Abstract
Hematopoietic stem cells (HSCs) are a major kind of pluripotent stem cells, which can give rise to all the other blood cells through the process of haematopoiesis and maintain the homeostasis of organism. HSCs are divided into three types based on their differentiation stage, including long-term self-renewing HSCs (LT-HSCs), short-term self-renewing HSCs (ST-HSCs) and multipotent progenitors (MPPs). These HSCs eventually differentiate into mature blood cells and immune cells after experiencing various common lymphoid progenitor (CLP) and common myeloid progenitor (CMP). The proliferation and differentiation of HSCs have been widely studied and revealed be controlled by various factors, molecules and transcription factors but litter is known about how microgravity affects HSCs. Our study was conducted in two flight programs, SJ-10 recoverable microgravity experimental satellite (SJ-10 satellite) program research and Tianzhou-1 cargo ship program, and mainly focuses on the maintaining and directed differentiation of hematopoietic stem cells. Our results revealed some new mechanisms for maintaining and directed differentiation under microgravity conditions, with the potential to boost immune system, and provide potential drugs for the prevention or treatment of immune system weakening in spaceflight.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Since the beginning of space travel, a number of reports regarding the deleterious effects of spaceflight on the human health emerge in endlessly. Life during spaceflight includes multiple stressed factors, such as microgravity, radiation, loss of light-dark cycle and confinement. Among all these factors, microgravity (between 10−3 and 10−5 g) exposure is the most important and stable factor reportedly affected human physiological and psychological functions (Grigor’ev 2007; Wichman 2005). It is clear from the last 50 years of space research that a series of changes occurred in human blood, including anemia, thrombocytopenia, a reduction in the numbers and suppressive function of lymphocytes, and structural abnormalities of the red blood cells (Davis et al. 1996; Blaber et al. 2010) during and post spaceflight. In recent years, the effects of microgravity on immune function have received more and more attentions, and have been investigated through analysis of blood leukocytes obtained from astronauts5, cells secured from space-flown mice and rats (Kraemer et al. 2004; Hwang et al. 2015) and in vitro cell culture (Paulsen et al. 2015). The composite data from International Space Station, Skylab and Shuttle flight suggest that deregulated immune-cell–mediated cytokine secretion is a major mechanism of spaceflight-associated immune dysfunction (Taylor et al. 1997; Tauber et al. 2017; Hughes-Fulford et al. 2015; Stowe et al. 2011; Crucian et al. 2008). Therefore, this chapter aims to summarize the latest progress related to the maintaining and directed differentiation of hematopoietic stem cells under microgravity in recent ten years and the scientific achievements in SJ-10 satellite research.
2 The Effects of Microgravity/Spaceflight on Immune System
Besides impact hematopoietic generation and hematopoietic system, spaceflight and microgravity also extensively altered various immune parameters and immune response. Changes in resistance to bacterial and viral infections in Apollo crew members have stimulated interest in the study of immunity and space flight. Results of studies from several laboratories in both humans and rodents have indicated alterations after space flight that include the following immunological parameters: thymus size, lymphocyte blastogenesis, interferon and interleukin production, natural killer cell activity, cytotoxic T-cell activity, leukocyte subset population distribution, response of bone marrow cells to colony stimulating factors, and delayed hypersensitivity skin test reactivity. The interactions of the immune system with other physiological systems, including muscle, bone, and the nervous system, may play a major role in the development of these immunological parameters during and after flight (Sonnenfeld 2002). There are studies about direct effects of space flight on immune responses. Data collected before and after 11 Shuttle space flights show that absolute lymphocyte numbers, lymphocyte blastogenic capability, and eosinophil percent in the peripheral blood of crewmembers are generally depressed postflight. 11 astronauts on shuttle flight 41B and 41D showed decreased circulating monocytes and B lymphocytes in peripheral blood post flight (Taylor et al. 1986). Researchers investigated spaceflight and microgravity on immune responses of rats flown on Biosputnik Cosmos 1887 and Cosmos 2044. The response to macrophage-colony stimulating factor (M-CSF) and granulocyte/monocyte colony-stimulating factor (GM-CSF) of rat bone marrow cells from Cosmos 1887 and 2044 were detected, respectively. The results are similar, which indicated that bone marrow cells from flown rats showed a decreased response to M-CSF and GM-CSF. Meanwhile, researchers also detected surface antigenic markers of immunocytes and they found that the percentages of suppressor T cells and helper T cells in spleens of flown rats on Cosmos 1887 and 2044 both increased (Sonnenfeld et al. 1990, 1992). Bone marrow cells from flown rats on Cosmos 2044 showed an increase in the percentage of cells expressing markers for helper T-cells in the myelogenous population and increased percentages of anti-asialo granulocyte/monocyte-1-bearing interleukin-2 receptor-bearing pan T- and helper T-cells in the lymphocytic population (Sonnenfeld et al. 1992).
During the nine-day spaceflight on the Space lab Life Science 1 (SLS-1) mission on the shuttle Columbia in 1991, experiments including 29 spaceflight male rats and 30 ground controls were conducted to research the effects of microgravity on the total and absolute leukocyte counts of flight rats and assess the lymphocyte subset immune competence in flight rats. Allebban et al. found there was a significant decrease in the number of total leukocytes (P < 0.0001) and absolute counts of lymphocytes (P < 0.0001) and monocytes (P < 0.0001) of flight animals compared with ground controls at the landing day. Meanwhile, there was a slight decrease in the absolute number of eosinophils and a slight increase in the number of neutrophils of flight animals at landing compared with the preflight count. However, the observed decrease in the number of leukocytes and lymphocytes at landing returned to the control levels after a nine-day recovery phase. Immunophenotyping of the peripheral blood and spleen lymphocytes of flight and control animals indicated that, on the day of landing, there was a decrease in the absolute number of CD4+ and CD8+ T cells and B lymphocytes. However, the relative percentages of peripheral blood CD4+, CD8+, and B cells were not found to be depressed. There were no differences in the percent reactivity of spleen lymphocytes of flight animals compared with controls (Allebban et al. 1994). Based on the different responses of peripheral blood and spleen lymphocyte to spaceflight, it seems that there is a compartmentalized response to microgravity, and this was consistent with previous study revealed microgravity effects on individual lymphatic tissues had tissue-specific effect (Nash and Mastro 1992).
Further, researchers carried out experiments more scientific on SLS-2 mission, the second flight of SLS series. Analyses on rats were performed prior to the flight, on recovery and at intervals following recovery. Meanwhile, unlike all other previous spaceflight missions, a group of rats on SLS-2 was killed and dissected by astronauts in space. The main objective of the SLS-2 study is to determine the effects of spaceflight on the hematopoietic system. Lchiki et al. analyzed the white blood cell (WBC) compositions and the bone marrow myeloid progenitor cell populations to ascertain adaptation to microgravity and subsequent readaptation to microgravity and subsequent readaptation to 1 G in rats flown on the 14-day SLS-2 mission. Researcher found that except neutrophilia, the WBC level in flight rats was normal at landing compared to ground control rats. Compared with ground control rats, numbers of colony forming units-granulocyte (CFU-G), colony forming units-granulocyte macrophage (CFU-GM) and colony forming units-macrophage (CFU-M) from flight rats at day 13 in flight were decreased when incubated with recombinant rat interleukin-3 (rrIL-3) alone or in combination with recombinant human erythropoietin (rhEpo). However, on recovery, flight rats had decreased numbers of total leukocytes and absolute numbers of lymphocytes and monocytes with elevated neutrophils compared with control rats. Meanwhile, flight rats had lower numbers of CD4, CD8, CD2, CD3 and B cells in the peripheral blood than ground controls, but no differences in spleen lymphocytes (Ichiki et al. 1996).
3 The Effects of Microgravity/Spaceflight on Hematopoietic Cells
Human have been exploring the space for more than 50 years. Compared to the normal earth environment, short- and long-duration spaceflight provides an abnormal environment for us. During a spaceflight, astronauts and laboratory animals are exposed to outer space environments which contain various factors harmful for organisms, and these factors include specific and unspecific factors. Specific factors are permanent factors of outer space, including microgravity, ionizing radiation, background radiation created by high-energy particles and factor influence circadian rhythms. While unspecific factors are produced in the process of spaceflight mission-launching and landing, including hypergravity, noise, vibrations and G force shock (Domaratskaya et al. 2002). Both the specific and unspecific factors are important for the health of astronauts. Microgravity is the largest effect in spaceflight, which affects physiological systems of human and experimental animal.
Short- and long-duration spaceflight could produce complex physiological changes, including fluid redistribution, neuro vestibular effects, muscle changes, bone demineralization and loss, immune dysregulation, and psychosocial changes in human (Williams et al. 2009). Similarly, spaceflight and microgravity also altered physiological systems of animal. Experiment carried on 45 rats also demonstrated microgravity could cause physiological, biochemical and morphological changes in the animal body in a 22 day’s spaceflight on biosatellite Cosmos-605 (Ilyin et al. 1975).
Lots of experimentations and studies have indicated that short- and long-duration spaceflight could alter a wide variety of hematopoietic and immunological responses, including decreased plasma and blood cell mass, altered blood flow, lymphocyte and eosinophil numbers, increased immunoglobulin A and M levels (Graebe et al. 2004; Vernikos 1996).
In order to better understand the effects of spaceflight on bone morrow and hemopoietic system, lots of study and experiments has been studied on the satellite, space shuttle and spaceship. Astronauts always showed anemia and extent of changes on hematopoietic system after experience shot- or long-during spaceflight, including thrombocytopenia, and abnormalities in red blood cell structure (Davis et al. 1996). Numerous studies have proved that exposure aboard a spacecraft leads to lots of changes in the peripheral blood of astronauts. Researchers detected red-cell mass of astronauts on two Skylab missions. The results showed there was a 14% mean decrease in red-cell mass after the 28-day mission and a 12% mean decrease after the 59-day mission. Meanwhile, plasma volume decreases after each mission, and it decreased greater after the 59-mission (Johnson et al. 1975). Iliukhin et al. observed cytokinetic and morphological changes in erythropoiesis of crews experienced 96-, 140- and 175-days spaceflight. The number of circulating erythrocytes decreased in flight and their life time reduced postflight (Iliukhin and Burkovskaia 1981).
