Abstract
Antibiotics have saved millions of lives. However, the overuse and misuse of antibiotics have contributed to a rapid emergence of antibiotic resistance worldwide. In addition, there is an unprecedented void in the development of new antibiotic classes by the pharmaceutical industry since the first introduction of antibiotics. This antibiotic crisis underscores the urgent and increasing necessity of new, innovative antibiotics. Enzybiotics are such a promising class of antibiotics. They are derived from endolysins, bacteriophage-encoded enzymes that degrade the bacterial cell wall of the infected cell at the end of the lytic replication cycle. Enzybiotics are featured by a rapid and unique mode-of-action, a high specificity to kill pathogens, a low probability for bacterial resistance development and a proteinaceous nature. (Engineered) endolysins have been demonstrated to be effective in a variety of animal models to combat both Gram-positive and Gram-negative bacteria and have entered different phases of preclinical and clinical trials. In addition, mycobacteriophage-encoded endolysins have been successfully used to inhibit mycobacteria in vitro. In this chapter we focus on the (pre)clinical progress of enzybiotics as potent therapeutic agent against human pathogenic bacteria.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Multidrug-resistant pathogens represent a major cause of morbidity and mortality and pose a serious threat on healthcare, both for humans and animals. In the United States alone, more than two million people are infected every year with bacteria that are resistant to conventional antibiotics. A significant proportion of 23,000 people dies (reported by the Centres for Disease Control and Prevention (US) 2013). Besides resistance development limiting the therapeutic potential of currently used antibiotics, there is an increasing awareness that the collateral damage of broad-spectrum antibiotics, i.e. killing of the benign commensal microbiome, causes harmful long-term effects such as an increased risk of secondary infections, asthma, obesity, diabetes type I and II (Langdon et al. 2016). Therefore, the development of therapeutics to treat (polymicrobial) drug-resistant infections without affecting the commensal microbiota is essential. However, since the 1980s, no new classes of antibiotics have entered the market, which further underlines the global need for novel strategies to combat multidrug- and pandrug-resistant pathogens.
Enzybiotics are a promising new class of enzyme-based antibacterials that may offer a response to this global call. They are safe, effective, fast-acting and highly specific. In addition, they weaken biofilms and have a low probability to provoke resistance development. They can be used alone or in combination with traditional antibiotics. Enzybiotics are derived from endolysins, bacteriophage-encoded enzymes that lyse the infected bacterial cell at the end of the lytic replication cycle. They enzymatically degrade the peptidoglycan (PG ) until the infected cell can no longer withstand the internal osmotic pressure and lyses, followed by dispersion of newly formed viral particles (i.e. “lysis form within”). Given the increasing incidence of antibiotic resistance and the lack of new alternative therapies, this unique and well-known feature of endolysins has spurred the idea to apply them exogenously to kill human pathogens (Nelson et al. 2001). Indeed, purified recombinant endolysins induce rapid osmotic lysis of Gram-positive bacteria through degradation of externally accessible PG with consequent cell death (i.e. “lysis from without”). The efficacy of endolysins has been demonstrated in numerous animal models of human disease (Nelson et al. 2001; Schmelcher et al. 2012a; Roach and Donovan 2015; Gerstmans et al. 2017). In addition, extensive engineering efforts have been done during the last two decades to enhance the potential of endolysins as therapeutic enzymes against Gram-positive bacteria and to extend their application to Gram-negative pathogens, which have a protective outer membrane. These endeavors have paved the path for recombinant endolysins to enter different phases in preclinical and clinical trials. Currently, different lead enzybiotics have entered the clinical phase with humans. The accelerating clinical advances and their high technical feasibility make phage endolysins as therapeutics the highest ranked alternatives to replace conventional antibiotics according to a recent pipeline investigation (Czaplewski et al. 2016).
11.2 Bacteriophages
11.2.1 From Phage Discovery Over Phage Therapy to Endolysin Therapy
In the early 20th century bacteriophages (or phages) were independently discovered by Twort (1915) and Felix d’Herelle (1917), but D’Herelle was the first to describe bacteriophages as bacterial viruses which have the ability to infect bacteria followed by bacterial lysis. Not long after their discovery, the therapeutic potential of phages to treat human and animal bacterial infections was recognized (Twort 1915; Sulakvelidze et al. 2001; Salmond and Fineran 2015). The first attempt to use bacteriophages therapeutically was the treatment of a 12-year-old boy suffering from severe dysentery. When the phage preparation was regarded as safe, a single dose was administered to the child. The boy, together with three additional patients fully recovered within a few days after a single administration (Sulakvelidze et al. 2001). Despite the successful outcome, the results of these studies were not published. In 1921, Richard Bruynoghe and Joseph Maisin have published the first study about the treatment of staphylococcal skin disease with phages. A regression of the infection was reported 24–48 h after treatment (Lavigne and Robben 2012). Several similar studies about phage therapy have followed and in early 1930 the commercialization of phages against an array of pathogens started. Despite these efforts, phage therapy for humans was abandoned in the Western medicine soon after the introduction of broad-spectrum antibiotics after World War II. Nowadays, the emergence of multidrug- and even pandrug-resistant pathogens has revitalized the interest in phages (Hanlon 2007). Nevertheless, phage therapy needs to overcome several technical hurdles such as regulation, narrow host range, bacterial resistance to phages, manufacturing, side effects of bacterial lysis, difficulties in the delivery of purified phage preparations, and the complicated pharmacokinetics of phages because of their self-replicating nature (Sandeep 2006; Hermoso et al. 2007). Using phage-encoded endolysins (or lysins) offer the potential to circumvent these obstacles typically related to intact phages (López et al. 2004; Hermoso et al. 2007).
11.2.2 Biological Function of Endolysin-Mediated Bacteriolysis
The biological role of endolysins is lysis of the infected bacterial cell at the end of the lytic cycle of bacteriophages in order to release progeny phage particles. The release is complicated by the presence of the bacterial cell wall, acting as a strong barrier. As phages co-evolved with their bacterial hosts, they have developed a variety of strategies to overcome this barrier (Young 2014). The most prevalent mechanism is the two component holin-endolysin system specific to the Caudovirales (tailed phages with dsDNA) and regulated by a timing mechanism to achieve lysis at an optimal time (Fig. 11.1). Endolysins accumulate in the cytoplasm as they cannot migrate through the cytoplasmic membrane (CM). The access to the PG in the periplasm is regulated by holins. Holins are small hydrophobic proteins that accumulate as homodimers in the CM in a uniformly distributed manner. At a genetically predetermined holin concentration, the holins aggregate into homo-oligomers and partially depolarize the CM by forming pores. The accumulated endolysins take advantage of these pores to gain access to the PG to degrade it. In addition, phage-encoded spanins weaken the outer membrane. When the impaired cell wall can no longer withstand the internal osmotic pressure, osmotic lysis and the release of the progeny (“lysis from within”) occur (Wang et al. 2000; White et al. 2011; Young 2014).
Schematic representation of the PG structure of S. aureus, the different cleavage specificities of endolysins and the simplified holin/endolysin lysis mechanism. (Bottom) When the critical holin concentration is achieved, holins depolarize the CM by forming pore lesions through which the endolysins accumulated in the cytoplasm gain access to PG. As a consequence, the PG is degraded and the cell wall cannot longer withstand the internal osmotic pressure and finally undergoes lysis. (Top) The glycan chains consist of β-1,4-linked, alternating monomers of N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc) residues and are further cross-linked via stem peptides that are attached to the MurNAc residues. The endolysins can be differentiated in three groups based on their cleavage specificity: (1) endopeptidases – targeting peptide bonds – including L-alanoyl-D-glutamate endopeptidase and interpeptide bridge endopeptidase, (2) amidases – targeting amide bonds – including the N-acetylmuramoyl-L-alanine amidases and (3) glycosidases – targeting glyosidic bonds – including N-acetyl-β-D-glucosaminidases, N-acetyl-β-D-muramidases and transglycosylases. (Hermoso et al. 2007)
11.3 The Structural and Biochemical Diversity of Endolysins
Although all endolysins have a conserved biological function, the constant evolutionary struggle between bacteriophage and bacterium has resulted in a huge biochemical and structural diversity among endolysins (Loessner 2005). Along with the high global abundance of phages, this diversity creates an enormous reservoir of specific endolysins that all have a medical potential.
11.3.1 Catalytic Specificity of Endolysins
The PG layer is a highly preserved layer that ensures the structural rigidity and integrity of the bacterial cells. PG consists of a polysaccharide of alternating N-acetylmuramic acid (MurNAc) and N-acetylglucosamine (GlcNAc) residues, linked by a β1-4 glycosidic bond. The glycan polymer is further stabilized by cross-links of the stem peptides, which are attached to MurNAc residues. Endolysins can recognize and digest a specific chemical bond of PG. They are classified into three different groups, according to the targeted bond (i.e. the amide, peptide or glycosidic bond) (Fig. 11.1) (Madigan et al. 2008).
