Abstract
In the last few decades, investigation on ecohydrological interaction including biogeochemical characteristics has been an important research topic in hydrology due to its role in natural resource management. Despite the research focus on this subject, quantifying the geochemical processes controlling the moisture flow and solute transport remains awaited especially under climate change conditions. At the same time, the wastewater treated in conventional wastewater treatment systems are reported with the inefficient removal of many emerging contaminants particularly in rural areas and remote communities in low socioeconomic conditions. As a result, in many instances, the wastewater may get disposed without appropriate advance treatment that further contaminate the soil-water system. Thus, there are urgent needs of the research to assess the risk posed to groundwater and to develop improved knowledge frame of hydrologic and biogeochemical processes and of geologic features controlling contaminant migration in the subsurface. Therefore, the main focus of this chapter is to present the different biogeochemical processes controlling reuse potential of treated wastewater in the subsurface under climate change conditions. The different geochemical process involved during the fate and transport in the subsurface are clearly elaborated and exemplified. Further, the role of varying climatic conditions on biogeochemical makeup and transport is discussed thoroughly. A state of the art of the different aspects of modeling and practical approaches to quantify the governing biogeochemical processes in the subsurface is reviewed comprehensively. Finally, the methodological framework is charted on the basis of the technical and socioeconomic aspect to implement the potential reuse of wastewater and remedial measures in the field. The outcomes of this chapter are of direct use in applying remediation technique in the field and for the decision-making related to the planning of (waste) water under varying environmental conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Treated wastewater
- Contaminant transport
- Subsurface Environment
- Bioremediation
- Groundwater resources
- Climate change
8.1 Introduction
Subsurface pollution has become a global concern due to the rapid growth of industrialization, urbanization, and modern development under climate change conditions (Gupta and Joshi 2017; Gupta et al. 2013, 2018a, b). Industries are key performers in the economy of developing countries but are also considered as one of the major polluters due to disposal of huge amount of untreated/partially treated wastewater into the environment, which creates serious soil and water pollution and causes severe toxic effects in living beings (Mustapha et al. 2018). The wastewater discharged from various industries carry various organic and inorganic pollutants, which are used in the processing of raw materials to obtain a good quality of products within a short period of time and in an economic way; however, their toxicity is usually ignored that poses a serious challenge for the safety of the environment and human health. Further, technological innovations in industries have given rise to new products and new pollutants in abundant level, which is above the self-cleaning capacity in the environment (Gupta and Sharma 2018; Ranjan et al. 2008).
A large number of impoverished communities around the world face challenges with respect to treatment of sewage and domestic wastewater. In particular, the rural areas and remote communities in low socioeconomic conditions may lack conventional centralized wastewater treatment systems. As a result, in many instances, the wastewater may get disposed without appropriate treatment that contaminates drinking water resources. Even if the communities choose a low-cost system for primary treatment, such as waste stabilization ponds that do not require much operation cost and skilled supervision, the existing wastewater does not undergo traditional secondary treatment for considerable reduction in biodegradable organic material, PPCPs, pathogens, nutrients, etc. from the wastewater. Treatment of wastewater is expensive, and in developing countries, the infrastructure for domestic wastewater treatment prior to disposal is poor in small cities and may be nonexistent in the rural and other remote communities. In developing nations, the discharge of untreated wastewater is the main cause for widespread pollution of surface and groundwater resources since there is often a large gap between generation and treatment of domestic wastewater. For example, according to a recent report (CPCB, India 2009), out of ~38 billion L/day of sewage/wastewater generated, treatment capacity exists for only ~12 billion L/day in India. The problem has reached a crisis proportion as both rural and urban communities may be suffering from pathogen-related health issues (Suthar et al. 2009) due to contamination of precious drinking water sources.
The land irrigated with (un)-treated wastewater containing emerging pollutants including PPCPs, biosolids, and petroleum hydrocarbons, during fertigation, has deteriorated large volumes of soil and groundwater (Clement et al. 2000). These emerging pollutants infiltrate into the subsurface along with soil moisture; it will move into the partially saturated zone. The advective-dispersive mechanisms cause spreading of these pollutants in the partially saturated zone and creates a redox environment in and around the polluted subsurface zones (Christensen et al. 2000). The redox environment controls the microbial numbers, diversity, and activity, which may decrease the natural attenuation of several emerging pollutants. Similarly, adsorption-desorption and or (de)attachment causes the alternation in biogeochemical behaviors of these pollutants in the subsurface.
Impacts of climate change on subsurface water quality are not investigated thoroughly, and very few studies were found relevant. Green et al. (2011a, b) showed that the indirect impacts of climate change are more likely to affect subsurface water quality issues. The geochemical makeup of subsurface environment is under direct influences of the surface or atmospheric conditions; thus, the variations in ambient temperatures, precipitations, stream flow, and other dominating variables affect the subsurface water quality (Klein and Nicholls 1999; Sharif and Singh 1999; Pierson et al. 2001; Ranjan et al. 2006; IPCC 2007a, b). At the same time, the pollution due to the release of several pollutants is a major concern to subsurface water quality. Natural attenuation of these pollutants is likely to be affected by environmental variability as microorganisms present in subsurface work differently under various environmental conditions. Further, rapid groundwater table fluctuations along with high pore water velocities are expected in shallow aquifers under climate change conditions (Dobson et al. 2007a, b) which ultimately affect subsurface water resources. Changing groundwater flow velocity and groundwater table dynamics causes stronger advective transport and enhances the dissolution rate of immiscible pollutants.
Thus, the objective of this book chapter is to present the subsurface processes controlling reuse potential of treated wastewater under climate change conditions. The information of this chapter will help in management and remediation of polluted sites under site prevailing conditions.
8.2 Treated Wastewater: As a Source of Subsurface Pollutants
The application of treated wastewater gains more attention due to water scarcity in most of the parts of the world and water conservation being so important at the present time (Kinney et al. 2008).The largest source of marginal water for agriculture is treated wastewater in many areas. Likewise, in urban areas, most of the treated wastewater is used for garden irrigation. Wastewater does not undergo higher treatment for considerable reduction in biodegradable organic material, TSS, salts (mainly Na, Cl, and bicarbonates), nutrients, microelements, emerging pollutants like pharmaceuticals and personal care products (PPCPs), nanoparticles, pathogens, etc. from the wastewater. Thus, higher quantities of these pollutants in the treated water than in fresh water are reported in most of the literature (Bernstein et al. 2006). To understand the suitability of treated wastewater in fertigation, a comparative account of physicochemical characteristics is presented in this section.
8.2.1 Electrical Conductivity (EC)
The US EPA recommended EC as a significant parameter for reclaimed water quality in different fields such as agriculture and industrial reuse (US EPA 2004). Treated wastewater from municipal and industrial treatment plants, especially pulp and paper industries, has been shown to have high concentrations of ions, which subsequently increases the EC. The high value of EC in treated wastewater than freshwater was reported in the literature. Bernstein et al. 2006 show that the EC value of treated wastewater was 2.0–2.5 dS m−1, which was higher than the potable water. The higher EC values in treated wastewater due to insufficient equalization of wastewater during primary/secondary treatment lead to less removal of total dissolved solids.
