Abstract
The renal tubules are the major component of the kidney and are vulnerable to a variety of injuries including ischemia, proteinuria, toxins, and metabolic disorders. It has long been believed that tubules are the victim of injury. In this review, we shift this concept to renal tubules as a driving force in the progression of kidney disease. In response to injury, tubular epithelial cells (TECs) can synthesize and secrete varieties of bioactive molecules that drive interstitial inflammation and fibrosis. Innate immune-sensing receptors on the TECs also aggravate immune responses. Necroinflammation, an auto-amplification loop between tubular cell death and interstitial inflammation, leads to the exacerbation of renal injury. Furthermore, TECs also play an active role in progressive renal injury via mechanisms associated with the conversion into collagen-producing fibroblast phenotype, cell cycle arrest at both G1/S and G2/M checkpoints, and metabolic disorder. Thus, a better understanding the mechanisms by which tubular injury drives AKI and CKD is necessary for the development of therapeutics to halt the progression of CKD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The renal tubules and tubulointerstitium make up a significant portion of the kidney and are the major sites in response to injuries. Increasing evidence shows that tubular epithelial cells (TECs) play diverse roles in renal repair or progression to chronic kidney disease (CKD). The innate immune characteristics demonstrate TECs as immune responders to a wide range of insults, with the consequent production and release of bioactive molecules that drive interstitial inflammation and fibrosis. Accumulating evidence shows that renal function decline correlates better with tubulointerstitial damage than that of glomerular injury (Risdon et al. 1968; Bohle et al. 1979; Mackensen-Haen et al. 1981). Maladaptive repair of injured tubules after acute kidney injury (AKI) also leads to the progression of CKD (Ferenbach and Bonventre 2015; Venkatachalam et al. 2015). Thus, TECs should be regarded not only as victims in the context of kidney disease but also as key inflammatory and fibrogenic cells that drive the progression from acute to chronic kidney disease, which will be the focus in this review. It should be noted that due to the length limitations, this review focuses on the emerging mechanisms by which TECs play a driving role in renal injury, whereas other potentially important factors/pathways not directly related to this topic are not discussed here.
2 Tubule-Derived Factors Associated with Tubulointerstitial Inflammation and Fibrosis
In response to stress and injury, TECs can be transformed into a secretory phenotype, with the consequent production and release of various bioactive molecules to favor the recruitment of inflammatory cells, the activation of fibroblasts, and the loss of endothelial cells, which eventually drive tubulointerstitial inflammation and fibrosis.
2.1 Pro-inflammatory Cytokines
In response to renal injury, TECs become activated and can actually facilitate the inflammatory response through induction of a variety of pro-inflammatory cytokines (e.g., interleukin, tumor necrosis factor, colony stimulating factor, and growth factor). After the first report of TNF-α and IL-6 produced by TEC following IL-1 stimulation (Jevnikar et al. 1991; Yard et al. 1992), a variety of cytokines produced by activated TECs are known including IL-1β, IL-18, IL-34, IL-16, CSF-1, TWEAK, VEGF, CTGF, and so on. In TECs, NLPR3 inflammasome activation causes the release of mature IL-1β and IL-18 during kidney injury (Leemans et al. 2014; Anders 2016). Observations by Menke and Wang showed that expression of CSF-1 is upregulated in TECs during kidney injury and may be responsible for the polarization of renal macrophages and recovery from AKI (Menke et al. 2009; Wang et al. 2015b; Huen et al. 2015). Baek et al. identified that TEC-derived IL-34 plays a key role in recruiting kidney macrophages and causing persistent kidney injury and the development of CKD (Baek et al. 2015).
2.2 Chemokines
Chemokines are a family of small molecular cytokines with chemotactic activity. TECs are rich sources of CCL subfamily (including MCP-1/CCL2, RANTES/CCL5, and MIP-1/CCL3) and CX3CL subfamily (fractalkine/CX3CL1), which have specific effects on monocytes and monocyte-derived lineages (Chung and Lan 2011). MCP-1/CCL2 is one of the most widely studied chemokines in AKI and CKD (Wang et al. 1997, 2000; Furuichi et al. 2003). CXCL8/IL-8 and CLCL12/SDF-1 are overexpressed after TECs injury and are chemotactic for a number of leukocyte populations (Li and Nord 2002, 2009; Zuk et al. 2014). A recent study reported that CXCL5 is increased in tubular cells following the induction of nephrotoxic nephritis and is responsible for the recruitment of neutrophils during acute renal tissue injury (Disteldorf et al. 2015).
2.3 ROS
It has become clear that oxidative stress contributes to CKD progression via myriad effects (Small et al. 2012; Massy et al. 2009; Nie and Hou 2012). Oxidative stress implies an increased production of reactive oxygen species (ROS), including superoxide anion (\( {{{\text{O}}_{2}}^{ - }} \)), hydrogen peroxide(H2O2), and hydroxyl anion (OH−). In response to multiple stimuli and agonists, mitochondrial dysfunction and NADPH oxidases have been recognized as the major contributors to ROS generation in TECs (Tang and Lai 2012; Sedeek et al. 2013). For instance, Ang II leads to tubular hypertrophy and TECs apoptosis via ROS-dependent mechanisms (Wolf et al. 2001; Leung et al. 2011). Albumin acts through epidermal growth factor receptor to stimulate NADPH oxidase and ROS production. ROS then activates NF-κB, which then ultimately leads to activation of ERK1/ERK2 pathway (Reich et al. 2005). In addition, albumin has also been shown to stimulate tubulointerstitial inflammation via the mROS-mediated activation of Nlrp3 inflammasome (Liu et al. 2014).
2.4 CRP
C-reactive protein (CRP) is an acute-phase protein, which is rapidly synthesized by the liver in response to infection, inflammation, and tissue damage. Besides its use as a biomarker of inflammation, CRP has been recognized as a pathogenic mediator in diabetic kidney disease (Liu et al. 2011), obstructive nephropathy (Li et al. 2011), and AKI (Pegues et al. 2013; Tang et al. 2014; Lai et al. 2016). CRP is also inducible by high glucose in human TECs and promotes renal inflammation and fibrosis through activation of TGF-β/SMAD and NF-κB signaling pathways under diabetic conditions and unilateral ureteral obstructive nephropathy (Liu et al. 2011; Li et al. 2011). Recent studies have demonstrated that CRP promotes AKI by causing TEC G1 cell-cycle arrest via CD32-Smad3–dependent p27-driven inhibition of the cyclin-dependent kinase 2/cyclin E mechanism (Tang et al. 2014; Lai et al. 2016).
2.5 Growth Factors
Transforming growth factor (TGF-β), connective tissue growth factor (CTGF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) are the best-described growth factors involved in the tubulointerstitial fibrosis, of which TGF-β derived from injured TECs has long been considered as one of the most important pro-fibrotic growth factors (Yang et al. 2010; Lan et al. 2012; Geng et al. 2012; Meng et al. 2016; Wu et al. 2013; Grande et al. 2015). Both Ang II exposure and Snail 1 overexpression can induce TGF-β1 production by TECs (Grande et al. 2015; Macconi et al. 2014). After the hypoxic injury, TECs undergo cell cycle arrest. Particularly when cells are under arrest in the G2/M phase, these cells produce large amounts of TGF-β1 (Yang et al. 2010). Increased TGF-β production by TECs can promote TIF through paracrine signaling to activate adjacent fibroblasts and pericytes transforming into myofibroblast-type cells (Wu et al. 2013; Ignotz et al. 1987; Roberts et al. 1986). Interestingly, TEC is also a target of TGF-β1. TGF-β1 can induce cultured TECs to differentiate into cells with distinct myofibroblast morphology and marked upregulation of collagen production (Zeisberg et al. 2003; Fan et al. 1999). Meanwhile, autocrine TGF-β signaling increases TEC production of PDGF-β and CTGF/CCN2 that can signal on neighboring fibroblasts (Geng et al. 2012).