(I) The first group of endolysins (N-acetylmuramoyl-L-alanine amidases; E.C 3.5.1.28) hydrolyses the amide bond between N-acetylmuramoyl residues and L-alanine, the first amino acid of the stem peptide. (II) The second group (endopeptidases; E.C 3.4.X.X) cleaves the peptide bond between two amino acids. L-alanoyl-D-glutamate endopeptidase cleaves the bond between L-alanine and D-glutamate in contrast to an interpeptide bridge-specific endopeptidase such as D-alanyl-glycyl endopeptidase that targets the cross-link between adjacent stem peptides. (III) Glycosidases are the final group and hydrolyze glyosidic bonds. Three subgroups can be differentiated. The first subgroup (N-acetyl-β-D-glucosaminidases; E.C 3.2.1.52) cleaves the N-acetylglucosaminyl-β-1-4-N-acetylmuramine bond on the reducing side of GlcNac. Both other subgroups, N-acetyl-β-D-muramidases (E.C 3.2.1.17, generically also termed lysozymes) and lytic transglycosylases (E.C. 4.2.2.n1, cleave the N-acetylmuramoyl-β-1-4-N-acetylmuramine bond on the reducing side of MurNAc. In contrary to all other groups, lytic transglycosylases are no hydrolases and cleave the β-1,4-glycosidic bond by an intramolecular reaction, resulting in a N-acetyl-1,6-anhydro-muramyl moiety (Hermoso et al. 2007; Nelson et al. 2012; Schmelcher et al. 2012a).
11.3.2 The Structural Properties and Function of Endolysins
Next to catalytic diversity, endolysins have a remarkable structural diversity, which has a large impact on both enzyme kinetics and specificity (Oliveira et al. 2013). The structural variety of endolysins is strongly related with the cell wall structure and differs for endolysins from phages infecting Gram-positive and Gram-negative species (Fig. 11.2). The Gram-negative cell wall is composed of a CM covered by one to three layers of PG and an additional outer membrane (OM). The OM is a complex asymmetric membrane featured by lipopolysaccharide (LPS) molecules composed of lipid A, core polysaccharide and O-specific polysaccharide. Lipid A generally consists of phosphorylated glucosamine disaccharide with fatty acid chains attached, providing hydrophobic stabilization of the OM. The anionic phosphates of the lipid A and the core polysaccharide moieties account for the negative charge of the OM and are stabilized by ionic interactions with divalent cations (Mg2+ and Ca2+). The LPS layer serves thus as a barrier for both hydrophobic and hydrophilic molecules larger than 600 Da. In contrary, Gram-positive bacteria only contain a thick PG layer decorated with (lipo)teichoic acids, which are linked with the PG and are responsible for the overall negative charge of the cell surface (reviewed by Nelson et al. 2012; Schmelcher et al. 2012a). The cell wall of mycobacteria is structurally different from Gram-positive and Gram-negative bacteria as it comprises an additional layer composed of mycolyl-arabinogalactan-PG complexes (Brennan 2003).
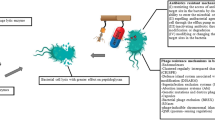
Antimicrobial activity of endolysins and Artilysin®s on different cell wall types. Left: The Gram-positive bacterial cell wall contains a CM and a thick PG layer. As a result, PG of the Gram-positive cell wall is readily accessible to exogenously added endolysins (blue) making these phage enzymes suitable for the treatment of Gram-positive infections. Middle: The Gram-negative cell wall is composed of a CM covered by one to three layers of PG, and an additional protective outer membrane (OM) hindering the access of endolysins to the PG. Artilysin®s (blue) can reach the PG through the fusion of an endolysin with an OMP-peptide. First, the OMP-peptide acts as a wedge by local and transient membrane destabilization, whereafter the Artilysin® acts similar as a native endolysin. Right: Mycobacteria consists of a mycolyl-arabinogalactan-PG complex. Mycobacterium phages produces two lytic enzymes, a PG hydrolase LysA (blue) and a lipase LysB (red) targeting the mycolic acids. (This figure was originally published in Biochem Soc Trans. Gerstmans et al. (2016) © Portland Press Limited)
Endolysins can be divided in globular or modular enzymes. Modular endolysins are generally encoded by phages infecting Gram-positive bacteria and mycobacteria, but also by some phages infecting Gram-negative bacteria. They are composed of multiple functional domains with separate activities: (1) an enzymatically active domain (EAD) and (2) a cell wall binding domain (CBD). The EAD is responsible for the catalytic action of the enzyme, digesting a specific chemical bond of PG. A CBD directs an endolysin to its substrate, i.e. the PG layer or a specific cell wall ligand such as (lipo)teichoic acids, resulting in an increased affinity and substrate proximity (Schmelcher et al. 2012a; Payne et al. 2013). Even after PG degradation, endolysins remain attached to the cell debris via their CBD. This may prevent neighboring, potential host cells from being damaged by diffusion of released endolysin molecules (Loessner et al. 2002). The most common architecture of modular endolysins is one or two N-terminal EADs and a C-terminal CBD, but the specific composition or order of the domains is not universally conserved and several exceptions have been reported: the CBD can be also located at the N-terminus (e.g. most modular endolysins from phages infecting Gram-negative bacteria), the CBD can be squeezed between two EADs, or can be absent. The domains are usually connected by a flexible inter-domain linker sequence, which can vary in size and ensures an autonomous function of both domains (Schmelcher et al. 2012a; Roach and Donovan 2015). A number of 89 different architectural modular organizations have been described, illustrating the high evolutionary diversity among endolysins (Oliveira et al. 2013).
Globular endolysins originating from Gram-negative infecting phages do not have a CBD and consist of a single domain functioning as an EAD (Briers et al. 2007). The presence of an OM preventing exogenous cleavage of the PG layer by released endolysins and the thin PG layer may eliminate the need for a CBD that directs endolysins to the cell wall. Notwithstanding, several Gram-negative infecting phages producing modular enzymes have already been discovered (Walmagh et al. 2012), especially in so-called large jumbo phages. These modular endolysins consist of N-terminal CBD specific for the conserved A1γ PG (conserved domains PG_binding_1 and PG_binding_3) fused to a C-terminal EAD (Briers et al. 2009) or two C-terminal EADs (Oliveira et al. 2013).
11.4 Preclinical Analysis of Enzybiotics as Antibacterials
The highly lytic nature of endolysins spurred the idea to use them as enzyme-based antibiotics (or enzybiotics). Nelson et al. (2001) were the first to report that purified recombinant endolysins are able to reduce high bacterial numbers in an animal infection model. In this study, complete eradication of bacteria from the oral mucosa was observed 2 h after an oral administration of purified C1 phage lysin to mice heavily colonized with group A streptococci. Since then, numerous preclinical studies have demonstrated their potential as therapeutic, whereas several concerns related to their proteinaceous nature have been rebutted. Whereas initially only Gram-positive pathogens were targeted because their PG is easily accessible, Gram-negative pathogens can meanwhile also be killed by engineered endolysins or endolysins with intrinsic antibacterial activity (Table 11.1).
Enzybiotics have a high specificity, often at genus, species or even serovar level. Specificity can be inferred from both domains. E.g. CBDs can bind specific ligands such as choline in the pneumococcal cell wall (Hermoso et al. 2003) or specific substituents of serotype-specific teichoic acids of Listeria monocytogenes (Eugster and Loessner 2012), whereas some EADs specifically cleave the pentaglycine crossbridge that only occurs in the PG of Staphylococcus aureus (Callewaert et al. 2011). Therefore, enzybiotics generally have a narrow spectrum of antibacterial activity, leaving commensal flora unaffected in contrast to the current, broad-spectrum antibiotics (e.g. penicillin and tetracycline) (Nelson et al. 2012). Moreover, endolysins have a unique and rapid mode-of-action holding several advantages compared to conventional antibiotics. Their ability to actively degrade the PG in seconds or minutes, depending on the concentration, makes them much faster than the existing antibiotics. The active degradation of the cell wall renders them also effective against persister cells (i.e. metabolically inactive cells) (Briers et al. 2014a; Gutierrez et al. 2014; Defraine et al. 2016). Enzybiotics do not require an active metabolism in contrast to antibiotics that target essential metabolic steps within actively growing bacteria. Compared to the rapid mode-of-action of enzybiotics, inhibition of such metabolic steps by small molecule antibiotics is usually a slow process, eventually leading to cell death after 24–48 h (Allison et al. 2011; Briers et al. 2014a).
Endolysin resistance has not been observed among strains from diverse sources and resistant strains can generally not be selected during in vitro experiments in which strains were repeatedly exposed to subinhibitory concentrations of (engineered) phage endolysins (Fischetti 2005; Pastagia et al. 2011; Briers et al. 2014a; Defraine et al. 2016). The high specificity and rapid action of endolysins, and the immutable nature of the PG layer may explain this low probability of resistance development against phage endolysins. This beneficial feature supports a prolonged use of enzybiotics, even under regimes of repeated use. The use of many broad-range antibiotics in contrary has led to the selection of resistant strains of both target pathogen and commensal bacteria, which is often accelerated by the distribution of resistant genes via horizontal gene transfer (Johnsborg and Håvarstein 2009). In spite of this overall low probability of resistance development, an exception of this rule has been reported for endolysins that target the pentaglycine crossbridge in S. aureus. The strain S. aureus Newman developed resistance against both endolysin LysK and bacteriocin lysostaphin. Serial exposure (i.e. ten rounds) of S. aureus Newman to subinhibitory doses of LysK and lysostaphin in liquid culture resulted in a 42-fold and 585-fold increases of the minimal inhibition concentration (MIC), respectively (Becker et al. 2016). Resistance is acquired by substitution of glycine residues of the pentaglycine bridge by serines (Climo et al. 1998). This indicates that endolysins targeting species-specific interpeptide crossbridges are susceptible to resistance development in contrast to endolysins targeting highly conserved bonds in the PG layer (i.e. the polysaccharide backbone and amide bond between the polysaccharide backbone and the stem peptide). Amidases and muramidases for example are the most prevalent endolysins targeting these highly conserved bonds.
