climate resilient plant breeding by applying next-generation artificial intelligence. Trend Biotechnol 37(11):1217–1235
Hart MM, Antunes PM, Chaudhary VB, Abbott LK (2018) Fungal inoculants in the field: is the reward greater than the risk? Funct Ecol 32(1):126–135
Hassani MA, Durán P, Hacquard S (2018) Microbial interactions within the plant holobiont. Microbiome 6(1):1–17
Higa T, Parr JF (1994) Beneficial and effective microorganisms for a sustainable agriculture and environment, vol 1. International Nature Farming Research Center, Atami, pp 1–16
Jayaraj R, Megha P, Sreedev PJIT (2016) Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip Toxicol 9(3-4):90
Kaminsky LM, Trexler RV, Malik RJ, Hockett KL, Bell TH (2019) The inherent conflicts in developing soil microbial inoculants. Trends Biotechnol 37(2):140–151
Kang D, Samuel J, Herschend J, Wei S, Nesme J, Sorensen SJ (2020) Construction of simplified microbial consortia to degrade recalcitrant materials based on enrichment and dilution-to-extinction cultures. Front Microbiol 10(3010):1–10
Katiyar P, Dubey RC, Maheshwari DK (2021) ACC deaminase-producing Ensifer adhaerens KS23 enhances proximate nutrient of Pisum sativum L. cultivated in high altitude. Arch Microbiol 203(5):2689–2698
Kshetri L, Nevita T, Pandey P (2015) Plant growth promoting rhizobacteria (PGPR) and their application for sustainable agriculture in north eastern region of India. ENVIS Bull Himal Ecol 23:41–47
Kshetri L, Pandey P, Sharma GD (2017) Solubilization of inorganic rock phosphate by rhizobacteria of Allium hookeri Thwaites and influence of carbon and nitrogen sources amendments. J Pure Appl Microbiol 11:1899–1908
Kumar A, Singh S, Gaurav AK, Srivastava S, Verma JP (2020) Plant growth-promoting bacteria: biological tools for the mitigation of salinity stress in plants. Front Microbiol 11(1216):1–41
Kumar A, Singh VK, Tripathi V, Singh PP, Singh AK (2018) Plant growth-promoting rhizobacteria (PGPR): perspective in agriculture under biotic and abiotic stress. In Crop improvement through microbial biotechnology, pp. 333–342
Kumar A, Verma JP (2018) Does plant-microbe interaction confer stress tolerance in plants: a review? Microbiol Res 207:41–52
Kumar H, Bajpai VK, Dubey RC, Maheshwari DK, Kang SC (2010) Wilt disease management and enhancement of growth and yield of Cajanus cajan (L) var. Manak by bacterial combinations amended with chemical fertilizer. Crop Prot 29(6):591–598
Kumar H, Dubey RC, Maheshwari DK (2017) Seed-coating fenugreek with Burkholderia rhizobacteria enhances yield in field trials and can combat Fusarium wilt. Rhizosphere 3:92–99
Lavazza A, Sironi VA (2019) Are we ready for a “microbiome-guided behaviour” approach? Camb Q Healthc Ethics 28(4):708–724
Layeghifard M, Hwang DM, Guttman DS (2017) Disentangling interactions in the microbiome: a network perspective. Trends Microbiol 25:217–228
Lee DJ, Show KY, Wang A (2013) Unconventional approaches to isolation and enrichment of functional microbial consortium–a review. Bioresour Technol 136:697–706
Lewis WH, Tahon G, Geesink P, Sousa DZ, Ettema TJ (2021) Innovations to culturing the uncultured microbial majority. Nat Rev Microbiol 19(4):225–240
Ma Q, Bi YH, Wang EX, Zhai BB, Dong XT, Qiao B, Yuan YJ (2019) Integrated proteomic and metabolomic analysis of a reconstructed three-species microbial consortium for one-step fermentation of 2-keto-L-gulonic acid, the precursor of vitamin C. J Ind Microbiol Biotechnol 46(1):21–31
Maheshwari DK, Kumar S, Kumar B, Pandey P (2010) Co-inoculation of urea and DAP tolerant Sinorhizobium meliloti and Pseudomonas aeruginosa as integrated approach for growth enhancement of Brassica juncea. Indian J Microbiol 50(4):425–431
