Abstract
Bacteriocin is a kind of antibiotic substance produced by bacteria in the metabolic process and has the function of resisting bacteria, fungi, or viruses. The intrinsic nature of bacteriocin is protein or polypeptide. For producing strain, bacteriocin is a biological weapon as it can inhibit or kill competitors in complex or harsh environments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
4.1 Bacteriocins: Introduction and Classification
Bacteriocin is a kind of antibiotic substance produced by bacteria in the metabolic process and has the function of resisting bacteria, fungi, or viruses. The intrinsic nature of bacteriocin is protein or polypeptide. For producing strain, bacteriocin is a biological weapon as it can inhibit or kill competitors in complex or harsh environments.
The history of bacteriocin can be traced back to the 1920s. In 1925, Gratia found that antagonism existed among Escherichia coli strains, which he believed was caused by a substance produced by the E. coli V strain. He further isolated the metabolite of the V strain. It seemed that it was a phage-like substance, which then was named as colicin. Later, it was found that not only gram-negative but also many gram-positive bacteria can produce similar substances. So these substances are known as bacteriocins altogether. Great differences exist between bacteriocins and antibiotics, another production generated by microorganisms. Bacteriocins are primary products synthesized by ribosome, encoded by genes, and can selectively inhibit or kill sensitive bacteria. Bacteriocins can be degraded into fragments by protease so that they do not change the normal intestinal flora. While antibiotics are secondary metabolites which are produced by certain microorganisms through enzymatic reactions. They are non-genetic encoding and kill all sensitive bacteria. In addition, the bacteriostatic spectrum of bacteriocins is relatively narrow and varied between each other, while the antibiotics are mostly broad spectrum.
To better distinguish bacteriocins from antibiotics, Konisky (1982) proposed the definition of bacteriocins as a class of bacteriostatic proteins or precursor polypeptides produced by certain bacteria through the ribosome mechanism. They can inhibit not only homologous bacteria. The producing strain is autoimmune to their bacteriocins (Konisky 1982).
The naming of bacteriocins follows a specific rule, usually end with “cin” or “in”. Before the suffix is the genus or species name of the bacterium. For example, the bacteriocin produced by E. coli is named colicin. Bacteriocin produced by Bacillus subtilis is named subtilin. Bacteriocin produced by Leuconostoc gelidum is named leucocin. And bacteriocin produced by Pediococcus acidilactici is named pediocin. Since some strains from the same species may produce different bacteriocins, they are distinguished by letters or numbers after cin or in. For example, lactacin F refers to the sixth bacteriocin reported in Lactobacillus, and bifilong Bb-42 is a bacteriocin produced by Bifidobacterium longum Bb-42 strain.
It is known that most bacteria in nature, including gram-positive and gram-negative bacteria, as well as certain archaea, can produce a variety of bacteriocins, such as lactic acid bacteria, B. subtilis, Staphylococcus aureus, E. coli, Halobacterium, and so on. Among them, the bacteriocins produced by lactic acid bacteria are nontoxic and diverse and has great application potential in the fields of food processing, agricultural production, and biomedicine. In particular, nisin, a bacteriocin produced by Lactococcus lactis, has been commercialized and is widely used as a natural food preservative in foods such as milk, meat, and canned foods.
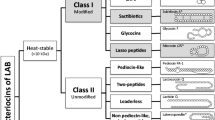
Researches on bacteriocins of lactic acid bacteria have attracted great attention since the discovery of nisin. Bacteriocins from lactic acid bacteria are peptides produced during acid fermentation, which have relatively low molecular weight and can inhibit or kill gram-positive pathogens and spoilage in foods. Some of them even have obvious inhibitory effects on gram-negative bacteria. According to the relative molecular mass, chemical structure, thermal stability, antibacterial spectrum, and other characteristics of amino acids, Klaenhammer (1993) classified lactic acid bacteria bacteriocins into four categories: the first class is lantibiotics, the typical representative is nisin; the second class is a kind of small molecule (relative molecular mass < 10,000), thermostable, membrane-active peptide, such as Pediocin PA-1; the third type is macromolecule (relative molecular mass > 30,000), thermolabile protein such as helveticin; the fourth type is a composite bacteriocin containing lipids or binding to proteins. The first three categories have been recognized, and the view on the fourth type of bacteriocin is still controversial.
4.1.1 The First Group of Bacteriocins
The first group of bacteriocins is lantibiotics. Lantibiotics are heat-stable, with a relative molecular weight of less than 50,000 and a length of 19–38-amino acid residues, and are named after its special amino acids, such as lanthionine. The producing bacteria are generally gram-positive, such as Lactococcus, Streptococcus, Micrococcus, Streptomyces, Lactobacillus, Bacillus, Staphylococcus, Enterococcus, and so on. The active sites of these bacteriocin molecules contain a large number of rare amino acids, including lanthionine (Lan), beta-methyllanthionine (MeLan), dehydroalanine (Dha), and dehydrobutyrine (Dhb). The amino acid sequence of lantibiotics is varied, but it has certain regularity in its cyclization, synthesis, and mechanism of action. According to the difference of the topological structure and action mode of these bacteriocins, lantibiotics can be divided into three subgroups: Type A is slender, with positive charge and amphiphilic, which can form potential-dependent holes on the bacterial plasma membrane. Type B is spherical, without charge or negative charge, and plays its role mainly by destroying the function of enzymes. Type C is a two-component lantibiotics, which contains two polypeptide chains playing a synergistic antibacterial role.
4.1.1.1 Type A Lantibiotics
All the bacteriocins produced by lactic acid bacteria belong to type A. Type A lantibiotics can be further subdivided into slender AI and N-terminal linear and C-terminal spherical AII, represented by nisin and lacticin 481, respectively.
The modification of class AI is accomplished by dehydratase LanB and cyclase LanC, which are then transported out of the cells by LanT and excised by protease LanP. Nisin is a typical representative of this type. It is produced by some strains of Lactococcus lactis subsp. lactis. As a natural antimicrobial peptide, nisin can effectively kill or inhibit most gram-positive bacteria and has a strong inhibitory effect on pathogenic bacteria such as Staphylococcus aureus, Clostridium botulinum, and Streptococcus hemolyticus. The isoelectric point of nisin was about 9. The stability increased with the decrease of pH. At pH 2, the antimicrobial activity of nisin was not lost after being treated at 121 °C for 30 minutes.
Up to now, five natural variants of nisin have been found in nature, namely, nisin A (Gross and Morell 1971), nisin Z (Mulders et al. 1991), nisin Q (Zendo et al. 2003), nisin F (de Kwaadsteniet et al. 2008), and nisin U (Wirawan et al. 2006). Among them, nisin A and nisin Z are the earliest and most studied ones. Their differences lie only in the amino acid at position 27, where nisin A is His, while nisin Z is Asn. But there is no difference in their antimicrobial activities. The structural gene of nisin encodes 57 amino acids, and 23 amino acids at N-terminal are removed during secretion. The mature nisin contains 34 amino acid residues and consists of 5 thioether bridges. Nisin has amphiphilic amino acid distribution. Its N-terminal is hydrophobic, which can interact with the phospholipid end of the target cell membrane. The C-terminal contains more hydrophilic amino acid, which shows hydrophilicity. The structure of nisin in solution was determined by NMR. It was found that there are two domains in nisin molecule: N-terminal domain and C-terminal domain. The former contains the first three thioether rings (A–C ring), and the latter consists of D and E thioether rings. A–C ring and D–E ring are connected by a flexible hinge region, which follows the C-terminal domain. It is a flexible tail consisting of six amino acids (van den Hooven et al. 1996). Nisin U, a variant of nisin A secreted by Streptococcus uberis, is the only bacteriocin not produced by Lactococcus lactis in the nisin family. Nisin U is composed of 31 amino acids, which is close to the antimicrobial spectrum of nisin A. However, the antimicrobial activity of nisin U to Streptococcus pyogenes and Lactococcus lactis is not as good as that of nisin A. Nisin U and nisin A have the same bridging mode, and the strains producing these two bacteriocin are cross immune. If nisin U and nisin A were added to the cultures of Lactococcus and Streptococcus, self-induction or mutual induction would occur between the two peptides, which indicated the similarity of their functions (Wirawan et al. 2006).
The dehydration and cyclization of class AII lantibiotics are accomplished by a bifunctional modifying enzyme LanM. The precursor peptides of class AII lantibiotics have conserved common sequences. Translocation and processing of the precursor are also completed by only one enzyme LanT. Lactacin 481, a kind of class AII bacteriocins, is composed of 27 amino acids which contain two lanthionine, MeLan and Dhb. The expression of lactacin 481 is regulated by adjusting the transcriptional level of the upstream promoter LctA gene (Hindre et al. 2004). Paik et al. found that the bacteriocin sublancin 168 produced by Bacillus subtilis has both stable disulfide bond and lantibiotics structure, which is the first bacteriocin with two types of structure (Paik et al. 1998).
4.1.1.2 Type B Lantibiotics
Type B lantibiotics is a compact spherical structure with no charge or negative charge in neutral environment. Mersacidin produced by Bacillus subtilis and cinnamycin secreted by Streptomyces cinnamomi are the representatives of type B lantibiotics.
Mersacidin consists of 20 amino acids with a relative molecular weight of 1825 (Chatterjee et al. 1992). It is the only lantibiotics whose structure is determined by X-ray (Schneider et al. 2000). Mersacidin mainly inhibits gram-positive bacteria, including penicillin-resistant Staphylococcus aureus. That is because mersacidin inhibits the synthesis of peptidoglycan by impacting on the diphosphate group of lipid II and mersacidin (Brotz et al. 1997). Mersacidin acts in a manner similar to vancomycin, so mersacidin has great potential in the development of drugs to treat drug-resistant superbacterial infections and can be used to inhibit methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus.
Cinnamycin contains 19 amino acids, with two beta-methylthionine residues linked to dehydrotyrosine via a nucleophilic cysteine at the N-terminal of the peptide chain. Its antibacterial activity can only be applied to a few strains, such as Bacillus subtilis. Cinnamycin destroys ATP-dependent calcium uptake and protein transport by increasing cell membrane permeability of sensitive bacteria. Moreover, the bacteriocin can bind to phosphatidylethanolamine on phospholipids, thereby inhibiting the competitive inhibition of phospholipase, preventing the conversion of phosphatidylethanolamine to lysophosphatidylethanolamine, and affecting the dissolution of red blood cells.