Antibacterial synergistic effects have been demonstrated between two or more endolysins or between endolysins and other antibacterial agents like traditional antibiotics. This synergetic effect implies an enhanced and faster degradation of PG resulting in an improved antibacterial efficiency, a requirement of smaller doses and a potentially reduced risk of resistance development (Schmelcher et al. 2012b). Synergy between two enzymes with different catalytic specificities (e.g. pneumococcal phage endolysins CpI-1 and Pal, and staphylococcal phage endolysins LysK and lysostaphin) can be explained by two possible mechanisms: either two endolysins simultaneously digest a different bond of the PG network, resulting in a more extensive degradation or one endolysin cleaves the first bond, concurrently improving the accessibility for the other endolysin (Loeffler and Fischetti 2003; Becker et al. 2008; Schmelcher et al. 2012b). The synergy mechanism between endolysins and antibiotics (e.g. the pneumococcal phage lysin Cpl-1 and gentamicin) remains unclear but it is proposed that partial PG degradation by the endolysin facilitates antibiotic uptake. In addition, it has been reported that the pneumococcal phage endolysin Cpl-1 can resensitize Streptococcus pneumoniae to penicillin against which they are resistant. As such, endolysins can slow down the emergence of antibiotic-resistant strains (Djurkovic et al. 2005; Viertel et al. 2014).
Since PG is a exclusively present in bacteria and not in mammalian cells, the risk on cytotoxic effects for humans and animals is minimized. However, the proteinaceous character of enzybiotics may provoke an immune response. In vitro and in vivo studies have shown that neutralizing antibodies can indeed be raised against endolysins after repeated exposure. These antibodies reduce the antibacterial activity of endolysins but do not completely neutralize them, while in other studies they do not have a significant impact at all (Fischetti 2010). Thus, endolysins can be used repeatedly to treat the same bacterial infection (Jado et al. 2003; Loeffler and Fischetti 2003; Hermoso et al. 2007; Rashel et al. 2007; Zhang et al. 2016). These observations may be explained by the high affinity of the CBD for its substrate (nanomolar range) that exceeds the affinity of endolysin-specific antibodies and the fast kinetics of these enzymes that outperform the hosts immune response (Loessner et al. 2002; Jado et al. 2003; Schmelcher et al. 2010). The proteinaceous nature of endolysins may potentially also induce an allergic reaction, but so far no allergic reactions have been reported against endolysins in two clinical phase I studies with SAL200 (ClinicalTrials.gov NCT01855048; Jun et al. 2017) and CF-301 (ClinicalTrials.gov NCT02439359; Cassino 2016).
Proteins, including endolysins, generally have a short half-life, which is estimated to be approximately between 4 and 40 min for endolysins (Loeffler and Fischetti 2003; Jun et al. 2017). In spite of the short half-life, the efficacy of endolysins has been demonstrated in various animal models with intravenous administration. Again, the fast mode-of-action of enzybiotics appears to be of pivotal importance to compensate the short half-life. Loeffler et al. (2003) has reported that due to the narrow window of action a repeated administration was required to make a therapeutic treatment successful.
The release of cellular debris of lysed bacteria upon systemic administration of enzybiotics in humans or animals may induce a pro-inflammatory response. This bacterial cell debris includes lipopolysaccharides (LPS), (lipo)teichoic acids and PG through membrane fragmentation and may provoke serious complications such as a septic shock (Fischetti 2010). Entenza et al. (2005) found that rats treated with a continuous intravenous infusion of Cpl-1 lysin (originating of Streptococcus pneumoniae phage) show an increased pro-inflammatory cytokine concentration in comparison with untreated rats. Witzenrath et al. (2009) instead reported that administration in 12-h intervals of the same enzyme reduces cytokine concentrations compared with untreated animals. The latter observation indicates that there is an optimal dosing for endolysins. The optimal dose should suffice to digest the PG and to kill the bacterial pathogen without additional fragmentation of the PG layer. An optimization of the dosing regimen when using endolysins in therapeutic applications is therefore inevitable (Entenza et al. 2005; Witzenrath et al. 2009; Fischetti 2010).
Initially, it was thought that pathogens that propagate and survive intracellularly to evade the immune system would be inaccessible for enzybiotics. However, recently, both native endolysins (PlyC) and chimeric endolysins (K-L) have been shown to eradicate intracellular pathogens such as Streptococcus pyogenes and S. aureus. Alternatively, the fusion of endolysins with protein transduction domains led to functional endolysins that get into mammalian cells and kill intracellular bacteria (Becker et al. 2016; Shen et al. 2016).
11.5 Endolysins as Therapeutics
11.5.1 Endolysins as Therapeutic Agents Against Gram-Positive Bacterial Infections
In 2001 the group of Vincent Fischetti demonstrated that purified recombinant endolysins can be used as a preventive and curative agent of streptococcal infections in mice. Since then, extensive efforts have been done to expand their potential as therapeutics including the administration of enzybiotics in complex environments (e.g., blood stream, mucous membranes,…) (Schmelcher et al. 2012a). Various in vitro and in vivo animal infection models of human diseases demonstrated that the administration of purified endolysin to animals infected by Gram-positive pathogens (such as Staphylococcus aureus, staphylococcus sp., streptococcus sp., Bacillus anthracis and Enterococcus sp.) rescues them from an otherwise deadly infection (Nelson et al. 2001, 2012; Schuch et al. 2002; see Haddad et al. 2017 for an overview of all animal models). In addition, several protein engineering strategies such as mutagenesis, truncation or domain swapping (i.e. chimeric endolysins) are applied to improve the antibacterial activity and/or modify the specificity and other features (reviewed by Gerstmans et al. 2017). This collection of successful reports on the use of recombinant endolysins to combat pathogens inspired to the term ‘enzybiotics’ (Hermoso et al. 2007). Below we focus on the in vitro and in vivo models of all (engineered) endolysins that are currently in the clinical development stage (Table 11.2).
11.5.1.1 Enzybiotics in the Clinical Pipeline
Enzybiotics are increasingly developed for human and veterinary pathogens as pharmaceutical product (clinical phase II trials are going on by ContraFect, Intron Biotechnology, GangaGen, Micreos), cosmetic (GladSkin series with StaphEfekt™ as functional compound, commercialized by Micreos) and wound care spray (Medolysin® based on Artilysins announced by Lysando).
11.5.1.1.1 CF-301
Endolysin CF-301 from the New York-based company Contrafect has a potent anti-staphylococcal activity for the treatment of S. aureus bloodstream infections including endocarditis and bacteremia. This endolysin, also referred to as PlySs2, was identified from a Streptococcus suis prophage (Gilmer et al. 2013). Schuch et al. (2014) demonstrated that CF-301 has a rapid antibacterial activity against S. aureus strains, an anti-biofilm activity, and acts synergistically in combination with standard antibiotics. CF-301 shows bactericidal activity in vitro against 250 S. aureus strains, including 120 MRSA isolates and 27 multidrug-resistant strains. A more than 3-log reduction in cell number within 30 min was observed in vitro in contrary to antibiotics that required 6–12 h to reach similar reduction levels. In vivo, a CF-301 treatment of mice with MRSA bacteremia resulted in a 2-log CFU reduction in bloodstream within 1 h. In addition, CF-301 (1x MIC) was able to eradicate a S. aureus biofilm in 2 h, whereas high doses of antibiotics (1000x MIC) fail. CF-301 also exhibits a potent in vitro synergy with daptomycin (DAP) (64- to 256-fold increased daptomycin susceptibility) and increased survival in bacteremia when combined with vancomycin (from 7–31% to 82–90% survival) or DAP (from 3% to 67% survival) (Schuch et al. 2014). Further, a strong post-antibiotic effect (PAE, time period to resume normal growth after the antibiotic treatment has stopped and the serum concentration is below the MIC), post-antibiotic sub-MIC effect (PA-SME, effect of sub-MICs on bacteria during PAE phase) and sub-MIC effect (SME, effect of sub-MICs on bacteria without previous exposure to suprainhibitory concentrations) has been demonstrated for CF-301. An in vitro experiment against a panel of 14 staphylococcal strains in human serum indicated a PAE of 4.8 h, a PA-SME up to 7.5 h and a SME up to 7.8 h. The in vivo PAE tested in a neutropenic mouse thigh model significantly exceeds the in vitro PAE, reaching 23–26 h (Schuch 2016). A follow-up study of the CF-301 SME reported that the exposure to subinhibitory levels of CF-301 as low as 0.004x MIC increases antibiotic susceptibility (DAP), reduces growth rates (tested in vitro and in a neutropenic mouse thigh infection model model), decreases biofilm formation and inhibits a virulence phenotype (Oh and Schuch 2017).