2.6 Intrarenal RAS
Renal local renin–angiotensin system (RAS) activation plays a pivotal role in the progression of CKD. Blockade of the RAS has become the mainstay therapy for the preservation of CKD (Hou et al. 2006). Ang II is the major bioactive product of the RAS driving renal fibrosis. There is substantial evidence that the major fraction of Ang II present in renal tissues is generated from angiotensinogen (AGT) and subsequently delivered to the kidney, as well as from AGT produced by the PTECs. Ang I delivered to the kidney can also be converted to Ang II (Kobori et al. 2007). Renin mRNA and renin-like activity have been observed in cultured PTECs (Henrich et al. 1996). The brush border membrane of proximal human kidney tubules also expresses abundant levels of angiotensin-converting enzyme (ACE) mRNA (Sibony et al. 1993) and protein (Vío and Jeanneret 2003). ACE has been detected in the proximal and distal tubular fluids (Casarini et al. 1997). Therefore, all of the major components required to generate Ang II are expressed within the renal tubules (Urushihara and Kagami 2017; Kobori and Urushihara 2013). And the upregulation of these RAS components may be in a Wnt/β-catenin-dependent manner (Zhou et al. 2015). Studies have demonstrated that Ang II stimulates TGF-β expression in cultured murine PTECs and upregulates specific receptors for TGF-β to further enhance its pro-inflammatory and fibrogenic action (Wolf et al. 1993, 1999; Liu et al. 2009). Ang II is also able to induce CTGF to mediate the fibrotic phenotype change (Liu et al. 2006, 2007; Chen et al. 2006). Moreover, we also proposed the interaction of Ang II and inflammation might be the critical node in the pathogenic tubuloglomerular feedback loop (Zhang and Liu 2011).
2.7 Wnt and Hh
The Wnt pathway has been implicated in the epithelial repair process, but an abundance of evidence also supports Wnt/β-catenin signaling in tubulointerstitial fibrosis (Kang et al. 2016; Kawakami et al. 2013; Tan et al. 2016; Edeling et al. 2016). There are 19 Wnt ligands, and all of them can bind to Frizzled and LRP5/6 receptors at the cell surface, leading to canonical signaling through β-catenin activation (Tan et al. 2014). Wnt proteins and receptors are upregulated after renal injury, and β-catenin activity appears to be increased in injured TECs (Zhou et al. 2012; He et al. 2009). Overexpression of Wnt1 in proximal tubules is sufficient to cause TIF and activate myofibroblasts to produce ECM, suggesting paracrine signaling (Maarouf et al. 2016). It is likely that injured TECs can produce Wnt ligands which then activate the neighboring fibroblasts to promote TIF (Gewin et al. 2017).
Hedgehog (Hh) signaling is a key mammalian developmental pathway and regulates tissue patterning, cell growth, and differentiation (Cain and Rosenblum 2011; Mao et al. 2010). Of three Hh ligands (Sonic Hh [Shh], Desert Hh [Dhh], and Indian Hh [Ihh]), Shh is well studied. Lineage tracing studies indicate that Shh and Ihh expression are upregulated in renal tubules after UUO (Fabian et al. 2012; Ding et al. 2012; Zhou et al. 2014). Interstitial fibroblasts and pericytes are the cells supposed to respond to these ligands. Shh induces fibroblast activation, manifested as an expression of α-SMA, fibronectin, collagen, and desmin (Ding et al. 2012).
2.8 Exosomes
Exosomes are small (30–100 nm in diameter), lipid bilayer membrane vesicles of endocytic origin. They can shuttle bioactive molecules including proteins, lipids, DNA, mRNA, and microRNAs (Zhang et al. 2016; Morrison et al. 2016). In kidneys, renal exosomes are produced and secreted by kidney cells which have been implicated in renal function and diseases via cell–cell communication (Krause et al. 2015). It is known that injured TECs can release exosomes containing TGF-β mRNA to activate fibroblasts, contributing to the development of renal fibrosis in post-AKI kidneys (Borges et al. 2013). We recently demonstrated that in the setting of proteinuric kidney disease, albumin triggered TECs to release exosomes packaged with CCL-2 mRNA, which was delivered to macrophages and led to interstitial inflammation (Lv et al. 2018). In addition, we also found that the HIF-1α-dependent release of miRNA-23a-enriched exosomes from hypoxic TECs activates macrophages to promote tubulointerstitial inflammation (Li et al. 2019).
3 Abnormal Repair of TECs: The Central Pathology Linking AKI to CKD
An increasing number of epidemiological studies have suggested that incomplete recovery from AKI can lead to progressive CKD (Waikar and Winkelmayer 2009; Okusa et al. 2009; Hsu 2012; Coca et al. 2012). This is supported by the finding that the incomplete tubular repair is tightly associated with persistent tubulointerstitial inflammation, proliferation of fibroblasts, and excessive deposition of extracellular matrix (Yang et al. 2010; Grgic et al. 2012). A number of recent studies have also demonstrated that tubule selective injury is sufficient to drive fibrosis, inflammation, and capillary rarefaction, which is making it to be a central link between AKI and CKD (Grgic et al. 2012; Takaori et al. 2016; Zhou et al. 2014; Humphreys et al. 2013).
In general, primary tubular injuries have a very good chance of recovery. The surviving cells dedifferentiate, migrate along the basement membrane, proliferate to restore cell number, and then restore the functional integrity of the nephron (Thadhani et al. 1996). However, some damaged TECs become atrophic or gain the fibrotic phenotype after AKI. This may be tightly associated with the abnormal repair process in response to the injuries. For example, in the initial repair phase after injury, TECs may become arrested in the G2/M phase, which may be associated with the activation of JNK signaling production of pro-fibrotic cytokine (Yang et al. 2010; Ferenbach and Bonventre 2015). This is confirmed by the ability of using pharmacological inhibition of G2/M-arrested cells with histone deacetylase inhibitors or p53 inhibition to block the process of fibrosis (Cianciolo Cosentino et al. 2013; Zhou et al. 2010). Recent studies also found that aging can sensitize TECs to be arrested at the cell cycle G2/M in response to cell stress and DNA damage, which provides a potential explanation for the increased risk of CKD progression after AKI in the elderly (Ferenbach and Bonventre 2015; Verzola et al. 2008; Liu et al. 2012; Yang and Fogo 2010). In addition, CRP-induced G1/S cell cycle arrest may also contribute to progressive TIF via the Smad3-p21/p27-dependent mechanism (Tang et al. 2014; Lai et al. 2016).