8.2.2 pH, Salt Composition, and Chemical Oxygen Demand (COD)
The low performances of conventional treatment resulted in highly dissolved organic matters and salt contents in treated wastewater, which altered the salt composition and pH values too. Generally, the high values of salt concentration and pH were found in previous studies. The highly dissolved organic matters containing treated wastewater resulted in high chemical oxygen demand. Bernstein et al. (2006) reported 86 mg/l of COD in treated wastewater. Similarly, Kushwah et al. (2012) show that the COD varied from 346.4–792.4 mg/l in the influent wastewater and 182.6–86.4 mg/l in the final treated wastewater at Kotra wastewater treatment plant. The pH, salt concentration, and COD of treated wastewater vary with sources of influent wastewater which originated from different industries.
8.2.3 Hydrocarbon Contaminants
Wastewater from petroleum refining and petrochemical industries contain BTEX compounds, polyaromatic hydrocarbons (PAHs), oil and grease, large amounts of suspended particulate matter, sulfides, ammonia, and phenol (Tobiszewski et al. 2012; Mustapha et al. 2015). These discharges are one of the major environmental hazards to human and animals (Seeger et al. 2011). Furthermore, oily wastewater can lead to the loss of biodiversity, destruction of breeding habitats of aquatic organisms, and hazard to the biota including humans (Mustapha et al. 2011). Traditional physicochemical, mechanical, and biological technologies cannot effectively treat the effluents from petroleum industries to meet strict effluent discharge regulations (Saien and Shahrezaei 2012; Wu et al. 2015).
8.2.4 Trace Elements
Research on the fate of heavy metals in treated wastewater shows significant residual occurrence due to low removal efficiencies of many trace elements by secondary as well as advance treatments (Karvelas et al. 2003; Sharma et al. 2007; Chary et al. 2008). Karvelas et al. (2003) investigated occurrence and fate of heavy metals in the wastewater treatment processes and found 47–63% of Cd, Cr, Pb, Fe, Ni, and Zn remains in treated wastewater effluent. Similarly, Sharma et al. (2007) investigated heavy metal contamination of soil resulting from wastewater irrigation in suburban areas of Varanasi, India. The results indicate that concentration of Cd was higher than the permissible limits of the Indian standard during summer, whereas Pb and Ni concentrations were higher in both summer and winter seasons. Furthermore, bioaccumulation/biomagnification enhanced further concentration in biomass. Thus, the reuse potential of wastewater depends on the concentration of these trace metals as well as their bioaccumulation/biomagnification rates (Anitha et al. 2012).
8.2.5 Emerging Pollutants
Emerging pollutants includes pharmaceuticals, and personal care products (PPCPs) designed to have biological effects even at low concentration and concern to cause ecological adverse effects. The main source of these pollutants is (un)-treated wastewater discharged from wastewater treatment plants/sewage treatment plants (WTPs/STPs) and land application of biosolids (Chefetz et al. 2008; Caliman and Gavirilescu 2009). Okuda et al. (2008) evaluated the removal efficiency of 66 pharmaceuticals during wastewater treatment process including conventional activated sludge (CAS) and biological nutrient removal (BNR) processes (Ebele et al. 2017). They show that the 30–80% removal efficiency was achieved, in which removal efficiencies of carbamazepine and crotamiton were less than 30% during wastewater treatment processes. Arye et al. (2010) reported about 50% removal of carbamazepine and other pharmaceuticals in soil irrigated with treated wastewater. These studies show that a lot of emerging pollutants remain in treated wastewater even after complete advance treatment processes. Thus, the land irrigated with (un)-treatment wastewater having a high risk of further contamination by emerging pollutants.
8.2.6 Microbial and Colloids/Nanoscale Pollutants
Treatment of wastewater is expensive, and in developing countries, the infrastructure for domestic wastewater treatment prior to disposal is poor in small cities and may be nonexistent in the rural and other remote communities. In developing nations, the discharge of untreated wastewater is the main cause for widespread pollution of surface and groundwater resources since there is often a large gap between generation and treatment of domestic wastewater. For example, according to a recent report (CPCB, India 2009), out of ~38 billion L/day of sewage/wastewater generated, treatment capacity exists for only ~12 billion L/day in India. The problem has reached a crisis proportion as both rural and urban communities may be suffering from pathogen-related health issues (Suthar et al. 2009). The pathogens of the major threat to the ecological health are viruses and the protozoa. The cryptosporidium and Giardia are well-known protozoa contaminates. Viruses are the most critical for the groundwater among the microbiological contamination; they are much smaller in size than others and are not filtrated out to the same extent in the porous soil matrix. Rose et al. (1996) reported that water from land irrigated with wastewater contains a significant number of pathogens including viruses and colloids. Likewise, Levantesi et al. (2010) quantify the pathogenic microorganisms at two wastewater reclamation sites and found a high concentration of Salmonella gene copies, Clostridium spores, and Giardia cysts in treated wastewater. Thus, the reuse potential of treated wastewater depends upon the degree of occurrences of these pollutants and their biochemical activation and deactivation characteristics.
Other than nZVI, the nanoparticles tested for remediation purpose are metal and their oxides (Ag, TiO2, ZrO2) and carbon-based nanomaterials (EPA 2008; Karn et al. 2009; Watlington 2005). TiO2 nanoparticles can serve both as reductive and oxidative catalysts and have been explored for the photo degradation of various pollutants that includes dyes, inorganic pollutants, and organic pollutants (Savage and Diallo 2005). After the discovery of carbon nanotubes (CNT) in 1991, its application in water purification has attracted great attention. Carbon nanotubes are basically the graphene sheets in tube form and that can be single-walled or double-walled depending upon the manufacturing conditions (Li et al. 2003).
8.3 Fate and Transport of Emerging Pollutants in Subsurface
The release of (un)-treated wastewater containing emerging pollutants including PPCPs, biosolids, and petroleum hydrocarbons, during fertigation, has deteriorated large volumes of soil and groundwater (Clement et al. 2000). If agriculture land irrigated with treated wastewater, emerging pollutants infiltrate into the subsurface along with soil moisture, it will move into the partially saturated zone (Gharaibeh et al. 2007). Because water and air already make a two-fluid system in the partially saturated soil-water system, the introduction of second liquid results in a three-fluid porous-medium system. In two-fluid systems, two fluids and one solid, and between these three substances, form three possible interface combinations: interfaces between the two fluids, interfaces between one fluid and the solid, and interfaces between a second fluid and the solid. When three fluids are present, there are six possible interface types (interface pairs), so the system becomes quite complex. An additional complication to the overall multi-fluid problem is the observation that immiscible organic fluid is often composed of a number of components. The multi-fluid porous-media systems are characterized by a solid phase within which interconnected pore space allows fluids to flow along with nonaqueous liquids, like petrochemical products. For example, gasoline has a number of components in it, some of which are lighter fractions like benzene, toluene, ethylbenzene, and xylene (BTEX).