4.1.1.3 Type C Lantibiotics
Type C is a multicomponent lantibiotic. The two independent transcription-modified peptides have no or very low activity, but they have significant synergistic antibacterial effect. Lacticin 3147 produced by Lactococcus lactis subsp. DPC3147 could be attributed to this kind of bacteriocin. It contains two peptides, LtnA1 and LtnA2, with relative molecular weights of 3306 and 2847, respectively. It is speculated that LtnA1 binds to lipid II, and the compound of LtnA2 and LtnA1-lipid II together can insert into the cell membrane of sensitive bacteria more effectively to form pores. The combined action activity of LtnA1 and LtnA2 is 30 times that of LtnA1 alone (Morgan et al. 2005). Lacticin 3147 mainly inhibits gram-positive bacteria, including Staphylococcus aureus, Enterococcus, Pneumococcus, Propionibacterium acnes, and Streptococcus mutans, and foodborne pathogens such as Listeria monocytogenes and Bacillus cereus. It can also inhibit gram-negative bacteria when cooperated with polymyxin (Draper et al. 2013). Smb (Yonezawa and Kuramitsu 2005) produced by Streptococcus mutans GS5 and cytolysin secreted by Enterococcus are also composed of two peptides. Cytolysin, an exotoxin, also known as streptococcal hemolysin, is the only lantibiotic that inhibits both bacteria and eukaryotic cells (Tyne et al. 2013).
4.1.2 The Second Group of Bacteriocins
The second group of bacteriocins does not contain noncoding amino acid residues such as lanthionine. They can be divided into three subgroups, class IIa, class IIb, and class IIc.
4.1.2.1 Class IIa
Class II bacteriocin is the largest and most widely studied subclass of bacteriocin in second categories. So far, all class IIa bacteriocins have strong anti-Listeria activities. Some class IIa bacteriocins can also inhibit other spoilage bacteria in food, such as Bacillus, Staphylococcus, Clostridium, and so on. More than 30 kinds of class a lactic acid bacteria have been found. Typical representatives are pediocin PA-1, pediocin AcH, sakacin A, sakacin P, leucocin A, curvacin A, enterocin A, etc. Lactobacillus, Pediococcus, Leuconostoc, Carnivora, and Enterococcus are the main producers. Leucocin A produced by Leuconostoc gelidum UAL 187 is the first found class IIa bacteriocin (Hastings et al. 1991). But pediocin PA-1 produced by Pediococcus lactis is the most studied class IIa bacteriocin.
Class IIa bacteriocins generally contain 37 to 48 amino acid residues and a conserved YGNGVXaaC group at the N-terminus, which is generally considered to be a recognition sequence for membrane-bound protein receptors. As more and more new class IIa bacteriocins are discovered, their N-terminal groups can also be expressed as YGNGVXaaCXaa(K/N)XaaXaaCXaaV(N/D)(W/K/R)Xaa(G/A/S) (A/N) (in parenthesis are the conservative residues, and Xaa represents residues with high frequency of variation). Another important feature of class IIa bacteriocins is that the N-terminal conserved region contains at least two cysteines forming a disulfide bond. In general, peptides containing two disulfide bonds are more bacteriostatic than bacteriocins containing one disulfide bond (Eijsink et al. 1998). Relative to the conservation of the N-terminus, the sequence similarity of the C-terminus of class IIa bacteriocin is only 34% to 80% and forms an α-helix, which acts as a transmembrane component when the cell membrane of the susceptible bacteria forms a pore. Class IIa bacteriocins have no posttranslational modifications other than the formation of disulfide bonds and are simpler in structure than other bacteriocins. Although the minimum inhibitory concentrations of these bacteriocins are different, there is a certain similarity in the inhibition spectrum (Fimland et al. 2005).
4.1.2.2 Class IIb
Class IIb bacteriocin is a two-component bacteriocin formed by two peptide oligomers. The complete activity requires two peptides to interact with each other, mainly including lactacin F, plantaricin S, plantaricin A, lactococcin C, and lactococcin MN (Garneau et al. 2002). Class IIb bacteriocins can also be subdivided into two categories, synergistic type (S type) and enhanced type (E type) (Marciset et al. 1997).
The antibacterial effect of S-type bacteriocin is formed by the interaction of two peptides. The single peptide has no inhibitory effect. Typical S-type bacteriocins are lactococcin G produced by Lactococcus lactis, lactacin F produced by Lactobacillus johnsonii, and lactacin 705 produced by Lactobacillus casei. Lactococcin G consists of an alpha chain and a beta chain, which contains 39 and 35 amino acids, respectively. The mode of action is to inhibit amino acid uptake, dissipate proton momentum, and reduce intracellular ATP levels.
The two peptides of type E bacteriocins both have antibacterial effects. The bacteriostatic effect is significantly enhanced when the two peptides act together. Thermophilin 13 produced by Streptococcus thermophilus, enterocin L50 (Cintas et al. 2000) produced by Enterococcus faecium, and ABP-118 (Flynn et al. 2002) produced by Lactobacillus saliva are typical representatives of E-type class IIb bacteriocins. The two polypeptides of plantaricin PlnJK have certain faint inhibitory effects on Escherichia coli and Listeria monocytogenes. The antibacterial effect is enhanced 1000 times when the two peptides are mixed with a concentration of 1:1 (Anderssen et al. 1998).
4.1.2.3 Class IIc
Class IIc bacteriocins include those non-lantibiotics that neither belong to class IIa nor class IIb and are generally considered to contain thiol-based activities and signal peptide encoding mechanisms (Cotter et al. 2005). Strains producing bacteriocin type IIc are various, and the diversity of bacteriocins is complex. Lactococcin B belongs to class IIc bacteriocins, which can be activated by mercaptan. The antimicrobial and immune proteins of sakacin P produced by Lactobacillus sake are coupled (Mathiesen et al. 2005). Class IIc bacteriocin also has some similar structures, such as GG sequence in leading sequence, ABC transporter, and some immune related-proteins. Some class IIc bacteriocins do not possess recognizable N-terminal signal peptides, such as enterocin Q produced by Enterococcus faecium, aureocin A53 produced by Streptomyces aureus, and BHT-B produced by Streptococcus rattus. Gassericin A is secreted by Lactobacillus gasseri LA39, which is isolated from human infant feces. It is encoded by chromosomes and has a very rare cyclic structure. It has inhibitory activity against many food pathogens, including Listeria monocytogenes, Bacillus cereus, and Staphylococcus aureus.
4.1.3 The Third Group of Bacteriocins
The third group of bacteriocins have a relatively high molecular weight (usually greater than 30,000). It is inactivated after heating at 100 °C for 30 minutes. These bacteriocins include helveticin J (Joerger and Klaenhammer 1986), lacticin A, lacticin B, caseicin 80 (Müller and Radler 1993), acidophilucin A, helveticin V-128, enterolysin A, etc. However, propionicin SM1 produced by Propionibacterium acnes is heat-resistant (Miescher et al. 2000). These bacteriocins can also be subdivided into two classes: lysozyme-like bacteriocins that inhibit bacteria through cell lysis and non-lysozyme antibacterial proteins. Only a few of the bacteriocins produced by Lactobacillus and Bifidobacterium have been reported. Only five of the third bacteriocins produced by lactic acid bacteria have been studied at the genetic level. They are lysostaphin produced by Staphylococcus simulans, helveticin J produced by Lactobacillus helveticus 481, zoocin A produced by Streptococcus zooepidemicus 4881, millericin B produced by Streptococcus milleri NMSCC 061, and enterolysin A produced by Enterococcus faecalis LMG 2333.
4.1.3.1 Lysozyme-Like Bacteriocins
Lysostaphin produced by Staphylococcus simulans is a representative of this kind of bacteriocin (King et al. 1980). Lysostaphin is a kind of metalloproteinase. Zn2+ is a necessary cofactor. It is composed of 246 amino acids, with the relative molecular weight of about 27,000. Lysostaphin is an extracellular enzyme. The producing strain first generates a 493-amino-acid proenzyme. The N-terminal of the enzyme has multiple repetitive sequences containing 13 amino acids. The N-terminal repeats are removed by cysteine protease after the proenzyme is secreted into the extracellular matrix, and then the mature lysostaphin is formed (Heinrich et al. 1987). Lysostaphin has many catalytic activity centers, of which endopeptidase, glycosidase, and amidase are related to the catalytic activity of hydrolyzing the cross-linked structure of bacterial cell wall peptidoglycan. Endopeptidase activity is the most important (Neumann et al. 1993). Endopeptidase can specifically hydrolyze Gly pentapeptide bridges in the cross-linked structure of bacterial cell wall peptidoglycans. Since this structure exists only in the cell wall of Staphylococcus and is most widely distributed in the cell wall of Staphylococcus aureus, lysostaphin can kill almost all staphylococci, but is invalid for other species of bacteria.
4.1.3.2 Non-lysozyme Antibacterial Proteins
These bacteriocins deplete ATP of target microorganisms through proton dynamics, leading to cell death. Dysgalacticin (relative molecular weight of 21,000) and streptococcin A-M57 (relative molecular weight of 17,000) are secreted by Streptococcus dysgalactiae subsp. equisimilis and Streptococcus pyogenes, respectively. The former has a narrow inhibitory spectrum, while the latter has a distinct inhibitory spectrum. It can inhibit non-streptococcal gram-positive bacteria such as Micrococcus luteus and Lactococcus lactis, most of Listeria, Bacillus megagenes and Staphylococcus simulans.
4.1.4 The Fourth Group of Bacteriocin
Some other proteins with bacteriostatic and bactericidal effects have been found during the research on bacteriocins. They not only inhibit gram-positive bacteria, but also inhibit gram-negative bacteria and fungi. These protein antagonists not fully conform to the definition of bacteriocins are called bacteriocin-like substances. Bacteriocin-like substances have wider application prospect than bacteriocins because of their stability in a wide pH range and broad-spectrum antibacterial properties.
Whitford et al. isolated a strain of Streptococcus LRC0255 from the rumen. Bacteriocin-like substance bovicin 255 produced by this strain was active at pH 1-12 and stable to heat. The activity of bovicin 255 was unchanged at 100 °C for 15 minutes. It inhibited gram-positive bacteria, but had no effect on gram-negative bacteria. It was sensitive to pronase and protease K but insensitive to pepsin and peptidase isolated from pig intestinal mucosa (Whitford et al. 2001).
Collado et al. isolated six strains of bifidobacteria from human feces, which not only inhibited gram-positive bacteria but also inhibited gram-negative bacteria and yeast. Bacteriocin-like substances produced by these bifidobacteria were stable at pH 3–10, and their activity remained unchanged at 100 °C for 10 min. They were resistant to alpha-amylase and phospholipase A but sensitive to protease. The relative molecular weight of the substances produced by BIR-0312 and BIR-0324 were 10,000-30,000, while those of the other four bacteriocins were all less than 10,000 (Collado et al. 2005a). Further studies found that these bacteriocins also inhibit Helicobacter pylori (Collado et al. 2005b).