Wnt/β-catenin signaling is a pathway involving the recovery from AKI. In the acute phase of injury, Wnt/β-catenin is likely to be protective. In both IRI and folic acid nephropathy, tubule-specific ablation of β-catenin has been shown to aggravate kidney injury by increasing TEC apoptosis (Zhou et al. 2012). Activation of Wnt-4/β-catenin signaling allows entry into the cell cycle via the upregulation of cyclin D1 and cyclin A, two of the most crucial proteins in regulating cell proliferation and cell cycle progression (Terada et al. 2003; Angers and Moon 2009; Clevers and Nusse 2012). Therefore, an early and appropriate activation of Wnt/β-catenin signaling is required for minimizing the initial renal damages after AKI (Zhou et al. 2016). However, persistent activation of Wnt signaling has a decisive role in driving AKI to CKD progression because sustained Wnt signaling causes uncontrolled fibroblast activation, RAS activation, inflammation, and excessive deposition of ECM (Tan et al. 2014; Xiao et al. 2016). It is well known that tissue injury and inflammation are closely linked and interact with each other (Wallach et al. 2014). While initial renal inflammation may be protective in favoring the repair process in response to AKI, unresolved and prolonged renal inflammation may cause progressive renal fibrosis. Thus, better understanding the mechanisms by which tubular injury drives interstitial inflammation and renal fibrosis is of paramount importance.
4 Emerging Mechanisms of Tubule Injury Driving the Progression of CKD
4.1 Inflammation: Innate Immune-Sensing Receptors in TECs Activation
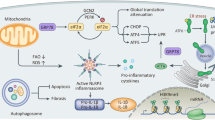
Uncontrolled or excessive inflammatory responses can lead to progressive kidney injury. In view of the immune characteristics of TECs, substantial information indicates that Toll-like receptors (TLRs), Nod-like receptors (NLRs) and the NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) inflammasome have important roles in the pathogenesis of multiple renal disorders (Leemans et al. 2014). TLRs are a family of transmembrane receptors and the signal transduction initiated from TLRs activates effector cells via several kinases and NF-κB-dependent mechanisms (Gluba et al. 2010). TLRs are widely expressed in TECs. For instance, TECs are known to express both TLR2 and TLR4, and both TLR2 and TLR4 signaling are activated during IRI (Wu et al. 2010; Allam et al. 2012; Wolfs et al. 2002), sepsis-induced AKI (El-Achkar and Dagher 2006; El-Achkar et al. 2006; Dear et al. 2006), diabetic nephropathy (Lin et al. 2012, 2013; Mudaliar et al. 2013; Devaraj et al. 2011), unilateral ureter obstruction (Pulskens et al. 2010; Leemans et al. 2009; Campbell et al. 2011; Skuginna et al. 2011). Necrotic tubular cells release high-mobility group box1 protein (HMGB1), histones, heat-shock proteins, and other DAMPs that activate TLR2 and TLR4 on renal parenchymal cells and drive inflammation (Wu et al. 2010; Allam et al. 2012; Leemans et al. 2005). We also found that albumin might serve as an endogenous DAMP to trigger the activation of TLR2-MyD88-NF-κB pathway and pro-inflammatory cytokine TNF-α and IL-6 secretion (Ding et al. 2015) (Fig. 11.1). NLRs are cytoplasmic receptors. Shigeoka and co-workers showed that Nod1 and Nod2 are present in TECs in both mouse and human kidneys and that the absence of these receptors can protect the kidney from AKI by inhibiting TEC apoptosis and inflammation (Shigeoka et al. 2010).
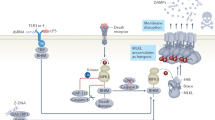
Landscape of interstitial inflammation caused by damaged TECs. In response to injury, damaged TECs release various kinds of DAMPs that activate innate immunity through identical pattern recognition receptors including TLRs and inflammasomes, with the consequent production and release of cytokines and chemokines to recruit inflammatory cell infiltration in the interstitium, which eventually drive interstitial inflammation and fibrosis. Some injury factors can also be seen as DAMPs (such as albumin). In addition, TNF-α and possibly other cytokines drive necroptosis as a secondary cell death category contributing to tubular necrosis and renal dysfunction. This sets up the auto-amplification loop of necroinflammation
In addition, emerging evidence suggests an important role for NLRP3 inflammasome and IL-1β/IL-18 in the pathogenesis of acute and chronic inflammation and tissue remodeling in the kidney (Anders and Muruve 2011; Chang et al. 2014) (Fig. 11.1). Upregulation of the NLRP3 inflammasome is demonstrated in both classical immune cells as well as in TECs in a wide variety of tubulointerstitial disease (Anders and Muruve 2011; Chang et al. 2014). We recently found that proteinuria causes NLRP3 inflammasome activation and IL-1β/IL-18 maturation in a time course and dose-dependent manner in the proximal tubules (Liu et al. 2014). Further investigation indicated that megalin/cubilin-mediated albumin retention and lysosomal rupture are involved in the activation of NLRP3 inflammasome and interstitial inflammation (Liu et al. 2015). Moreover, Ang II has also been shown to induce NLRP3 inflammasome activation in TECs, which is associated with mitochondrial dysfunction or ER stress (Wang et al. 2015a; Wen et al. 2016). Thus, activation of the inflammasome pathway may represent a new mechanism of tubulointerstitial inflammation.
4.2 Necroinflammation: An Auto-Amplification Loop Between Tubular Injury and Tubulointerstitial Inflammation
Necroinflammation is a new pathological auto-amplification loop driven by necrosis (defined by cell death involving rupture of the plasma membrane) and inflammation (defined by cytokine release, increased vascular permeability, and recruitment of immune effector cells) (Linkermann et al. 2014; Mulay et al. 2016b). Following this pathological process, ischemia, toxins, and proteinuria can trigger tubulointerstitial inflammation, and in turn, tubulointerstitial inflammation causes TECs injury, which leads to an aggravation of interstitial inflammation (Fig. 11.1).
How TECs necrosis induces tubulointerstitial inflammation? In the last decade, it was unraveled that injured cells release DAMPs that activate innate immunity through identical pattern recognition receptors including TLRs and inflammasomes (Anders and Schaefer 2014). As mentioned above, this process is also involved in kidney inflammation and immunopathology (Anders and Muruve 2011; Anders et al. 2004; Anders 2010). AKI is most frequently associated with cell necrosis that implies DAMPs release. For example, ischemic, septic, or toxic forms of tubular necrosis can induce HMGB1, histones, heat-shock proteins, and other DAMPs release, which activate TLR2 and TLR4 on renal parenchymal cells and inflammatory cells to drive inflammation (Allam et al. 2012; Leelahavanichkul et al. 2011; Rabadi et al. 2012; Wu et al. 2010; Arumugam et al. 2009). Deficiency of receptor-interacting protein kinase 3 (RIPK3) or mixed lineage kinase domain-like (MLKL), two core proteins of the necroptosis pathway, blocks oxalate crystal-induced AKI and inflammation (Mulay et al. 2016a).
How tubulointerstitial inflammation induces TECs necrosis? DAMPs released by dying cells activate the pattern recognition receptors of infiltrating immune cells and intrinsic renal parenchymal cells and induce the release of numerous pro-inflammatory mediators. In particular, TNF-α and IFN-γ can induce necroptosis via two distinct pathways (Dannappel et al. 2014; Takahashi et al. 2014; Vanden Berghe et al. 2014). Mulay et al. showed that oxalate crystal formation inside tubules induced TNF-α secretion, which could activate the RIPK1, RIPK3, and MLKL pathway of necroptosis via TNFR1. And blocking either TNF-α or TNFR1 could abrogate kidney injury and dysfunction (Mulay et al. 2016a). Furthermore, the NLRP3 inflammasome activation not only triggers cytokine release but also pyroptosis, as a consequence of inflammasome-driven caspase-11 activation (Bergsbaken et al. 2009; Case et al. 2013). But if pyroptosis can occur in TECs is under debate (Krautwald and Linkermann 2014; Yang et al. 2014) (Fig. 11.1).