Mercury, Gemini, and Russian manned orbital flights gave us the first opportunity to evaluate the actual effects of spaceflight on human being. Various blood indices including plasma volume, red blood cell mass and erythrocyte survival were detected on the Gemini astronauts before and after Gemini orbital flights IV (1965), V (1965) and VII (1965). Though the three spaceflight were affected by different stresses, investigators found plasma volume changes associated with the shorter flights, and it may have been compensated for in the longer Gemini VII flight. At the same time, the survival of erythrocytes and red blood cell mass in Gemini V and VII decreased and there was no compensation for the RBC mass even in the longer flight (Fischer et al. 1967). However, the mechanistic and the cause is unknown.
Because some data could not be obtained in human studies, there also carried out animal experiments during spaceflight to thoroughly study the effects of spaceflight and microgravity. Lots of experiments have proved that spaceflight and microgravity could change the hemopoietic system of rats. On the Soviet biosatellite Cosmos 782, which was launched in 1972, rats experienced a 19.5 day of weightless spaceflight. Based on the output of radioactive CO, survival parameters of erythrocytes were evaluated upon return from orbit. The results indicated that all survival factors and indexes of erythrocytes were altered in flight rats when compared to the ground control rats. Compared to vivarium control rats, the mean potential lifespan, measured size of erythrocytes in flight rats decreased. Meanwhile, random hemolysis was increased three-fold in the flight rats (Leon et al. 1978). During the 18–22 day’s spaceflight aboard on Cosmos biosatellites, Shvets et al. investigated the histogenesis of the hemopoietic tissue at the level of stem cells, the results showed microgravity could inhibit the erythropoiesis in various skeletal sites (Shvets et al. 1984). A life science module housing young and mature rats was flown on shuttle mission Spacelab 3 (SL-3) to research the effects of spaceflight and microgravity on blood. The results of hematology studies of flight and control rats indicated the hematocrit, red blood cell counts, and hemoglobin determinations were significantly increased in flight animals. Meanwhile, the flight rats also accompany with a mild neutrophilia and lymphopenia. Lange et al. first performed the erythropoietin assays and clonal studies. There were no significant changes in bone marrow and spleen cell differentials or erythropoietin determinations. Clonal assays demonstrated an increased erythroid colony formation of flight animal bone marrow cells at erythropoietin doses of 0.02 and 1.0 U/ml but not 0.20 U/ml (Lange et al. 1987).
The recovery of normal blood count and the maintenance of hemopoiesis at a constant level are provided for by clonogenic hemopoietic cells of the stem cell and the committed progenitor cell compartments. Hence, changes observed in the peripheral blood can reflect more profound processes occurring in the hemopoietic tissues under the effect of spaceflight factors. In order to study the effects of microgravity on physiological parameters of organisms, Spacelab Life Sciences-1 (SLS-1) was launched aboard Space Shuttle Orbiter Columbia (STS-40) by NASA in 1995. During the spaceflight on SLS-1 mission, researches conducted a comprehensive examination of erythropoiesis and blood volume regulation in rats during space travel. Researchers assessed RBCM (red blood cell mass), PV (plasma volume), reticulocyte counts, marrow erythropoietin levels, iron utilization and storage, RBC survival, and RBC shape. On landing day (R+0), they found RBCM and PV adjusted for body mass were significantly lower in the flight animals than controls. Their results also suggested the decrease in RBCM exposure to microgravity during spaceflight was not due to hemolysis or splenic sequestration. The flight animals had lower reticulocyte counts during early recovery, which indicated diminished erythropoiesis during the post-flight study period. Meanwhile, researchers also conducted clonal assays to determine the hematopoietic generating capacity. The flight animals had a significantly decreased number of BFU-e’s and CFU-e’s recovered from their morrows on landing day at various levels of exogenous EPO, which indicated the numbers of bone morrow erythroid progenitors were decreased in flight animals. However, the result is contradict with the experiments on SL-3 (Lange et al. 1987). Besides the experiments above-mentioned, researchers also detected the RBC counts, Hgb, the number of RBC with atypical shapes and serum EPO, while there were no significant differences in these indexes between spaceflight and ground control animals (Udden et al. 1995). Further, there are study investigate the effects of microgravity and increased gravity on bone marrow of rats. Experiments were carried out in microgravity on rats flown on Soviet Biosatellite 2044 and in hypergravity by centrifugation at 2 × g. Lange et al. investigated bone marrow cell differential counts, clonal studies of RBC colony formation, and plasma erythropoietin determinations of spaceflight rats and ground control rats. The results showed bone marrow cells of spaceflight rats formed fewer CFU-e than ground control rats, which is consistent with previous results conducted on SLS-1 but contradict with the experiments on SL-3 (Lange et al. 1987; Udden et al. 1995). Furthermore, rats in hypergravity formed more CFU-e than ground control rats, which suggested that the decreased proliferative potential directly produced by microgravity could be reversed by hypergravity (Lange et al. 1994).
Consistently, Vacek et al. found the decreased colony forming of hematopoietic cells during spaceflight was caused by specific factor, mainly microgravity. To prove and differentiate the effects of specific and unspecific spaceflight factors on the hemopoietic tissue, researches designed three groups of animals, including the flight group (F), the synchronous control group (SC) and the vivarium ground control group (VC). The flight group was exposed to the entire spaceflight factors (specific and unspecific) aboard the satellite; the synchronous control group was exposed to unspecific spaceflight factors simulated on land; the vivarium control group was kept under normal laboratory conditions. Researchers found the number of CFU-s decreased significantly in bone marrow, spleen, and liver of flown rats on Cosmos-1129, Cosmos-1514, Cosmos-1667 and Cosmos-1887, and the phenomenon was irrespective of sex of rats (Vacek et al. 1985; Domaratskaya et al. 2002). These results revealed that the numbers of CFU-s in bone marrow of flight group rats significantly decreased compared to that of SC and VC group rats. Therefore, it was the effect of specific spaceflight factors lead to the decrease of CFU-s content in the bone marrow. Meanwhile, the numbers of CFU-s contents in the spleen of flight group rats were comparable to those in SC rats. So, the changes of CFU-s content in the spleen of flight group to vivarium control group were caused by unspecific factors. It also demonstrated the decrease of CFU-s content in the main hemopoietic organ of adult animals-bone marrow, was not because of the redistribution of these cells to the spleen or liver. Vacek et al. also found that a significant decrease was revealed in the contents of progenitor cells of the granulocyte and erythrocyte lineages in the rat bone marrow after a 14-days spaceflight. The number of CFU-gm was about 3–6-fold lower, and the numbers of BFU-e and CFU-e was about of 2.5 and 1.5-fold lower, respectively, than in rats of the VC and SC groups. Vacek et al. also found in the bone marrow of F rats, the number of CFU-f (progenitors of stromal fibroblasts) became almost 15-fold lower than in SC and VC rats, and no differences in this parameter between the two control groups were revealed (Vacek et al. 1990). These data suggest that bone marrow CFU-f is sensitive to the specific spaceflight factors. Hodgson et al. thought that the number of CFU-s naturally decreases because of their differentiation into more “advanced” cell forms, and it appears that the loss of CFU-s during spaceflight is also not counter balanced by the input of “younger” and more potent cells from the stem cell compartment (Hodgson and Bradley 1979).
Spaceflight/microgravity both impacts the proliferation and differentiation of hematopoietic progenitor cells. Davis et al. found that spaceflight/microgravity reduced proliferation and differentiation. During the space shuttle missions STS-63 (Discovery) and STS-69 (Endeavour), researchers investigated the in vitro effects of spaceflight on hematopoietic cell proliferation and differentiation (Davis et al. 1996). CD34+ bone marrow progenitor cells were cultured in a culture system with hematopoietic supportive stromal cells under microgravity/spaceflight or normal gravity, respectively. After 11–13 days of culture, they found the total cell number under microgravity/spaceflight was significantly less than that under normal gravity (57–84% decrease). As for the specific progenitor cell types, microgravity/spaceflight significantly decreased the number of myeloid progenitor cell number and erythroid progenitor cell number, especially erythroid progenitor cell number. These results indicate that spaceflight has a direct effect on hematopoietic progenitor cell proliferation and differentiation and those specific aspects of in vitro hematopoiesis, particularly erythropoiesis.
Besides human and rats, the effects of spaceflight/microgravity on hemopoietic tissues and cells have also been proved on lower vertebrates. Michurina et al. adapted the method of hemopoietic cell transplantation into irradiated recipients for analyzing the effect of spaceflight factors on clonogenic hemopoietic cells of newts. On the biosatellites Bion-10 and Bion-11, they first transplant hemopoietic cell into irradiated recipients for analyzing the effect of spaceflight factors on clonogenic hemopoietic cells. After newts were exposed on board the Bion 11 satellite, the hemopoietic cells from these news were transplanted into irradiated recipients and were analyzed after 22 days. Histological analysis results revealed the number and size of the foci of poorly differentiated hemopoietic cells in the spleens of recipients received and the liver hemopoietic cells of flight group newts were significantly smaller compared to that in spleens of recipients received the liver hemopoietic cells of SC group newts. The absence of hemopoietic recovery in the liver, and the decreased size and number of spleen colonies in the recipients of hemopoietic cells was reported from newts of the group flown. This suggest that spaceflight factors have a specific effect on colony-forming (clonogenic) hemopoietic cells of newts (Michurina et al. 1996). Analysis of morphologically unrecognizable clonogenic hemopoietic cells in lower vertebrates is possible to perform and newts can be adequately used as experimental animals for studying the effects of spaceflight factors on hemopoiesis on board a biosatellite.