CF-301 is formulated for intravenous injection. This endolysin has completed a Phase I trial in healthy human volunteers. In the phase I clinical trial (clinicaltrials.gov NCT02439359), a placebo-controlled, dose-escalating study (from 0.04 to 0.4 mg/kg/dose) has been conducted to examine the safety and tolerability of single intravenous dose of CF-301 in healthy human subjects. The clinical data have demonstrated that CF-301 is well tolerated and has a good safety profile. The latter implies that adverse clinical safety signals (hypersensitivity or serious adverse events), acute cardiovascular and inflammatory responses were absent (Cassino 2016; Jandourek et al. 2017a, b). To investigate the inflammatory response on CF-301, a range of inflammatory markers such as the high-sensitivity C-reactive protein, the erythrocyte sedimentation rate and complement factors Bb, C3a, C5a and CH50 were analyzed. No differences between placebo and CF-301 injected human subjects have been reported (Jandourek et al. 2017b). In addition, no clinically relevant changes in systolic and diastolic blood pressure, heart rate and QT were found in PK/PD models based on the clinical phase I data (Ghahramani et al. 2017).
11.5.1.1.2 N-Rephasin®SAL200
SAL200 with anti-staphylococcal activity (commercial name: N-Rephasin®) is developed by Intron Biotechnology. SAL200 is the recombinant variant of endolysin SAL-1 that is derived from the staphylococcus phage SAP-1. A stabilizing formulation containing 0.01 M L-histidine (pH 6.0), 5% (w/v) sorbitol, 10 mM CaCl2 and 0.1% (w/v) Poloxamer 188 was developed for human application. Initial in vitro experiments with the formulated SAL200 demonstrated a rapid and effective antibacterial activity against a panel of clinical and biofilm-forming S. aureus isolates. A daily intravenous administration of formulated SAL200 during 3 days in mice infected with MRSA significantly increased their survival rate. No bacteria could be detected in blood and splenic tissue of mice treated with SAL200 in contrary to the control group (Jun et al. 2013). In addition, GLP-compliant safety evaluation studies of intravenously administered SAL200 have been executed on rats and dogs, including toxicity, central nervous system, respiratory, and cardiovascular function tests. Both, single-dose and repeated-dose (one dose per day for a period of 4 weeks) toxicity tests in rats does not result in adverse clinical effects related with the administration. Similar to rats, repeated dose toxicity tests were conducted in dogs. After a 2 week treatment, dogs showed no changes in body weight, food consumption, ophthalmology, electrocardiography, hematology, serum biochemistry, organ weight or urinalysis. However, from 10 days after the first treatment, clinical signs such as subdued behavior, prone position, irregular respiration, and vomiting have been reported in dogs but were transient and mild. These clinical signs were resolved 30 min to 1 h after each new injection. In the safety pharmacology studies, no adverse effects were observed in the central nervous, respiratory and function tests. Also in the cardiovascular function tests there were no adverse events or laboratory abnormalities observed by the first and second administration. However, mild and transient changes were observed upon the third and fourth injection but again, the signs were resolved 6 h after administration. Further investigation has demonstrated that a repeated administration of SAL200 elicit an immune response in both dogs (14 days) and rats (28 days), observed by the presence of anti-SAL-1 antibodies (Jun et al. 2014). In addition, a reduction in blood C3 complement was observed in the exposed dogs. C3 complement proteins support antibodies and phagocytic cells by killing foreign invaders and thus play an important role in the innate immune system. However, it is unclear whether this response was due to the residual lipopolysaccharide endotoxin in the recombinant protein preparation or to the enzyme itself. After these preclinical tests, the pharmacokinetics of SAL200 have been studied by an intravenous administration in monkeys. SAL200 was well tolerated and no adverse events or laboratory abnormalities were observed when injected as a single dose administration (up to 80 mg/kg body weight) or as a 5-day multiple-dose administration (up to 40 mg/kg per day) (Jun et al. 2016).
Based on these data, SAL200 entered phase 1 clinical trial (ClinicalTrials.gov NCT01855048, results published in Jun et al. 2017) to evaluate the safety, pharmacokinetics and pharmacodynamics of intravenous medication SAL200 in healthy men. After an intravenous infusion of single ascending doses, SAL 200 was well tolerated and no severe adverse events or clinically significant values were observed and all present clinical signs including fatigue, rigors, headache, and myalgia were transient, self-limiting, and mild. However, as expected the humoral immune response was induced and antibodies ranging from 2 to 12 μg/ml were formed against the recombinant endolysin SAL200. Also the pharmacokinetics and pharmacodynamics analysis support the potential use of SAL200 as new endolysin-based therapeutic drug. The antibacterial activity in blood has been assessed with an ex vivo blood assay using blood samples collected from all active pharmaceutical ingredient-treated participants 1 h after injection. These blood samples were spotted on a lawn of S. aureus bacteria and compared with a standard series of SAL200. These studies ensure that a dosing regimen of more than 1 mg/kg of SAL200 is a viable treatment because of the following observations: (I) The blood SAL200 concentration was greater than 0.078 μg/ml in all collected blood samples 1 h after injection, which is the minimum bactericidal concentration to kill a bacterial population of 1 × 106 CFU/ml in serum environment. (II) The time to reduce the optical density of the initial bacterial suspension with 50% (TOD50, equivalent to one-half log drop in initial viable bacteria) was less than 10 min (Jun et al. 2017).
11.5.1.1.3 P128
The company Gangagen (India) created the engineered enzybiotic P128 for intranasal use against S. aureus. P128 is a chimeric protein that combines the phage tail-associated catalytic domain Lys16 of staphylococcus phage K with the well-known staphylococcal cell wall binding SH3b domain from lysostaphin. In an initial in vitro experiment, S. aureus was found to be effective (>99% reduction of cell numbers) against a panel of S. aureus clinical strains, including MRSA, methicillin-sensitive S. aureus (MSSA), and a mupirocin-resistant S. aureus (Paul et al. 2011; Vipra et al. 2012). George et al. (2012) confirmed the stable activity of P128 in body fluids such as blood, plasma and normal and hyperimmune sera and demonstrated that P128 has no cytotoxic effects on mammalian cells, indicating the potency of P128 as antibacterial agent. To evaluate the in vivo efficiency, P128 was formulated as a hydrogel and tested in a nasal rat colonization model using MRSA USA300. Rats treated with the P128 hydrogel were either completely decolonized (four out of the nine rats) or the bacterial cell numbers were significantly reduced (Paul et al. 2011). A study on dogs diagnosed with a canine staphylococcal pyoderma skin infection further demonstrated the clinical efficiency of P128. Nearly 5–6 log reduction was seen in vitro upon P128 treatment on canine pyoderma isolates. Further, a case study with 17 dogs suffering from canine pyoderma were treated twice daily for 8 days with hydrogel P128. All lesions of the dogs under treatment healed completely after treatment and no recurrence of the symptoms occurred within 2 months (Junjappa et al. 2013). In addition, two studies have reported P128 as effective antibiofilm agent against sinus-derived clinical S. aureus isolates (Drilling et al. 2016) and coagulase-negative staphylococci (CoNS), the major cause of catheter-related bloodstream infections (Poonacha et al. 2017). The latter study revealed that P128 has a potent efficiency against both planktonic cells, biofilms and persister cells of three CoNS species S. epidermidis, S. haemolyticus, and S. lugdunensis. Moreover, the combination of P128 and the selected antibiotics (vancomycin, daptomycin, linezolid) showed a high synergistic inhibition of these staphylococcal strains (Poonacha et al. 2017). A renal abscess rat model was used to evaluate the efficiency of P128 against S. aureus bacteremia and its potential hypersensitivity reactions. Rats injected with a single intravenous dose of P128 up to 12.0 mg/kg and re-injected after a 15 day resting period showed no abnormal clinical signs of Type I hypersensitivity (anaphylaxis) in contrary to the positive control group injected with ovalbumin, an agent that causes anaphylaxis. In addition, no P128-related tissue injury (vasculitis or glomerulonephritis) typical for type III hypersensitivity was observed. As expected, low titers of anti-P128 antibodies were raised in the P128-dosed animals. Notwithstanding this, the concentration of a single intravenous bolus dose of 2.5 mg/kg in rats remains above the minimal inhibitory concentration (4 μg/mL) for 15 min. In addition, a single dose of P128 (2.5 mg/kg) was efficacious in rescuing animals from fatal MRSA USA300 bacteremia and prevents formation of renal abscesses (Channabasappa et al. 2017). Based on these data, a second preclinical study was started to evaluate pharmacokinetics and efficacy of P128 in a neutropenic mouse model of bacteremia. A single bolus (10, 30 and 60 mg/kg) of P138 was intravenously administered and caused a rapid and dose-dependent antibacterial activity. A maximum bactericidal effect was detected for all test dose levels after 30 mins and the cell numbers remain low after 24 h. Finally, the half-life was determined between 5.2 h (30 mg/kg dose) – 5.6 h (60 mg/kg dose) (Sriram et al. 2017).
11.5.1.1.4 Staphefekt™
In 2013, Micreos Human Health BV (the Netherlands) launched Staphefekt™, a recombinant staphylococcal phage endolysin, which is the functional compound in the cetomacrogol-based cream and the gel-based Gladskin series. These products are promoted by Micreos for the treatment of various inflammatory skin infections provoked by S. aureus by humans such as eczema, rosacea, skin irritation and inflammatory acne. The Gladskin products are currently registered as a (class I) medical device and are prescription-free available on the market in Europe. In a case study, three human objects suffering from S. aureus-related dermatoses were treated with Staphefekt™ resulting in a clinically relevant reduction of S. aureus on the skin. However, the clinical symptoms quickly recurred after stopping the treatment with Staphefekt™ (Totté et al. 2017).