4.3 Partial Epithelial–Mesenchymal Transition (EMT)
TECs might directly contribute to renal fibrosis via EMT, a phenotypic conversion program that is characterized by the loss of epithelial markers (such as E-cadherin, zonula occludens-1 [ZO-1] and cytokeratin) and gain of mesenchymal features (including vimentin, α-smooth muscle actin [α-SMA], fibroblast-specific protein-1 [FSP1], interstitial matrix components type I collagen, and fibronectin) (Liu 2004; Strutz 2009). Historical data and recent new findings have suggested that renal fibrosis might occur as a result of the tubular epithelial cells injury. In response to this, TECs produce various chemokines and cytokines around peritubular compartments to attract and direct the influx of inflammatory cells to the tubulointerstitial space. Infiltrating cells in turn activate and produce a mixture of soluble factors, including pro-inflammatory, pro-fibrotic cytokines, and MMPs. Altered microenvironment contributes to the reshaping of the mesenchymal cell phenotype, and rendering TECs adaptable to changing cell phenotype for the sake of escaping apoptosis (Prunotto et al. 2012; Liu 2010).
However, the precise contribution of the EMT to kidney fibrosis remains a subject of debate, as studies using genetic cell lineage tracing could not find evidence of a direct contribution of epithelial cells to the myofibroblast population in the fibrotic kidney (Humphreys et al. 2010). Two studies recently addressed this dispute and offered new insights into the potential role of tubular EMT in the development and progression of renal fibrosis (Ovadya and Krizhanovsky 2015; Zhou and Liu 2016). The transcription factors Snail 1 and Twist are the main regulators of the EMT program. Grande et al. (2015) focus on Snail 1, whereas Lovisa et al. (2015) carried out experiments with both Snail 1 and Twist. By conditional deletion of Snail 1 or Twist in TECs, the EMT is specifically inhibited. As a result, fibrosis is reduced in several CKD models, including unilateral ureter obstruction, nephrotoxic serum-induced nephritis, and folic acid-induced nephropathy. And improvement of renal fibrosis also led to the preservation of tubular cell integrity and function. Interestingly, both studies found that TECs undergo incomplete EMT during renal fibrosis—the cells express markers of both epithelial and mesenchymal cells and remain associated with their basement membrane. In this respect, these observations are in harmony with earlier genetic cell lineage tracing studies and demonstrate that partial EMT is sufficient to induce tubular function impairment, triggering cell cycle arrest, and promoting the release of critical fibrogenic cytokines, although evidence for partial EMT in human CKD is rare.
4.4 Cell Cycle Arrest
A series of elegant studies have identified that G1/S and G2/M arrest in TECs is an important driver of maladaptive TECs repair and renal fibrosis, providing a link between AKI and CKD (Yang et al. 2010; Cianciolo Cosentino et al. 2013; Tang et al. 2013). Yang et al. demonstrated a causal association between epithelial cell cycle G2/M arrest and a fibrotic outcome in toxic and obstructive models of AKI. G2/M-arrested PTECs activate JNK signaling, which acts to upregulate pro-fibrotic cytokine (TGF-β1 and CTGF) production (Yang et al. 2010). Canaud et al. further identified PTECs in the G2/M phase form target of rapamycin–autophagy spatial coupling compartments, which facilitate pro-fibrotic secretion similar to the senescence-associated secretory phenotype (Canaud et al. 2019). Targeting the G2/M checkpoint to maintain the proper progression of TECs through the cell cycle during the injury phase has been proposed as an attractive therapeutic target to prevent the progression of CKD (Canaud and Bonventre 2015). Cianciolo Cosentino et al. reported that a histone acetylase inhibitor could reduce the number of cells in G2/M arrest and reduce post-injury tubular atrophy and interstitial fibrosis (Cianciolo Cosentino et al. 2013). Jenkins et al. suggested that miR-192 has an important role in aristolochic acid-induced G2/M arrest (Jenkins et al. 2014). Interestingly, the induction of a transient G0/G1 arrest in TECs with the CDK4/6 inhibitor PD0332991 before IRI ameliorated kidney injury by preventing apoptosis and pro-fibrotic cytokine production (DiRocco et al. 2014).
As previously discussed, the functional consequences of EMT during fibrotic injury are the induction of the G2 phase arrest of TECs (Lovisa et al. 2015). Genetic inhibition of EMT by knocking out Twist and Snail 1, resulted in a substantial decrease in the G2/M-arrested TECs. In vitro induction of EMT with TGF- β1 also induced G2/M arrest in TECs (Wu et al. 2013; Lovisa et al. 2015). Furthermore, it was found that the G2 arrest was mediated by the cell cycle inhibitor p21 (Lovisa et al. 2015). And it is in line with a finding that p21 in kidney proximal tubules mediates fibrosis (Megyesi et al. 2015).
4.5 Metabolic Disorder
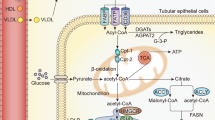
The intracellular accumulation of excess non-esterified fatty acid (NEFA) and metabolites in TECs, namely lipotoxicity, can result in renal dysfunction, especially in the context of diabetic nephropathy (Schelling 2016; Kimmelstiel and Wilson 1936; Oliver et al. 1954; Herman-Edelstein et al. 2014). Several groups have shown that proximal tubule uptake of filtered NEFAs is the source of tubular toxicity in case of glomerular damage. Tubulointerstitial damage can be induced in rats by infusion of NEFA-loaded albumin and in vitro incubation with albumin-bound NEFAs stimulate PTEC apoptosis (Thomas et al. 2002; Kamijo et al. 2002; van Timmeren et al. 2005). Tubular cells have a high level of energy demand and the ATP that they use is mostly produced by fatty acid oxidation. New findings indicate that dysregulation of fatty acid oxidation followed intracellular lipid accumulation profoundly affects the fate of TECs, by promoting EMT, inflammation, and eventually interstitial fibrosis (Kang et al. 2015). They also investigated the mechanisms behind the depressed metabolic pathways in fibrotic kidney disease and further demonstrated that TGF-β1 inhibits the expression of carnitine palmitoyltransferase 1 (CPT1), the rate-limiting enzyme in FAO, and thereby decreases fatty acid metabolism (Kang et al. 2015). Furthermore, miR-21 is shown to be implicated in the regulation of metabolic pathways recently (Trionfini et al. 2015; Chau et al. 2012). miR-21 promotes tubular injury and fibrosis by downregulating PPARα, with consequent alterations of TEC lipid metabolism. Inhibition of miR-21 reduces TGF-β-induced fibrogenesis and inflammation, preserves tubular integrity, as a result of enhanced PPARα/RXR activity and improved mitochondrial function (Gomez et al. 2015).
5 Conclusion
In this review, we shift TECs from the victim of injury to a driving force in the progression from AKI to CKD. Damaged TECs can contribute directly to interstitial inflammation and fibrosis through various kinds of mechanisms (Fig. 11.2). Thus, protecting tubules from repeated injury and restoring healthy tubular function may be the priority of treatment of kidney diseases. Although the mechanisms of tubular injury remain to be elucidated, the G1/S and G2/M cell cycle arrest may be a pivotal obstacle to the adaptive repair of injured TECs and targeting the G1/S and G2/M checkpoint to maintain the proper cell cycle transition may be an attractive therapeutic target to prevent the progression of CKD.