Nevertheless, there are studies showed spaceflight and microgravity didn’t impact the properties of hematopoietic cells and the hematopoietic generating potential. Kozinets et al. use morphological, interferometric and electron microscopic techniques examined the morphofunctional properties of peripheral blood cells of Cosmos-936 rats and found the changes of microgravity on rat bone morrow cell composition; cell structure and function were reversible. Rats showed symptoms of a stress reaction immediately at landing and disappeared after three days. The percentage of bone marrow cell distribution was shifted towards enhanced myelopoiesis and diminished erythropoiesis. However, after the readaptation period the ratio of bone marrow cell composition returned to the normal level, which seems spaceflight and microgravity seems doesn’t intrinsically change properties of hematopoietic system (Kozinets et al. 1983).
Besides the effects of spaceflight/microgravity on bone marrow and hemopoietic system, researchers also investigated the effects of non-specific spaceflight factors on HSCs. An experiment conducted onboard the biosatellite Cosmos-1514 demonstrated that the effects of spaceflight on the proliferative capacity of HSCs also partly owing to the non-specific flight factors, such as launching effect and landing effect (Vacek et al. 1985). After a 5-day stay under microgravity onboard Cosmos-1514, Vacek et al. found synchronous rats also exhibited a decrease in the number of haemopoietic stem cells (CFUs) in bone marrow and spleens compared to ground control rats. This suggested that non-specific flight factors also decrease the number of CFU-s in the spleens of flight in the experiments conducted onboard the Cosmos-2044 Biosatellite of 14-day flight, Vacek et al. investigated the effects of microgravity on the proliferation ability of progenitors in bone marrow. The results of clonal assay demonstrated that the number of progenitors of erythrocytes (erythroid burst-forming units, BFU-e) and of granulocytes and macrophages (colony forming units-granulocyte macrophage, CFU-GM) in bone marrow was decreased in flight rats exposed to microgravity during a 14-day flight onboard the Cosmos-2044 biosatellite when compared to ground control rats. However, the number of progenitors of both lineages of haemopoiesis was also decreased in synchronous control rats, thus suggesting that the pool of progenitors is influenced also by the action of the nonspecific space flight factors (Vacek et al. 1991). The results are similar with previously results conducted on biosatellite Cosmos-1514 (Vacek et al. 1985).
However, there are fewer studies about the mechanisms of the effects of spaceflight/microgravity on hematopoietic system and cells. The reasons that lead to the suppression of hemopoiesis in animals during the post flight period can be attributed to not only the decreased number of different clonogenic hemopoietic cells, but also to changes in the stroma of hemopoietic organs, such as bone marrow, which provide suitable environment for hemopoiesis (Davis et al. 1996). For spaceflight/microgravity could decrease in the number of fibroblast progenitor cells (CFU-f) was accompanied by a simultaneous reduction in the number of hemopoietic progenitors of granulocytic and macrophagal lineages, which depend on the cytokine (CSF-gm) produced by fibroblasts. Therefore, spaceflight factors may have an adverse effect on the stem cells of both hemopoietic and stromal tissues.
4 The Effects of Simulated Microgravity on Hematopoietic Cells
Human space flight missions have resulted in some hematologic anomalies. Therefore, the effects of microgravity on the hematologic function have drawn the attention of researchers to the health of astronauts. However, because of the rare less chances and very expensive price of spaceflight experiments, researchers imagining conduct experiments to simulate the microgravity effects in spaceflight on ground. NASA designed a rotating wall vessel (RWV) bioreactor that could produce simulated microgravity on ground. Researchers always adopt NASA’s RWV bioreactor to investigate the effects of microgravity on cells. The RWV bioreactor rotates the vessel wall and cell culture media at the same speed, which continuously randomized the gravitational vector and maintaining the cells relatively motionless in the fluid and produced the effect of simulated microgravity (Schwarz et al. 1992; Tsao et al. 1992). Simulated microgravity provides a methodology for researching the effects of microgravity on various kinds of cells. Over the past few decades, there are many studies targeting the effects of simulated microgravity on these properties of hematopoietic cells. These researches revealed that simulated microgravity extensively impact the properties of hematopoietic cells, not only the proliferation capacity, but also the differentiation and migration.
CD34 antigen is surface glycophospho protein expressed on developmentally early lymph hematopoietic stem and progenitor cells and is known as hematopoietic progenitor cell antigen (Krause et al. 1996). Bone marrow CD34+ cells contain hematopoietic stem and progenitor cells and they could differentiate into all the various blood cell types. Plett et al. cultured adult human BM CD34+ cells in RWV bioreactors under either stationary state (1 × g control) or rotated-produced simulated microgravity for 4–6 days. They examined cellular expansion, hematopoietic potential, retention of primitive cell phenotype, apoptotic cell content and adhesion molecule status of these BM CD34+ cells. The results showed that the number of CD34+ cells nearly didn’t increased when cultured under simulated microgravity, while cells cultured under normal gravity proliferated up to 3 fold, which demonstrated that simulated microgravity could significantly decreased the proliferative ability of BM CD34+ cells. One possible explanation maybe that simulated microgravity decreased the exit G0/G1 phase of cell cycle and made these BM CD34+ cells at a greater degree of hematopoietic potential. Meanwhile, compared cells cultured under normal gravity, BM CD34+ cells under simulated microgravity condition produced greater numbers of cells and progenitors, and these cells keep survival for a longer time. However, simulated microgravity did not affect expression of adhesion molecules and induction of apoptosis of BM CD34+ cells (Plett et al. 2001). Further, they also investigated the effects of simulated microgravity on the migration potential, cell-cycle kinetics and progenitor differentiation of BM CD34+ cells and found that simulated microgravity significantly inhibits the migration potential, cell-cycle progression, and differentiation patterns of primitive BM CD34+ cells (Plett et al. 2004). BM CD34+ cells were cultured under simulated microgravity (μg) by using rotating wall vessels (RWV) or under normal gravity in control cultures for 2–18 days. Simulated microgravity significantly decreased the migration potential and this effect may be caused by a significant reduction of stromal cell-derived factor 1 (SDF-1α), which correlated with decreased expression of F-actin and important for cell-directed migration. Simulated microgravity also altered cell-cycle kinetics by prolonged S phase and reduced cyclin A expression. Compared to be cultured under normal gravity, BM CD34+ cells cultured under simulated microgravity condition favored differentiated into more CD33+ myeloid cells (65.8% vs. 6.9%) and less Gly-A+ erythroid cells (26.0% vs. 61.4%). Chiu et al. investigated the effects of simulated microgravity on the differentiation and proliferation human umbilical cord blood stem cells (CBSC). CD34+ mononuclear cells were isolated from waste human umbilical cord blood samples and stimulated with vascular endothelial growth factor (VEGF) under simulated microgravity for 14 days. Simulated microgravity significantly increased cellular proliferation with three-dimensional (3D) tissue-like aggregates. Meanwhile, CD34+ cells cultured under microgravity without microcarrier beads (MCB) developed vascular tubular assemblies and exhibited endothelial phenotypic markers. These results suggest that CD34+ human umbilical cord blood progenitors are capable of trans-differentiation into vascular endothelial cell phenotype and assemble into 3D tissue structures (Chiu et al. 2005).
Simulated microgravity also significantly influences properties of erythroid progenitor-like K562 leukemia cells. Yi et al. investigated effects of simulated microgravity on proliferation, cell death, cell cycle progress and cytoskeleton of erythroid progenitor-like K562 leukemia cells. The results showed the cell densities cultured in Rotary Cell Culture System (RCCS) were only 55.5%, 54.3%, 67.2% and 66.4% of the flask-cultured control cells when cultured for 24 h, 48 h, 72 h, and 96 h, respectively. RCCS culture induced an accumulation of cell number at S phase and a decrease at G0/G1 and G2/M phases at 12 h, which concomitant with the changes of intercellular cyclins levels that with a decrease in cyclin A and a decrease in cyclin B, D1 and E. However, simulated microgravity seemed didn’t impact apoptosis in their experiments (Yi et al. 2009). Consistently, Long et al. found simulated microgravity environment significantly decreased the proliferation rate of K562 cells. Consistently, simulated microgravity environment also significantly inhibited cell cycle progression and made the cell cycle arrested in G0/G1 phase. Further mechanism study suggested that the decreased proliferation rate and the cell cycle arrested in G0/G1 phase under simulated microgravity environment may be caused by decreased expression of ERK1/2 phosphorylation (Long et al. 2011). These results demonstrated that simulated microgravity inhibit proliferation and cell cycle of K562 leukemia cells.
Migration, proliferation, and differentiation of bone marrow (BM) hematopoietic stem cells (HSC) are important factors in maintaining hematopoietic homeostasis. Besides impact the proliferation, differentiation and migration of BM HSCs and erythroid progenitor-like K562 leukemia cells, simulated microgravity also change the properties of erythroid lineage. Arthur J. Sytkowski and Kerry L. Davis reported simulated microgravity significantly inhibit erythroid growth and erythropoietin (Epo)-induced differentiation. Logarithmic growth of rauscher murine erythroleukemia cells was observed when cultured under normal gravity or simulated microgravity condition by using RWA bioreactor (Sytkowski and Davis 2001). Cells in simulated microgravity grew more slowly and its doubling time was nearly two times of cells cultured under normal gravity (24 h vs. 14.4 h). The percentage of Epo-induced cell differentiation under simulated microgravity was significantly lower than cells cultured under normal gravity (25% vs. 12%). However, simulated microgravity didn’t impact cell apoptosis, which are consistent with the result that simulated microgravity had no effect on apoptosis of BM CD34+ cells (Plett et al. 2001). Zou et al. found simulated microgravity not only significantly inhibit cellular proliferation rate but also induced cell apoptosis of the human erythropoietin (EPO)-dependent megakaryoblastic leukemia cell line UT-7/EPO. Meanwhile, simulated microgravity could downregulated the expression of erythropoietin receptor (EPOR), which is crucial for the survival, proliferation, and differentiation of erythroid progenitors (Sytkowski and Davis 2001). Further, when transferred human EPOR gene into UT-7/EPO cells, which increased the expression (approximately 61%) of EPOR on the surface of UT-7/EPO cells, they found that simulated microgravity-induced apoptosis markedly decreased in these UT-7/EPO-EPOR cells (Zou et al. 2011).