In partially saturated zones, pollutants fractionalized and cause partitioning into air phase, aqueous phase, solid phase, and pure phase itself. The remaining mass of pollutants moves downward through the partially saturated zone, in which lighter pollutants like LNAPLs are generally retained by the water table due to their lighter density to water, while dense pollutants like DNAPLs penetrate the water table and move downward till they are retained by an impermeable layer (USEPA 1995). At the water table, LNAPL starts dissolving with groundwater and subsequently moves to surrounding downgradient locations due to advection, diffusion, and dispersion mechanisms of mass transport (Dobson et al. 2007a, b; Powers et al. 1991).
Advection, dispersion, and liquid diffusion contribute to contaminant spreading in the water-saturated area, with the last being the slowest process. Advective transport driven by groundwater flow depends on groundwater velocity. Mechanical dispersion causes migration in both a parallel as well as in a perpendicular direction with respect to the main groundwater flow direction. Dispersion dominates over diffusion at groundwater velocities higher than 0.1 m day-1 (McCarthy and Johnson 1993). Diffusion, driven by solute concentration gradients over space, may dominate at low groundwater velocities.
Let us consider the subsurface contaminant mass balance in an elementary root zone layer of Δz thickness shown in Fig. 8.1. The contaminant influx into the layer is denoted by J(z) (mg per unit area per unit time) [ML−2S−1], and the outflux from the layer is J(z + Δz). S C is the sink term denoting the amount of solute taken up by plant roots and degradation of the contaminant by microbial biomass [ML−3S−1]. Then, the change in total contaminant concentration C t over time t can be written as:
Diffusive Flux
Diffusion is mathematically described by Fick’s law as the net rate of contaminant transport proportional to the negative gradient of its concentration and can be modified for the unsaturated porous medium as:
where τ is the tortuosity factor (dimensionless) which accounts for the increased distance of transport due to tortuous path of the solute particle in a porous media. D o and D m are the free-water diffusivity and molecular diffusion coefficients, respectively [L2 T−1].
Dispersive Flux
The dispersion at a microscopic scale occurs due to the variation of velocity within the pores and due to the tortuous movement of the fluid around the soil grains. Macroscopic dispersion refers to the dispersion resulting from the interfingering of materials of different permeability. Mechanical dispersion is mathematically described in the same way as molecular diffusion by using the Fick’s law as:
where α L is the longitudinal dispersivity of the porous media in the direction of flow [L] and v is the pore velocity. Now by adding all the abovementioned fluxes, we get the resultant flux as in Eq. 8.4.
where \( D=\tau {D}_{\mathrm{O}}+{\alpha}_L\frac{q}{\theta }=\tau {D}_{\mathrm{O}}+{\alpha}_{\mathrm{L}}\nu \) here; D is the diffusion-dispersion or hydrodynamic dispersion coefficient, which is the pore water velocity-dependent function [L2 T−1]. The modified form of advection-dispersion as:
Biodegradation/Natural Attenuation
A general expression of organic contaminants like PPCPs, hydrocarbons depletion in soil, in which microbial densities and contaminant concentration determine the degradation kinetics, can be written as (Yadav and Hassanizadeh 2011):
where μ max is the maximum growth rate, C is the contaminant concentration at time t, C 0 is the initial contaminant concentration, X 0 corresponds to the contaminant required to produce initial microbial density, and K s is the half saturation constant also known as a growth-limiting concentration. The above equation reflects a linear relationship for changes in microbial density and the nonlinear relationship of changes in contaminant concentration on the rate of contaminant degradation. Furthermore, different simplified degradation kinetic models can be approximated considering extreme ratios of initial contaminant concentration (C 0) to K s or initial microbial densities (X 0) to C 0 in Eq. 8.6.
Adsorption/Desorption
Adsorption/desorption is an important geochemical process controlling the fate and transport of pollutants in the subsurface. The solid phase mass partition, i.e., adsorption is governed by:
where S is the mass of adsorbed contaminants, C is the mass of aqueous phase, and K d is the distribution coefficient product of organic carbon partitioning coefficient (K oc) and organic carbon content of the soil f oc as:
The retardation factor (R) is mainly used to estimate the adsorption in subsurface, and it is calculated using following equation:
where R, retardation factor; ρ b , soil bulk density (g/cm3); n, porosity; and K d, soil distribution coefficient.
Volatilization
Mass transfer between the air, water, or pure phases, commonly known as volatilization, is an important geochemical process controlling the fate and transport of emerging pollutants in the subsurface. Between air and water phases, Henry’s law is used to estimate the volatilization rate, given by:
where p is the partial pressure of the contaminant in the gas phase, k H is Henry’s law constant (a function of temperature), and C w is the aqueous concentration of the contaminant.
8.4 Subsurface Processes Controlling Reuse Potential of Treated Wastewater
8.4.1 Redox Environment and Redox Buffering
Sufficient organic matter and other reduced pollutants introduced in subsurface due to heavy irrigation using wastewater create a redox environment in and around the polluted subsurface zones (Christensen et al. 2000). The redox system of contaminant plumes which originated from the application of (un)-treated wastewater in subsurface may involve gases (O2, N2, CH4, CO2), and dissolved components (NO3 −, NH4 +, CH2O, Fe2+, Mn2+, SO4 2−, HS−, H+) as well as solids (FeOOH, MnO2) and components Fe2+, Mn2+, NH4 + associated with the solids by ion exchange. The redox conditions of a contaminant plume constitute an important part of the chemical framework controlling the behavior of the contaminants in the plume. The redox conditions developed in plume zone is dominating by O2 reduction by aerobic respiration/denitrification; iron (III), Mn (IV), and sulfate reduction; and methanogenic production as shown in Fig. 8.2. Within this zone and downgradient from it, sulfate reduction may take place. Iron reduction takes place further downgradient, where the conditions are less reducing (Albrechtsen and Christensen 1994). Zones of manganese and nitrate reduction have been observed, sometimes overlapping the iron-reducing zone. Finally, aerobic conditions may exist in the outskirts of the reduced plume, if the pristine aquifer is oxidized and contains significant amounts of dissolved oxygen (Barcelona and Holm 1991). In this process, the hydrolysis of the organic pollutant takes places, which produces the sufficient amount of CO2 (Borden et al. 1995).
The set of reactions that create the complex redox environment of contaminants plumes originated from the application of wastewater consists of combinations of two half-reactions: oxidation half-reaction and reduction half-reaction. During organic matter oxidation, it is evident that when all electron acceptors are present, oxygen will be used first, followed by nitrate, manganese, iron, and sulfate. Finally, methanogenesis and fermentation reactions dominate, when the most favorable electron acceptors are depleted. The numbers, diversity, and ability of microorganisms to attenuate different organic substrates are reduced under these conditions. Therefore, the natural attenuation of several emerging pollutants is reported to be quite slow under redox conditions. Thus, redox environment which originated from the application of (un)-treated wastewater may lead high risk and vulnerability of subsurface natural resource pollution.