McCarty NS, Ledesma-Amaro R (2019) Synthetic biology tools to engineer microbial communities for biotechnology. Trends Biotechnol 37(2):181–197
Meena M, Divyanshu K, Kumar S, Swapnil P, Zehra A, Shukla V, Upadhyay RS (2019) Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon 5(12):1–20
Metcalfe DB, Fisher RA, Wardle DA (2011) Plant communities as drivers of soil respiration: pathways, mechanisms, and significance for global change. Biogeosciences 8(8):2047–2061
Millard P, Singh BK (2010) Does grassland vegetation drive soil microbial diversity? Nutr Cycl Agroecosyst 88(2):147–158
Mohr SE, Smith JA, Shamu CE, Neumüller RA, Perrimon N (2014) RNAi screening comes of age: improved techniques and complementary approaches. Nat Rev Mol Cell Biol 15(9):591–600
Molina-Romero D, Juárez-Sánchez S, Venegas B, Ortíz-González CS, Baez A, Morales-García YE, Muñoz-Rojas J (2021) A bacterial consortium interacts with different varieties of maize, promotes the plant growth, and reduces the application of chemical fertilizer under field conditions. Front Sustain Food Syst 4(616757):1–14
Mueller UG, Linksvayer TA (2022) Microbiome breeding: conceptual and practical issues. Trends Microbiol 30(10):997–1011
Ng WL, Bassler BL (2009) Bacterial quorum-sensing network architectures. Annu Rev Genet 43:197–222
Niu B, Paulson JN, Zheng X, Kolter R (2017) Simplified and representative bacterial community of maize roots. Proc Natl Acad Sci 114(12):2450–2459
Nuti M, Giovannetti G (2015) Borderline products between bio-fertilizers/bio-effectors and plant protectants: the role of microbial consortia. J Agric Sci Technol 5:305–315
Overbeek W, Jeanne T, Hogue R, Smith DL (2021) Effects of microbial consortia, applied as fertilizer coating, on soil and rhizosphere microbial communities and potato yield. Front Agron 3(717400):1–13
Pandey P, Aeron A, Maheshwari DK (2010) Sustainable approaches for biological control of fusarium wilt in pigeon pea (Cajanus cajan L. Millspaugh). Plant Growth Health Promoting Bacteria 18:231–249
Pandey P, Bisht S, Sood A, Aeron A, Sharma GD, Maheshwari DK (2012) Consortium of plant-growth-promoting bacteria: future perspective in agriculture. Bacteria Agrobiol 9:185–200
Pandey P, Kang SC, Gupta CP, Maheshwari DK (2005) Rhizosphere competent Pseudomonas aeruginosa GRC1 produces characteristic siderophore and enhances growth of Indian mustard (Brassica campestris). Curr Microbiol 51(5):303–309
Pandit A, Adholeya A, Cahill D, Brau L, Kochar M (2020) Microbial biofilms in nature: unlocking their potential for agricultural applications. J Appl Microbiol 129(2):199–211
Panke-Buisse K, Lee S, Kao-Kniffin J (2017) Cultivated sub-populations of soil microbiomes retain early flowering plant trait. Microb Ecol 73(2):394–403
Panke-Buisse K, Poole AC, Goodrich JK, Ley RE, Kao-Kniffin J (2015) Selection on soil microbiomes reveals reproducible impacts on plant function. ISME J 9(4):980–989
Pérez-Jaramillo JE, Carrión VJ, de Hollander M, Raaijmakers JM (2018) The wild side of plant microbiomes. Microbiome 6(1):1–6
Pierson EA, Weller DM (1994) To suppress take-all and improve the growth of wheat. Phytopathology 84(1):940–947
Puentes-Téllez PE, Salles JE (2018) Construction of effective minimal active microbial consortia for lignocellulose degradation. Microb Ecol 76:419–429
Rana SS, Rana MC (2011) Cropping system. Department of Agronomy, College of Agriculture, CSK Himachal Pradesh Krishi Vishvavidyalaya, Palampur, p 80
Roell GW, Zha J, Carr RR, Koffas MA, Fong SS, Tang YJ (2019) Engineering microbial consortia by division of labor. Microb Cell Factories 18:1–11
Rosier A, Medeiros FH, Bais HP (2018) Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil 428(1):35–55