Migration, proliferation, and differentiation of bone marrow (BM) hematopoietic stem cells (HSC) are important factors in maintaining hematopoietic homeostasis. Simulated microgravity have a board impact on hematopoietic homeostasis. The results obtained from simulated microgravity may partly reflect the effects of microgravity/spaceflight on hemopoietic cells and hemopoietic system. Simulated microgravity provides an effective alternative method to carry out microgravity experiments for majority of researchers. All these results provided some possible explanation of the hematologic abnormalities observed in humans during space flight. However, the mechanistic of simulated microgravity on hemopoietic system has not been clarified and further researches are needed.
5 Regulation of HSCs Self-renewal in BM
5.1 Stem Cells
Stem cells are a kind of undifferentiated biological cells. They possess two primary characteristics: self-renew and differentiation. They can both experience indefinite cycles of cell division while maintaining the undifferentiated state and differentiate into one or more kinds of functionally mature cells of particular tissues, such as cardiomyocytes, haematopoietic cells, chondrocytes, adipocytes and endothelial cells. Based on the differentiation potential, also known as the number of mature cell types to which they can give rise, stem cells can be divide into totipotent stem cells, pluripotent stem cells, multipotent stem cells, oligopotent stem cells and unipotent cells (Hans 2007). Totipotent stem cells can differentiate into embryonic and extraembryonic cell types. Pluripotent stem cells are the descendants of totipotent cells and can differentiate into nearly all cells. Multipotent stem cells can differentiate into a number of cell types. Oligopotent stem cells can differentiate into only a few cell types. Unipotent cells are cells with lowest differentiation capacity, which can produce only one cell type, their own. However, they have the property of self-renewal, which distinguishes them from non-stem cells, such as progenitor cells. According to the developmental stages, stem cells could be divided into two types in mammals: embryonic stem cells and adult stem cells. Embryonic stem (ES) cells are isolated from the inner cell mass of pre-implantation blastocyst of mammals, the properties of ES cells identified them as being highly suitable for the generation in vitro of specific cell lineages. While adult stem cells, also named tissue-specific stem cells, are existed in various niches throughout the body, such as bone marrow, brain, liver and skin. Adult stem cells including neural stem cells, hematopoietic stem cells, mesenchymal stem cells, epidermal stem cells and so on. They primarily perform functions as tissue maintenance, growth and repair in later life (Rippon and Bishop 2004; Bishop et al. 2002). Because stem cells process plasticity, so theoretically, adult stem cells could be obtained from patients, then differentiated in vitro and transplanted back to the same individual for tissue repair without the need for immunosuppression (Hans 2007). Stem cells can provide appropriate cell source for tissue engineering and therapeutic application.
5.2 Hematopoietic Stem Cells
Hematopoietic stem cells (HSCs) are a kind of pluripotent stem cells, which can give rise to all the other blood cells through the process of haematopoiesis and maintain the homeostasis of organism. HSCs can produce adequate production of blood cells, including all myeloid lineages blood cells (monocytes, macrophages, neutrophils, basophils, eosinophils, erythrocytes, dendritic cells, and megakaryocytes or platelets) and lymphoid lineages blood cells (include T cells, B cells, and natural killer cells). Most blood cells have relatively short lifespans after birth, from several hours like granulocytes to several years like memory T cells. Hence, in order to maintain haematopoiesis, HSCs continuously differentiate into multiple lineages of different types of blood cell and are responsible for blood cell renewal. HSCs are rare in organism. In adult mammals, most of these cells are derived from mesoderm and located in the red bone marrow (BM), which is contained in the core of most bones. In bone marrow, HSCs are at a very low percentage, generally from 0.01 to 0.05%. In fetal, HSCs predominantly reside in fetal liver (FL). Besides, a small number of HSCs can also be found in the peripheral blood (PB) and umbilical cord blood (UCB).
5.3 Types of HSCs
Generally, HSCs can be divided into three types: long-term self-renewing HSCs (LT-HSCs), short-term self-renewing HSCs (ST-HSCs) and multipotent progenitors (MPPs). In the three types of HSCs, LT-HSCs are thought to self-renew for the whole lifespan of an organism, which have an ability to engraft and repopulate a host hematopoietic system. ST-HSCs and MPPs have a shorter duration, and are able to restore hematopoiesis in a lethally irradiated mouse only for up to four months. Meanwhile, MPPs are not with the ability to self-renewing (Reya et al. 2001). When LT-HSCs differentiated to ST-HSCs and then to MPPs, the ability of self-renewal lost gradually and the ability of mitotic activity increased slowly. After LT-HSCs becomes the non-self-renewing MPPs, these cells continue differentiated to either one of the two hematopoietic lineages, including common lymphoid progenitor (CLP) and common myeloid progenitor (CMP). During the process of differentiation, HSCs first loses self-renewal capacity, and then lose lineage potential step-by-step. CLP finally give raise to mature functional lymphoid lineage cells, including mature nature killer (NK) cells, B cells, T cells and dendritic cells (DCs). Meanwhile, CMP finally differentiated into functional myeloid lineage cells, including eosinophils, basophils, neutrophils and monocytes/macrophages, erythrocytes, megakaryocytes and platelets (Forsberg et al. 2006; Cabrita et al. 2003). CMP can also differentiated into mature DCs (Seita and Weissman 2010). Through differentiation, HSCs can give raise to all kinds of blood cells (Fig. 1). With the advancement of new technologies of multi-color fluorescence flow cytometry, the phenotypes of these cells in the process of HSCs differentiation are defined by lots of specific surface molecular markers, which make it possible to identify and separate them from organism, and these molecular markers are listed in Table 1.
The hematopoietic lineages of differentiation. The curved arrow indicated self-renewing ability. The LT-HSCs resides at the top of the hierarchy, which are defined as the cells that have both the self-renewal capacity and the potential to give rise to all other hematopoietic cell types. Through differentiation, HSCs first lose self-renewal capacity, then loses lineage potential step-by-step as them commit to become all mature functional blood cells. CLP: common lymphoid progenitor, CMP: common myeloid progenitor, MEP: Megakaryocyte/erythrocyte progenitor, GMP: granulocyte/macrophage progenitor, MkP: Megakaryocyte progenitor, EP: erythrocyte progenitor, GP: granulocyte progenitor, MacP: macrophage progenitor, DC: dendritic cell, NK: natural killer
5.4 Molecular Mechanisms Regulating HSCs Self-renewal in BM
In order to maintain the life-long haematopoiesis and homeostasis of mammalian organism, HSCs must replicate themselves to maintain the constant HSCs pool in BM. The process of HSCs replication through mitosis is called self-renewal. Recently, lots of advanced have been achieved about the molecular signature to define the stemness and self-renewal of stem cells and HSCs. The fate choice of HSCs to either self-renew or differentiate is determined by a complex interplay between intrinsic mechanisms and extrinsic signals from the surrounding environment or stem cell niches (Moore and Lemischka 2006).
In adult BM, the number of HSCs stays relatively constant and BM HSCs are retaining quiescent under normal conditions. Previous results showed the majority of BM HSCs slowly, constant incorporation of nucleotide analogues, and demonstrated that the majority of these cells divide regularly (Bradford et al. 1997; Cheshier et al. 1999). It seems that the cell-cycle state of HSCs correlates to their multipotency. When LT-HSCs were engrafted to irradiated recipients, these cells are in the G0 phase of the cell cycle (Passegue et al. 2005). It is widely assumed that the dividing HSCs in BM undergo asymmetric cell division, in which an individual HSC gives rise to a non-identical daughter cell (keeping the HSC identity) and the other becoming a differentiated progenitor cell.
External environmental signals integrate with intrinsic molecular pathways controlled the fates of HSCs. Gain and loss of functional studies have found several transcription factors are implicated in there gulation of HSCs self-renewal. The transcription factor translocation Ets leukemia (Tel) has been demonstrated to be required HSCs self-renewal. Inactivation of Tel leads to the depletion of HSCs in BM without influencing their committed progenitors (Hock et al. 2004b). HoxB4, a member of the homeobox gene family, encodes a set of transcription factors which usually regulate embryonic body patterning and organogenesis. Sauvageau has proved that both in vitro and in vivo, overexpression of HoxB4 in HSCs has been shown to enhance the self-renewal of HSCs (Sauvageau et al. 2004). Furthermore, it has been described that there is a remarkable growth advantage in HoxB4-transduced HSCs over untransduced ones when cultured in vitro (Antonchuk et al. 2002). While HoxB4 knockout mice only exhibit a mild defect in the proliferative potential of HSCs and normal hematopoiesis. The discrepancy may due to the redundant function of other Hox family members (Brun et al. 2004). Two studies independently identified Growth factor independence 1 (Gfi1), which is a zinc finger-containing transcriptional repressor, as a positive regulator of HSCs self-renewal that functions by restraining HSC proliferation. Gfi1 knockout HSCs exhibit increased proliferation and decreased capability for repopulation of irradiated recipients (Zeng et al. 2004; Hock et al. 2004a). Gfi1 might function through upregulating the cell-cycle inhibitor p21, since p21 expression is greatly decreased in Gfi1 knockout HSCs. Consistent with this hypothesis is the observation that p21-deficient HSCs also show impaired repopulation capability compared to wild-type HSCs (Cheng et al. 2000). p18, which is another cell-cycle inhibitor, has an opposite effect on HSCs self-renewal compared to p21. Compared to wild-type HSCs, p18-deficient HSCs increased their repopulation capability (Yuan et al. 2004; Yu et al. 2006). c-Myc protein plays an important role in the homeostasis of HSCs. Conditional decreased the activity of c-Myc in the bone marrow (BM) results in severe cytopenia and accumulation of HSCs in situ, which were caused by HSCs couldn’t initiate their normal differentiation. While enforced c-Myc expression in HSCs leading to loss of self-renewal activity at the expense of differentiation (Wilson et al. 2004). Gain and loss of function studies have shown that both Stat5 and Stat3 are important to HSCs self-renewal. They are positive regulator and can promote the ability of self-renewal of HSCs (Kato et al. 2005; Chung et al. 2006). These results indicate that HSCs self-renewal is delicate controlled by lots of transcription factors and molecular pathways regulating cell proliferation and cell-cycle repression.