8.4.2 Adsorption/Desorption Processes
Sorption and desorption are the major processes influencing the fate (uptake, biodegradation, chemical degradation, photodegradation, and mobility) of organic contaminants in the environment (Petersen et al. 1996; Jeribi et al. 2002; Sparks 2003). Generally, adsorption of the pollutants depends on the K d values, which correlated with organic carbon content (f or) of soil. The organic carbon content (f oc) of soil increases as it is irrigated with treated wastewaters. Furthermore, the changing pH values cause the ionization of complex organic pollutants, PPCPs, which reduce their lipophilicity (K ow). Ternes et al. (2007) investigated the adsorption of pharmaceuticals and musk fragrances in soil irrigated with treated wastewater in Braunschweig, Germany. They estimated the K d values of different pharmaceuticals and musk fragrances compounds and show that adsorption is not more effective for pharmaceutical compounds due to its corresponding low K d values. Whereas, comparatively high adsorption was found for Musk fragrances having high K d values. Chefetz et al. (2008) investigated sorption and mobility of pharmaceutical compounds in soil irrigated with treated wastewater. The results of the adsorption-desorption batch experiment show that the adsorption isotherms were more linear for soil irrigated with treated wastewater than freshwater. They suggested that the adsorption isotherm of the pollutants depends upon the soil organic matter (SOM). They also suggest that the higher mobility of PPCPs in soil column systems indicates that their residues in soils irrigated with treated wastewater can leach from the root zone and be transported to the groundwater after rain events. Thus, the reuse potential of the treated wastewater depends on the degree of SOM present in wastewater as well as its mobility in the subsurface. It is generally observed that the humification of the SOM as well as the level of clay and organo-clay complexes affect significantly the adsorption of several pollutants originated from the application of treated wastewater (Senesi and Plaza 2007).
8.4.3 Attachment and Detachment Processes
Land irrigated with treated wastewater contains a significant amount of nanoscale emerging pollutants like viruses/colloids. The behavior of these nanoscale pollutants in subsurface depends upon their interaction with solids to be the result of differences in the electrical charge and the hydrophobicity of pollutant surface. Solids having high isoelectric points are better virus adsorbents than those with low isoelectric points (Bales et al. 1991). Thus, the electrostatic conditions of soil matrix control the attachment/detachment of nanoscale pollutants, especially viruses/colloids (Yates et al. 1987). Attachment of viruses is more in soil having higher cation exchange capacity (Burge and Enkiri 1978), exchangeable iron, and iron oxides (Lipson and Stotzky 1984; Chu et al. 2003). Moore et al. (1982) reported that the attachment shows more in media having higher specific surface areas. Generally, granular soils are weaker adsorbents than clays and minerals (Sobsey et al. 1980; Moore et al. 1981). Clays may have surfaces that have a very heterogeneous charge distribution. Vilker et al. (1983) suggested that poliovirus 1 be attached to the edges of montmorillonite particles in regions of positive charges due to the presence of aluminum ions. Farrah and Preston (1993) show that viruses/colloids are detached more in soil modified with precipitation of metallic salts than unmodified sand. Furthermore, the higher pH than 3.9 creates a negative charge, and less pH than 3.9 creates a positive charge, which may control the attachments and de-attachment behaviors of these nanoscale pollutants (Schijven and Hassanizadeh 2000). Similarly, high electrical conductivity due to the application of treated wastewater may increase the attachment rate, which acts as long-term residual pollutant in the subsurface. An increasing organic contents by regular application of treated wastewater may reduce the attachment sites on soil solids, which may result in additional pollutants load on underlying groundwater resources. Thus, attachment and de-attachment behavior of nanoscale pollutants control the reuse potential of treated wastewater (Sinton et al. 1997).
8.4.4 Mobile–Immobile Regions
The nonuniform flow of water which takes place leads to the non-equilibrium conditions in the variably saturated zone. Water mostly flows from macropores by passing the rest of the soil matrix resulting in a nonuniform wetting of the soil as flowing water has very less time to equilibrate with the soil matrix. These non-equilibrium conditions were termed to be most frustrating processes in terms of hampering accurate predictions of contaminant transport in soils and fractured rocks by Simunek et al. (2003). Preferential flow and transport hasten the movement of contaminants like fertilizers, pesticides, pathogens, and trace elements originating from the application of treated wastewater to the underlying vadose zone (Gardenas et al. 2006, Wang 2008).
Studies for predicting the water flow in subsurface rely on the assumption of continuity, i.e., hydraulic properties over the domain are uniform. Since subsurface is composed of various soil materials like sand, clay, loam etc., hydraulic properties of the soil vary with space (Botros et al. 2009). Many researchers had studied the effects of preferential flow paths in soil on fluid and solute transport (Harter et al. 2005) and tried to model this phenomenon using various approaches (Simunek and Genuchten 2009). Because of preferential flow of water and solutes through the macropores, there is very less time for water and solutes to equilibrate with the rest of the soil matrix creating non-equilibrium conditions in the soil matrix.
Dual-porosity and dual-permeability models have been extensively used for modeling preferential flow phenomenon, where flow region is divided into two: mobile and immobile regions. Simunek et al. (2003) extensively reviewed various conceptual models which can be used for modeling non-equilibrium flow and transport of water and solute in the heterogeneous vadose zone. To investigate the spatial and temporal distribution of contaminants originated from the application of treated wastewater, Richard’s equation and advection-dispersion equations are mostly used for simultaneous water and solute transport considering mobile-immobile regions. Total moisture content (θ) in the soil domain is considered to be the sum of moisture content in fractures (θ m) and matrix (θ im). The moisture flow equation in its mixed form coupled by a nonuniform sink function for water uptake by plants can be written in 3D form as:
where θ is the volumetric water content defined as the volume of water per unit volume of soil (dimensionless), h is the pressure head of mobile region [L], S is a sink function that represents the water extraction by surface vegetation [T−1], z is the depth of vadose zone measured positive upward [L], Γw is the water transfer rate between mobile and immobile region [T−1], K is the hydraulic conductivity of the soil [LT−1], and t is the time [T]. The water transfer Γw rate between mobile and the immobile region is assumed to be proportional to the difference in effective saturation between mobile and immobile regions.
where ω is a first-order water transfer coefficient and S e,m and S e,im are the effective saturation of the mobile and immobile regions defined as:
Similarly, the solute transfer function Γs between mobile and the immobile region is considered to be proportional to the difference in solute concentration between mobile and immobile regions.
The following texts describe factors affecting mobility-immobility of contaminants originated due to land application of treated wastewater in the subsurface.
-
Subsurface heterogeneity creates mobile-immobile zones, which may cause the complexity in water and solute flow regions. Thus, the subsurface zone having a high degree of formations of heterogeneity holds more pollutants in pore spaces (Simunek et al. 2003).
-
Similarly, the pollutant heterogeneity due to the application of treated wastewater having trace metals, hydrocarbons, etc. causes more vulnerability to soil and underlying groundwater resources (Yadav and Junaid 2014).
-
The bulk density, particle size distribution, pH, redox conditions, ionic exchange capacity, organic matter contents, types and the amount of metallic oxides, and types and the amount of clay minerals significantly affect mobile-immobile regions of water flow and solute transport in the subsurface.