There are relatively few reports on bacteriocin-like substances which can inhibit fungi. Magnusson and Schnürer from the Department of Microbiology of Swedish Agricultural University isolated a Lactobacillus coryniformis Si3 strain from silage. This strain produced a broad-spectrum inhibitory fungal protein. It has strong inhibitory effect on fungi including Aspergillus, Penicillium, Mucor, and Fusarium and also has weak inhibitory effect on yeast including Kluyveromyces marxianus and Saccharomyces cerevisiae. The activity was highest at pH 3.0–4.5 and was lost when pH was higher than 6, but it could be restored when pH was returned to 3.6. The activity was stable at 121 °C for 15 min. It was sensitive to protease K, trypsin, and pepsin. Relative molecular weight was 30,000 (Magnusson and Schnürer 2001).
4.2 Species and Structure of Bacteriocin of Lactic Acid Bacteria
4.2.1 Bacteriocins Produced by Lactobacillus
Many different Lactobacillus spp. were isolated from natural fermented dairy products, nondairy products, starters, animals, plants, and human intestines. The Lactobacillus spp. produce a wide range of bacteriocins, which differ greatly in molecular weight, biochemical characteristics, sensitive range, and mode of action. In addition to producing bacteriocins, Lactobacillus often produces acids, hydrogen peroxide, diacetyl, and other substances, which can antagonize certain microorganisms. Therefore, it is necessary to remove the above compounds from the samples before studying the bacteriostatic effect of Lactobacillus. Currently known Lactobacillus bacteriocins are more than 30 kinds, including plantaricin A, plantaricin B, plantaricin C, sakacin A, sakacin M, sakacin P, lactocin S, etc.
4.2.1.1 Plantaricin
Bacteriocin produced by Lactobacillus plantarum is collectively referred to as plantaricin. Most of the plantaricins have a broad spectrum of inhibition, and the inhibited bacteria include lactic acid bacteria and other gram-positive bacteria such as Listeria, Staphylococcus aureus, and Listeria monocytogenes. Plantaricin is complex in classification; plantaricin LR14 and plantaricin C belong to class I. Some belong to class II, such as plantaricin S and plantaricin NC8, due to their conserved YYGNGV/C region at the N-terminal, which conforms to the characteristics of class IIa bacteriocins. The yield of plantaricin mainly depends on the genetic characteristics and culture conditions of the producing bacteria. It is generally believed that plantaricin begins to synthesize and secrete in the middle of the exponential growth period and reaches the maximum yield at the stationary phase. Therefore, in the process of plantaricin production, conditions such as culture time, temperature, initial pH of the fermentation broth, and medium composition should be well controlled.
4.2.1.2 Gassericin A
Gassericin A is secreted by Lactobacillus gasseri LA 39. Isoleucine is connected to N-terminal, and alanine is connected to C terminal of Gassericin A. A total of 58 amino acids are linked to form a ring structure with a relative molecular weight of 5652 (Kawai et al. 2009). Gassericin A is thermostable and insensitive to pH. It is still active in the pH range of 2–12. It can inhibit some Lactobacillus and some foodborne spoilage bacteria such as Listeria monocytogenes, Bacillus cereus, and Staphylococcus aureus (Nakamura et al. 2013). Another strain of Lactobacillus gasseri LA158 isolated from infant feces can synthesize bacteriocin Gassericin T, which is also thermostable and has a bacteriostatic spectrum similar to that of Gassericin A. However, Gassericin T is a two-component bacteriocin and belongs to lacticin F group bacteriocins (Arakawa et al. 2009a, 2009b).
4.2.1.3 Sakacin
Sakacin A is secreted by Lactobacillus sake 706 and inhibits many Lactobacillus and gram-positive bacteria. The synthesized gene cluster is located on the plasmids. Holck et al. determined the protein sequence of sakacin A by Edman degradation reaction. The bacteriocin contained 41 amino acids, and its relative molecular weight was about 4308.7 (Mathiesen et al. 2005).
4.2.1.4 Lactacin
Lactacin B is produced by Lactobacillus acidophilus N2. It is a kind of thermostable, catalase-insensitive bacteriocin, the relative molecular weight of about 65,000. It can inhibit Enterococcus faecalis and homologous Lactobacillus delbrueckii, Lactobacillus bulgaricus, and Lactobacillus helveticus (Dobson et al. 2007). Lactacin F, secreted by Lactobacillus acidophilus 11088, is a hydrophobic peptide with 54–57 amino acids and a relative molecular weight of about 63,000. Lactacin F is the first nonlantibiotic bacteriocin produced by lactic acid bacteria with known DNA and protein sequence information. Both lactacin B and F are heat-stable and maintained antimicrobial activity at 121 °C for 15 min. Their solubility was similar, and both belonged to class II bacteriocins (Muriana and Klaenhammer 1991). Although the similarity between the two bacteriocins is very high, there is a certain difference in their antibacterial spectrum. It is shown that the antibacterial spectrum of lactacin B is obviously narrower than that of lactacin F.
4.2.1.5 Lactocin
Lactocin S is a thermostable bacteriocin produced by Lactobacillus sakei. Its activity decreased by only half when it was treated at 100 °C for 1 h (Mortvedt et al. 1991). Lactocin 27 is a thermostable class II bacteriocin produced by Lactobacillus helveticus. It contains large amounts of glycine and alanine. It is sensitive to trypsin and streptomycin and insensitive to fig protease (Upreti and Hinsdill 1975).
4.2.1.6 Helveticin
Helveticin J is synthesized and secreted by Lactobacillus helveticus 481. Its regulatory gene cluster is located on chromosome and has a narrow inhibitory range. It belongs to class III bacteriocins. Its molecular weight is about 37,511. It is unstable and inactivated after 30 minutes at 100 °C (Joerger and Klaenhammer 1986).
4.2.2 Bacteriocins Produced by Bifidobacterium
Bifidobacterium is an important component of human intestinal symbiotic bacteria, accounting for 3–7% of the total amount of microorganisms in adults and 91% in newborns. Bifidobacterium produces a lot of lactic acid and acetic acid in the metabolic process. The acidic environment formed by Bifidobacterium can inhibit the colonization and growth of pathogenic bacteria and has a positive effect on host health. Unlike Lactobacillus, only a limited number of studies have shown the production of bacteriostatic substances or bacteriocins in the strains of Bifidobacterium. Bifidobacterium bifidum, B. longum, B. infantum, and B. thermophilus have been reported to produce bacteriocins.
4.2.2.1 Bifidin
In 1984, Anand et al. reported that B. bifidum 1452 could produce bacteriocin bifidin, which was thermostable and had no loss of activity after heating for 30 minutes at 100 °C. Partially purified bifidin was obtained by ethanol-acetone precipitation and molecular sieve. The crude extract remained stable in refrigerator for 3 months at 5–8 °C. Sequence analysis showed that the antimicrobial peptide contained two kinds of amino acids, phenylalanine and glutamic acid, as well as trace amounts of aspartate, threonine, serine, glycine, isoleucine, and leucine (Anand et al. 1984). In vitro experiments confirmed that bifidin could inhibit the propagation of Micrococcus flavus and Staphylococcus aureus.
Bifidin I is an antibacterial substance isolated from the metabolites of B. infantum BCRC 14602 (Cheikhyoussef et al. 2010). It can inhibit lactic acid bacteria and some gram-positive bacteria such as Staphylococcus, Streptococcus, and Bacillus. It also inhibits gram-negative bacteria such as Salmonella, Shigella, and Escherichia coli, but it is not effective on yeast. The relative molecular weight was about 30,000. The maximum activity (1600 AU/mL) can be obtained after 16–20 h culture in MRS medium. The thermal stability is not affected in the range of pH 4–10.
4.2.2.2 Bifilong
Bifilong Bb-46 is produced by B. longum Bb-46. It can inhibit E. coli, Salmonella typhimurium, Bacillus cereus, and Staphylococcus aureus. It is sensitive to trypsin and pepsin, but not heat-resistant. The activity of 121 °C loses after heating for 15 min and is stable between pH 4 and 7 but decreases rapidly above pH 9.
4.2.2.3 Bifidocin B
Bifidocin B is the first isolated bifidobacterial bacteriocin, with a molecular weight of 33,000, belonging to class IIa bacteriocins (Yildirim and Johnson 1998). Bifidocin B, synthesized and secreted by B. bifidum NCFB 1454, was sensitive to protease but resistant to organic solvents, heat, and pH. Bifidocin B was not inactivated by heating for 15 min at 121 °C, and its activity was stable in the range of pH 2 to 10. Bifidocin B can inhibit some foodborne pathogens and spoilage bacteria, such as Listeria, Enterococcus, Bacillus, Leuconostoccus, Phanerococcus, and so on, but has no effect on other gram-positive bacteria and gram-negative bacteria. Bifidocin B is produced during exponential phase and reaches the maximum yield (3, 200 AU/mL) at the beginning of the stable period.
4.2.2.4 Thermophilicin B67
B. thermophilum RBL67 is isolated from infant feces. In a reaction system simulating the composition and activity of bifidobacteria in the proximal colon of infants, the addition of B. thermophilus RBL67 resulted in a sharp decrease in the number of Bifidobacterium although the composition and activity of intestinal flora did not change much. This may be due to the killing of related microorganisms by thermophilicin B67 produced by B. thermophilus RBL67 (Zihler et al. 2011). When B. thermophilus RBL67 was mixed together with Pediococcus acidilactici UVA1, a bacteriocin-producing bacterium isolated from infant feces, the culture system was stable, and cell growth and bacteriocin production and activity were not affected (Mathys et al. 2009). In the reaction system simulating the microbial composition of pig intestinal tract, when mixing B. thermophilus RBL67 with prebiotics oligosaccharides and galactooligosaccharides, the growth of Salmonella enteric subsp. enterica serotype N-15 could be inhibited, which presumably not only related to acetic acid but also to the bacteriocin produced by B. thermophiles (Tanner et al. 2014).
4.2.3 Bacteriocins Produced by Lactococcus, Enterococcus, and Other Species
Lactococcus is widely used in dairy industry as a starter. Bacteriocins produced by Lactococcus lactis include diplococcin, lactococcin, lacticin, and nisin. It is also found that some Lactococcus will produce a large amount of non-lantibiotics.
4.2.3.1 Nisin
Nisin is produced by L. lactis, which consists of 34 amino acid residues as introduced before. Nisin contains five rare amino acids, namely, ABA, DHA, DHB, ALA-S-ALA, and ALA-S-ABA. They form five inner rings through thioether bonds. Nisin has hydrophilic and hydrophobic properties, showing the characteristics of cationic peptides, isoelectric point in the alkaline range. The solubility of Nisin increases with the decrease of pH. It is almost insoluble in alkaline condition. The stability of Nisin is related to its solubility at different pH values. In hydrochloric acid solution with pH 2.5 or lower, even boiling does not affect the activity. The activity remains stable after heating for 30 minutes at pH 2 and 121 °C. However, when pH exceeds 4, nisin inactivates rapidly in aqueous solution.