References
Allam R, Scherbaum CR, Darisipudi MN, Mulay SR, Hägele H, Lichtnekert J et al (2012) Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol 23:1375–1388
Anders HJ (2010) Toll-like receptors and danger signaling in kidney injury. J Am Soc Nephrol 21:1270–1274
Anders HJ (2016) Of Inflammasomes and alarmins: IL-1β and IL-1α in kidney disease. J Am Soc Nephrol 27:2564–2575
Anders HJ, Muruve DA (2011) The inflammasomes in kidney disease. J Am Soc Nephrol 22:1007–1018
Anders HJ, Schaefer L (2014) Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol 25:1387–1400
Anders HJ, Banas B, Schlöndorff D (2004) Signaling danger: toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol 15:854–867
Angers S, Moon RT (2009) Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10:468–477
Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM (2009) Toll-like receptors in ischemia-reperfusion injury. Shock 32:4–16
Baek JH, Zeng R, Weinmann-Menke J, Valerius MT, Wada Y, Ajay AK et al (2015) IL-34 mediates acute kidney injury and worsens subsequent chronic kidney disease. J Clin Invest 125:3198–3214
Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7:99–109
Bohle A, Christ H, Grund KE, Mackensen S (1979) The role of the interstitium of the renal cortex in renal disease. Contrib Nephrol 16:109–114
Borges FT, Melo SA, Özdemir BC, Kato N, Revuelta I, Miller CA et al (2013) TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol 24:385–392
Cain JE, Rosenblum ND (2011) Control of mammalian kidney development by the Hedgehog signaling pathway. Pediatr Nephrol 26:1365–1371
Campbell MT, Hile KL, Zhang H, Asanuma H, Vanderbrink BA, Rink RR et al (2011) Toll-like receptor 4: a novel signaling pathway during renal fibrogenesis. J Surg Res 168:e61–e69
Canaud G, Bonventre JV (2015) Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol Dial Transplant 30:575–583
Canaud G, Brooks CR, Kishi S, Taguchi K, Nishimura K, Magassa S et al (2019) Cyclin G1 and TASCC regulate kidney epithelial cell G2-M arrest and fibrotic maladaptive repair. Sci Transl Med 11
Casarini DE, Boim MA, Stella RC, Krieger-Azzolini MH, Krieger JE, Schor N (1997) Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol 272:F405–F409
Case CL, Kohler LJ, Lima JB, Strowig T, de Zoete MR, Flavell RA et al (2013) Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc Natl Acad Sci U S A 110:1851–1856
Chang A, Ko K, Clark MR (2014) The emerging role of the inflammasome in kidney diseases. Curr Opin Nephrol Hypertens 23:204–210
Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G et al (2012) MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med 4:121ra18
Chen L, Liu BC, Zhang XL, Zhang JD, Liu H, Li MX (2006) Influence of connective tissue growth factor antisense oligonucleotide on angiotensin II-induced epithelial mesenchymal transition in HK2 cells. Acta Pharmacol Sin 27:1029–1036
Chung AC, Lan HY (2011) Chemokines in renal injury. J Am Soc Nephrol 22:802–809
Cianciolo Cosentino C, Skrypnyk NI, Brilli LL, Chiba T, Novitskaya T, Woods C et al (2013) Histone deacetylase inhibitor enhances recovery after AKI. J Am Soc Nephrol 24:943–953
Clevers H, Nusse R (2012) Wnt/β-catenin signaling and disease. Cell 149:1192–1205
Coca SG, Singanamala S, Parikh CR (2012) Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 81:442–448
Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L et al (2014) RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 513:90–94
Dear JW, Yasuda H, Hu X, Hieny S, Yuen PS, Hewitt SM et al (2006) Sepsis-induced organ failure is mediated by different pathways in the kidney and liver: acute renal failure is dependent on MyD88 but not renal cell apoptosis. Kidney Int 69:832–836
Devaraj S, Tobias P, Kasinath BS, Ramsamooj R, Afify A, Jialal I (2011) Knockout of toll-like receptor-2 attenuates both the proinflammatory state of diabetes and incipient diabetic nephropathy. Arterioscler Thromb Vasc Biol 31:1796–1804
Ding H, Zhou D, Hao S, Zhou L, He W, Nie J et al (2012) Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol 23:801–813
Ding LH, Liu D, Xu M, Wu M, Liu H, Tang RN et al (2015) TLR2-MyD88-NF-κB pathway is involved in tubulointerstitial inflammation caused by proteinuria. Int J Biochem Cell Biol 69:114–120
DiRocco DP, Bisi J, Roberts P, Strum J, Wong KK, Sharpless N et al (2014) CDK4/6 inhibition induces epithelial cell cycle arrest and ameliorates acute kidney injury. Am J Physiol Renal Physiol 306:F379–F388
Disteldorf EM, Krebs CF, Paust HJ, Turner JE, Nouailles G, Tittel A et al (2015) CXCL5 drives neutrophil recruitment in TH17-mediated GN. J Am Soc Nephrol 26:55–66
Edeling M, Ragi G, Huang S, Pavenstädt H, Susztak K (2016) Developmental signalling pathways in renal fibrosis: the roles of Notch, Wnt and Hedgehog. Nat Rev Nephrol 12:426–439
El-Achkar TM, Dagher PC (2006) Renal Toll-like receptors: recent advances and implications for disease. Nat Clin Pract Nephrol 2:568–581
El-Achkar TM, Huang X, Plotkin Z, Sandoval RM, Rhodes GJ, Dagher PC (2006) Sepsis induces changes in the expression and distribution of Toll-like receptor 4 in the rat kidney. Am J Physiol Renal Physiol 290:F1034–F1043
Fabian SL, Penchev RR, St-Jacques B, Rao AN, Sipilä P, West KA et al (2012) Hedgehog-Gli pathway activation during kidney fibrosis. Am J Pathol 180:1441–1453
Fan JM, Ng YY, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC et al (1999) Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int 56:1455–1467
Ferenbach DA, Bonventre JV (2015) Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 11:264–276
Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H et al (2003) CCR39 signaling contributes to ischemia-reperfusion injury in kidney. J Am Soc Nephrol 14:2503–2515
Geng H, Lan R, Singha PK, Gilchrist A, Weinreb PH, Violette SM et al (2012) Lysophosphatidic acid increases proximal tubule cell secretion of profibrotic cytokines PDGF-B and CTGF through LPA2- and Gαq-mediated Rho and αvβ6 integrin-dependent activation of TGF-β. Am J Pathol 181:1236–1249
Gewin L, Zent R, Pozzi A (2017) Progression of chronic kidney disease: too much cellular talk causes damage. Kidney Int 91:552–560
Gluba A, Banach M, Hannam S, Mikhailidis DP, Sakowicz A, Rysz J (2010) The role of Toll-like receptors in renal diseases. Nat Rev Nephrol 6:224–235
Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S et al (2015) Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J Clin Invest 125:141–156
Grande MT, Sánchez-Laorden B, López-Blau C, De Frutos CA, Boutet A, Arévalo M et al (2015) Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med 21:989–997
Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T et al (2012) Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int 82:172–183