8.5 Role of Climate Change In Biogeochemical Processes
Observational records and climate projections provide abundant evidence that like other ecosystems, subsurface processes have dynamic interactions with the ground surface and its prevailing environmental conditions. Climate variability affects subsurface processes both directly by altering surface water and heat flux and indirectly via changes in groundwater extraction patterns. Subsurface water resources are vulnerable and have the potential to be strongly affected by climate change with wide-ranging consequences (Green et al. 2011a, b). Impacts of climate change to the subsurface natural resources are further compounded due to accelerating demands arising from increasing population, urbanization, deforestation, and intensification of agriculture. Subsurface recharge and increased groundwater discharge due to climate change significantly disturb the moisture flow pattern, which further affects the biogeochemical characteristics of the subsurface system (Green et al. 2011a, b). In this situation, land irrigated with treated wastewater causes additional pollution load on subsurface natural resources. The following text describes the effects of climate change on biogeochemical processes controlling reuse potential of treated wastewater in the subsurface.
-
Temperature is a key factor in reaction kinetics and dissolved oxygen concentrations, and hence, a small change in subsurface water temperatures could have a significant impact on subsurface water quality (Gunawardhana and Kazama 2012).The increased subsurface temperature may alter the geochemical processes (particularly redox reactions) that can exert control on the dissolved concentration and mobility of a wide variety of chemical contaminants (e.g., nutrients, trace metals, iron, and manganese) released by application of treated wastewater (Destouni and Darracq 2009). This may be of particular concern for supply wells that derive their water from riverbank infiltration. Rising temperatures could potentially increase soil mineralization rates of organic nitrogen to nitrate, leading to an increase in the potential for nitrate leaching to the water table in agricultural areas that irrigated with treated wastewater (Stuart et al. 2011).
-
Modifications in biogeochemical interactions take place due to subsurface water warming by increasing temperature-propagated heat flux to sufficient depth, which can affect (co)-metabolic actions of soil microbiotas responsible for degrading subsurface pollutants (Gupta and Yadav 2017).
-
Likewise, temperature plays a significant role in controlling the nature and extent of microbial metabolisms that are responsible for degradation of several pollutants (Yadav and Hassanizadeh 2011). Bioavailability and solubility of pollutants are also temperature-dependent. Low-temperature conditions usually result in increased viscosity, reduced volatilization, and decreased water solubility of pollutants, and thus delayed the onset of biodegradation process.
-
Alternation in subsurface physical and functional interactions is mostly caused by the increasing evapotranspiration (ET) resulting from rising temperature. The increasing ET accelerates the subsurface moisture losses, which directly affects microbial cellular water loss in the subsurface. In particular, biodegradation process is strongly affected by the soil moisture contents. Thus, lands irrigated with treated wastewater in the semiarid and arid region are more vulnerable to underlying natural resources (Dettinger et al. 2004).
-
Furthermore, in semiarid and arid regions, climate variability and changes accelerate evapotranspiration rates and cause the significant subsurface water losses, which ultimately lead to the high salinity problems (Earman and Dettinger 2011).
-
The ratio of soil moisture and air in unsaturated zone has a direct effect on pollutants fate and transport under varying climatic conditions (Yadav and Hassanizadeh 2011). The low soil moisture content results in greater air-filled porosity, which should improve oxygen mass transfer to the pollutant-degrading microbial assemblage. However, there is likely to be a trade-off between improved oxygen availability and soil moisture content (Arora et al. 1982; Alvarez and Illman 2006).
-
However, pollutant movement is getting retarded by adsorption on organic and/or mineral components of soil solids when air-filled porosity increases at low water contents. Thus, irrigation with treated wastewater reduces air-filled porosity and causes more downward movement of pollutants (Petersen et al. 1994).
-
Extreme rainstorm intensity related to climate change may increase the downward flux of pollutants (e.g., nitrate, chloride) introduced by application of treated wastewater in vadose zone (Taylor et al. 2013). Alternatively, intense storms could produce precipitation rates that quickly exceed soil infiltration capacities and may flush additional pollutants mass to underlying groundwater table.
-
The increase in rainfall intensity and surface flooding expected to accompany under climate change may drive the expansion of irrigated wastewater with runoff to the subsurface. This could potentially increase the amount of dissolved toxic chemicals and nutrients in runoff ultimately increasing the vulnerability of shallow aquifers to contaminations (Clifton et al. 2010; Green et al. 2011a, b).
-
The continuous irrigation by treated wastewater causes accelerated advective and dispersive flux, which increases pore water velocities in partially saturated zones. Rapid groundwater table fluctuations along with high pore water velocities can enhance the mobilization of pollutants considerably (Dobson et al. 2007a, b).
-
Large subsurface zones are polluted by advective and dispersive flux under high groundwater flow velocities caused by climatic variations. The fast groundwater velocity also enhances dissolution of pollutants, which may increase the pollution load in downgradient locations (Gupta and Yadav 2017).
-
The dynamics of groundwater table accelerate (up)-downward movement of the pollutants causing their entrapment in pore space, which ultimately increases their coverage area. Pollutants entrapped in the form of isolated blobs or ganglia in pore spaces increase water interfacial area responsible for their enhanced dissolution rates in the aqueous phase (Soga et al. 2004). Thus, pollutants trapped in the porous media act as long-lasting sources of groundwater pollution (Yadav and Hassanizadeh 2011).
-
The wide coverage of pollutants due to advective and dispersive flux resulted by rapid groundwater table and pore water velocities creates large redox environment in the subsurface. Underlying redox environment may reduce the degradation of upcoming pollutants from treated wastewater applied at the land surface.
-
To sum up, the climatic variation response to dynamic soil moisture flow, nature of underlying groundwater flow, and ambient temperature profile and pollutant regimes not only affect the microbial degradation rate but also the transport of substrate throughout the variably saturated zone. Thus, the climate change significantly affects the biogeochemical processes controlling reuse potential of treated wastewater in the subsurface.
8.6 Groundwater Pollution Load Resulted from Land Application of Treated Wastewater: A Case Study
Commonly, groundwater aquifer vulnerability has been assessed by data-based models like DRASTIC, modified DRASTICA, etc. incorporating the major geohydrological factors that affect and control the groundwater contamination movements. But these models are not effectively able to implement the contamination characteristics and biogeochemical behaviors to evaluate the major risk to underlying resources. Thus, an effort has been made to map the vulnerability of shallow groundwater to (sub)-surface pollutants resulting from land irrigated with wastewater of study area, using soil moisture flow and contaminant transport modeling.
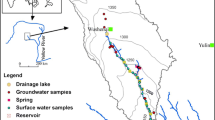
8.6.1 Study Area
Kishanghrah Tehsil of Ajmer district, Rajasthan, is selected for this study (Fig. 8.3). The study area is a semiarid climatic zone in the map of India. The area is dry throughout the years except for monsoon or rainy season. The mean annual rainfall (1957–2012) of the district is 323 mm. Almost 95% of the total annual rainfall is received during the southwest monsoon, which enters the district in the last week of June and withdraws in the middle of September. The annual potential evapotranspiration in the district is 1565.6 mm and is the highest (243 mm) in the month of May (CGWB 2008).