4.2.3.2 Lactococcin
Lactococcin A is produced by Lactococcus lactis. The producing bacteria can also produce an immune protein with an amphiphilic alpha helix and a molecular weight of 11,000. This immune protein interacts with Lactococcin A receptor protein to prevent bacteriocin from inserting into the cell membrane, so that lactococcin has no inhibitory effect on the producing bacteria (Hui et al. 1995).
4.2.3.3 Enterocin
Many enterococci produce antimicrobial peptides called enterocins, such as enterocin A produced by Lactococcus lactis MG1614, enterocin AS-48 produced by Enterococcus faecalis, and enterocin B produced by Enterococcus faecalis W3. Enterocin A belongs to class IIa bacteriocins. It contains 47 amino acids and 2 disulfide bonds. Enterocin B has a wide spectrum of antimicrobial activity and a stable thermostability (Aymerich et al. 1996). It also inhibits Listeria monocytogenes, Enterococcus faecalis, Clostridium, and Staphylococcus aureus (Casaus et al. 1997). Enterocin 1146 is also a bacteriocin produced by Enterococcus faecalis, which inhibits all Listeria, most Clostridium butyricum, and Clostridium perfringens. Enterocin 1146 can inhibit the growth of Listeria without affecting the fermentation of starter and thus has broad application prospects in fermentation industry (Parente and Ricciardi 1994).
4.2.3.4 Pediocin
Pediocin is produced by Pediococcus, which is often used to ferment vegetables, cheese, meat, and sausages. Most of the Pediococcus can be isolated from plants and plant products but also from pickles, fermented sausages, and so on. Similar to Lactococcus, the condition of nutritional demand of Pediococcus is severe. Pediocin is produced by Pediococcus acidilactici, Pediococcus cerevisiae, and Pediococcus pentosaceus. Pediocin A, pediocin PA-1, pediocin AcH, pediocin JD, pediocin Bac, and pediocin SJ-1 were found to be the bacteriocins produced by Pediococcus lactis. The amino acid sequences of most of the pediocins are highly homologous and share some common structural features, such as highly conserved N-terminus and poorly polarized C-terminus. The relative molecular weight of pediocin is about thousands of Dalton and are thermally stable.
Etchells et al. (1964) used both Pediococcus cerevisiae FBB-61 and Lactobacillus plantarum FBB-67 in pickled cucumber, and the results showed that the growth lag of Lactobacillus plantarum FBB-67 was as long as 10 days. In fact, Lactobacillus plantarum is more acid-resistant than Pediococcus cerevisiae. It is speculated that the product of Pediococcus cerevisiae may have certain inhibitory effect on Lactobacillus plantarum. Rueckert et al. (1979) found that the product of FBB-61 was a bacteriocin, which was stable at high temperature (100 °C for 60 min) and freezing point and sensitive to pronase and could not penetrate the semipermeable membrane. The product was then named as pediocin A. This bacteriocin has a wide range of bacteriostasis, which can be compared with nisin to a certain extent, including lactic acid bacteria, Clostridium botulinum, Clostridium perfringens, and Staphylococcus aureus. But it has no effect on gram-negative bacteria.
Pediocin PA-I is the most studied bacteriocin produced by lactic acid bacteria except nisin. It was encoded by a 9.3 kb plasmid. Pediocin SJ-1 is encoded by a 6.1 kb plasmid, which is very similar to pediocin PA-I in bacteriostatic spectrum, molecular weight, and genetic characteristics, in addition to being sensitive to alpha-amylase. AcH is produced by Lactococcus lactis H. The molecular weight of AcH is 2700. It is thermostable and sensitive to trypsin, papain, protease K, and chymotrypsin. It has a broad inhibition spectrum and can inhibit Lactobacillus, Leuconostoc, Staphylococcus aureus, Listeria monocytogenes, and so on. The inhibiting mechanism is to inhibit ATP synthesis, reduce energy supply, damage transport system, and eventually lead to cell death of sensitive bacteria.
4.2.3.5 Enterolysin
Enterolysin is secreted by Enterococcus during growth. Enterolysin A, produced by Enterococcus faecalis LMG 2333, is a class III bacteriocin with unstable heat and broad antibacterial spectrum (Nilsen et al. 2003). The precursor protein of enterolysin A contains 343 amino acids. After the removal of 27 amino acids, the final secretion of mature enterolysin A contains 316 amino acids. These bacteriocins can destroy the cell membrane of sensitive bacteria and have antibacterial effect on Lactobacillus, Lactococcus, Pediococcus, Enterococcus, Bacillus, Listeria, and Staphylococcus.
4.2.3.6 Leucocin
Leuconostoc is widely found in many food raw materials, dairy products, and wine fermentation. Leucocin is produced by Leuconostoc isolated from goat milk, cheddar cheese, retail mutton, and vacuum packaged meat. Mesenterocin 5, Leucocin A, Leucocin S, and carnocin have been reported, but they are only descriptions of general characteristics and lack of complete biochemical and genetic information. For example, mesenterocin 5 produced by Leuconostoc mesenteroides isolated from cheddar cheese is relatively heat-resistant (it can survive 30 minutes at 100 °C), and the relative molecular weight is about 4500. When Leuconostoc mesenteroides was inoculated on the medium for about 6 hours, mesenterocin 5 was produced, and the maximum yield was achieved in 10 hours. Mesenterocin 5 can inhibit the growth of Listeria monocytogenes but has no effect on the fermentation starter such as Lactobacillus. Leucocin A, a peptide bacteriocin with a relative molecular weight of 3930.3, can be produced during the whole incubation period (about 7 days) of Leuconostoc gelidum in the range of pH 4.0–6.5 at 25 °C.
4.3 Biosynthesis and Genetic Regulation of Typical Bacteriocin Produced by Lactic Acid Bacteria
Bacteriocin-producing lactic acid bacteria synthesize and secrete some bacteriocins in the logarithmic phase. Bacteriocin is usually transported to the medium by means of cell membrane permeation, but some types of bacteriocins remain in the cell under certain conditions. The production of bacteriocin is synchronous with the growth of bacteria, and the yield is closely related to the number of producing strain. Optimizing culture conditions can effectively increase the output of bacteriocin, such as adding sugar, vitamins, nitrogen, and other stimulants in the medium or adjusting pH and temperature to the optimal range. The production of bacteriocin can be increased by increasing the number of bacteriocin-producing lactic acid bacteria (Abbasiliasi et al. 2011; Espeche et al. 2014). PH condition of culture medium is one of the important factors affecting bacteriocin production, but different kinds of bacteriocins have different pH requirements (de Arauz et al. 2012). For Lactococcus, the Elliker medium without buffering ability is more conducive to the production of bacteriocin than the M17 medium with high buffering ability. During fermentation, high yield can be achieved by regulating pH. Different strains produce bacteriocins at different stages. For example, pediocin AcH is produced in large quantities at pH 5.0 or below 5.0 after entering a stable growth stage. On the contrary, nisin and leuconocin formed in large quantities during the logarithmic growth period, and the pH value was high.
Bacteriocin biosynthesis is regulated by genes that are generally located on chromosomes or plasmids of the producing bacteria (Nes et al. 1996). Operators of thioether antibiotics and bacteriocins II are mostly located on chromosomes, such as plantaricins EF and plantaricins NC8, produced by Lactobacillus plantarum 8P-A3. The regulatory genes of these two bacteriocins are located on chromosomes, and the size of the gene fragments is about 20 kb. Most of the gene clusters that regulate bacteriocin production are located on plasmids. Mandal et al. (2010, 2011) reported a new bacteriocin pediocin NV5 produced by Lactococcus lactis LAB 5, and plasmid elimination experiments showed that the pediocin NV5 gene cluster was located on a plasmid about 5 kb in size.
4.3.1 Biosynthesis and Regulation of Nisin
The gene controlling biosynthesis, immunity, and regulation of nisin is located on a 70 KB conjugated transposon named Tn5276. The regulatory synthesis gene cluster of nisin involves structural genes, mature genes, immune genes and regulatory genes which are controlled by gene cluster nisA/ZBTCIPRKFEG, and adjacent genes related to sucrose metabolism. The 11 genes of the gene cluster consist of 4 transcription units, namely, nisABTCIPRK, nisI, nisRK, and nisFEG. Among them, nisI and nisRK are constitutive transcription units, while nisABTCIPRK and nisFEG are regulated by nisRK. The functions of each gene in the nisin synthetic gene cluster have been identified (Riley and Chavan 2007a):
-
1.
The structural gene nisA encodes a propeptide containing 57 amino acids.
-
2.
The posttranslational modification gene nisB: nisB encodes the dehydratase NisB, which is located on the cell membrane and is responsible for dehydrating specific Ser and Thr in the propeptide to form Dha and Dhb. If the nisin precursor peptides were fused with a variety of non-wool thiobacillins and co-expressed with the nisBTC gene, the modified polypeptides could be produced, which indicated that the activity of NisB was not limited to the precursor of nisin, but could dehydrate Ser and Thr in a variety of amino acid sequences. Ser and Thr in nisin precursor peptides are never dehydrated, suggesting that NisB has some flexibility in substrate selection, but the specific mechanism is still unclear.
-
3.
The posttranslational modification gene nisC: nisC encodes cyclizase NisC, which is also located on the cell membrane. The nisin precursor dehydrated by NisB is further modified by NisC to form five intramolecular thioether rings. Deletion of nisC gene results in no formation of the wool sulfur ring, and the precursor cannot be secreted into the extracellular domain.
-
4.
The transport gene nisT: NisT belongs to the ATP-binding cassette transporter family. A typical ABC transporter contains two transmembrane domains and two ATP-binding domains. While NisT contains only one transmembrane domain and one ATP-binding domain, so two NisT molecules form a complete ABC transporter. The homologous dimer form acts as a transporter. NisT is located on the cell membrane and is responsible for transporting the dehydrated and cyclized nisin precursor to the cell membrane. Deletion or inactivation of NisT results in the inability of nisin to secrete extracellularly and accumulate in the cytoplasm. In terms of substrate selection, NisT is not only capable of transporting fully modified nisin but also capable of transporting partially modified or unmodified nisin.
-
5.
The precursor peptide excision gene nisP: NisP is an extracellular serine protease. Excision of the precursor sequence of nisin is the last step in the biosynthesis of nisin. Heterologous expression of NisP can also remove the precursor peptide from nisin precursor and produce active nisin molecules.