11.5.2 Enzybiotics Against Gram-Negative Bacteria
Gram-negative bacteria have a protective outer membrane (OM) that shields the access to the cell for both hydrophobic and hydrophilic molecules, including endolysins. The outer membrane comprises a lipopolysaccharide (LPS) layer which is typically stabilized by electrostatic interactions between divalent cations and anionic phosphates and hydrophobic stacking of the fatty acids of the lipid A moiety of the LPS molecules. The presence of this membrane generally excludes Gram-negative bacteria from being killed by exogenously added endolysins.
Notwithstanding this largely impermeable barrier, some endolysins can permeate to a certain extent the OM of Gram-negative bacteria. Especially Acinetobacter baumannii appears to be sensitive to a number of such endolysins including LysAB3 and LysAB4 (Lai et al. 2013), PlyAB1 (Huang et al. 2014), PlyF307 (Lood et al. 2015), LysABP-01 (Thummeepak et al. 2016), ABgp46 (Oliveira et al. 2016) and LysPA26 (Guo et al. 2017). However, higher concentrations of those endolysins appear to be needed to kill Gram-negative bacteria (100–500 μg/ml) compared to Gram-positive bacteria (<10 μg/ml), indicating that this OM still hinders the passage of endolysin (Lim et al. 2014; Thummeepak et al. 2016; Shavrina et al. 2016). PlyF307 has a high bactericidal activity (>5 log reduction in cell number) against all tested clinical A. baumannii strains. Further, a treatment with PlyF307 (100 μg/ml) resulted in a significant reduction of planktonic and biofilm A. baumannii (~2-log reduction in colonizing bacteria) both in vitro and in vivo. Finally, PlyF307 increased survival of mice with lethal A. baumannii bacteremia with 50% (Lood et al. 2015). Recent studies focusing on antimicrobial peptides (AMPs) reported that several endolysin-derived peptides have physicochemical properties to disrupt and penetrate the Gram-negative OM and function as potent antibacterials (Thandar et al. 2016). Further, different compounds facilitate the passage of endolysins through the OM. Aromatic essential oils (carvacrol) disintegrate the OM by inducing LPS release (Diez-Martinez et al. 2013), chelating agents (EDTA) capture the stabilizing divalent cations from their binding sites (Briers et al. 2007) and polycationic compounds (polymyxin E) competitively displace the divalent cations (Mg2+ and Ca2+) (Vaara 1993; Thummeepak et al. 2016). Another approach is the use of high hydrostatic pressure (HHP) to permeabilize the OM for endolysins (Briers et al. 2008). However, this approach may only find application in the food sector for products that cannot be pasteurized (e.g. oysters, guacamole) and are mostly not suitable for therapeutic applications.
We reported the development of Artilysin®s (Briers et al. 2014a). Artilysins are protein engineered endolysins that kill Gram-negative bacteria. The Artilysin® structure is based on a fusion of a selected endolysin and a specific outer membrane permeabilizing (OMP-)peptide. This OMP-peptide has physicochemical properties that interferes with the stabilizing forces of the LPS layer in the OM. OMP-peptides may comprise cationic and hydrophobic amino acids, giving them a polycationic or amphipathic nature. The OMP-peptide locally and transiently destabilizes the outer membrane for endolysin passage to the periplasm. Once the Artilysin® reaches the periplasm, they act similarly to a native endolysin: the CBD binds its target (specifically, the PG_1 and PG_3 CBDs bind PG with chemotype A1γ) and the EAD cleaves specific bonds of the PG, eventually resulting in osmotic lysis of the bacterial cell. OM permeabilizers, such as EDTA, can further enhance the antibacterial activity of Artilysin®s (Briers et al. 2014a, b).
The best described Artilysin® is Artilysin-175 (Art-175). Art-175 is a fusion of the broad-spectrum sheep myeloid-29 acid (SMAP-29) peptide to the Gram-negative specific endolysin KZ144. The endolysin moiety has been further modified by site-specific mutations of three cysteines to serines to create a more stable and active Artilysin®. Art-175 has a high bactericidal effect (>4 log reduction in cell number after 30 min) against all tested P. aeruginosa strains, including environmental, clinical and multidrug-resistant strains. Art-175 is also highly bactericidal against multidrug-resistant Acinetobacter baumannii, including their persisters, resulting in a complete eradication of bacterial cultures (up to 8 log reduction) (Defraine et al. 2016). To assess potential resistance development, three different P. aeruginosa strains were serially exposed to subinhibitory doses of Art-175. Similar to endolysins, the highly selective pressure of the subinhibitory doses did not lead to the recovery of resistant variants after 20 cycles in contrast to when control antibiotics are used. In addition, no cross-resistance against Art-175 could be observed with thirteen prevalent resistance mechanisms, including colistin (Schirmeier et al. 2018). Art-175 shows no cytotoxicity against mouse connective tissue fibroblasts. These data indicate that Art-175 is well suited for a broad range of applications in hygiene, veterinary and humane medicine, including persister-driven chronic infections. Persisters are insensitive to traditional antibiotics, because they have shut down essential metabolic processes targeted by those antibiotics. Metabolically inactive persisters cells likely remain susceptible to Art-175 because of its active mode of action (i.e. enzymatic PG degradation), while traditional antibiotics rely on a passive inhibition mechanism.
In vivo efficiency of Art-085 (a fusion of OMP-peptide SMAP-29 and endolysin KZ144, similar to Art-175 but without mutations) has been reported in two dogs with otitis that not could be healed with standard antibiotics. Three different P. aeruginosa strains and one β-hemolytic Streptococcus sp. were identified in both ears of the first dog diagnosed with otitis externa. The dog recovered completely after a systematic treatment of Art-085 (three doses of Art-085 the 1st day followed by one daily dose during 6 days) without relapse. Similar results have been observed with the second dog suffering from media purulenta. Two different P. aeruginosa strains and one Proteus mirabilis strain were identified in both ears and a 3-week treatment with marbofloxacin showed no observable effect. Again, systematic treatment of Art-085 (seven treatments with Art-085 within 1 day and administration of three additional doses at day 7 and 8) was effective. After 2 weeks no relapse was observed (Briers and Lavigne 2015).
LoGT-008, which comprises a polycationic nonapeptide fused to the endolysin PVP-SE1gp146, was evaluated in two infection models. Human keratinocytes were cultured in vitro and infected by P. aeruginosa, mimicking barrier-disrupted, infected skin wounds as in burns. LoGT-008 could fully protect the cultured human keratinocytes monolayer infected with the highly virulent P. aeruginosa strain PA14, which is otherwise lethal. No cytotoxic effects were observed. Antibacterial killing of P. aeruginosa strain PA14 was confirmed using a simple in vivo Caenorhabditis elegans infection model. Treatment with LoGT-008 led to an increased survival rate (63%) in comparison with the untreated worms (10%) and ciprofloxacin (45%) (Briers et al. 2014b).
Lysando AG commercializes Artilysin®s and reports about the development of a wound care spray. The spray supports the healing process by creating an optimum moisture and a protective film and has bactericidal activity against pathogens. In a study to determine the efficacy of the wound spray, a group of human subjects was daily treated for a long period (>30 days). For 90% of the patients, the wound healing process started immediately after application and the wound sizes were reduced. Within 30 days, complete healing of the wounds was detected for 40% of the human subjects. One of the patients that has been treated with the wound spray was a coma patient with severe decubitus that was chronically infected by MRSA. After a few days, signs of healing were described and complete wound healing appeared after 10 months (Lysando 2017).
Artilysation or modifying the properties of an endolysin by fusion with a specific peptide can also be used to improve the properties of Gram-positive-specific endolysins. Artilysin-240 (Art-240), a fusion of a polycationic nonapeptide fused to the C-terminus of endolysin λSa2lys has an increased in vitro antibacterial activity against stationary streptococci cells. A 0.5–1.5 higher log reduction in bacterial cell number was observed compared to λSa2lys. Moreover, Art-240 has an increased enzymatic activity over a broader range of pH values and NaCl conditions compared to endolysin λSa2lys. Further experiments demonstrate that Art-240 has a twofold higher killing rate causing a 1.7-log reduction in cell number after 5 min whereas λSa2lys needs 30 min to cause the same reduction in cell number. In addition, a 4 to 12-fold reduced dose of Art-240 is required to achieve the same bactericidal effect as the native endolysin (e.g. 12.5 nM of Art-240 and 150 nM of λSa2lys are needed for a 2-log reduction). The positive charges of the fused polycationic nonapeptide, acting as an additional and local positive anchor, likely strengthen the interactions of Art-240 with the anionic phosphate groups in teichoic acids present on the cell surface (Rodríguez-Rubio et al. 2016).