8.6.2 Methodology
HYDRUS-1D is used to model the solute transport to the underlying groundwater resource. The classical advection-dispersion equation coupled with Richard’s equation is numerically simulated at different point locations for assessing the intrinsic vulnerability. The governing flow and transport equations (refer Eq. 8.9) are solved by Galerk in finite element scheme. Simulations are carried for two approaches: equilibrium approach and non-equilibrium approach using dual porosity approach. The methodology follows the physics of the soil moisture and contaminant movement in variably saturated porous media. This work demonstrates the potential vulnerability of shallow aquifer regions irrigated with treated wastewater in Kishangarh, Rajasthan. The soil moisture flow and solute transport regimes of the vadose zone associated with specific hydrogeological conditions play a crucial role in pollution risk assessment of the underlying groundwater resources. The role of soil type, slope, and the land-use cover is considered for estimating the transient flux at the top boundary from daily precipitation and evapotranspiration data of the study area. A constant flux of 150 mL/hr. of BTEX, a mixture of hydrocarbons, generally found in treated wastewater is applied as solute flux at top boundary.
8.6.3 Results and Discussion
The computed solute transport profiles of varying hydrogeological conditions associated with different locations in the study area are shown in Fig. 8.4. The intrinsic vulnerability is assessed by comparing the transit time for the solute peak to reach the saturated zone. Figure 8.3 shows the variation in hydrocarbon concentrations with time for a constant flux boundary condition for all locations. The N5 line in each graph represents the concentration of solute at the water table, and the N1 lines represent the same at the observation point located at the top boundary and remaining (N2-N4) in the middle of the vadose zone. Results show that the high vulnerability of groundwater in the area irrigated with wastewater in the Kishangarh city, whereas low vulnerability is observed in the rural area of Kishangarh. The study may assist in decision-making related to the planning of application of treated wastewater and the sustainable water resource development of the selected semiarid area.
8.7 Conclusion and Future Prospects
Land application of treated wastewater results in more vulnerability of underlying subsurface resources. This is due to insufficient treatment capacities of wastewater treatment plants as well as the presence of PPCPs, BTEX, nanoscale particles, viruses, and other low-degradable emerging pollutants. The natural attenuation of these pollutants is quite slow in the subsurface and acts as long-term source of pollution. The sufficient amount of these pollutants is introduced in the subsurface when land is irrigated with treated wastewater. Thereafter, these pollutants start moving downward due to gravity and reach to groundwater table. The wide area coverage by the pollutants creates a redox environment in and around the polluted subsurface zones. Similarly, the mass partitioning takes place in the partially saturated zone by adsorption/desorption and or attachment/attachments to solid particles. Further, the mobile-mobile regions of subsurface control the reuse potential of treated wastewater. On the other hand, the climatic variables affect the solubility of different pollutants present in treated wastewater. In this book chapter, the physicochemical properties of a typical treated wastewater are presented. Then, the fate and transport processes of emerging pollutants are described in depth. Thereafter, the subsurface process that controls the reuse potential of treated wastewater is elaborated. Similarly, the role of climate change on underlying geochemical processes is highlighted. A case study on this topic is presented here to demonstrate the potential vulnerability of shallow aquifer regions irrigated with treated wastewater in Kishangarh, Rajasthan. In the future, there is a need to improve the performance of the wastewater treatment plants. Furthermore, developing some appropriate in situ bioremediation techniques is required to decontaminate the polluted soil-water resources under climate change conditions.
References
Albrechtsen H-J, Christensen TH (1994) Evidence for microbial iron reduction in a landfill leachate-polluted aquifer Vejen, Denmark. Appl Environ Microbiol 60:3920–3925
Alvarez PJJ, Illman WA (2006) Bioremediation and natural attenuation, process fundamentals and mathematical models. Wiley-Interscience. ISBN-10 0-471-65043-9
Anitha K, Bolan NS, Müller K, Laurenson S, Naidu R, Kim WI (2012) The influence of wastewater irrigation on the transformation and bioavailability of heavy metal(loid)s in soil. Adv Agron 115:215–297
Arora HS, Cantor RR, Nemeth JC (1982) Land treatment: a viable and successful method of treating petroleum industry wastes. Environ Int 7:285–291
Arye G, Dror I, Berkowitz B (2010) Fate and transport of carbamazepine in soil aquifer treatment (SAT) infiltration basin soils. Chemosphere 82(2011):244–252
Bales RC, Hinkle SR, Kroeger TW, Stocking K, Gerba CP (1991) Bacteriophage adsorption during transport through porous media: chemical perturbations and reversibility. Environ Sci Technol 25:2088–2095
Barcelona MJ, Holm TR (1991) Oxidation–reduction capacities of aquifer solids. Environ Sci Technol 25:1565–1572
Bernstein N, Bartal A, Friedman H, Rot I, Snir P, Chazan A, Ioffe M (2006) Application of treated wastewater for cultivation of roses (Rosa hybrida) in soil-less culture. Sci Hortic 108:185–193
Borden RC, Gomez CA, Becker MT (1995) Geochemical indicators of intrinsic bioremediation. Groundwater 33(2):180–189
Botros FE, Harter T, Onsoy YS, Tuli A, Hopmans JW (2009) Spatial variability of hydraulic properties and sediment characteristics in a deep alluvial unsaturated zone. Vadose Zone J 8(2):276–289
Burge WD, Enkiri MK (1978) Virus adsorption by five soil. J Environ Qual 7:73
Caliman FA, Gavirilescu M (2009) Pharmaceuticals, personal care products and endocrine disrupting agents in the environment – a review. Clean 37:277–303
Central Ground Water Board, Ministry of Water Resources, Government of India (2008) Groundwater Scenario Ajmer District Rajasthan. District Groundwater Brochure, Western Region Jaipur
Chary NS, Kamala CT, Raj DSS (2008) Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol Environ Safe 69:513–524
Cheftez B, Mualem T, Ben-Ari J (2008) Sorption and mobility of pharmaceutical compounds in soil irrigated with reclaimed wastewater. Chemosphere 73:1335
Christensen TH et al (2000) Characterization of redox conditions in groundwater contaminant plumes. J Contam Hydrol 45(3–4):165–241
Chu Y, Jin Y, Baumann T, Yates MV (2003) Effect of soil properties on saturated and unsaturated virus transport through columns. J Environ Qual 32:2017–2025