-
6.
Immune-related genes nisI and nisFEG: Because nisin has strong antibacterial and bactericidal activities, Lactococcus lactis expressing nisin needs a specific mechanism to protect itself from nisin attacks. This function is achieved through NisI and NisFEG systems. When the two systems work alone, they can only provide weak nisin immunity for Lactococcus lactis, which shows that there is a strong synergy between the two systems. NisI exists in the form of membrane-bound lipoprotein or membrane-unbounded free form. Both forms of proteins can interact with nisin molecules and prevent them from contacting the lipid bilayer, acting as the “front guard” of the guard cell portal. NisF is a cytoplasmic ATP-binding protein; NisG and NisE are membrane proteins. These three proteins form ABC-type transporters. It is speculated that NisFEG proteins are responsible for clearing the nisin molecules that invade the membrane and returning them to the extracellular space.
-
7.
Regulate genes nisR and nisK: They encode response regulators and histidine kinases, respectively, forming a two-component regulatory system for nisin biosynthesis. The sequence of NisR protein was similar to that of the transcriptional regulatory protein in the two-component regulatory system. The inactivation of NisR protein coding gene results in the inability of Lactococcus lactis to produce nisin. NisK is located on the cell membrane and can sense and bind mature nisin. When mature nisin exists outside the cell, NisK binds to nisin and initiates autophosphorylation of histidine, which activates specific signaling pathways, transfers phosphate groups to NisR, and activates nisin synthesis and the transcription of immune-related genes. Inactivation of nisK gene did not induce nisA gene transcription.
Based on the existing research, Kuipers et al. put forward a model of nisin synthesis and regulation in 1993 (Kuipers et al. 1993). First, NisK is activated and self-phosphorylated after induction of extracellular signals (nisin); NisK, as a conducting protein, transfers phosphoryl groups to the response regulator NisR; phosphorylated NisR acts as a transcription activator to activate the transcription of nisA/Z and nisF promoters, leading to downstream gene transcription and synthesis of unmodified nisin precursors and progenitors; NisB and NisC are responsible for dehydration of pronisin to form thioether bonds and other posttranslational modifications; NisT is responsible for transporting the modified pronisin to the extracellular membrane; NisP is responsible for extracellular processing of the modified pronisin, removing the leading sequence and releasing the active mature nisin (Huo 2007).
4.3.2 Biosynthesis and Regulation of Pediocin
Pediocin belongs to class IIa bacteriocins. Its biosynthesis requires at least four genes: structural gene, immune gene, transporter coding gene, and transmembrane protein gene (Venema et al. 1995). The structural genes mainly encode the precursor peptides of pediocin, while the immune genes encode the immune proteins to protect producing strain from the attack of the secreted pediocin. The coding genes of the ABC transporters encode proteins responsible for the transmembrane transport of pediocin (Rodriguez et al. 2002). The secretion process is as follows: the binding of the precursor peptide’s guiding sequence with the hydrolytic domain of ABC transporter triggers the release of energy from ATP hydrolysis, and the conformation of the transporter changes, so that the guiding sequence is separated from the precursor peptide. At the same time, mature pediocins are transported across the cytoplasmic membrane. An auxiliary protein is needed during the secretion process. As ABC transporters do not have the ability to hydrolyze proteins from the N-terminal, the separation of the guiding sequence from the precursor peptide is accomplished by a specific protease.
The regulation of pediocin depends on the synergism of inducible peptide, transmembrane histidine kinase (HK), and reaction regulator. HK is a receptor for the mature inducing peptide of pediocin. It releases phosphoryl groups through autophosphorylation inside the cytosol, and phosphoryl groups activate the response regulator into a transcription-activating factor that regulates gene transcription related to the biosynthesis of pediocin, including pediocin, immunity proteins, regulatory factors, etc. The synthesis of pediocin is controlled by transmembrane transport system. Biologically inactive precursors of pediocin are firstly synthesized in ribosomes. Then the precursors are cleaved at a specific site and the guided sequence is removed. Finally, bioactive tablets are formed and secreted out of cells. The amino acids of the ABC transmembrane transporter of pediocin have high homology. The C-terminal is a conserved ATP binding site, and the N-terminal is a target cell membrane binding region, containing a specific 150 amino acid extension. This structural region plays an important role in the removal of the lead peptide sequence and is a recognition signal for the removal of the precursor and the transmembrane transport of mature pediocin molecules out of the cytoplasm.
Take pediocin PA-1 as an example. This bacteriocin is encoded and secreted by plasmid. The synthetic gene cluster of pediocin PA-1 is composed of pedA, pedB, pedC, and pedD. The pedA gene regulates and encodes precursors containing 62 amino acids. PedB is a regulatory gene for immune-related proteins. The proteins encoded by pedC and pedD genes are involved in the translocation, processing, and secretion of bacteriocins. The transmembrane transport of class IIa bacteriocins, including Pediocin PA-1, is accomplished by the ABC transporter and an auxiliary protein. They are two transmembrane proteins that work together to form their own transport system (Havarstein et al. 1994). The number of amino acids in ABC transporters ranged from 715 to 724, with high homology at N and C ends. Venema et al. (1995) demonstrated that the N-terminal of PedD (the ABC transporter of Pediocin PA-1) plays a role in the removal of precursor peptides, and it does not participate in the secretion process (Rodriguez et al. 2002). The leader peptide is not only the signal of the propeptide but also responsible of the transmembrane transport of mature molecules. The protease region of the ABC transporter binds to the precursor peptide and then activates the hydrolysis of ATP and the conformational changes of the transporter protein, resulting in the removal of the precursor peptide and the transport of mature bacteriocin through the cytoplasmic membrane. The deletion mutation of any gene can lead to the failure to produce normal bacteriocin.
4.3.3 Biosynthesis and Regulation of Plantaricin
Lactobacillus plantarum C11 is the first strain to describe the synthesis and regulation of plantaricin at the gene level. In addition to Lactobacillus plantarum C11, similar bacteriocin biosynthesis gene clusters were found in the genomes of Lactobacillus plantarum V90, J51, J23, NC8, and WCFS1. These clusters are about 18–19 kb in length and about 25 genes, consisting of 5–6 operons. The conserved regions of the gene clusters are bacteriocin operons (plnEF1) and transport operons (plnGHSTMVW); the relatively conserved regions include one regulator and two to three bacteriocin operons. Each gene cluster also contains one or two unknown operons with relatively low conservatism.
The gene cluster of Lactobacillus plantarum C11 has five inducible operons. They are plnABCD, plnEF1, plnJKLR, plnGHSTMVW, and plnMNOP. plnABCD is a quorum-sensing transcription regulator, which can not only activate its own transcription but also control the transcription of other operons. plnABCD encodes a signal transduction system. plnA encodes a self-inducible peptide, plnB encodes a transmembrane protein-histidine protein kinase, and plnCD encodes two highly homologous reaction regulators. PlnC activates the target protein expression, and PlnD inhibits the target protein expression (Diep et al. 1994). plnEF1 and plnJKLR synthesize immune proteins, so that the peptides have antibacterial activity. Transport operon plnGHSTMVW encodes the bacteriocin secretion pathway and works together to control bacteriocin secretion. The plnH encodes a transporter. plnSTMVW is highly homologous in this operon and encodes proteins belonging to the class II CAAX aminoproteinase family. This class of CAAX genes is very rare in other bacteriocin gene clusters. So far, their roles in bacteriocin biosynthesis are not obvious. plnMNOP encodes four hypothetical proteins. The N-terminal of the lead sequence of plnN transcription contains a double glycine structure. The plnO-encoded proteins are highly homologous to the glycosyltransferase family, while the plnP-encoded proteins are highly homologous to the type II aminotransferase family.
4.4 The Mechanism of Bacteriocin
Bacteriocin of lactic acid bacteria can achieve bacteriostatic or bactericidal effects through different mechanisms. The target of bacteriocin is the cell membrane of sensitive bacteria, which changes the cell membrane structure by utilizing different electrovalence in its protein structure (Reeves 2012). The effect of bacteriocin on target cells is divided into two steps. First, bacteriocin binds to specific and non-specific receptors on the surface of cell membrane of sensitive bacteria, at which stage bacteriocin still shows sensitivity to proteolytic enzymes. Irreversible specific changes occur on this step, such as formation holes on the membrane of susceptible cells, leading to the loss of ATP and K+ ions or the obstacle of biochemical reaction. The balance of osmotic pressure inside and outside the cell is destroyed, and then the target cell is killed.
4.4.1 Antibacterial Mode of Nisin
Nisin is a typical type A lantibiotics. Type A lantibiotics inhibit bacteria mainly through two ways, inhibiting cell wall synthesis and forming pores in cell membrane. Lipid II is an important carrier in the process of cell membrane synthesis, responsible for transporting peptidoglycan components to the cell wall. Nisin can bind to lipid II and inhibit the formation of cell wall, which hinders the synthesis of cell membrane and phospholipid compounds and causes the release of intracellular substances, then leading to cell lysis (Riley and Chavan 2007b). This hypothesis has also been confirmed by experiments. As the adsorption of nisin on the surface of pathogenic bacteria is a prerequisite for its sterilization, it was found that adding activated carbon and other adsorbents in the system would significantly prolong the bacteriostatic time of nisin. Nisin has a strong adsorption effect, but it is obviously affected by pH. When the pH is 6.5, the adsorption rate can reach 100%, while at pH 4.5, the adsorption rate is only 43%. Nisin is positively charged and combines with sensitive bacterial cells to increase the permeability of cell membranes, which leads to the loss of nutrients and cell lysis. Therefore, there is a theory that the bacteriostatic mechanism of nisin is similar to that of cationic surfactant.
Nisin-induced pore-formation is a deeply studied mechanism. There are two mature theories: barrel plate model and wedge model. The first three steps of the two models are similar. Firstly, the N-terminal of nisin binds to the peptide glycan precursor lipid II and inserts into the membrane. At this time, the C-terminal of nisin crosses the cell membrane and switches the peptide to the transmembrane direction. When the transmembrane potential of nisin molecule is high enough inside and outside the cell, the position of nisin molecule can be changed perpendicular to the plane of the membrane. When the nisin molecules are overturned across the membrane, they are arranged in a circle like a barrel plate. This is barrel plate model. While the wedge model considers that when the transmembrane potential is high enough, the nisin molecule can change its position perpendicular to the plane of the membrane, resulting in the lipid surface bending, forming a wedge-like pore (Twomey et al. 2002). Pores allow hydrophilic molecules with relative molecular weight less than 500 to pass through, leading to potassium ion outflow from the cytoplasm, cell membrane depolarization and ATP leakage, extracellular water molecules inflow, whole cell wall degradation, cell autolysis, and death. This reaction mainly occurs in the compartment of differentiated daughter cells. There are two kinds of cell wall hydrolases involved in the reaction, N-ethylphthalide-L-alanine phthaliminase and N-ethylphthalide-glucosidase, which are strong cationic proteins that bind to negatively charged substances in the cell wall through electrostatic interaction. Cationic peptides and intramural inhibitors replace the latter by a process similar to cation exchange, and then enzymes activate and rapidly cleave cells.