Besides transcription factors, other proteins also have been found can regulate HSCs self-renewal. Bmi-1, which is polycomb group (PcG) protein that forms the Polycomb repression complex 1 (PRC1) with other members of the PcG family and can modulate gene expression, have been reported could regulate HSCs self-renewal. Bmi1-deficient mice have exhibited the progressive hematopoietic defect with increase of age (Lessard and Sauvageau 2003). In BM HSCs, knockout Bmi-1 can destruct the long-term proliferate ability, while overexpress of Bmi-1 can strengthen the expansion ability of HSCs in vitro and enhance the proliferate ability in vivo (Iwama et al. 2004; Park et al. 2003). All this demonstrated Bmi1 plays a crucial role in maintenance of proliferation capacities of HSCs and progenitors.
Environmental signals, such as Notch, Wnt, BMP and Sonic hedgehog (Shh) signals, also play important roles in regulate the gene expression related with HSCs self-renewal. Duncan reported that Notch signaling is active in HSCs, and its expression was downregulated after HSCs differentiation. It has been reported Notch signaling played important role in lymphoid lineage commitment between T cell and B cell. Notch signaling pathway is always active in HSCs, and the activity is attenuated when HSCs differentiate. When HSCs was treated with Notch signaling inhibitor, the differentiation ability of HSCs was increased and the number of HSCs was decreased (Duncan et al. 2005). Besides, overexpress Notch1 in HSCs increased self-renewal capability (Stier et al. 2002). However, who found that the self-renewal ability of HSCs was not affected when Notch1 signaling was deficient in BM-HSCs (Mancini et al. 2005). Wnt signaling has also been found in the regulation of HSCs self-renewal. Wnt family includes several secreted protein and the receptors of Wnt proteins are the Frizzled family and LDL-receptor-related proteins. Several Wnt proteins have been successfully purified and Wnt3a protein has thus been shown to act on HSCs as a growth factor (Willert et al. 2003). β-catenin is one of the most target molecular downstream of Wnt signal. Reya found that overexpression of activated β-catenin in HSCs lead to expansion of the pool of immature cells in vitro, which can reconstitute irradiated recipients (Reya et al. 2003). The in vivo repopulation capability of HSCs was lost when treated with Wnt signaling inhibitor. Wnt3A has been found to promote self-renewal of HSCs (Willert et al. 2003). However, who found that β-catenin-deficient mice had no defects on HSCs and the self-renewal capability of β-catenin-deficient HSCs was normal (Cobas et al. 2004).
6 Molecular Mechanisms Regulating Differentiation of HSCs to Microphage
Besides the potent proliferative and self-renewal capacity to produce new hematopoietic cells, HSCs can give raise to various lineages of function cells of blood. As showed in Fig. 1, HSCs differentiated macrophages should experience a few selections. The first choice should be experienced is to differentiate into myeloid or lymphoid lineage cells. After they have become CMPs, they will face the second choice that whether to differentiate into GMPs or MEPs. Only when they have differentiate into GMPs, then they can finally differentiate into macrophage. Before they eventually become macrophages, they should experience the stages of CMPs and GMPs. During this process, several transcription factors and molecular pathways have been reported played important roles in this process: the commitment of monocytes/macrophages from HSCs.
During the process of differentiation, primitive HSCs continuously divide and give raise to differentiated daughter cells, and these cells will become a single lineage that possess a fixed genetic program. Differential expression of transcription factors triggers the determination of HSCs fate: commitment to either CLPs or CMPs. With the development of lots of biotechnologies, several candidate transcription factors determine the initial destiny of primitive HSCs have been found, including. But these transcription factors have not been tested in clearly interpretable in vivo models.
PU.1, which is an Ets family transcription factor, plays an important role in both innate and adaptive immune system, it can control the development of granulocytes, macrophages, B cells and T cells in a cell-intrinsic manner. PU.1 is highly expressed in HSCs, CLPs and a part of CMPs (Nutt et al. 2005), which is accordance with its important roles in the development and differentiation of HSCs.
The transcription factor PU.1 is the most studied transcription factor during the initial differentiation of primitive HSCs. In all the studied transcription factors during this process, the most studied transcription factor is PU.1. The expression of PU.1 is one of the earliest events controlling HSCs to lineage commitment. Conditional disruption of PU.1 in bone marrow HSCs inhibit the transition of HSCs to CLP and CMPs, which means PU.1 is important for the generation of initial myeloid and lymphoid progenitors from HSCs (Iwasaki et al. 2005). HSCs upregulated PU.1 and GATA-1 will differentiate into CMPs. Meanwhile, PU.1 is also vital for CLPs for it is consistently expressed in CLPs (Scott et al. 1994). Further, PU.1 can regulate the mRNA expression of IL-7 receptor and M-CSF receptor, which are important for normal developments of lymphoid and myeloid lineages, respectively (DeKoter and Singh 2000; DeKoter et al. 1998). Overexpression of PU.1 in HSCs has been shown to be able to commit multipotent hematopoietic progenitors to monocytic/granulocytic direction (Nerlov and Graf 1998). It seems that expression level of PU.1 determine the differentiation of HSCs. DeKoter found that PU.1 controlled the lineage commitment of HSCs to myeloid or lymphoid cells are dosage-related, for a higher concentration favoring HSCs commitment to myeloid cells (DeKoter and Singh 2000). However, the molecular mechanisms regulating the expression of key transcription factors PU.1 are nearly unknown. The ‘extrinsic theory’ consider that the stable expression of transcriptional programs might be subject to instruction from outside signals, which has been proved that exogenous anti-TGF-β1 antibody could significantly upregulate PU.1 and GATA-1 expression level in the in vitro culture system of human CLRPP (cytokine low-responding proliferating progenitors) cells.
Kondo found low but detectable level of GM-CSF receptors were expressed on HSCs but not CLPs. It seems that the expression of GM-CSF receptor was down-regulated during the process of HSCs differentiated into CLPs. When GM-CSF or M-CSF receptor was overexpressed in CLPs, these cells were reprogramed to monocytes or granulocyts by GM-CSF or M-CSF in vitro (Kondo et al. 2000). Further, Evans found that signals mediated by cytoplasmic domain of GM-CSFR induced multipotent FDCP-mix cells differentiated into granulocytic/monocytic cells (Evans et al. 1999).
Meanwhile, during the differentiation process, how these differentiation-inducing transcription factors predominate over self-renewal-maintaining factors is also unknown. The expression of intrinsic transcription factors controlling the differentiation of HSCs might be instructed by outside signals. Kondo found low but detectable levels of GM-CSF receptor were expressed on HSCs but not CLPs. During the process of HSCs differentiated into CLPs, the expression of GM-CSF receptor was down-regulated. When GM-CSF or M-CSF receptor was overexpressed in CLPs, these cells were reprogramed to monocytes or granulocyts by GM-CSF or M-CSF in vitro (Kondo et al. 2000).
After the cells have differentiated into CMPs, these cells will experience the second choice that differentiated into MEPs or GMPs. PU.1 and GATA-1 play important roles in controlling this process, for they are co-expressed in CMPs and one of them exclusively expressed will determine the direction of differentiation. It has been proved that PU.1 is vital for monocytic and B lymphocytic development. During the process of CMPs differentiated to GMPs, PU.1 can inhibit the transcriptional activity of GATA-1, which predominately regulate CMPs differentiate into erythroid and megakaryocytic development. Overexpress of PU.1 could inhibit the binding of GATA-1 to its target gene promoter (Zhang et al. 2000). Matsumura found ectopic express PU.1 in erythroid/megakaryocytic leukemia cell K562 could redirect them to differentiate into granulocytes and monocytes, while overexpression of GATA-1 in granulocytic progenitor 32D cells redirects them to megakaryopoiesis (Matsumura et al. 2000). Similarly, Iwasaki found disruption of PU.1 in CMPs blocks myelomonocytic differentiation in vitro (Iwasaki et al. 2005). PU.1 functions may through regulate the expression of M-CSF/M-CSFR or GM-CSF/GM-CSFR, for it has been reported that PU.1 can regulate the expression of M-CSFRα and GM-CSFR (DeKoter et al. 1998; Anderson et al. 1998). Further, M-CSF receptor ectopically expressed in murine multiple-potent EML cells can increase their differentiation potential to granulocyte/monocyte and decrease their differentiation potential to erythroid lineage (Pawlak et al. 2000). However, other transcription factors may also function in this process. Overexpression of c-Myb could reprogram the K562 cells differentiate to granulocytic/monocytic lineage (Matsumura et al. 2000). Egr-1 (early growth response gene-1) was found to promote macrophage production in the expense of erythroid and granulocytic development (Krishnaraju et al. 2001). However, SCL could promote productions of erythroid and megakaryocytic colonies when expressed in CD34+ cells (Elwood et al. 1998). TGF-β1 signal could enhance the commitment of erythroid while inhibit the development of granulocyte/monocyte (Drexler et al. 1998; Krystal et al. 1994).