Clement TP, Johnson CD, Sun Y, Klecka GM, Bartlett C (2000) Natural attenuation of chlorinated ethene compounds: model development and field-scale application at the Dover site. J Contam Hydrol 42(2–4):113–140
Clifton C et al. (2010) Water and climate change: impacts on groundwater resources and adaptation options. Water Working Notes, World Bank Group Water Sector Board Note No 25, 76 p
CPCB (2009) Status of water supply, wastewater generation and treatment in Class I cities and Class II towns of India. Central Pollution Control Board
Destouni G, Darracq A (2009) Nutrient cycling and N2O emissions in a changing climate: the subsurface water system role. Environ Res Lett 4(3):035008
Dettinger MD, Cayan DR, Meyer MK, Jeton AE (2004) Simulated hydrologic responses to climate variations and change in the Merced, Carson, and American River Basins, Sierra Nevada, California, 1900–2099. Clim Chang 62:283–317
Dobson R, Schroth MH, Zeyer J (2007a) Effect of water-table fluctuation on dissolution and biodegradation of a multi-component, light nonaqueous-phase liquid. J Contam Hydrol 94:235–248
Dobson R, Schroth MH, Zeyer J (2007b) Effect of water-table fluctuation on dissolution and biodegradation of a multi-component, light nonaqueous-phase liquid. J Contam Hydrol 94:235–248
Earman S, Dettinger M (2011) Potential impacts of climate change on groundwater resources – a global review. J Water Climate Change 02(4):213–229 http://www.iwaponline.com/jwc/002/jwc0020213.htm
Ebele AJ, Abou-Elwafa Abdallah M, Harrad S (2017) Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg Contam 3(2017):1–16
EPA (2008) Nanotechnology for site remediation fact sheet. U.S. Environmental Protection Agency, (October), pp 1–17
Farrah SR, Preston DR (1993) Adsorption of viruses to sand modified by in situ precipitation of metallic salts. Wiener Mitteilungen Wien 12:25–29
Gardenas A, Simunek J, Jarvis NJ, van Genuchten MT (2006) Two-dimensional modelling of preferential water flow and pesticide transport from a tile-drained field. J Hydrol 329:647–660
Gharaibeh MA, Eltaif NI, Al-Abdullah B (2007) Impact of field application of treated wastewater on hydraulic properties of vertisols. Water Air Soil Pollut 184:347–353
Green TR et al (2011a) Beneath the surface of global change: impacts of climate change on groundwater. J Hydrol 405:532–560
Green TR et al (2011b) Beneath the surface of global change: impacts of climate change on groundwater. J Hydrol 405:532–560
Gunawardhana LN, Kazama S (2012) Statistical and numerical analysis of the influence of climate variability on aquifer water levels and groundwater temperatures: the impacts of climate change on aquifer thermal regimes. Global Planet Chang 86–87:66–78
Gupta PK, Shashi R, Yadav BK (2013) BTEX Biodegradation in soil-water system having different substrate concentrations. Int J Eng 2(12)
Gupta PK, Yadav BK (2017) Chapter 8: Bioremediation of Non-Aqueous Phase Liquids (NAPLS) polluted soil and water resources. In: Environmental pollutants and their bioremediation approaches. CRC Press, Taylor and Francis Group, Boca Raton. ISBN 9781138628892
Gupta PK, Joshi P (2017) Assessing groundwater resource vulnerability by coupling GIS based DRASTIC and solute transport model in Ajmer District, Rajasthan. Journal of Geological Society of India (Springer), DOI: https://doi.org/10.1007/s12594-018-0958-y
Gupta PK, Sharma D (2018) Assessments of hydrological and hydro-chemical vulnerability of groundwater in semi-arid regions of Rajasthan, India. Sustainable Water Resources Management, 1-15. https://doi.org/10.1007/s40899-018-0260-6.
Gupta PK, Ranjan S, Kumar D (2018a) Groundwater pollution by emerging industrial pollutants and its remediation techniques. Chapter 2, In Recent advances in environmental management, CRC Press Taylor & Francis Group, ISBN 9780815383147, Vol 1
Gupta PK, Abhishek YBK (2018b) Impact of hydrocarbon pollutants on partially saturated soil media in batch system: Morphological analysis using SEM techniques. Chapter 5, water quality management; Water Sci Technol, ISBN: 978-981-10-5794-6, Vol. 79, Springer
Harter T, Ginn TR, Onsoy YS, Horwath WR (2005) Spatial variability and transport of nitrate in a deep alluvial vadose zone. Vadose Zone J 4:41–54
IPCC (2007a) Climate change (2007). The physical science basis. In: Solomon S et al (eds) Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge/New York, p 996
IPCC (2007b) Climate change (2007). Impacts, adaptation and vulnerability. In: Parry ML, Canziani OF, Palutikof JP, Linden PJVD, Hanson CE (eds) Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge/New York
Jeribi M, Almir-Assad B, Langevin D, Henaut I, Argillier JF (2002) Adsorption kinetics of asphaltenes at liquid interfaces. J Colloid Interface Sci 256(2):268–272
Karn B, Kuiken T, Otto M (2009) Nanotechnology and in situ remediation: a review of the benefits and potential risks. Environ Health Perspect 117(12):1823–1831
Karvelas M, Katsoyiannis A, Samara C (2003) Occurrence and fate of heavy metals in the wastewater treatment process. Chemosphere 53(10):1201–1210
Kinney CA, Furlong ET, Kolpin DW, Burkhardt MR, Zaugg SD, Werner SL, Bossio JP, Benotti MJ (2008) Bioaccumulation of pharmaceuticals and other anthropogenic waste indicators in earthworms from agricultural soil amended with biosolid or swine manure. Environ Sci Technol 42:1863–1870
Klein RJT, Nicholls RJ (1999) Assessment of coastal vulnerability to climate change. Ambio 28(2):182–187
Kushwah SK, Malik S, Singh A (2012) Water quality assessment of raw sewage and final treated water with special reference to waste water treatment plant Bhopal, MP, India. Res J Recent Sci 1(ISC-2011):185–190
Levantesi C, La Mantia R, Masciopinto C, Böckelmann U, Ayuso-Gabella MN, Salgot M et al (2010) Quantification of pathogenic microorganisms and microbial indicators in three wastewater reclamation and managed aquifer recharge facilities in Europe. Sci Total Environ 408(21):4923–4930
Li YH, Wang S, Luan Z, Ding J, Xu C, Wu D (2003) Adsorption of cadmium(II) from aqueous solution by surface oxidized carbon nanotubes. Carbon 41(5):1057–1062
Lipson SM, Stotzky G (1984) Effect of proteins on Reovirus adsorption to clay minerals. Appl Environ Microbiol 8:525–530
McCarthy KA, Johnson RL (1993) Transport of volatile organic compounds across the capillary fringe. Water Resour Res 29(6):1675–1683
Moore RS, Taylor DH, Sturman LS, Reddy MM (1981) Poliovirus adsorption by 34 minerals and soils. Appl Environ Microbiol 42:963–975
Moore RS, Taylor DH, Reddy MM, Sturman LS (1982) Adsorption of reovirus by minerals and soils. Appl Environ Microbiol 44:852–859
Mustapha HI, Rousseau D, van Bruggen J, Lens P (2011) Treatment performance of horizontal subsurface flow constructed wetlands treating inorganic pollutants in simulated refinery effluent. In: 2nd Biennial Engineering Conference. School of Engineering and Engineering Technology, Federal University of Technology, Minna