11.5.3 Endolysins Active Against Mycobacteria
Mycobacterium tuberculosis, the causative agent of tuberculosis, poses a major threat because of the emergence of drug-resistant mycobacteria and the lack of effective therapies. The mycobacterial outer membrane possesses a mycolyl-arabinogalactan-PG complex composed of an inner PG layer (first barrier) that is covalently attached to arabinogalactan (AG), which in turn is esterified with a mycolic acid rich layer. The latter layer provides mycobacteria of a second, lipid barrier (Brennan 2003). In nature, mycobacteriophages attack both barriers from within by producing two cell wall hydrolytic enzymes, LysA and LysB. LysA is a PG hydrolase that degrades the PG layer and LysB is an enzyme with lipolytic activity that completes lysis by cleaving the linkage between the mycolic acids and the arabinogalactan layer (Gil et al. 2008; Payne et al. 2009). The presence of this mycolyl-arabinogalactan layer partly restricts access of endolysins (LysA) to the PG layer when added exogenously (Payne et al. 2009; Grover et al. 2014).
The past few years, mycobacteriophage-derived endolysins were isolated and tested against cell wall components of different mycobacteria strains. Catalão et al. (2011) examined the endolysin LysA derived from mycobacteriophage Ms6. Close inspection of the lysA gene revealed a shorter open reading frame entirely embedded in the same reading frame, encoding a second functional PG hydrolase. E. coli crude extracts that contain one of both lysins were spotted onto a bacterial lawn of test strains and inhibited bacterial growth of Gram-positive bacteria and mycobacteria. Grover et al. (2014) have demonstrated the bacteriostatic activity of LysB (isolated from two different mycobacteriophages Bxz2 and Ms6) against M. smegmatis in combination with surfactants. The presence of surfactants eliminates artifacts due to cell aggregation and facilitates the activity of LysB. The bacteriostatic activity of LysB was highest in the presence of Tween 80. This additional anti-bacterial effect can be explained by the oleic acid release due to LysB-mediated hydrolysis of Tween 80 (Grover et al. 2014).
11.6 Conclusion
The rise of multidrug-resistant and pandrug-resistant pathogens and the lack of new antibiotics has accelerated the research focus towards enzybiotics as potent antibiotic alternatives. Their high antibacterial activity against multidrug-resistant strains and persisters, low resistance development profile, anti-biofilm activity and synergy with antibiotics makes enzybiotics a promising candidate to use as novel therapeutics for human and animal health. Numerous preclinical studies have already demonstrated the potential of (engineered) endolysins to combat Gram-positive and Gram-negative pathogens and initial research has been done to extend enzybiotics towards mycobacteria. Currently, different lead enzybiotics are in the clinical development stage. Considering the fact that different clinical trials are still in progress, we expect that new clinical developments will be revealed in the near future.
Abbreviations
- AG:
-
Arabinogalactan
- Art:
-
Artilysin
- AMP:
-
Antimicrobial peptide
- CBD:
-
Cell wall binding domain
- CM:
-
Cytoplasmic membrane
- CoNS:
-
Coagulase-negative staphylococci
- DAP:
-
Daptomycin
- EAD:
-
Enzymatically active domain
- EDTA:
-
Ethylenediaminetetraacetic acid
- GlcNAc:
-
N-acetylglucosamine
- GLP:
-
Good laboratory practice
- HHP:
-
High hydrostatic pressure
- LAL:
-
Limulus amoebocyte lysate
- LPS:
-
Lipopolysaccharide
- MA:
-
Mycolic acids
- MIC:
-
Minimal inhibitory concentration
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- MSSA:
-
Methicillin-sensitive Staphylococcus aureus
- MurNAc:
-
N-acetylmuramic acid
- NA:
-
Not applicable
- ND:
-
Not defined
- OM:
-
Outer membrane
- OMP:
-
Outer membrane permeabilizing
- PAE:
-
Post-antibiotic effect
- PA-SME:
-
Post-antibiotic sub-MIC effect
- PG:
-
Peptidoglycan
- SME:
-
Sub-MIC effect
References
Allison KR, Brynildsen MP, Collins JJ (2011) Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473:216–220. https://doi.org/10.1038/nature10069
Becker SC, Foster-Frey J, Donovan DM (2008) The phage K lytic enzyme LysK and lysostaphin act synergistically to kill MRSA. FEMS Microbiol Lett 287:185–191. https://doi.org/10.1111/j.1574-6968.2008.01308.x
Becker SC, Roach DR, Chauhan VS et al (2016) Triple-acting lytic enzyme treatment of drug-resistant and intracellular Staphylococcus aureus. Sci Rep 6:25063. https://doi.org/10.1038/srep25063
Brennan PJ (2003) Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis 83:91–97. https://doi.org/10.1016/S1472-9792(02)00089-6
Briers Y, Lavigne R (2015) Breaking barriers: expansion of the use of endolysins as novel antibacterials against gram-negative bacteria. Future Microbiol 10:377–390. https://doi.org/10.2217/fmb.15.8
Briers Y, Volckaert G, Cornelissen A et al (2007) Muralytic activity and modular structure of the endolysins of Pseudomonas aeruginosa bacteriophages phiKZ and EL. Mol Microbiol 65:1334–1344. https://doi.org/10.1111/j.1365-2958.2007.05870.x
Briers Y, Cornelissen A, Aertsen A et al (2008) Analysis of outer membrane permeability of Pseudomonas aeruginosa and bactericidal activity of endolysins KZ144 and EL188 under high hydrostatic pressure. FEMS Microbiol Lett 280:113–119. https://doi.org/10.1111/j.1574-6968.2007.01051.x
Briers Y, Schmelcher M, Loessner MJ et al (2009) The high-affinity peptidoglycan binding domain of Pseudomonas phage endolysin KZ144. Biochem Biophys Res Commun 383:187–191. https://doi.org/10.1016/j.bbrc.2009.03.161
Briers Y, Walmagh M, Grymonprez B et al (2014a) Art-175 is a highly efficient antibacterial against multidrug-resistant strains and persisters of Pseudomonas aeruginosa. Antimicrob Agents Chemother 58:3774–3784. https://doi.org/10.1128/AAC.02668-14
Briers Y, Walmagh M, Van Puyenbroeck V et al (2014b) Engineered endolysin-based “Artilysins” to combat multidrug-resistant gram-negative pathogens. MBio 5:e01379–e01314. https://doi.org/10.1128/mBio.01379-14
Callewaert L, Walmagh M, Michiels CW, Lavigne R (2011) Food applications of bacterial cell wall hydrolases. Curr Opin Biotechnol 22:164–171. https://doi.org/10.1016/j.copbio.2010.10.012
Cassino C (2016) Results of the first in human study of lysin CF-301 evaluating the safety, tolerability and pharmacokinetic 1248 profile in healthy volunteers. In: 26th ECCMID, April 9. http://c.eqcdn.com/_aa148792491891c86783e37af306cef5/contrafect/db/257/1148/pdf/ContraFect+CF-301+ECCMID+2016+Poster.pdf. Accessed 20 Dec 2017
Catalão MJ, Milho C, Gil F, et al (2011) A second endolysin gene is fully embedded in-frame with the lysA gene of mycobacteriophage Ms6. PLoS One 6:e20515. doi: 10.1371/journal.pone.0020515
Centres for Disease Control and Prevention (US) (2013) Antibiotic resistance threats in the United States, 2013. Centres for Disease Control and Prevention, US Department of Health and Human Services
Channabasappa S, Chikkamadaiah R, Durgaiah M, et al (2017) Preclinical studies of anti-staphylococcal ectolysin P128 for potential systemic hypersensitivity and evaluation of efficacy in Staphylococcus aureus bacteremia with renal abscesses in rats. In: ASM Microbe, June. http://www.gangagen.com/GangaGen-News/presentation_poster/ASM 2017 Rat final.pdf. Accessed 28 Dec 2017
Climo MW, Patron RL, Goldstein BP, Archer GL (1998) Lysostaphin treatment of experimental methicillin-resistant Staphylococcus aureus aortic valve endocarditis. Antimicrob Agents Chemother 42:1355–1360
Czaplewski L, Bax R, Clokie M et al (2016) Alternatives to antibiotica – a pipeline portfolio review. Lancet Infect Dis 16:239–251. https://doi.org/10.1016/S1473-3099(15)00466-1
Defraine V, Schuermans J, Grymonprez B et al (2016) Efficacy of artilysin Art-175 against resistant and persistent Acinetobacter baumannii. Antimicrob Agents Chemother 60:3480–3488. https://doi.org/10.1128/AAC.00285-16
D’Herelle F. (1917) Sur un microbe invisible antagoniste des bacilles dysentériques. C. R. Acad. Sci. 165:373–375
Diez-Martinez R, de Paz HD, Bustamante N et al (2013) Improving the lethal effect of cpl-7, a pneumococcal phage lysozyme with broad bactericidal activity, by inverting the net charge of its cell wall-binding module. Antimicrob Agents Chemother 57:5355–5365. https://doi.org/10.1128/AAC.01372-13