After CMPs have differentiated into GMPs, these bipotent precursor will choice whether to differentiate into granulocytes or monocytes/macrophages. Several transcription factors have been found play important roles in this process. PU.1 plays important regulatory roles in the HSCs differentiation. Rodney et al. found mutation of PU.1 gene could cause a severe reduction in myeloid (granulocyte/macrophage) progenitors. PU.1-deficiency myeloid progenitors can proliferate in vitro in response to the multilineage cytokines interleukin‐3 (IL‐3), IL‐6 and stem cell factor but are unresponsive to the myeloid‐specific cytokines granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), G‐CSF and M‐CSF. PU.1-deficiency myeloid progenitors could not induce detectable macrophage differentiation. Retroviral transduction of PU.1 into mutant progenitors restores responsiveness to myeloid‐specific cytokines and development of mature granulocytes and macrophages (DeKoter et al. 1998). PU.1 disruption in GMPs resulting in only myeloblast colony formation, and these myeloblast colonies did not express CD11b (Iwasaki et al. 2005).
Overexpress Egr-1 (early growth response gene-1), also known as Zif268, in myeloid-enriched cells could promote macrophage differentiation and inhibit granulocyte differentiation (Krishnaraju et al. 2001). The interferon consensus sequence binding protein (ICSBP/IRF-8), which can interact with PU.1, was found could inhibit the production of neutrophils from GMCSF-dependent ICSBP−/− cell line Tot2 and granulocytic progenitor 32D cells (Tamura et al. 2000). Meanwhile, express of ICSBP into Tot2 cells could also upregulate the expression of transcription factor Egr-1. ICSBP−/−mice developed granulocytosis along with atypical macrophages, which resemble a chronic myelogenous leukemia (CML) -like disease (Holtschke et al. 1996). Furthermore, overexpress ICSBP also could inhibit myelo proliferation both in vitro and in vivo (Hao and Ren 2000; Schmidt et al. 1998).
When multipotent progenitor cells differentiated to macrophage, Maf B, the bZip transcription factor, was upregulated successively. Meanwhile, overexpress Maf B in transformed myeloblasts could lead to the differentiation of monocytes/macrophages independent of overexpression of PU.1 (Kelly et al. 2000).
Tamura et al. found a specific protein FMIP, which could interact with the cytoplasmic domain of M-CSF receptor c-fms and inhibit the c-fms signaling. Overexpression of FMIP in bipotent FDC-P1Mac11 could prevent the M-CSF-induced monocytic differentiation and made all cells differentiated into granulocytes (Tamura et al. 1999). They also found ICSBP could induce macrophage differentiation (Tamura et al. 2000). HoxA10 have been proved could regulate monocytic differentiation. When HoxA10 was overexpressed in U937 cells, it could activate p21 and induced cell cycle arrest, which could promote monocytic differentiation from U937 cells (Bromleigh and Freedman 2000). Overexpression of GATA-2 in FDCP cell line could also induce these cells to monocytic differentiation and accompanied with cell cycle arrest (Heyworth et al. 1999).
Besides, DNA methyltransferases Dnmt3a and Dnmt3b have been proved that they could promote the differentiation of embryonic stem cells. Recently, Grant et al. found Dnmt3a played an important role in HSCs self-renewal and differentiation. Dnmt3a loss progressively impairs HSCs differentiation over serial transplantation, while simultaneously expanding HSCs number in the bone marrow. Meanwhile, Dnmt3a-null HSCs upregulate multipotency genes and downregulate differentiation factors (Dnmt3a is essential for hematopoietic stem cell differentiation).
7 The Scientific Achievements in SJ-10 Satellite Research
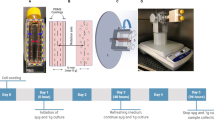
SJ-10 recoverable satellite is the first microgravity scientific satellite of China, which was launched on April 6, 2016 and mainly carries out microgravity science and space life science. During the 12 days spaceflight, we studied the effects and mechanisms of spaceflight/microgravity on the proliferation of HSPCs and macrophage differentiation. Meanwhile, we also investigated the effects and simulated microgravity of simulated microgravity on the proliferation and macrophage differentiation by using the RWV bioreactor (Fig. 2). About the proliferation of murine hematopoietic stem/progenitor cells, we found that both spaceflight/microgravity and simulated microgravity inhibited the proliferation of HSPCs. Meanwhile, cell cycle analysis demonstrated simulated microgravity inhibited the G1/S transition. RNA-seq and bioinformatics analysis revealed microgravity inhibited proliferation of HSPCs through regulating cell proliferation and cell apoptosis signaling pathway. Further, we also found that HSPCs proliferated under simulated microgravity for 12 days could re-differentiate into more macrophages. About macrophage differentiation, we elucidate the cellular and molecular mechanism of microgravity-affected macrophage differentiation. We found that microgravity significantly inhibits maturation of macrophages and impedes their further M1/2 polarization after in vitro differentiation comparing with the normal gravity. We firstly propose that the two microgravity-driven signaling pathways and metabolic alteration are important in macrophage differentiation affected by microgravity (Fig. 5).
7.1 Spaceflight/Microgravity Decreased the Proliferation of Murine Hematopoietic Stem/Progenitor Cells
Exposure to spaceflight/microgravity can cause a series of physiological and psychological changes, such as bone loss, cardiovascular dysfunction, and immune dysfunction. Anemia and hematopoietic disorder are always observed in astronauts when experienced short- or long-duration of spaceflight. Hematopoietic stem and progenitor cells (HSPCs), which could self-renew and give rise to all the other blood cells, play vital role in the haematopoiesis and homeostasis. However, the effects and mechanism of microgravity on the proliferation of HSPCs are remaining unclear. To study the effect and mechanism of spaceflight/microgravity and simulated microgravity on the proliferation of HSPCs, HSPCs were cultured in incubator which experienced a 12-days spaceflight on SJ-10 satellite (with cell unit in Fig. 3a) and Tianzhou-1 cargo ship (with cell unit in Fig. 3b), and in rotating wall vessel (RWV) bioreactor, respectively. Both spaceflight and simulated all significantly decreased the number of proliferated HSPCs (Fig. 4). Simulated microgravity blocked the cell cycle of HSPCs at G1/S transition and promoted cell apoptosis. RNA-Seq and bioinformatics analysis revealed that microgravity inhibited proliferation and promote apoptosis of HSPCs through regulating cell proliferation and cell apoptosis signaling pathways-associated genes. Meanwhile, microgravity through CSF and cAMP pathways regulated cell proliferation and cell cycle. Furthermore, HSPCs proliferated under simulated microgravity had the same the ability to re-differentiated into macrophages as that cultured under normal gravity. These results directly proved the inhibitory effect of microgravity on proliferation of HSPCs in vitro and preliminary revealed the regulatory mechanism of inhibition of microgravity on proliferation of HSPCs.
Cell culture systems during space flight and control experiment. a Cell culture unit used in SJ-10 satellite, in which 2 × 106 cells with 2.5 ml medium were placed in the cell culture chamber separated by two pieces of polycarbonate filter membrane in each end of the unit. The nutrient solution enters the culture unit from the nutrient bag connected at one end, infiltrates the filter membrane, enters the cell culture chamber, and then passes from the other end of the culture unit to the waste liquid bag. The cells cultured in this unit will eventually return to the ground for recycling and further analysis. b Cell observation unit used in Tianzhou-1 cargo ship, in which cells attached to micro-carriers were cultured with 1.8 ml medium in the middle cell culture chamber of the unit. The camera photographed cells from the glass observation window in the unit and collected the image information. Two units are connected in series, one is 10× magnifications of cells for images collection, and the other is 20×. The nutrient flows from one end of the nutrient solution bag to the other end of the waste liquid bag, and provides nutrient for cells in the cell culture chamber
Cell number and morphology of hematopoietic stem cells (HSPCs) after culturing in different gravity conditions for 12 days. a The number change of HSPCs in 12 days culturing in normal gravity and modeled microgravity. b Cell morphology of HSPCs after culturing in normal gravity and modeled microgravity for 12 days, observed in microscope at low magnification and magnification
7.2 Microgravity Suppresses Macrophage Differentiation
Space flight-associated immune system weakening ultimately precludes the expansion of human presence beyond the Earth’s orbit, so it is an urgent need to understand the relevant mechanisms caused by microgravity. To figure out the effect of microgravity on the microgravity-caused changes of cell state and transcriptome profiling during the differentiation of macrophages, we differentiated hematopoietic progenitor cells (HPCs) of mouse bone marrow in the presence of macrophage colony stimulating factor (M-CSF) for 12 days under spaceflight and simulated microgravity conditions, respectively, and performed gene expression analysis by next-generation sequencing (NGS). Results showed that microgravity significantly reduced the proliferation and differentiation of macrophages and even potentially impaired functional polarization of those macrophages. RNA-Seq data indicated that the genes related cell proliferation and macrophage differentiation were down-regulated while the genes related apoptosis and repair process were up-regulated under microgravity. Among paramount gene signature, we identified the major microgravity-regulated pathways during macrophage development (Fig. 5).