Sanchez-Gorostiaga A, Bajić D, Osborne ML, Poyatos JF, Sanchez A (2019) High-order interactions distort the functional landscape of microbial consortia. PLoS Biol 17(12):1–34
Santoyo G, Guzmán-Guzmán P, Parra-Cota F, Santos-Villalobos SDL, Orozco-Mosqueda MDC, Glick BR (2021) Plant growth stimulation by microbial consortia. Agronomy 11(219):1–24
Sarma BK, Yadav SK, Singh S, Singh HB (2015) Microbial consortium-mediated plant defense against phytopathogens: readdressing for enhancing efficacy. Soil Biol Biochem 87:25–33
Sathya A, Vijayabharathi R, Gopalakrishnan S (2017) Plant growth promoting Actinobacteria: a new strategy for enhancing sustainable production and protection of grain legumes. 3Biotech 7:102–102
Schikora A, Schenk ST, Hartmann A (2016) Beneficial effects of bacteria-plant communication based on quorum sensing molecules of the N-acyl homoserine lactone group. Plant Mol Biol 90:605–612
Schultenkämper K, Brito LF, Wendisch VF (2020) Impact of CRISPR interference on strain development in biotechnology. Biotechnol Appl Biochem 67(1):7–21
Shahzad SM, Arif MS, Riaz M, Iqbal Z, Ashraf M (2013) PGPR with varied ACC-deaminase activity induced different growth and yield response in maize (Zea mays L.) under fertilized conditions. Eur J Soil Biol 57:27–34
Shanmugam V, Senthil N, Raguchander T, Ramanathan A, Samiyappan R (2002) Interaction of Pseudomonas fluorescens with Rhizobium for their effect on the management of peanut root rot. Phytoparasitica 30:169–176
Sharma CK, Bishnoi VK, Dubey RC, Maheshwari DK (2018) A twin rhizospheric bacterial consortium induces systemic resistance to a phytopathogen Macrophomina phaseolina in mung bean. Rhizosphere 5:71–75
Sindhu SS, Gupta SK, Dadarwal KR (1999) Antagonistic effect of Pseudomonas spp. on pathogenic fungi and enhancement of growth of green gram (Vigna radiata). Biol Fertil Soils 29(1):62–68
Singh BK, Trivedi P, Egidi E, Macdonald CA, Delgado-Baquerizo M (2020) Crop microbiome and sustainable agriculture. Nat Rev Microbiol 18(11):601–602
Singh G, Singh N, Marwaha TS (2009) Crop genotype and a novel symbiotic fungus influences the root endophytic colonization potential of plant growth promoting rhizobacteria. Physiol Mol Biol Plants 15(1):87–92
Singh R, Ryu J, Kim SW (2019) Microbial consortia including methanotrophs: some benefits of living together. J Microbiol 57:939–952
Singha KM, Singh B, Pandey P (2021) Host specific endophytic microbiome diversity and associated functions in three varieties of scented black rice are dependent on growth stage. Sci Rep 11(1):1–17
Skariyachan S, Patil AA, Shankar A, Manjunath M, Bachappanavar N, Kiran S (2018) Enhanced polymer degradation of polyethylene and polypropylene by novel thermophilic consortia of Brevibacillus sps. and Aneurinibacillus sp. screened from waste management landfills and sewage treatment plants. Polym Degrad Stab 149:52–68
Skariyachan S, Setlur AS, Naik SY, Naik AA, Usharani M, Vasist KS (2017) Enhanced biodegradation of low and high-density polyethylene by novel bacterial consortia formulated from plastic-contaminated cow dung under thermophilic conditions. Environ Sci Pollut Res 24:8443–8457
Song H, Ding MZ, Jia XQ, Ma Q, Yuan YJ (2014) Synthetic microbial consortia: from systematic analysis to construction and applications. Chem Soc Rev 43(20):6954–6981
Stockwell VO, Johnson KB, Sugar D, Loper JE (2011) Mechanistically compatible mixtures of bacterial antagonists improve biological control of fire blight of pear. Phytopathology 101:113–112
Stringlis IA, Zhang H, Pieterse CM, Bolton MD, de Jonge R (2018) Microbial small molecules–weapons of plant subversion. Nat Prod Rep 35(5):410–433