Djurkovic S, Loeffler JM, Fischetti VA (2005) Synergistic killing of Streptococcus pneumoniae with the bacteriophage lytic enzyme Cpl-1 and penicillin or gentamicin depends on the level of penicillin resistance. Antimicrob Agents Chemother 49:1225–1228. https://doi.org/10.1128/AAC.49.3.1225-1228.2005
Drilling AJ, Cooksley C, Chan C et al (2016) Fighting sinus-derived Staphylococcus aureus biofilms in vitro with a bacteriophage-derived muralytic enzyme. Int Forum Allergy Rhinol 6:349–355. https://doi.org/10.1002/alr.21680
Entenza JM, Loeffler JM, Grandgirard D et al (2005) Therapeutic effects of bacteriophage Cpl-1 lysin against Streptococcus pneumoniae endocarditis in rats. Antimicrob Agents Chemother 49:4789–4792. https://doi.org/10.1128/AAC.49.11.4789-4792.2005
Eugster MR, Loessner MJ (2012) Wall teichoic acids restrict access of bacteriophage endolysin Ply118, Ply511, and PlyP40 cell wall binding domains to the Listeria monocytogenes peptidoglycan. J Bacteriol 194:6498–6506. https://doi.org/10.1128/JB.00808-12
Fischetti VA (2005) Bacteriophage lytic enzymes: novel anti-infectives. Trends Microbiol 13:491–496. https://doi.org/10.1016/j.tim.2005.08.007
Fischetti VA (2010) Bacteriophage endolysins: a novel anti-infective to control gram-positive pathogens. Antimicrob Agents Chemother 300:357–362. https://doi.org/10.1016/j.ijmm.2010.04.002
George SE, Chikkamadaiah R, Durgaiah M et al (2012) Biochemical characterization and evaluation of cytotoxicity of antistaphylococcal chimeric protein P128. BMC Res Notes 5:280. https://doi.org/10.1186/1756-0500-5-280
Gerstmans H, Rodríguez-Rubio L, Lavigne R, Briers Y (2016) From endolysins to Artilysin®s: novel enzyme-based approaches to kill drug-resistant bacteria. Biochem Soc Trans 44:123–128. https://doi.org/10.1042/BST20150192
Gerstmans H, Criel B, Briers Y (2017) Synthetic biology of modular endolysins. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2017.12.009
Ghahramani P, Khariton T, Jones S, et al (2017) Population pharmacokinetic-pharmacodynamic assessment of cardiac safety endpoints for CF-301, a first-in-class antibacterial lysin. In: ASM Microbe, June 3. https://www.contrafect.com/news/posters-and-presentations
Gil F, Catalao MJ, Moniz-Pereira J et al (2008) The lytic cassette of mycobacteriophage Ms6 encodes an enzyme with lipolytic activity. Microbiology 154:1364–1371. https://doi.org/10.1099/mic.0.2007/014621-0
Gilmer DB, Schmitz JE, Euler CW, Fischetti VA (2013) Novel bacteriophage lysin with broad lytic activity protects against mixed infection by Streptococcus pyogenes and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:2743–2750. doi: 10.1128/AAC.02526-12
Grover N, Paskaleva EE, Mehta KK et al (2014) Growth inhibition of Mycobacterium smegmatis by mycobacteriophage-derived enzymes. Enzym Microb Technol 63:1–6. https://doi.org/10.1016/j.enzmictec.2014.04.018
Guo M, Feng C, Ren J et al (2017) A novel antimicrobial endolysin, LysPA26, against Pseudomonas aeruginosa. Front Microbiol 8:293. https://doi.org/10.3389/fmicb.2017.00293
Gutierrez D, Ruas-Madiedo P, Martinez B et al (2014) Effective removal of staphylococcal biofilms by the endolysin LysH5. PLoS One 9:e107307. https://doi.org/10.1371/journal.pone.0107307
Haddad KH, Schmelcher M, Sabzalipoor H, et al (2017) Recombinant endolysins as potential therapeutics against antibiotic-resistant Staphylococcus aureus: current status of research and novel delivery strategies. Clin Microbiol Rev 31. doi: https://doi.org/10.1128/CMR.00071-17
Hanlon GW (2007) Bacteriophages: an appraisal of their role in the treatment of bacterial infections. Int J Antimicrob Agents 30:118–128. https://doi.org/10.1016/j.ijantimicag.2007.04.006
Hermoso JA, Monterroso B, Albert A et al (2003) Structural basis for selective recognition of pneumococcal cell wall by modular endolysin from phage Cp-1. Structure 11:1239–1249. https://doi.org/10.1016/j.str.2003.09.005. showArticle Info
Hermoso JA, García JL, García P (2007) Taking aim on bacterial pathogens: from phage therapy to enzybiotics. Curr Opin Microbiol 10:461–472
Huang G, Shen X, Gong Y et al (2014) Antibacterial properties of Acinetobacter baumannii phage Abp1 endolysin (PlyAB1). BMC Infect Dis 14:681. https://doi.org/10.1186/s12879-014-0681-2
Jado I, López R, García E et al (2003) Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J Antimicrob Chemother 52:967–973. https://doi.org/10.1093/jac/dkg485
Jandourek A, Boyle J, Cassino C, et al (2017a) Long term immunology results of a phase 1 placebo controlled dose escalating study to examine the safety of CF-301 in human volunteers. In: 27th ECCMID, April 22. http://c.eqcdn.com/_aa148792491891c86783e37af306cef5/contrafect/db/257/1164/pdf/ECCMID+2017+Immunogenicity.pdf. Accessed 16 Dec 2017
Jandourek A, Boyle J, Murphy G, Cassino C (2017b) Inflammatory markers in a phase 1 placebo controlled dose escalating study of intravenous doses of CF-301 in human subjects. In: ASM Microbe, June 2. http://c.eqcdn.com/_aa148792491891c86783e37af306cef5/contrafect/db/257/1167/pdf/ASM+2017+CF-301-102+Phase+1+Inflammatory+Markers+Poster+FINAL+POSTER.pdf. Accessed 16 Dec 2017
Johnsborg O, Håvarstein LS (2009) Regulation of natural genetic transformation and acquisition of transforming DNA in Streptococcus pneumoniae. FEMS Microbiol Rev 33:627–642
Jun SY, Jung GM, Yoon SJ et al (2013) Antibacterial properties of a pre-formulated recombinant phage endolysin, SAL-1. Int J Antimicrob Agents 41:156–161. https://doi.org/10.1016/j.ijantimicag.2012.10.011
Jun SY, Jung GM, Yoon SJ et al (2014) Preclinical safety evaluation of intravenously administered SAL200 containing the recombinant phage endolysin SAL-1 as a pharmaceutical ingredient. Antimicrob Agents Chemother 58:2084–2088. https://doi.org/10.1128/AAC.02232-13
Jun SY, Jung GM, Yoon SJ et al (2016) Pharmacokinetics of the phage endolysin-based candidate drug SAL200 in monkeys and its appropriate intravenous dosing period. Clin Exp Pharmacol Physiol 43:1013–1016. https://doi.org/10.1111/1440-1681.12613
Jun SY, Jang IJ, Yoon S et al (2017) Pharmacokinetics and tolerance of the phage endolysin-based candidate drug SAL200 after a single intravenous administration among healthy volunteers. Antimicrob Agents Chemother 61:e02629–e02616. https://doi.org/10.1128/AAC.02629-16
Junjappa RP, Desai SN, Roy P et al (2013) Efficacy of anti-staphylococcal protein P128 for the treatment of canine pyoderma: potential applications. Vet Res Commun 37:217–228. https://doi.org/10.1007/s11259-013-9565-y
Lai M-J, Soo P-C, Lin N-T et al (2013) Identification and characterisation of the putative phage-related endolysins through full genome sequence analysis in Acinetobacter baumannii ATCC 17978. Int J Antimicrob Agents 42:141–148. https://doi.org/10.1016/j.ijantimicag.2013.04.022
Langdon A, Crook N, Dantas G (2016) The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med 8:39. https://doi.org/10.1186/s13073-016-0294-z
Lavigne R, Robben J (2012) Professor Dr. Richard Bruynoghe: a 1951 overview of his bacteriophage research spanning three decades. Bacteriophage 2:1–4. https://doi.org/10.4161/bact.20024
Lim J-A, Shin H, Heu S, Ryu S (2014) Exogenous lytic activity of SPN9CC endolysin against gram-negative bacteria. J Microbiol Biotechnol 24:803–811. https://doi.org/10.4014/jmb.1403.03035
Loeffler JM, Fischetti VA (2003) Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and-resistant Streptococcus pneumoniae strains. Antimicrob Agents Chemother 47:375–377
Loeffler JM, Djurkovic S, Fischetti VA (2003) Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect Immun 71:6199–6204. https://doi10.1128/iai.71.11.6199-6204.2003
Loessner MJ (2005) Bacteriophage endolysins – current state of research and applications. Curr Opin Microbiol 8:480–487
Loessner MJ, Kramer K, Ebel F, Scherer S (2002) C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol Microbiol 44:335–349
Lood R, Winer BY, Pelzek AJ et al (2015) Novel phage lysin capable of killing the multidrug-resistant gram-negative bacterium Acinetobacter baumannii in a mouse bacteremia model. Antimicrob Agents Chemother 59:1983–1991. https://doi.org/10.1128/AAC.04641-14
López R, Garcíia E, García P (2004) Enzymes for anti-infective therapy: phage lysins. Drug Discov Today Ther Strateg 1:469–474
Lysando (2017) Topical applications in humans. https://www.lysando.com/topical-applications.html. Accessed 14 Jan 2017