Nisin mainly killed or suppressed gram-positive bacteria and spores, but had no obvious inhibitory effect on negative bacteria. This is because the cell wall of gram-positive bacteria is thick and its composition is relatively simple. While the cell wall of gram-negative bacteria is complex and compact, allowing only molecules with relative molecular mass below 600 to pass through. The relative molecular weight of nisin is about 3510, so it cannot pass through the cell wall and contact the cell membrane of gram-negative bacteria. When nisin is combined with chelating agent EDTA or surfactant, gram-negative bacteria such as Salmonella begin to become sensitive and can be inhibited or killed by nisin. The inhibitory effect of nisin was concentration dependent, which was related to the concentration of nisin and the cell concentration of sensitive bacteria. In addition, the physiological state of the sensitive bacteria also has some influence. The vegetative reproductive cells in the energy state are more likely to be killed than the vegetative reproductive cells in the static state. The sensitivity of spores to nisin is higher than that of bacterial cells, and the inhibition occurs before the expansion and growth of spores, which is very important in the preservation of hot-processed foods.
In conclusion, nisin mainly inhibits the microorganisms by adsorbing to the surface of cell membranes. At the same time, pH, ion concentration, lactic acid concentration, and nitrogen source types are also some factors affecting the adsorption of nisin. Nisin has two mechanisms; nisin-induced pore formation leads to the loss of proton motility (PMF), ion leakage, and ATP hydrolysis of sensitive bacteria and then to cell death. Nisin can also combine with lipid II, interfere with cell wall synthesis, and inhibit cell growth or survival. The dual mechanism of nisin enables it to work at nanomolar concentration.
4.4.2 Antibacterial Mode of Pediocin
The bacteriostasis mechanism of pediocin also mainly owes to the formation of pores on the cell membrane of sensitive bacteria. However, the adsorption and binding of pediocin to cell membranes are caused by electrostatic interaction. There is no need for the existence of membrane receptor proteins, so there is no selectivity. After being adsorbed on the surface of cell membrane, the hydrophobic region of the C-terminal of the pediocin peptide chain interacts hydrophobically with the tail of the phospholipid bilayer of cell membrane, which enables the C-terminal to insert into the cell membrane of sensitive bacteria, thus forming a pore (Bhunia et al. 1991).
4.4.3 Antibacterial Mode of Plantaricin
The antimicrobial mechanism of lantibiotics-type plantaricin is similar to that of nisin. Plantaricin of non-lantibiotics type mainly forms a hydrophilic channel on the cell membrane of susceptible bacteria. The formation of the channel is related to the “coupling molecule groups” on the surface of target cell membrane. However, this hydrophilic channel is self-directed by specific receptor proteins on the membrane and is not related to membrane potential, so it is also called non-energy-dependent. Compared with lantibiotics-type plantaricin, the function of this bacteriocin depends on destroying the stability of cell membrane function (such as energy conduction), rather than forming pores on the cell membrane. The inhibitory effect is related to the lipid composition and culture pH. In addition, the coupling molecular groups on the surface of the target cell membrane make it easier for the bacteriocin to interact with the target cell, thus improving the bacteriostatic effectiveness.
4.5 The Application of Lactic Acid Bacteria Bacteriocin
The food industry is a major production system in China. During food preservation, human or environmental factors can lead to corruption and deterioration, which not only cause economic losses but also affect health and even cause irreversible physical damage. Although some antioxidants and preservatives are added in food production to inhibit the growth of microorganisms and retard spoilage, most of these additives are synthetic compounds, and improper or excessive consumption will lead to physical damage. Therefore, it is urgent to develop a natural, nontoxic, harmless, and effective bacteriostatic agent. Bacteriocin produced by lactic acid bacteria is a kind of polypeptide substance. One advantage of this substance is that it can be degraded and digested by protease in digestive tract without residues. On the other hand, bacteriocin can inhibit most spoilage bacteria and foodborne pathogens (Hoover and Steenson 2014). The most widely used bacteriocin is nisin. Nisin has been widely used in food production, which effectively inhibits the growth of harmful bacteria, improves the quality of food processing, and prolongs the storage time of food.
4.5.1 Application of Nisin in Food Industry
Nisin is a highly effective and nontoxic polypeptide antimicrobial substance extracted from the Lactococcus lactis. Nisin is a natural preservative and antimicrobial agent recognized and used by most countries (Tolonen et al. 2004). Hirsch et al. first applied nisin to food preservation research. They found that nisin inhibited the growth of Clostridium in cheese production but had some adverse effects on the growth of fermentation medium and the ripening process of cheese. In 1952, McClintock et al. tried to add a certain amount of nisin to the mixture in cheese production and found that the quality of cheese had been greatly improved. On this basis, researchers gradually tried to apply nisin into other food production. In 1953, nisin was first commercially produced in the United Kingdom with the trade name of Nisaplin. In 1969, the Food Additives Committee (FAC) and the World Health Organization (WHO) evaluated the safety of nisin and confirmed that nisin could be used as a food additive in food. At present, more than 50 countries and regions around the world have approved nisin as a safe biological preservative, mainly used in processing cheese, milk products, and canned products (Jia 2009).
4.5.1.1 Application in Meat Products
In traditional meat production, nitrate and nitrite are commonly used as chromogenic agents to produce salted red and salted flavor and to inhibit the growth of Clostridium botulinum. However, nitrite can be converted to nitrosamines under specific conditions, and nitrosamines are carcinogens, so nitrate and nitrite in meat products have carcinogenic risks. Studies have shown that nisin can effectively control the growth of Clostridium botulinum, and nisin itself is acidic, which can reduce the pH value of surrounding media, thus reducing the residual nitrite content and the formation of nitrosamines (Rayman et al. 1983).
Rayman et al. found that nisin could also be used as an effective substitute to reduce the amount of nitrate and nitrite used in ham (Rayman et al. 1981). After adding 3000 IU/mL nisin to ham, the nitrate content decreased from 0.02% to 0.003%, while the quality of ham remained unchanged. In addition, in the traditional meat processing process, excessive heat treatment can significantly change the texture and appearance of meat products. However, the storage life of meat products with nisin can be prolonged by only 45% of the original heat treatment, and the final product has little difference in color, aroma, and taste compared with the traditional processed products. Nisin can also increase the sensitivity of some bacteria to heat and have some auxiliary bactericidal action. After soaking meat products in a certain concentration of nisin aqueous solution, reheating and sterilizing can ensure that the sterilization temperature is reduced, the sterilization time is shortened, and the original flavor of meat products is maintained on the premise of reaching the shelf life. When the addition of nisin was 0.3 g/kg in sausage processing, the overwhelming majority of gram-positive bacteria were inhibited, and the product color, aroma, and taste were not affected. Pork silk processing with 0.48 g/kg nisin could replace the preservative potassium sorbate and improve product quality.
Nisin is often used in combination with other preservatives to expand the scope and enhance the effect of bacteriostasis in practical production. The method is to mix preservatives into a solution, then mix them directly with meat products, or inject into meat products. It can also be coated on the surface of meat products. The operation is simple and convenient.
4.5.1.2 Application in Milk Products
Nisin has been successfully used in hard cheese, pasteurized milk, canned concentrated milk, yoghurt, butter, milk dessert, ice cream, and other products. Dairy products are rich in nutrients and are extremely susceptible to spoilage. Pasteurization and refrigeration can prolong the shelf life, but the spores of sporogenous bacteria are not killed and can germinate under suitable conditions. The shelf life of dairy products can be prolonged by adding 30–50 mg/kg nisin, which can be doubled at 35 °C. The addition of 80–100 mg/kg nisin in canned refined milk can reduce the sterilization time by 10 minutes. The addition of 20 mg/kg nisin in UHT milk can completely inhibit the growth of spore-producing bacteria in sterilized milk (Maisnierpatin et al. 1992). Adding 40 IU/mL nisin to yoghurt can delay the post-acidification process for 3 days and keep the number of live bacteria above 107 CFU/mL. The sensory quality of yoghurt is good.
Nisin is the first antiseptic used in cheese. The mixed application of nisin-resistant bacteria and nisin-producing bacteria in cheese starter can increase the quality of cheese by more than 90% compared with 41% by conventional methods. In processing solid and semisolid cheese, nisin helps to reduce the swelling caused by butyric acid. When soft cheese is stored under uncontrollable temperature, adding nisin can inhibit the growth of anaerobic bacteria and prolong the storage time of products (Davies et al. 1997).
4.5.1.3 Application in Pickles
Bottled pickles are a kind of food with long storage period and convenient consumption. Generally, pasteurization is used in bottled pickles. Clostridium and a few heat-resistant gram-positive bacteria will remain in food. Traditional methods of inhibiting bacteria mainly depend on high osmotic pressure (high salt and sugar), hypoxic environment, and chemical preservatives. At present, salt content in all kinds of pickles is high, but high salt foods are easy to induce hypertension and other diseases. The addition of 100 mg/kg nisin in some pickles not only inhibits the re-fermentation of lactic acid bacteria and the growth of Staphylococcus and Bacillus but also reduces the use of salt and the risk of high salt (Tolonen et al. 2004). In addition, adding nisin to pickles is better than adding conventional sodium benzoate and potassium sorbate. Some countries forbid sodium benzoate to be used in food. Therefore, it is of great economic significance to use nisin for preservation and bacteriostasis of pickles.
4.5.1.4 Application in Cans
Nisin is soluble and stable and has high bacteriostatic activity under acidic conditions, so it can be used for preservation of canned foods with high acidity (pH < 4.6). For example, after sterilization of canned tomatoes, there are still a small number of acid-resistant gram-positive bacteria such as Clostridium pasteurianum and Bacillus leaching, which can cause product spoilage. But adding 100–200 IU/g of nisin can effectively inhibit the growth and reproduction of these bacteria. In canned potatoes and mushrooms with low acidity (pH > 4.6), some thermophilic bacteria still survive after high heat treatment, which leads to canned spoilage. And high heat treatment has a certain adverse effect on the sensory quality of food. Adding appropriate amount of nisin can not only effectively inhibit the growth of spores but also reduce the heat treatment time of cans, maintain their freshness, and prolong their shelf life. For example, the common spoilage bacteria in canned mushrooms are Bacillus stearothermophilus and Clostridium nigrificans. The former increases the acidity of the contents, while the latter produces gas and makes the lid of the cans inedible. If sterilization is only carried out by heating high temperature or prolonging time, the color of the content will be darkened, and the elasticity of the tissue will become worse. The sensory properties of canned food can be greatly improved by the combination of heating and adding nisin, even though the storage period at room temperature remains unchanged for 2 years.