He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y (2009) Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20:765–776
Henrich WL, McAllister EA, Eskue A, Miller T, Moe OW (1996) Renin regulation in cultured proximal tubular cells. Hypertension 27:1337–1340
Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U (2014) Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res 55:561–572
Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR et al (2006) Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med 354:131–140
Hsu CY (2012) Yes, AKI truly leads to CKD. J Am Soc Nephrol 23:967–969
Huen SC, Huynh L, Marlier A, Lee Y, Moeckel GW, Cantley LG (2015) GM-CSF promotes macrophage alternative activation after renal ischemia/reperfusion injury. J Am Soc Nephrol 26:1334–1345
Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV et al (2010) Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176:85–97
Humphreys BD, Xu F, Sabbisetti V, Grgic I, Movahedi Naini S, Wang N et al (2013) Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest 123:4023–4035
Ignotz RA, Endo T, Massagué J (1987) Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem 262:6443–6446
Jenkins RH, Davies LC, Taylor PR, Akiyama H, Cumbes B, Beltrami C et al (2014) miR-192 induces G2/M growth arrest in aristolochic acid nephropathy. Am J Pathol 184:996–1009
Jevnikar AM, Brennan DC, Singer GG, Heng JE, Maslinski W, Wuthrich RP et al (1991) Stimulated kidney tubular epithelial cells express membrane associated and secreted TNF alpha. Kidney Int 40:203–211
Kamijo A, Kimura K, Sugaya T, Yamanouchi M, Hase H, Kaneko T et al (2002) Urinary free fatty acids bound to albumin aggravate tubulointerstitial damage. Kidney Int 62:1628–1637
Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F et al (2015) Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med 21:37–46
Kang HM, Huang S, Reidy K, Han SH, Chinga F, Susztak K (2016) Sox9-Positive progenitor cells play a key role in renal tubule epithelial regeneration in mice. Cell Rep 14:861–871
Kawakami T, Ren S, Duffield JS (2013) Wnt signalling in kidney diseases: dual roles in renal injury and repair. J Pathol 229:221–231
Kimmelstiel P, Wilson C (1936) Intercapillary lesions in the glomeruli of the kidney. Am J Pathol 12:83–98
Kobori H, Urushihara M (2013) Augmented intrarenal and urinary angiotensinogen in hypertension and chronic kidney disease. Pflugers Arch 465:3–12
Kobori H, Nangaku M, Navar LG, Nishiyama A (2007) The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 59:251–287
Krause M, Samoylenko A, Vainio SJ (2015) Exosomes as renal inductive signals in health and disease, and their application as diagnostic markers and therapeutic agents. Front Cell Dev Biol 3:65
Krautwald S, Linkermann A (2014) The fire within: pyroptosis in the kidney. Am J Physiol Renal Physiol 306:F168–F169
Lai W, Tang Y, Huang XR, Ming-Kuen Tang P, Xu A, Szalai AJ et al (2016) C-reactive protein promotes acute kidney injury via Smad3-dependent inhibition of CDK2/cyclin E. Kidney Int 90:610–626
Lan R, Geng H, Polichnowski AJ, Singha PK, Saikumar P, McEwen DG et al (2012) PTEN loss defines a TGF-β-induced tubule phenotype of failed differentiation and JNK signaling during renal fibrosis. Am J Physiol Renal Physiol 302:F1210–F1223
Leelahavanichkul A, Huang Y, Hu X, Zhou H, Tsuji T, Chen R et al (2011) Chronic kidney disease worsens sepsis and sepsis-induced acute kidney injury by releasing High Mobility Group Box Protein-1. Kidney Int 80:1198–1211
Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ et al (2005) Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest 115:2894–2903
Leemans JC, Butter LM, Pulskens WP, Teske GJ, Claessen N, van der Poll T et al (2009) The role of Toll-like receptor 2 in inflammation and fibrosis during progressive renal injury. PLoS ONE 4:e5704
Leemans JC, Kors L, Anders HJ, Florquin S (2014) Pattern recognition receptors and the inflammasome in kidney disease. Nat Rev Nephrol 10:398–414
Leung JC, Chan LY, Tang SC, Lam MF, Chow CW, Lim AI et al (2011) Oxidative damages in tubular epithelial cells in IgA nephropathy: role of crosstalk between angiotensin II and aldosterone. J Transl Med 9:169
Li H, Nord EP (2002) CD40 ligation stimulates MCP-1 and IL-8 production, TRAF6 recruitment, and MAPK activation in proximal tubule cells. Am J Physiol Renal Physiol 282:F1020–F1033
Li H, Nord EP (2009) IL-8 amplifies CD40/CD154-mediated ICAM-1 production via the CXCR-1 receptor and p38-MAPK pathway in human renal proximal tubule cells. Am J Physiol Renal Physiol 296:F438–F445
Li ZI, Chung AC, Zhou L, Huang XR, Liu F, Fu P et al (2011) C-reactive protein promotes acute renal inflammation and fibrosis in unilateral ureteral obstructive nephropathy in mice. Lab Invest 91:837–851
Li ZL, Lv LL, Tang TT, Wang B, Feng Y, Zhou LT et al (2019) HIF-1α inducing exosomal microRNA-23a expression mediates the cross-talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int 95:388–404
Lin M, Yiu WH, Wu HJ, Chan LY, Leung JC, Au WS et al (2012) Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol 23:86–102
Lin M, Yiu WH, Li RX, Wu HJ, Wong DW, Chan LY et al (2013) The TLR4 antagonist CRX-526 protects against advanced diabetic nephropathy. Kidney Int 83:887–900
Linkermann A, Stockwell BR, Krautwald S, Anders HJ (2014) Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat Rev Immunol 14:759–767
Liu Y (2004) Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15:1–12
Liu Y (2010) New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 21:212–222
Liu BC, Chen L, Sun J, Huang HQ, Ma KL, Liu H et al (2006) Connective tissue growth factor-mediated angiotensin II-induced hypertrophy of proximal tubular cells. Nephron Exp Nephrol 103:e16–e26
Liu XC, Liu BC, Zhang XL, Li MX, Zhang JD (2007) Role of ERK1/2 and PI3-K in the regulation of CTGF-induced ILK expression in HK-2 cells. Clin Chim Acta 382:89–94
Liu BC, Gao J, Li Q, Xu LM (2009) Albumin caused the increasing production of angiotensin II due to the dysregulation of ACE/ACE2 expression in HK2 cells. Clin Chim Acta 403:23–30
Liu F, Chen HY, Huang XR, Chung AC, Zhou L, Fu P et al (2011) C-reactive protein promotes diabetic kidney disease in a mouse model of type 1 diabetes. Diabetologia 54:2713–2723
Liu J, Yang JR, He YN, Cai GY, Zhang JG, Lin LR et al (2012) Accelerated senescence of renal tubular epithelial cells is associated with disease progression of patients with immunoglobulin A (IgA) nephropathy. Transl Res 159:454–463
Liu D, Xu M, Ding LH, Lv LL, Liu H, Ma KL et al (2014) Activation of the Nlrp3 inflammasome by mitochondrial reactive oxygen species: a novel mechanism of albumin-induced tubulointerstitial inflammation. Int J Biochem Cell Biol 57:7–19
Liu D, Wen Y, Tang TT, Lv LL, Tang RN, Liu H et al (2015) Megalin/cubulin-lysosome-mediated albumin reabsorption is involved in the tubular cell activation of NLRP3 inflammasome and tubulointerstitial inflammation. J Biol Chem 290:18018–18028