Madigan MT, Martinko JM, Dunlap PV, Clark DP (2008) Brock biology of microorganisms 12th edn. Int Microbiol 11:65–73
Nelson D, Loomis L, Fischetti VA (2001) Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc Natl Acad Sci U S A 98:4107–4112. https://doi.org/10.1073/pnas.061038398
Nelson DC, Schmelcher M, Rodriguez-Rubio L et al (2012) Endolysins as antimicrobials. Adv Virus Res 83:299. https://doi.org/10.1016/B978-0-12-394438-2.00007-4
Oh J, Schuch R (2017) The sub-MIC effect of lysin CF-301 on Staphylococcus aureus (S. aureus). In: ASM Microbe, June 2. http://c.eqcdn.com/_aa148792491891c86783e37af306cef5/contrafect/db/257/1169/pdf/A%0A1548 SM+2017+PAE+Final+Version+Poster.pdf. Accessed 20 Dec 2017
Oliveira H, Melo LDR, Santos SB et al (2013) Molecular aspects and comparative genomics of bacteriophage endolysins. J Virol 87:4558–4570. https://doi.org/10.1128/JVI.03277-12
Oliveira H, Vilas Boas D, Mesnage S et al (2016) Structural and enzymatic characterization of ABgp46, a novel phage endolysin with broad anti-gram-negative bacterial activity. Front Microbiol 7:208. https://doi.org/10.3389/fmicb.2016.00208
Pastagia M, Euler C, Chahales P et al (2011) A novel chimeric lysin shows superiority to mupirocin for skin decolonization of methicillin-resistant and-sensitive Staphylococcus aureus strains. Antimicrob Agents Chemother 55:738–744. https://doi.org/10.1128/AAC.00890-10
Paul VD, Rajagopalan SS, Sundarrajan S et al (2011) A novel bacteriophage Tail-Associated Muralytic Enzyme (TAME) from phage K and its development into a potent antistaphylococcal protein. BMC Microbiol 11:226. https://doi.org/10.1186/1471-2180-11-226
Payne K, Sun Q, Sacchettini J, Hatfull GF (2009) Mycobacteriophage lysin B is a novel mycolylarabinogalactan esterase. Mol Microbiol 73:367–381. https://doi.org/10.1111/j.1365-2958.2009.06775.x
Payne CM, Resch MG, Chen L et al (2013) Glycosylated linkers in multimodular lignocellulose-degrading enzymes dynamically bind to cellulose. Proc Natl Acad Sci 110:14646–14651. https://doi.org/10.1073/pnas.1309106110
Poonacha N, Nair S, Desai S, et al (2017) Efficient killing of planktonic and biofilm-embedded coagulase-negative staphylococci by bactericidal protein P128. Antimicrob Agents Chemother 61. doi: https://doi.org/10.1128/AAC.00457-17
Rashel M, Uchiyama J, Ujihara T et al (2007) Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage φMR11. J Infect Dis 196:1237–1247. https://doi.org/10.1086/521305
Roach DR, Donovan DM (2015) Antimicrobial bacteriophage-derived proteins and therapeutic applications. Bacteriophage 5:e1062590. https://doi.org/10.1080/21597081.2015.1062590
Rodríguez-Rubio L, Chang W-L, Gutiérrez D et al (2016) “Artilysation” of endolysin λSa2lys strongly improves its enzymatic and antibacterial activity against streptococci. Sci Rep 6:35382. https://doi.org/10.1038/srep35382
Salmond GPC, Fineran PC (2015) A century of the phage: past, present and future. Nat Rev Microbiol 13:777–786. https://doi.org/10.1038/nrmicro3564
Sandeep K (2006) Bacteriophage precision drug against bacterial infections. Curr Sci 90:631–633
Schirmeier E, Zimmermann P, Hofmann V et al (2018) Inhibitory and bactericidal effect of Artilysin®Art-175 against colistin-resistant mcr-1-positive Escherichia coli isolates. Int J Antimicrob Agents. https://doi.org/10.1016/j.ijantimicag.2017.08.027
Schmelcher M, Shabarova T, Eugster MR et al (2010) Rapid multiplex detection and differentiation of Listeria cells by use of fluorescent phage endolysin cell wall binding domains. Appl Environ Microbiol 76:5745–5756. https://doi.org/10.1128/AEM.00801-10
Schmelcher M, Donovan DM, Loessner MJ (2012a) Bacteriophage endolysins as novel antimicrobials. Future Microbiol 7:1147–1171. https://doi.org/10.2217/fmb.12.97
Schmelcher M, Powell AM, Becker SC et al (2012b) Chimeric phage lysins act synergistically with lysostaphin to kill mastitis-causing Staphylococcus aureus in murine mammary glands. Appl Environ Microbiol 78:2297–2305. https://doi.org/10.1128/AEM.07050-11
Schuch R (2016) Post-antibiotic effects of lysin CF-301 against Staphylococcus aureus in human serum. http://c.eqcdn.com/_aa148792491891c86783e37af306cef5/contrafect/db/257/1150/pdf/Post-Antibiotic+Effects+of+Lysin+CF-301+Against+Staphylococcus+aureus+in+Human+Serum.pdf. Accessed 15 Jan 2017
Schuch R, Nelson D, Fischetti VA (2002) A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 418:884–889. https://doi.org/10.1038/nature01026
Schuch R, Lee HM, Schneider BC et al (2014) Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia. J Infect Dis 209:1469–1478. https://doi.org/10.1093/infdis/jit637
Shavrina MS, Zimin AA, Molochkov NV et al (2016) In vitro study of the antibacterial effect of the bacteriophage T5 thermostable endolysin on Escherichia coli cells. J Appl Microbiol 121:1282–1290. https://doi.org/10.1111/jam.13251
Shen Y, Barros M, Vennemann T et al (2016) A bacteriophage endolysin that eliminates intracellular streptococci. Elife 5:e13152
Sriram B, Channabasappa S, Chikkamadaiah R, et al (2017) Pharmacokinetics and efficacy of ectolysin P128 in a mouse model of systemic Methicillin Resistant Staphylococcus aureus (MRSA) infection. In: ASM Microbe, June. http://www.gangagen.com/GangaGen-News/presentation_poster/ASM 2017-Mousefinal.pdf. Accessed 18 Dec 2017
Sulakvelidze A, Alavidze Z, Morris JG (2001) Bacteriophage therapy. Antimicrob Agents Chemother 45:649–659. https://doi.org/10.1128/AAC.45.3.649-659.2001
Thandar M, Lood R, Winer BY et al (2016) Novel engineered peptides of a phage lysin as effective antimicrobials against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 60:2671–2679. https://doi.org/10.1128/AAC.02972-15
Thummeepak R, Kitti T, Kunthalert D, Sitthisak S (2016) Enhanced antibacterial activity of Acinetobacter baumannii bacteriophage ØABP-01 endolysin (LysABP-01) in combination with colistin. Front Microbiol 7:1402. https://doi.org/10.3389/fmicb.2016.01402
Totté JEE, van Doorn MB, Pasmans SGMA (2017) Successful treatment of chronic Staphylococcus aureus-related dermatoses with the topical endolysin Staphefekt SA. 100: a report of 3 cases. Case Rep Dermatol 9:19–25. https://doi.org/10.1159/000473872
Twort FW (1915) An investigation on the nature of ultra-microscopic viruses. Lancet 186:1241–1243
Vaara M (1993) Outer membrane permeability barrier to azithromycin, clarithromycin, and roxithromycin in gram-negative enteric bacteria. Antimicrob Agents Chemother 37:354–356
Viertel TM, Ritter K, Horz H-P (2014) Viruses versus bacteria-novel approaches to phage therapy as a tool against multidrug-resistant pathogens. J Antimicrob Chemother 69:2326–2336. https://doi.org/10.1093/jac/dku173
Vipra AA, Desai SN, Roy P et al (2012) Antistaphylococcal activity of bacteriophage derived chimeric protein P128. BMC Microbiol 12:41. https://doi.org/10.1186/1471-2180-12-41
Walmagh M, Briers Y, Dos Santos SB et al (2012) Characterization of modular bacteriophage endolysins from Myoviridae phages OBP, 201φ2-1 and PVP-SE1. PLoS One 7:e36991. https://doi.org/10.1371/journal.pone.0036991
Wang IN, Smith DL, Young R (2000) Holins: the protein clocks of bacteriophage infections. Annu Rev Microbiol 54:799–825. https://doi.org/10.1146/annurev.micro.54.1.799
White R, Chiba S, Pang T et al (2011) Holin triggering in real time. Annu Rev Microbiol 108:798–803. https://doi.org/10.1073/pnas.1011921108
Witzenrath M, Schmeck B, Doehn JM et al (2009) Systemic use of the endolysin Cpl-1 rescues mice with fatal pneumococcal pneumonia. Crit Care Med 37:642–649. https://doi.org/10.1097/CCM.0b013e31819586a6
Young R (2014) Phage lysis: three steps, three choices, one outcome. J Microbiol 52:243–258. https://doi.org/10.1038/srep29344
Zhang L, Li D, Li X et al (2016) LysGH15 kills Staphylococcus aureus without being affected by the humoral immune response or inducing inflammation. Sci Rep 6:29344. https://doi.org/10.1038/srep29344
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Dams, D., Briers, Y. (2019). Enzybiotics: Enzyme-Based Antibacterials as Therapeutics. In: Labrou, N. (eds) Therapeutic Enzymes: Function and Clinical Implications. Advances in Experimental Medicine and Biology, vol 1148. Springer, Singapore. https://doi.org/10.1007/978-981-13-7709-9_11
Download citation
DOI: https://doi.org/10.1007/978-981-13-7709-9_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7708-2
Online ISBN: 978-981-13-7709-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)