Although Nisin has good bacteriostasis performance, its essence is a kind of biological polypeptide substance, which will be destroyed in varying degrees when sterilized at high temperature and will degrade continuously with the prolongation of storage period. If the concentration of nisin is lower than 0.002%, its effect will be significantly weakened. Therefore, the stability and residues in cans should be noticed, so as to ensure that nisin is always within the effective concentration range during the shelf life of cans.
4.5.1.5 Application in Alcohol Production and Beverages
Lactobacillus and Pediococcus cerevisiae are common contaminating bacteria in brewage. The abnormal growth and reproduction of Lactobacillus will lead to beer turbidity, acidification and stickiness. While Pediococcus cerevisiae makes the agglutination of Saccharomyces cerevisiae worse. Almost all the spoilage bacteria in beer can be inhibited when the concentration of nisin is above 100 IU/mL. 100 IU/mL of Nisin can inhibit the spoilage of lactic acid bacteria, but the yeast is almost unaffected. Therefore, in the production of alcoholic beverages such as beer, fruit wine, and strong ethanol, in addition to the addition of yeast, adding appropriate amount of nisin can be used to inhibit the growth of gram-positive bacteria. Like the application of nisin in other food production, the addition of nisin not only reduces the time and temperature of pasteurization and the damage to product quality but also prolongs the shelf life of alcoholic products such as beer, which can be doubled. This effect is more obvious for non-pasteurized alcoholic products.
Malic acid-lactic acid fermentation caused by lactic acid bacteria often occurs in wine making, that is, the transformation of malic acid in wine into a biochemical reaction of lactic acid and CO2. Generally, the measure of inhibiting malic acid-lactic acid fermentation is to add enough SO2 (up to 100 mg/L) in wine. But SO2 will destroy the typical flavor of wine, and it is strictly restricted to use due to the inhibition of normal fermentation bacteria growth. It has been reported that the addition of nisin in the brewing process can replace part of the role of SO2 and not only will not affect the composition and flavor of the finished wine but also can inhibit unnecessary malic acid-lactic acid fermentation and eliminate the adverse effects caused by lactic acid bacteria.
Adding appropriate amount of nisin to fruit juice products before pasteurization can not only reduce the heat processing strength and increase the residue of nisin but also prevent the growth of miscellaneous bacteria such as Bacillus, thus preventing the corruption of fruit juice products.
4.5.2 Application of Bacteriocin in Medical Treatment
More than 2500 kinds of antibiotics have been found now. Most of them have adverse effects, only dozens of antibiotics commonly used in clinic. Antibiotics mainly interfere with the metabolic process of bacteria to inhibit their growth and reproduction or directly kill them. The extensive use of antibiotics and burst of bacterial resistance bring new hidden dangers to human health. Therefore, alternatives to antibiotics will become a new hotspot in drug research. Bacteriocin fills this gap because of its good bacteriostatic effect and safety characteristics.
Bacteriocins has a narrow antimicrobial spectrum and has a certain specificity and targeting. Methicillin-resistant Staphylococcus aureus (MRSA) is an important pathogen of nosocomial infection. Studies have found that some bacteriocins can inhibit MRSA (Aunpad and Na-Bangchang 2007). Bacteriocin is also effective in some skin diseases such as acne. Oral cavity is a necessary place for pathogenic bacteria in food. The special environment of oral cavity can easily lead to dental caries. Therefore, the oral mouth wash containing bacteriocin provides a new possibility to prevent this kind of oral disease. Bacteriocin also has great potential in the prevention and treatment of cow mastitis. At present, bacteriocin or bacteriocin-producing strains are usually used as an adjuvant therapy at animal level. So far, no pure bacteriocin can be directly used as a drug in clinical practice. For Helicobacter pylori and Neisseria, the efficacy of mutacin B-Ny266 and nisin A is comparable to that of vancomycin and oxacillin. Mersacidin, a B-type wool thiobacillin, is very effective in the treatment of staphylococcal infections and shows the ability to eliminate Staphylococcus aureus in rat models.
4.5.3 Application of Bacteriocin in Feed Industry
The application of bacteriocin in feed industry is an extension of food industry application. Bacteriocins can be added as an additive to the fodder. They have good thermal stability and therefore can tolerate high-temperature sterilization in feed processing. At the same time, bacteriocin can inhibit the exogenous pathogenic bacteria in animal intestinal tract, but has no killing effect on the inherent bacteria in animal intestinal tract, reduce the harm of pathogenic bacteria to animals, promote the healthy growth of animals, and improve the utilization rate of feed. In pig feed research, it is found that adding of bacteriocin in feed can reduce the diarrhea rate of piglets and increase the survival rate of piglets, which has significant economic benefits.
4.5.4 Application Prospect of Bacteriocin
Nisin is a highly effective, safe, and nontoxic natural preservative, which meets the development requirements of food preservatives in the future. It can be widely used in food, especially in food which needs heat treatment. It not only has good antiseptic and bacteriostatic effect but also can weaken the intensity of heat treatment, reduce processing costs, and improve the flavor and quality of products. In recent years, the combination of nisin and non-heat treatment technology - high hydrostatic pressure or pulsed electric field, has become a research hotspot in food and other industries. Nonthermal compound bacteriostasis technology not only shows good bacteriostasis effect but also prolongs the shelf life of food, so that the nutrition, taste, and other varieties of food are maintained.
Nisin in food can be affected by some external factors or food media, which will lead to the decline or even disappearance of bacteriostasis. For example, if food is not processed by heat or the heat treatment is not enough, the protease of microorganisms, plants, or animal organisms in food can degrade nisin during shelf life, which is below the minimum inhibitory concentration and will lose its effect. Nisin has better bacteriostasis effect in liquid and homogeneous food, but it is less effective in solid and heterogeneous food. Therefore, it is necessary to increase the dosage of nisin in solid and heterogeneous food. If Nisin is combined with other antibacterial substances to form a compound preservative, it can play a broad-spectrum antibacterial effect. From a single type to a compound preservative, the application prospect of nisin will be broader.
References
Abbasiliasi S et al (2011) Effect of medium composition and culture condition on the production of bacteriocin-like inhibitory substances (blis) by lactobacillus paracasei la07, a strain isolated from BUDU. Biotechnol Biotechnol Equip 25(4):2652–2657
Anand SK, Srinivasan RA, Rao LK (1984) Antibacterial activity associated with Bifidobacterium bifidum. Cult Dairy Prod J 19:6–8
Anderssen EL et al (1998) Antagonistic activity of lactobacillus plantarum C11: two new two-peptide bacteriocins, plantaricins EF and JK, and the induction factor plantaricin a. Appl Environ Microb 64(6):2269–2272
Arakawa K et al (2009a) Effects of gassericins a and T, bacteriocins produced by lactobacillus gasseri, with glycine on custard cream preservation. J Dairy Sci 92(6):2365–2372
Arakawa K et al (2009b) Negative effect of divalent metal cations on production of gassericin T, a bacteriocin produced by lactobacillus gasseri, in milk-based media. Int Dairy J 19(10):612–616
Aunpad R, Na-Bangchang K (2007) Pumilicin 4, a novel bacteriocin with anti-MRSA and anti-VRE activity produced by newly isolated bacteria Bacillus pumilus strain WAPB4. Curr Microbiol 55(4):308–313
Aymerich T et al (1996) Biochemical and genetic characterization of enterocin a from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl Environ Microbiol 62(5):1676–1682
Bhunia AK et al (1991) Mode of action of pediocin AcH from Pediococcus acidilactici H on sensitive bacterial strains. J Appl Bacteriol 70(1):25–33
Brotz H et al (1997) The lantibiotic mersacidin inhibits peptidoglycan biosynthesis at the level of transglycosylation. Eur J Biochem 246(1):193–199
Casaus P et al (1997) Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin a. Microbiology-Uk 143:2287–2294
Chatterjee S et al (1992) Mersacidin, a new antibiotic from Bacillus fermentation, isolation, purification and chemical characterization. J Antibiot 45(6):832–838
Cheikhyoussef A et al (2010) Bifidin I–A new bacteriocin produced by Bifidobacterium infantis BCRC 14602: purification and partial amino acid sequence. Food Control 21(5):746–753
Cintas LM et al (2000) Biochemical and genetic evidence that Enterococcus faecium L50 produces enterocins L50A and L50B, the sec-dependent enterocin P, and a novel bacteriocin secreted without an N-terminal extension termed enterocin Q. J Bacteriol 182(23):6806–6814
Collado MC, Hernandez M, Sanz Y (2005a) Production of bacteriocin-like inhibitory compounds by human fecal Bifidobacterium strains. J Food Prot 68(5):1034–1040
Collado MC et al (2005b) Antimicrobial peptides are among the antagonistic metabolites produced by Bifidobacterium against helicobacter pylori. Int J Antimicrob Agents 25(5):385–391
Cotter PD, Hill C, Ross RP (2005) Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3(10):777–788
Davies EA, Bevis HE, Delves–Broughton J (1997) The use of the bacteriocin, nisin, as a preservative in ricotta–type cheeses to control the food–borne pathogen Listeria monocytogenes. Lett Appl Microbiol 24(5):343–346
de Arauz LJ et al (2012) Culture medium of diluted skimmed milk for the production of nisin in batch cultivations. Ann Microbiol 62(1):419–426
de Kwaadsteniet M, ten Doeschate K, Dicks LMT (2008) Characterization of the structural gene encoding Nisin F, a new lantibiotic produced by a Lactococcus lactis subsp lactis isolate from freshwater catfish (Clarias gariepinus). Appl Environ Microbiol 74(2):547–549
Diep DB et al (1994) The gene encoding plantaricin a, a bacteriocin from lactobacillus plantarum C11, is located on the same transcription unit as an agr-like regulatory system. Appl Environ Microbiol 60(1):160–166