Liu BC, Tang TT, Lv LL, Lan HY (2018) Renal tubule injury: a driving force toward chronic kidney disease. Kidney Int 93:568–579
Lovisa S, LeBleu VS, Tampe B, Sugimoto H, Vadnagara K, Carstens JL et al (2015) Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med 21:998–1009
Lv LL, Feng Y, Wen Y, Wu WJ, Ni HF, Li ZL et al (2018) Exosomal CCL2 from tubular epithelial cells is critical for albumin-induced tubulointerstitial inflammation. J Am Soc Nephrol 29:919–935
Maarouf OH, Aravamudhan A, Rangarajan D, Kusaba T, Zhang V, Welborn J et al (2016) Paracrine Wnt1 drives interstitial fibrosis without inflammation by tubulointerstitial cross-talk. J Am Soc Nephrol 27:781–790
Macconi D, Remuzzi G, Benigni A (2014) Key fibrogenic mediators: old players. Renin-angiotensin system. Kidney Int Suppl 4:58–64
Mackensen-Haen S, Bader R, Grund KE, Bohle A (1981) Correlations between renal cortical interstitial fibrosis, atrophy of the proximal tubules and impairment of the glomerular filtration rate. Clin Nephrol 15:167–171
Mao J, Kim BM, Rajurkar M, Shivdasani RA, McMahon AP (2010) Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development 137:1721–1729
Massy ZA, Stenvinkel P, Drueke TB (2009) The role of oxidative stress in chronic kidney disease. Semin Dial 22:405–408
Megyesi J, Tarcsafalvi A, Li S, Hodeify R, Seng NS, Portilla D et al (2015) Increased expression of p21WAF1/CIP1 in kidney proximal tubules mediates fibrosis. Am J Physiol Renal Physiol 308:F122–F130
Meng XM, Nikolic-Paterson DJ, Lan HY (2016) TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 12:325–338
Menke J, Iwata Y, Rabacal WA, Basu R, Yeung YG, Humphreys BD et al (2009) CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. J Clin Invest 119:2330–2342
Morrison EE, Bailey MA, Dear JW (2016) Renal extracellular vesicles: from physiology to clinical application. J Physiol 594:5735–5748
Mudaliar H, Pollock C, Komala MG, Chadban S, Wu H, Panchapakesan U (2013) The role of Toll-like receptor proteins (TLR) 2 and 4 in mediating inflammation in proximal tubules. Am J Physiol Renal Physiol 305:F143–F154
Mulay SR, Desai J, Kumar SV, Eberhard JN, Thomasova D, Romoli S et al (2016a) Cytotoxicity of crystals involves RIPK3-MLKL-mediated necroptosis. Nat Commun 7:10274
Mulay SR, Linkermann A, Anders HJ (2016b) Necroinflammation in kidney disease. J Am Soc Nephrol 27:27–39
Nie J, Hou FF (2012) Role of reactive oxygen species in the renal fibrosis. Chin Med J (Engl) 125:2598–2602
Okusa MD, Chertow GM, Portilla D, Acute Kidney Injury Advisory Group of the American Society of Nephrology (2009) The nexus of acute kidney injury, chronic kidney disease, and World Kidney Day 2009. Clin J Am Soc Nephrol 4:520–522
Oliver J, MacDowell M, Lee YC (1954) Cellular mechanisms of protein metabolism in the nephron. I. The structural aspects of proteinuria; tubular absorption, droplet formation, and the disposal of proteins. J Exp Med 99:589–604
Ovadya Y, Krizhanovsky V (2015) A new twist in kidney fibrosis. Nat Med 21:975–977
Pegues MA, McCrory MA, Zarjou A, Szalai AJ (2013) C-reactive protein exacerbates renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 304:F1358–F1365
Prunotto M, Budd DC, Gabbiani G, Meier M, Formentini I, Hartmann G et al (2012) Epithelial-mesenchymal crosstalk alteration in kidney fibrosis. J Pathol 228:131–147
Pulskens WP, Rampanelli E, Teske GJ, Butter LM, Claessen N, Luirink IK et al (2010) TLR4 promotes fibrosis but attenuates tubular damage in progressive renal injury. J Am Soc Nephrol 21:1299–1308
Rabadi MM, Ghaly T, Goligorksy MS, Ratliff BB (2012) HMGB1 in renal ischemic injury. Am J Physiol Renal Physiol 303:F873–F885
Reich H, Tritchler D, Herzenberg AM, Kassiri Z, Zhou X, Gao W et al (2005) Albumin activates ERK via EGF receptor in human renal epithelial cells. J Am Soc Nephrol 16:1266–1278
Risdon RA, Sloper JC, De Wardener HE (1968) Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 2:363–366
Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM et al (1986) Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA 83:4167–4171
Schelling JR (2016) Tubular atrophy in the pathogenesis of chronic kidney disease progression. Pediatr Nephrol 31:693–706
Sedeek M, Nasrallah R, Touyz RM, Hébert RL (2013) NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J Am Soc Nephrol 24:1512–1518
Shigeoka AA, Kambo A, Mathison JC, King AJ, Hall WF, da Silva Correia J et al (2010) Nod1 and nod2 are expressed in human and murine renal tubular epithelial cells and participate in renal ischemia reperfusion injury. J Immunol 184:2297–2304
Sibony M, Gasc JM, Soubrier F, Alhenc-Gelas F, Corvol P (1993) Gene expression and tissue localization of the two isoforms of angiotensin I converting enzyme. Hypertension 21:827–835
Skuginna V, Lech M, Allam R, Ryu M, Clauss S, Susanti HE et al (2011) Toll-like receptor signaling and SIGIRR in renal fibrosis upon unilateral ureteral obstruction. PLoS ONE 6:e19204
Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC (2012) Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology (Carlton) 17:311–321
Strutz FM (2009) EMT and proteinuria as progression factors. Kidney Int 75:475–481
Takahashi N, Vereecke L, Bertrand MJ, Duprez L, Berger SB, Divert T et al (2014) RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature 513:95–99
Takaori K, Nakamura J, Yamamoto S, Nakata H, Sato Y, Takase M et al (2016) Severity and frequency of proximal tubule injury determines renal prognosis. J Am Soc Nephrol 27:2393–2406
Tan RJ, Zhou D, Zhou L, Liu Y (2014) Wnt/β-catenin signaling and kidney fibrosis. Kidney Int Suppl 4:84–90
Tan RJ, Zhou D, Liu Y (2016) Signaling crosstalk between tubular epithelial cells and interstitial fibroblasts after kidney injury. Kidney Dis (Basel) 2:136–144
Tang SC, Lai KN (2012) The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrol Dial Transplant 27:3049–3056
Tang J, Liu N, Tolbert E, Ponnusamy M, Ma L, Gong R et al (2013) Sustained activation of EGFR triggers renal fibrogenesis after acute kidney injury. Am J Pathol 183:160–172
Tang Y, Huang XR, Lv J, Chung AC, Zhang Y, Chen JZ et al (2014) C-reactive protein promotes acute kidney injury by impairing G1/S-dependent tubular epithelium cell regeneration. Clin Sci (Lond) 126:645–659
Terada Y, Tanaka H, Okado T, Shimamura H, Inoshita S, Kuwahara M et al (2003) Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J Am Soc Nephrol 14:1223–1233
Thadhani R, Pascual M, Bonventre JV (1996) Acute renal failure. New Engl J Med 334:1448–1460
Thomas ME, Harris KP, Walls J, Furness PN, Brunskill NJ (2002) Fatty acids exacerbate tubulointerstitial injury in protein-overload proteinuria. Am J Physiol Renal Physiol 283:F640–F647