Abbreviations
- CBSC:
-
Cord blood stem cells
- CFU-G:
-
Colony forming units-granulocyte
- CFU-GM:
-
Colony forming units-granulocyte macrophage
- CFU-M:
-
Colony forming units-macrophage
- CLP:
-
Common lymphoid progenitor
- CMP:
-
Common myeloid progenitor
- DC:
-
Dendritic cell
- EP:
-
Erythrocyte progenitor
- GM-CSF:
-
Granulocyte/monocyte colony-stimulating factor
- GMP:
-
Granulocyte/macrophage progenitor
- GP:
-
Granulocyteprogenitor
- HSCs:
-
Hematopoietic stem cells
- HSPCs:
-
Hematopoietic stem and progenitor cells
- LT-HSCs:
-
Long-term self-renewing HSCs
- MacP:
-
Macrophage progenitor
- M-CSF:
-
Macrophage-colony stimulating factor
- MEP:
-
Megakaryocyte/erythrocyte progenitor
- MkP:
-
Megakaryocyte progenitor
- MPPs:
-
Multipotent progenitors
- NGS:
-
Next-generation sequencing
- NK:
-
Natural killer
- RBCM:
-
Red blood cell mass
- RWV:
-
Rotating wall vessel
- SJ-10 satellite:
-
SJ-10 recoverable microgravity experimental satellite
- SL-3:
-
Spacelab 3
- SLS-1:
-
Splace lab Life Science 1
- ST-HSCs:
-
Short-term self-renewing HSCs
- STS-40:
-
Space Shuttle Orbiter Columbia
- WBC:
-
White blood cell
References
Allebban Z, Ichiki AT, Gibson LA et al (1994) Effects of spaceflight on the number of rat peripheral blood leukocytes and lymphocyte subsets. J Leukoc Biol 55(2)a: 209–213
Anderson KL, Smith KA, Conners K et al (1998) Myeloid development is selectively disrupted in PU.1 null mice. Blood 91(10): 3702–3710
Antonchuk J, Sauvageau G, Humphries RK (2002) HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell 109(1):39–45
Bishop AE, Buttery LD, Polak JM (2002) Embryonic stem cells. J Pathol 197(4):424–429
Blaber E, Marcal H, Burns BP (2010) Bioastronautics: the influence of microgravity on astronaut health. Astrobiology 10(5):463–473
Bradford GB, Williams B, Rossi R et al (1997) Quiescence, cycling, and turnover in the primitive hematopoietic stem cell compartment. Exp Hematol 25(5):445–453
Bromleigh VC, Freedman LP (2000) p21 is a transcriptional target of HOXA10 in differentiating myelomonocytic cells. Genes & Dev 14(20):2581–2586. https://doi.org/10.1101/Gad.817100
Brun AC, Bjornsson JM, Magnusson M et al (2004) Hoxb4-deficient mice undergo normal hematopoietic development but exhibit a mild proliferation defect in hematopoietic stem cells. Blood 103(11):4126–4133
Cabrita GJ, Ferreira BS, da Silva CL et al (2003) Hematopoietic stem cells: from the bone to the bioreactor. Trends Biotechnol 21(5):233–240
Cheng T, Rodrigues N, Shen H et al (2000) Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287(5459):1804–1808
Cheshier SH, Morrison SJ, Liao X et al (1999) In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci USA 96(6):3120–3125
Chiu B, Wan JZ, Abley D et al (2005) Induction of vascular endothelial phenotype and cellular proliferation from human cord blood stem cells cultured in simulated microgravity. Acta Astronaut 56(9–12):918–922
Chung YJ, Park BB, Kang YJ et al (2006) Unique effects of Stat3 on the early phase of hematopoietic stem cell regeneration. Blood 108(4):1208–1215
Cobas M, Wilson A, Ernst B et al (2004) Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J Exp Med 199(2):221–229
Crucian BE, Stowe RP, Pierson DL et al (2008) Immune system dysregulation following short- vs long-duration spaceflight. Aviat Space Environ Med 79(9):835–843
Davis TA, Wiesmann W Kidwell W et al (1996) Effect of spaceflight on human stem cell hematopoiesis: suppression of erythropoiesis and myelopoiesis. J Leukoc Biol 60(1):69–76
DeKoter RP, Singh H (2000) Regulation of B lymphocyte and macrophage development by graded expression of PU.1. Science 288(5470):1439–1441
DeKoter RP, Walsh JC, Singh H (1998) PU.1 regulates both cytokine-dependent proliferation and differentiation of granulocyte/macrophage progenitors. EMBO J 17(15):4456–4468
Domaratskaya EI, Michurina TV, Bueverova EI et al (2002) Studies on clonogenic hemopoietic cells of vertebrate in space: problems and perspectives. Adv Space Res 30(4):771–776
Drexler HG, Meyer C, Zaborski M et al (1998) Growth-inhibitory effects of transforming growth factor-beta 1 on myeloid leukemia cell lines. Leuk Res 22(10):927–938
Duncan AW, Rattis FM, DiMascio LN et al (2005) Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol 6(3):314–322
Elwood NJ, Zogos H, Pereira DS et al (1998) Enhanced megakaryocyte and erythroid development from normal human CD34(+) cells: consequence of enforced expression of SCL. Blood 91(10):3756–3765
Evans CA, Pierce A, Winter SA et al (1999) Activation of granulocyte-macrophage colony-stimulating factor and interleukin-3 receptor subunits in a multipotential hematopoietic progenitor cell line leads to differential effects on development. Blood 94(5):1504–1514
Fischer CL, Johnson PC, Berry CA (1967) Red blood cell mass and plasma volume changes in manned space flight. J Am Med Assoc 200(7):579
Forsberg EC, Bhattacharya D, Weissman IL (2006) Hematopoietic stem cells: expression profiling and beyond. Stem Cell Rev 2(1):23–30
Graebe A, Schuck EL, Lensing P et al (2004) Physiological, pharmacokinetic, and pharmacodynamic changes in space. J Clin Pharmacol 44(8):837–853
Grigor’ev AI (2007) Physiological problems of manned mission to Mars. Ross Fiziol Zh Im I M Sechenova 93(5):473–484
Hans RS (2007) The potential of stem cells: an inventory. In: Human biotechnology as social challenge, England. Ashgate Publishing, Ltd., p 28
Hao SX, Ren R (2000) Expression of interferon consensus sequence binding protein (ICSBP) is downregulated in Bcr-Abl-induced murine chronic myelogenous leukemia-like disease, and forced coexpression of ICSBP inhibits Bcr-Abl-induced myeloproliferative disorder. Mol Cell Biol 20(4):1149–1161
Heyworth C, Gale K, Dexter M, May G, Enver T (1999) A GATA-2/estrogen receptor chimera functions as a ligand-dependent negative regulator of self-renewal. Genes & Dev 13(14):1847–1860. https://doi.org/10.1101/gad.13.14.1847
Hock H, Hamblen MJ, Rooke HM et al (2004a) Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature 431(7011):1002–1007
Hock H, Meade E, Medeiros S et al (2004b) Tel/Etv6 is an essential and selective regulator of adult hematopoietic stem cell survival. Genes Dev 18(19):2336–2341
Hodgson GS, Bradley TR (1979) Properties of haematopoietic stem cells surviving 5-fluorouracil treatment: evidence for a pre-CFU-S cell? Nature 281(5730):381–382
Holtschke T, Lohler J, Kanno Y et al (1996) Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell 87(2):307–317
Hughes-Fulford M, Chang TT, Martinez EM et al (2015) Spaceflight alters expression of microRNA during T-cell activation. FASEB J 29(12):4893–4900
Hwang SA, Crucian B, Sams C et al (2015) Post-spaceflight (STS-135) mouse splenocytes demonstrate altered activation properties and surface molecule expression. PLoS ONE 10(5):e0124380
Ichiki AT, Gibson LA, Jago TL et al (1996) Effects of spaceflight on rat peripheral blood leukocytes and bone marrow progenitor cells. J Leukoc Biol 60(1):37–43
Iliukhin AV, Burkovskaia TE (1981) Cytokinetic evaluation of erythropoiesis on prolonged orbital flights. Kosm Biol Aviakosm Med 15(6):42–46
Ilyin EA, Serova LV, Portugalov VV et al (1975) Preliminary results of examinations of rats after a 22-day flight aboard the Cosmos-605 biosatellite. Aviat Space Environ Med 46(3):319–321
Iwama A, Oguro H, Negishi M et al (2004) Enhanced self-renewal of hematopoietic stem cells mediated by the polycomb gene product Bmi-1. Immunity 21(6):843–851
Iwasaki H, Somoza C, Shigematsu H et al. (2005) Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood 106(5):1590–1600
Johnson PC, Kimzey SL, Driscoll TB (1975) Postmission plasma volume and red-cell mass changes in the crews of the first two Skylab missions. Acta Astronaut 2(3–4):311–317
Kato Y, Iwama A, Tadokoro Y et al (2005) Selective activation of STAT5 unveils its role in stem cell self-renewal in normal and leukemic hematopoiesis. J Exp Med 202(1):169–179
Kelly LM, Englmeier U, Lafon I et al (2000) MafB is an inducer of monocytic differentiation. EMBO J 19(9):1987–1997
Kondo M, Scherer DC, Miyamoto T et al (2000) Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature 407(6802):383–386
Kozinets GI, Korol’kov VI, Britvan II et al (1983) Morphofunctional properties of the peripheral blood and bone marrow cells of rats following a flight on board the Kosmos-936 biosatellite. Kosm Biol Aviakosm Med 17(2):61–65
Kraemer WJ, Mastro AM, Gordon SE et al (2004) Responses of plasma proenkephalin peptide F in rats following 14 days of spaceflight. Aviat Space Environ Med 75(2):114–117
Krause DS, Fackler MJ, Civin CI et al (1996) CD34: structure, biology, and clinical utility. Blood 87(1):1–13
Krishnaraju K, Hoffman B, Liebermann DA (2001) Early growth response gene 1 stimulates development of hematopoietic progenitor cells along the macrophage lineage at the expense of the granulocyte and erythroid lineages. Blood 97(5):1298–1305
Krystal G, Lam V, Dragowska W et al (1994) Transforming growth factor beta 1 is an inducer of erythroid differentiation. J Exp Med 180(3):851–860
Lange RD, Andrews RB, Gibson LA et al (1987) Hematological measurements in rats flown on Spacelab shuttle, SL-3. Am J Physiol 252(2 Pt 2):R216–R221