Mustapha HI, van Bruggen JJ, Lens PN (2015) Vertical subsurface flow constructed wetlands for polishing secondary Kaduna refinery wastewater in Nigeria. Ecol Eng 84:588–559. https://doi.org/10.1016/j.ecoleng.2015.09.060
Mustapha IH, Gupta PK, Yadav BK, van Bruggen JJA, Lens PNL (2018) Performance evaluation of duplex constructed wetlands for the treatment of diesel contaminated wastewater. In: Chemosphere. https://doi.org/10.1016/j.chemosphere.2018.04.036
Okuda T, Kobayashi Y, Nagao R, Yamashita N, Tanaka H, Tanaka S, Fujii S, Konishi C, Houwa I (2008) Removal efficiency of 66 pharmaceuticals during wastewater treatment process in Japan. Water Sci Technol 57(1):65–71
Petersen LW, Rolston DE, Moldrup P, Yamaguchi T (1994) Volatile organic vapor diffusion and adsorption in soils. J Environ Qual 23:799–805
Petersen LW, El-Farhan YH, Moldrup P, Rolston DE, Yamaguchi T (1996) Transient diffusion, adsorption, and emission of volatile organic vapors in soils with fluctuating low water contents. J Environ Qual 25:1054–1063
Pierson WL, Nittim R, Chadwick MJ, Bishop KA, Horton PR (2001) Assessment of changes to saltwater/freshwater habitat from reductions in flow to the Richmond river estuary, Australia. Water Sci Technol 43(9):89–97
Powers SE, Loureiro CO, Abriola LM, Weber WJ (1991) Theoretical study of the significance of non-equilibrium dissolution of nonaqueous-phase liquids in subsurface systems. Water Resour Res 27(4):463–477
Ranjan SP, Kazama S, Sawamoto M (2006) Effects of climate and land use changes on groundwater resources in coastal aquifers. J Environ Manag 80(1):25–35
Ranjan S, Gupta PK, Yadav BK (2018) Application of nano-materials in subsurface remediation techniques – challenges and future prospects. Chapter 6, In Recent advances in environmental management, CRC Press Taylor & Francis Group, ISBN 9780815383147, Vol 1
Rose JB, Dickson LJ, Farrah SR, Carnahan RP (1996) Removal of pathogenic and indicator microorganisms by a full-scale water reclamation facility. Water Res 30:2785–2797
Saien J, Shahrezaei F (2012) Organic pollutants removal from petroleum refinery wastewater with nanotitania photocatalyst and UV Light Emission. Int J Photoenergy 2012:1–5
Savage N, Diallo MS (2005) Nanomaterials and water purification: opportunities and challenges. J Nanopart Res 7(4–5):331–342
Schijven JF, Hassanizadeh SM (2000) Removal of viruses by soil passage: overview of modeling, processes, and parameters. CRC Crit Rev Environ Sci Technol 30:49–127
Seeger EM, Kuschk P, Fazekas H, Grathwohl P, Kaestner M (2011) Bioremediation of benzene-, MTBE- and ammonia-contaminated groundwater with pilot-scale constructed wetlands. Environ Pollut 199:3769–3776
Senesi N, Plaza C (2007) Role of humification processes in recycling organic wastes of various nature and sources as soil amendments. Clean-Soil Air Water 35:26–41. https://doi.org/10.1002/clen.200600018
Sharma RK, Agrawal M, Marshall F (2007) Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol Environ Saf 66(2):258–266
Sherif MM, Singh VP (1999) Effect of climate change on sea water intrusion in coastal aquifers. Hydrol Process 13(8):1277–1287
Simunek J, van Genuchten MT (2009) Modelling nonequilibrium flow and transport processes using HYDRUS. Vadose Zone J 7:782–797
Simunek J, Jarvis NJ, van Genuchten MT, Gardenas A (2003) Review and comparison of models for describing non-equilibrium and preferential flow and transport in the vadose zone. J Hydrol 272:14–35
Sinton LW, Finlay RK, Pang L, Scott DM (1997) Transport of bacteria and bacteriophages in irrigated effluent into and through an alluvial gravel aquifer. Water Air Soil Pollut 98:17–42
Sobsey MD, Dean CH, Knuckles ME, Wagner RA (1980) Interactions and survival of enteric viruses in soil materials. Appl Environ Microbiol 40:92–101
Soga K, Page JWE, Illangasekare TH (2004) A review of NAPL source zone remediation efficiency and the mass flux approach. J Hazard Mater 110(1–3):13–27
Sparks DL (2003) Environmental soil chemistry. Academic/China Translation & Printing Services Ltd., Amsterdam/Hong Kong
Stuart M et al (2011) A review of the impact of climate change on future nitrate concentrations in groundwater of the UK. Sci Total Environ 409(15):2859–2873
Suthar S, Chhimpa V, Singh S (2009) Bacterial contamination in drinking water: a case study in rural areas of northern Rajasthan, India. Environ Monit Assess 159:43–50
Taylor RG et al (2013) Ground water and climate change. Nat Clim Chang 3:322–329
Ternes TA, Bonerz M, Herrmann N, Teiser B, Andersen HR (2007) Irrigation of treated wastewater in Braunscheug, Germany: an option to remove pharmaceuticals and musk fragrances. Chemosphere 66:894–904
Tobiszewski M, Tsakovski S, Simeonov V, Namieśnik J (2012) Chlorinated solvents in a petrochemical wastewater treatment plant: an assessment of their removal using self-organising maps. Chemosphere 87:87. https://doi.org/10.1016/j.chemosphere.2012.01.057
US EPA (2004) Guidelines for water reuse, EPA/625/R-04/108 September
USEPA (U.S. Environmental Protection Agency) (1995) Light nonaqueous phase liquids. Office of solid waste and emergency response, Washington, DC. EPA/540/S-95/500
Vilker VL, Meronek GC, Bulter PC (1983) Interaction of poliovirus with montmorillonite clay in phosphate-buffered saline. Eviron Sci Technol 17:631–634
Wang F (2008) Modeling the spatial distribution of nitrogen leaching from dairy farmland. Vadose Zone J 7(2):439–452
Watlington K (2005) Emerging nanotechnologies for site remediation and wastewater treatment. Environmental Protection Agency, Washington DC
Wu S, Wallace S, Brix H, Kuschk P, Kirui WK, Masi FD (2015) Treatment of industrial effluents in constructed wetlands: Challenges, operational strategies and overall performance. Environ Pollut 201:107–120
Yadav BK, Hassanizadeh SM (2011) An overview of biodegradation of LNAPLs in coastal (semi)-arid environment. Water Air Soil Pollut 220:225. https://doi.org/10.1007/s11270-011-0749-1
Yadav BK, Junaid S (2014) Groundwater vulnerability assessment to contamination using soil moisture flow and solute transport modelling. J Irrig Drain Eng 141:04014077-1. https://doi.org/10.1061/(ASCE)IR.1943-4774.0000841
Yates MV, Yates SR, Wagner J, Gerba CP (1987) Modeling virus survival and transport in the subsurface. J Contam Hydrol 1:329–345
Acknowledgment
The University Grant Commission (UGC) Fellowship received by Mr. Pankaj K Gupta is duly acknowledged.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gupta, P.K., Yadav, B.K. (2019). Subsurface Processes Controlling Reuse Potential of Treated Wastewater Under Climate Change Conditions. In: Singh, R., Kolok, A., Bartelt-Hunt, S. (eds) Water Conservation, Recycling and Reuse: Issues and Challenges. Springer, Singapore. https://doi.org/10.1007/978-981-13-3179-4_8
Download citation
DOI: https://doi.org/10.1007/978-981-13-3179-4_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-3178-7
Online ISBN: 978-981-13-3179-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)