Trionfini P, Benigni A, Remuzzi G (2015) MicroRNAs in kidney physiology and disease. Nat Rev Nephrol 11:23–33
Urushihara M, Kagami S (2017) Role of the intrarenal renin-angiotensin system in the progression of renal disease. Pediatr Nephrol 32:1471–1479
van Timmeren MM, Bakker SJ, Stegeman CA, Gans RO, van Goor H (2005) Addition of oleic acid to delipidated bovine serum albumin aggravates renal damage in experimental protein-overload nephrosis. Nephrol Dial Transplant 20:2349–2357
Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P (2014) Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 15:135–147
Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK (2015) Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol 26:1765–1776
Verzola D, Gandolfo MT, Gaetani G, Ferraris A, Mangerini R, Ferrario F et al (2008) Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am J Physiol Renal Physiol 295:F1563–F1573
Vío CP, Jeanneret VA (2003) Local induction of angiotensin-converting enzyme in the kidney as a mechanism of progressive renal diseases. Kidney Int Suppl 86:S57–S63
Waikar SS, Winkelmayer WC (2009) Chronic on acute renal failure: long-term implications of severe acute kidney injury. JAMA 302:1227–1229
Wallach D, Kang TB, Kovalenko A (2014) Concepts of tissue injury and cell death in inflammation: a historical perspective. Nat Rev Immunol 14:51–59
Wang Y, Chen J, Chen L, Tay YC, Rangan GK, Harris DC (1997) Induction of monocyte chemoattractant protein-1 in proximal tubule cells by urinary protein. J Am Soc Nephrol 8:1537–1545
Wang Y, Rangan GK, Goodwin B, Tay YC, Harris DC (2000) Lipopolysaccharide-induced MCP-1 gene expression in rat tubular epithelial cells is nuclear factor-kappaB dependent. Kidney Int 57:2011–2022
Wang J, Wen Y, Lv LL, Liu H, Tang RN, Ma KL et al (2015a) Involvement of endoplasmic reticulum stress in angiotensin II-induced NLRP3 inflammasome activation in human renal proximal tubular cells in vitro. Acta Pharmacol Sin 36:821–830
Wang Y, Chang J, Yao B, Niu A, Kelly E, Breeggemann MC et al (2015b) Proximal tubule-derived colony stimulating factor-1 mediates polarization of renal macrophages and dendritic cells, and recovery in acute kidney injury. Kidney Int 88:1274–1282
Wen Y, Liu Y, Tang T, Lv L, Liu H, Ma K et al (2016) NLRP3 inflammasome activation is involved in Ang II-induced kidney damage via mitochondrial dysfunction. Oncotarget 7:54290–54302
Wolf G, Mueller E, Stahl RA, Ziyadeh FN (1993) Angiotensin II-induced hypertrophy of cultured murine proximal tubular cells is mediated by endogenous transforming growth factor-beta. J Clin Invest 92:1366–1372
Wolf G, Ziyadeh FN, Stahl RA (1999) Angiotensin II stimulates expression of transforming growth factor beta receptor type II in cultured mouse proximal tubular cells. J Mol Med (Berl) 77:556–564
Wolf G, Wenzel U, Hannken T, Stahl RA (2001) Angiotensin II induces p27(Kip1) expression in renal tubules in vivo: role of reactive oxygen species. J Mol Med (Berl) 79:382–389
Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS et al (2002) In vivo expression of Toll- like receptor 2 and 4 by renal epithelial cells: IFN-γ and TNF-α mediated up-regulation during inflammation. J Immunol 168:1286–1293
Wu H, Ma J, Wang P, Corpuz TM, Panchapakesan U, Wyburn KR et al (2010) HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol 21:1878–1890
Wu CF, Chiang WC, Lai CF, Chang FC, Chen YT, Chou YH et al (2013) Transforming growth factor β-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am J Pathol 182:118–131
Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF et al (2016) Sustained activation of Wnt/β-catenin signaling drives AKI to CKD progression. J Am Soc Nephrol 27:1727–1740
Yang H, Fogo AB (2010) Cell senescence in the aging kidney. J Am Soc Nephrol 21:1436–1439
Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV (2010) Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16:535–543
Yang JR, Yao FH, Zhang JG, Ji ZY, Li KL, Zhan J et al (2014) Ischemia-reperfusion induces renal tubule pyroptosis via the CHOP-caspase-11 pathway. Am J Physiol Renal Physiol 306:F75–F84
Yard BA, Daha MR, Kooymans-Couthino M, Bruijn JA, Paape ME, Schrama E et al (1992) IL-1 alpha stimulated TNF alpha production by cultured human proximal tubular epithelial cells. Kidney Int 42:383–389
Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F et al (2003) BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9:964–968
Zhang JD, Liu BC (2011) Angiotensin II, a missing node in new pathogenic glomerulotubular feedback loop. Med Hypotheses 77:595–597
Zhang W, Zhou X, Zhang H, Yao Q, Liu Y, Dong Z (2016) Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Renal Physiol 311:F844–F851
Zhou D, Liu Y (2016) Renal fibrosis in 2015: understanding the mechanisms of kidney fibrosis. Nat Rev Nephrol 12:68–70
Zhou L, Fu P, Huang XR, Liu F, Lai KN, Lan HY (2010) Activation of p53 promotes renal injury in acute aristolochic acid nephropathy. J Am Soc Nephrol 21:31–41
Zhou D, Li Y, Lin L, Zhou L, Igarashi P, Liu Y (2012) Tubule-specific ablation of endogenous β-catenin aggravates acute kidney injury in mice. Kidney Int 82:537–547
Zhou D, Li Y, Zhou L, Tan RJ, Xiao L, Liang M et al (2014) Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J Am Soc Nephrol 25:2187–2200
Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J et al (2015) Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling. J Am Soc Nephrol 26:107–120
Zhou D, Tan RJ, Fu H, Liu Y (2016) Wnt/β-catenin signaling in kidney injury and repair: a double-edged sword. Lab Invest 96:156–167
Zuk A, Gershenovich M, Ivanova Y, MacFarland RT, Fricker SP, Ledbetter S (2014) CXCR4 antagonism as a therapeutic approach to prevent acute kidney injury. Am J Physiol Renal Physiol 307:F783–F797
Acknowledgements
This chapter was modified from a paper reported by our group in Kidney Int (Liu et al. 2018). The related contents are reused with permission.
This study was supported by grants from the National Key Research and Development Program of China (2018YFC1314004), the National Natural Scientific Foundation (No. 81720108007, 81130010, 81470997, and 81670696), and the Clinic Research Center of Jiangsu Province (No. BL2014080).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Liu, BC., Tang, TT., Lv, LL. (2019). How Tubular Epithelial Cell Injury Contributes to Renal Fibrosis. In: Liu, BC., Lan, HY., Lv, LL. (eds) Renal Fibrosis: Mechanisms and Therapies. Advances in Experimental Medicine and Biology, vol 1165. Springer, Singapore. https://doi.org/10.1007/978-981-13-8871-2_11
Download citation
DOI: https://doi.org/10.1007/978-981-13-8871-2_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8870-5
Online ISBN: 978-981-13-8871-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)