Lange RD, Gibson LA, Driscoll TB et al (1994) Effects of microgravity and increased gravity on bone marrow of rats. Aviat Space Environ Med 65(8):730–735
Leon HA, Serova LV, Cummins J et al (1978) Alterations in erythrocyte survival parameters in rats after 19.5 days aboard Cosmos 782. Aviat Space Environ Med 49(1 Pt 1):66–69
Lessard J, Sauvageau G (2003) Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 423(6937):255–260
Long XX, Zhong TY, Ping BH (2011) Impacts of simulated microgravity on proliferation of K562 Cell. Chin J Microcirc 1:010
Mancini SJ, Mantei N, Dumortier A et al (2005) Jagged1-dependent Notch signaling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood 105(6):2340–2342
Matsumura I, Kawasaki A, Tanaka H et al (2000) Biologic significance of GATA-1 activities in Ras-mediated megakaryocytic differentiation of hematopoietic cell lines. Blood 96(7):2440–2450
Michurina TV, Domaratskaya EI, Nikonova TM et al (1996) Blood and clonogenic hemopoietic cells of newts after the space flight. Adv Space Res 17(6–7):295–298
Moore KA, Lemischka IR (2006) Stem cells and their niches. Science 311(5769):1880–1885
Nash PV, Mastro AM (1992) Variable lymphocyte responses in rats after space flight. Exp Cell Res 202(1):125–131
Nerlov C, Graf T (1998) PU.1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev 12(15):2403–2412
Nutt SL, Metcalf D, D’Amico A et al (2005) Dynamic regulation of PU.1 expression in multipotent hematopoietic progenitors. J Exp Med 201(2):221–231
Park IK, Qian D, Kiel M et al (2003) Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423(6937):302–305
Passegue E, Wagers AJ, Giuriato S et al (2005) Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med 202(11):1599–1611
Paulsen K, Tauber S, Dumrese C et al (2015) Regulation of ICAM-1 in cells of the monocyte/macrophage system in microgravity. Biomed Res Int 2015:538786
Pawlak G, Grasset MF, Arnaud S et al (2000) Receptor for macrophage colony-stimulating factor transduces a signal decreasing erythroid potential in the multipotent hematopoietic EML cell line. Exp Hematol 28(10):1164–1173
Plett PA, Frankovitz SM, Abonour R et al (2001) Proliferation of human hematopoietic bone marrow cells in simulated microgravity. Vitro Cell Dev Biol Anim 37(2):73–78
Plett PA, Abonour R, Frankovitz SM et al (2004) Impact of modeled microgravity on migration, differentiation, and cell cycle control of primitive human hematopoietic progenitor cells. Exp Hematol 32(8):773–781
Reya T, Morrison SJ, Clarke MF et al (2001) Stem cells, cancer, and cancer stem cells. Nature 414(6859):105–111
Reya T, Duncan AW, Ailles L et al (2003) A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423(6938):409–414
Rippon HJ, Bishop AE (2004) Embryonic stem cells. Cell Prolif 37(1):23–34
Sauvageau G, Iscove NN, Humphries RK (2004) In vitro and in vivo expansion of hematopoietic stem cells. Oncogene 23(43):7223–7232
Schmidt M, Nagel S, Proba J et al (1998) Lack of interferon consensus sequence binding protein (ICSBP) transcripts in human myeloid leukemias. Blood 91(1):22–29
Schwarz RP, Goodwin TJ, Wolf DA (1992) Cell culture for three-dimensional modeling in rotating-wall vessels: an application of simulated microgravity. J Tissue Cult Methods 14(2):51–57
Scott EW, Simon MC, Anastasi J et al (1994) Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science 265(5178):1573–1577
Seita J, Weissman IL (2010) Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med 2(6):640–653
Shvets VN, Vatsek A, Kozinets GI et al (1984) Hemopoietic status of rats exposed to weightlessness. Kosm Biol Aviakosm Med 18(4):12–16
Sonnenfeld G (2002) The immune system in space and microgravity. Med Sci Sports Exerc 34(12):2021–2027
Sonnenfeld G, Mandel AD, Konstantinova IV et al (1990) Effects of spaceflight on levels and activity of immune cells. Aviat Space Environ Med 61(7):648–653
Sonnenfeld G, Mandel AD, Konstantinova IV et al (1992) Spaceflight alters immune cell function and distribution. J Appl Physiol (1985) 73(2 Suppl):191S–195S
Stier S, Cheng T, Dombkowski D et al (2002) Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood 99(7):2369–2378
Stowe RP, Sams CF, Pierson DL (2011) Adrenocortical and immune responses following short- and long-duration spaceflight. Aviat Space Environ Med 82(6):627–634
Sytkowski AJ, Davis KL (2001) Erythroid cell growth and differentiation in vitro in the simulated microgravity environment of the NASA rotating wall vessel bioreactor. Vitro Cell Dev Biol Anim 37(2):79–83
Tamura T, Mancini A, Joos H, Koch A, Hakim C, Dumanski J et al (1999) FMIP, a novel Fms-interacting protein, affects granulocyte/macrophage differentiation. Oncogene 18(47):6488–6495. https://doi.org/10.1038/sj.onc.1203062
Tamura T, Nagamura-Inoue T, Shmeltzer Z, Kuwata T, Ozato K (2000) ICSBP directs bipotential myeloid progenitor cells to differentiate into mature macrophages. Immunity 13(2):155–165
Tauber S, Lauber BA, Paulsen K et al (2017) Cytoskeletal stability and metabolic alterations in primary human macrophages in long-term microgravity. PLoS ONE 12(4):e0175599
Taylor GR, Neale LS, Dardano JR (1986) Immunological analyses of U.S. Space Shuttle crewmembers. Aviat Space Environ Med 57(3):213–217
Taylor GR, Konstantinova I, Sonnenfeld G et al (1997) Changes in the immune system during and after spaceflight. Adv Space Biol Med 6:1–32
Tsao YD, Goodwin TJ, Wolf DA et al (1992) Responses of gravity level variations on the NASA/JSC bioreactor system. Physiologist 35(1 Suppl):S49–S50
Udden MM, Driscoll TB, Gibson LA et al (1995) Blood volume and erythropoiesis in the rat during spaceflight. Aviat Space Environ Med 66(6):557–561
Vacek A, Serova LV, Rotkovska D et al (1985) Changes in the number of haemopoietic stem cells (CFUs) in bone marrow and spleens of pregnant rats after a short space flight onboard the Cosmos-1514 biosatellite. Folia Biol (Praha) 31(5):361–365
Vacek A, Bueverova EI, Michurina TV et al (1990) Decrease in the number of progenitors of fibroblasts (CFUf) in bone marrow of rats after a 14-day flight onboard the Cosmos-2044 biosatellite. Folia Biol (Praha) 36(3–4):194–197
Vacek A, Michurina TV, Serova LV et al (1991) Decrease in the number of progenitors of erythrocytes (BFUe, CFUe), granulocytes and macrophages (GM-CFC) in bone marrow of rats after a 14-day flight onboard the Cosmos-2044 Biosatellite. Folia Biol (Praha) 37(1):35–41
Vernikos J (1996) Human physiology in space. BioEssays 18(12):1029–1037
Wichman HA (2005) Behavioral and health implications of civilian spaceflight. Aviat Space Environ Med 76(6 Suppl):B164–B171
Willert K, Brown JD, Danenberg E et al (2003) Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423(6938):448–452
Williams D, Kuipers A, Mukai C et al (2009) Acclimation during space flight: effects on human physiology. CMAJ 180(13):1317–1323
Wilson A, Murphy MJ, Oskarsson T et al (2004) c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev 18(22):2747–2763
Yi ZC, Xia B, Xue M et al (2009) Simulated microgravity inhibits the proliferation of K562 erythroleukemia cells but does not result in apoptosis. Adv Space Res 44(2):233–244
Yu H, Yuan Y, Shen H et al (2006) Hematopoietic stem cell exhaustion impacted by p18 INK4C and p21 Cip1/Waf1 in opposite manners. Blood 107(3):1200–1206
Yuan Y, Shen H, Franklin DS et al (2004) In vivo self-renewing divisions of haematopoietic stem cells are increased in the absence of the early G1-phase inhibitor, p18INK4C. Nat Cell Biol 6(5):436–442
Zeng H, Yucel R, Kosan C et al (2004) Transcription factor Gfi1 regulates self-renewal and engraftment of hematopoietic stem cells. EMBO J 23(20):4116–4125
Zhang P, Zhang X, Iwama A et al (2000) PU.1 inhibits GATA-1 function and erythroid differentiation by blocking GATA-1 DNA binding. Blood 96(8):2641–2648
Zou LX, Cui SY, Zhong J et al (2011) Upregulation of erythropoietin receptor in UT-7/EPO cells inhibits simulated microgravity-induced cell apoptosis. Adv Space Res 48(2):390–394
Acknowledgements
The authors sincerely thank Dr. Shujin Sun from Institute of Mechanics, Chinese Academy of Sciences for free helping and supporting in the supply of microgravity instruments, and Prof. Enkui Duan and Dr. Xiaohua Lei from Institute of Zoology, Chinese Academy of Sciences for valuable opinions and suggestions in the design of our experiment and manuscript. The authors also appreciated Dr. Lu Shi from Institute of Zoology, Chinese Academy of Sciences for proofreading and editing our manuscript. This work was supported by grant from the National Natural Science Foundation of China (NSFC U1738111).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Science Press and Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Wang, P., Qian, J., Tian, H., Zhao, Y. (2019). The Maintaining and Directed Differentiation of Hematopoietic Stem Cells Under Microgravity. In: Duan, E., Long, M. (eds) Life Science in Space: Experiments on Board the SJ-10 Recoverable Satellite. Research for Development. Springer, Singapore. https://doi.org/10.1007/978-981-13-6325-2_9
Download citation
DOI: https://doi.org/10.1007/978-981-13-6325-2_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-6324-5
Online ISBN: 978-981-13-6325-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)