Dobson AE, Sanozky-Dawes RB, Klaenhammer TR (2007) Identification of an operon and inducing peptide involved in the production of lactacin B by lactobacillus acidophilus. J Appl Microbiol 103(5):1766–1778
Draper LA et al (2013) The two peptide lantibiotic lacticin 3147 acts synergistically with polymyxin to inhibit gram negative bacteria. BMC Microbiol 13(1):1–8
Eijsink VGH et al (1998) Comparative studies of class IIa bacteriocins of lactic acid bacteria. Appl Environ Microbiol 64(9):3275–3281
Espeche MC et al (2014) Physicochemical factors differentially affect the biomass and bacteriocin production by bovine Enterococcus mundtii CRL1656. J Dairy Sci 97(2):789–797
Etchells JL et al (1964) Pure culture fermentation of brined cucumbers. Appl Microbiol 12(6):523–535
Fimland G et al (2005) Pediocin-like antimicrobial peptides (class IIa bacteriocins) and their immunity proteins: biosynthesis, structure, and mode of action. J Pept Sci 11(11):688–696
Flynn S et al (2002) Characterization of the genetic locus responsible for the production of ABP-118, a novel bacteriocin produced by the probiotic bacterium lactobacillus salivarius subsp salivarius UCC118. Microbiology-Sgm 148:973–984
Garneau S, Martin NI, Vederas JC (2002) Two-peptide bacteriocins produced by lactic acid bacteria. Biochimie 84(5-6):577–592
Gross E, Morell JL (1971) Structure of nisin. J Am Chem Soc 93(18):4634–4635
Hastings JW, Sailer M, Johnson K, Roy KL, Vederas JC, Stiles ME (1991) Characterization of Leucocin A-UAL 187 and cloning of the Bacteriocin Cene from Leuconostoc gelidum. J Bacteriol 173(23):7491–7501
Havarstein LS, Holo H, Nes IF (1994) The leader peptide of colicin V shares consensus sequences with leader peptides that are common among peptide bacteriocins produced by gram-positive bacteria. Microbiology-Uk 140:2383–2389
Heinrich P et al (1987) The molecular organization of the lysostaphin gene and its sequences repeated in tandem. Mol Gen Genet MGG 209(3):563–569
Hindre T et al (2004) Regulation of lantibiotic lacticin 481 production at the transcriptional level by acid pH. FEMS Microbiol Lett 231(2):291–298
Hoover DG, Steenson LR (2014) Bacteriocins of lactic acid bacteria. Academic Press, New York
Hui FM, Zhou LX, Morrison DA (1995) Competence for genetic transformation in Streptococcus pneumoniae: organization of a regulatory locus with homology to two lactococcin a secretion genes. Gene 153(1):25–31
Huo, G. C. Research and application of lactic acid bacteria. 2007
Jia SR (2009) Biological preservatives. China Light Industry Press, Beijing
Joerger MC, Klaenhammer TR (1986) Characterization and purification of helveticin J and evidence for a chromosomally determined bacteriocin produced by lactobacillus helveticus 481. J Bacteriol 167(2):439–446
Kawai Y et al (2009) DNA sequencing and homologous expression of a small peptide conferring immunity to Gassericin a, a circular Bacteriocin produced by lactobacillus gasseri LA39. Appl Environ Microbiol 75(5):1324–1330
King BF, Biel ML, Wilkinson BJ (1980) Facile penetration of the Staphylococcus aureus capsule by lysostaphin. Infect Immun 29(3):892–896
Klaenhammer TR (1993) Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev 12(1-3):39–85
Konisky J (1982) Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol 36(1):125–144
Kuipers OP et al (1993) Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Eur J Biochem 216(1):281–291
Magnusson J, Schnürer J (2001) Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-Spectrum Proteinaceous antifungal compound. Appl Environ Microbiol 67(1):1–5
Maisnierpatin S et al (1992) Inhibition of listeria-monocytogenes in camembert cheese made with a nisin-producing starter. Lait 72(3):249–263
Mandal V, Sen SK, Mandal NC (2010) Assessment of antibacterial activities of pediocin produced by Pediococcus acidilactici lab 5. J Food Saf 30(3):635–651
Mandal V, Sen SK, Mandal NC (2011) Isolation and characterization of pediocin NV 5 producing Pediococcus acidilactici LAB 5 from vacuum-packed fermented meat product. Indian J Microbiol 51(1):22–29
Marciset O et al (1997) Thermophilin 13, a nontypical antilisterial poration complex bacteriocin, that functions without a receptor. J Biol Chem 272(22):14277–14284
Mathiesen G et al (2005) Characterization of a new bacteriocin operon in sakacin P-producing lactobacillus sakei, showing strong translational coupling between the bacteriocin and immunity genes. Appl Environ Microbiol 71(7):3565–3574
Mathys S, Meile L, Lacroix C (2009) Co-cultivation of a bacteriocin-producing mixed culture of Bifidobacterium thermophilum RBL67 and Pediococcus acidilactici UVA1 isolated from baby faeces. J Appl Microbiol 107(1):36–46
Miescher S et al (2000) Propionicin SM1, a bacteriocin from Propionibacterium jensenii DF1: isolation and characterization of the protein and its gene. Syst Appl Microbiol 23(2):174–184
Morgan SM et al (2005) Sequential actions of the two component peptides of the lantibiotic lacticin 3147 explain its antimicrobial activity at nanomolar concentrations. Antimicrob Agents Chemother 49(7):2606–2611
Mortvedt CI et al (1991) Purification and amino-acid-sequence of lactocin-S, a bacteriocin produced by lactobacillus-sake-L45. Appl Environ Microbiol 57(6):1829–1834
Mulders JWM et al (1991) Identification and characterization of the Lantibiotic Nisin-Z, a natural Nisin variant. Eur J Biochem 201(3):581–584
Müller E, Radler F (1993) Caseicin, a bacteriocin from Lactobacillus casei. Folia Microbiol 38(6):441–446
Muriana PM, Klaenhammer TR (1991) Purification and partial characterization of lactacin F, a bacteriocin produced by lactobacillus acidophilus 11088. Appl Environ Microbiol 57(1):114–121
Nakamura K et al (2013) Food preservative potential of gassericin A-containing concentrate prepared from cheese whey culture supernatant of lactobacillus gasseri LA39. Anim Sci J 84(2):144–149
Nes IF et al (1996) Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Van Leeuwenhoek 70(2-4):113–128
Neumann VC et al (1993) Extracellular proteolytic activation of bacteriolytic peptidoglycan hydrolases of Staphylococcus simulans biovar staphylolyticus. FEMS Microbiol Lett 110(2):205–212
Nilsen T, Nes IF, Holo H (2003) Enterolysin a, a cell wall-degrading bacteriocin from Enterococcus faecalis LMG 2333. Appl Environ Microbiol 69(5):2975–2984
Paik SH, Chakicherla A, Hansen JN (1998) Identification and characterization of the structural and transporter genes for, and the chemical and biological properties of, sublancin 168, a novel lantibiotic produced by Bacillus subtilis 168. J Biol Chem 273(36):23134–23142
Parente E, Ricciardi A (1994) Influence of pH on the production of enterocin 1146 during batch fermentation. Lett Appl Microbiol 19(1):12–15
Rayman MK, Aris B, Hurst A (1981) Nisin: a possible alternative or adjunct to nitrite in the preservation of meats. Appl Environ Microbiol 41(2):375–380
Rayman K, Malik N, Hurst A (1983) Failure of nisin to inhibit outgrowth of Clostridium botulinum in a model cured meat system. Appl Environ Microbiol 46(6):1450–1452
Reeves P (2012) The bacteriocins, vol 11. Springer, New York
Riley MA, Chavan MA (2007a) Bacteriocins. Springer, Berlin/Heidelberg
Riley MA, Chavan MA (2007b) Bacteriocins: ecology and evolution. Springer, Berlin/Heidelberg
Rodriguez JM, Martinez MI, Kok J (2002) Pediocin PA-1, a wide-spectrum bacteriocin from lactic acid bacteria. Crit Rev Food Sci Nutr 42(2):91–121
Rueckert PW et al (1979) Mammalian and microbial cell-free conversion of anthracycline antibiotics and analogs. J Antibiot 32(2):141–147
Schneider TR et al (2000) Ab initio structure determination of the lantibiotic mersacidin. Acta Crystallograph Sect D-Biol Crystallograph 56:705–713
Tanner SA et al (2014) Synergistic effects of Bifidobacterium thermophilum RBL67 and selected prebiotics on inhibition of Salmonella colonization in the swine proximal colon PolyFermS model. Gut Pathog 6(1):44
Tolonen M et al (2004) Formation of nisin, plant-derived biomolecules and antimicrobial activity in starter culture fermentations of sauerkraut. Food Microbiol 21(2):167–179
Twomey D et al (2002) Lantibiotics produced by lactic acid bacteria: structure, function and applications. Anton Leeuw Int J Gen Mol Microbiol 82(1-4):165–185
Tyne DV, Martin MJ, Gilmore MS (2013) Structure, function, and biology of the Enterococcus faecalis Cytolysin. Toxins 5(5):895–911
Upreti GC, Hinsdill RD (1975) Production and mode of action of lactocin 27: bacteriocin from a homofermentative lactobacillus. Antimicrob Agents Chemother 7(2):139–145
van den Hooven HW et al (1996) Surface location and orientation of the lantibiotic nisin bound to membrane-mimicking micelles of dodecylphosphocholine and of sodium dodecylsulphate. Eur J Biochem 235(1–2):394–403
Venema K et al (1995) Functional analysis of the pediocin operon of Pediococcus acidilactici PAC1. 0: PedB is the immunity protein and PedD is the precursor processing enzyme. Mol Microbiol 17(3):515–522
Whitford MF et al (2001) Identification of bacteriocin-like inhibitors from rumen Streptococcus spp. and isolation and characterization of bovicin 255. App Environ Microbiol 67(2):569–574
Wirawan RE et al (2006) Molecular and genetic characterization of a novel nisin variant produced by Streptococcus uberis. Appl Environ Microbiol 72(2):1148–1156
Yildirim Z, Johnson MG (1998) Characterization and antimicrobial spectrum of bifidocin B, a bacteriocin produced by Bifidobacterium bifidum NCFB 1454. J Food Prot 61(1):47–51
Yonezawa H, Kuramitsu HK (2005) Genetic analysis of a unique bacteriocin, Smb, produced by Streptococcus mutans GS5. Antimicrob Agents Chemother 49(2):541–548
Zendo T et al (2003) Identification of the lantibiotic Nisin Q, a new natural nisin variant produced by Lactococcus lactis 61-14 isolated from a river in Japan. Biosci Biotechnol Biochem 67(7):1616–1619
Zihler A et al (2011) Protective effect of probiotics on Salmonella infectivity assessed with combined in vitro gut fermentation-cellular models. BMC Microbiol 11:264
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd. and Science Press
About this chapter
Cite this chapter
Zhang, Q. (2019). Lactic Acid Bacteria and Bacteriocins. In: Chen, W. (eds) Lactic Acid Bacteria. Springer, Singapore. https://doi.org/10.1007/978-981-13-7283-4_4
Download citation
DOI: https://doi.org/10.1007/978-981-13-7283-4_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-7282-7
Online ISBN: 978-981-13-7283-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)