Subhashchandrabose SR, Ramakrishan B, Megharaj M, Venkatesawrlu K, Naidu R (2011) Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnol Adv 29(6):896–907
Tabacchioni S, Passato S, Ambrosino P, Huang L, Caldara M, Cantale C, Schlüter A (2021) Identification of beneficial microbial consortia and bioactive compounds with potential as plant biostimulants for a sustainable agriculture. Microorganisms 9(2):426
Tshikantwa TS, Ullah MW, He F, Yang G (2018) Current trends and potential applications of microbial interactions for human welfare. Front Microbiol 9:1156
Van Ittersum MK, Cassman KG, Grassini P, Wolf J, Tittonell P, Hochman Z (2013) Yield gap analysis with local to global relevance—a review. Field Crops Res 1(143):4–17
Vandana UK, Rajkumari J, Singha LP, Satish L, Alavilli H, Sudheer PD, Pandey P (2021) The endophytic microbiome as a hotspot of synergistic interactions, with prospects of plant growth promotion. Biology 10(2):101
Verbruggen E, van der Heijden MGA, Rillig MC, Kiers ET (2013) Mycorrhizal fungal establishment in agricultural soils: factors determining inoculation success. New Phytol 197:1104–1109
Voges M (2019) Molecular principles to engineer plant microbiomes. Stanford University, Stanford, CA, pp 1–24
Vorholt JA, Vogel C, Carlström CI, Müller DB (2017) Establishing causality: opportunities of synthetic communities for plant microbiome research. Cell Host Microbe 22(2):142–155
Wang CJ, Yang W, Wang C, Gu C, Niu DD, Liu HX (2012) Induction of drought tolerance in cucumber plants by a consortium of three plant growth-promoting rhizobacterium strains. PLoS One 7(12):1–10
Wicaksono WA, Jones EE, Casonato S, Monk J, Ridgway HJ (2018) Biological control of Pseudomonas syringae pv. actinidiae (Psa), the causal agent of bacterial canker of kiwifruit, using endophytic bacteria recovered from a medicinal plant. Biol Control 116:103–112
Woo SL, Pepe O (2018) Microbial consortia: promising probiotics as plant biostimulants for sustainable agriculture. Front Plant Sci 9(1801):1–13
Xia Y, Farooq MA, Javed MT, Kamran MA, Mukhtar T, Ali J, Chaudhary HJ (2020) Multi-stress tolerant PGPR Bacillus xiamenensis PM14 activating sugarcane (Saccharum officinarum L.) red rot disease resistance. Plant Physiol Biochem 151:640–649
Xue D, Christenson R, Genger R, Gevens A, Lankau RA (2018) Soil microbial communities reflect both inherent soil properties and management practices in Wisconsin potato fields. Am J Potato Res 95:696–708
York A (2018) Pick of the crop microbiome. Nat Rev Microbiol 16(10):583–583
Zhang J, Liu YX, Zhang N, Hu B, Jin T, Xu H, Guo X (2019a) NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat Biotechnol 37(6):676–684
Zhang LN, Wang DC, Hu Q, Dai XQ, Xie YS, Li Q, Liu HM, Guo JH (2019b) Consortium of plant growth-promoting rhizobacteria strains suppresses sweet pepper disease by altering the rhizosphere microbiota. Front Microbiol 10(1668):1–10
Acknowledgments
PP acknowledges DBT, Govt of India; and AD acknowledges DST-INSPIRE, Govt of India for financial assistances.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Maheshwari, D.K., Das, A., Dheeman, S., Pandey, P. (2023). An Overall Insight Into the Attributes, Interactions, and Future Applications of “Microbial Consortium” for Plant Growth Promotion with Contemporary Approaches. In: Maheshwari, D.K., Dheeman, S. (eds) Sustainable Agrobiology. Microorganisms for Sustainability, vol 43. Springer, Singapore. https://doi.org/10.1007/978-981-19-9570-5_1
Download citation
DOI: https://doi.org/10.1007/978-981-19-9570-5_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-9569-9
Online ISBN: 978-981-19-9570-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)