Abstract
Circular RNAs (circRNAs) represent a special group of noncoding single-stranded highly stable ribonucleic acid entities abundant in the eukaryotic transcriptome. These circular forms of RNAs are significantly enriched in human brain and retinal tissues. However, the biological evolution and function of these circRNAs are poorly understood. Recent reports showed circRNA to be an important player in the development of neurodegenerative diseases like Alzheimer’s disease. With the progression of age, circRNA level increases in the brain and also in age-associated neurological disorder like Alzheimer’s disease (AD), Parkinson’s disease, inflammatory neuropathy, nervous system neoplasms, and prion diseases. One highly represented circRNA in the human brain and retina is a ciRS-7 (CDR1as) which acts as an endogenous, anticomplementary miRNA inhibitor or “sponge” to quench the normal functioning of miRNA-7. Low CDR1as level can lead to increase in miR-7 expression which downregulates the activity of ubiquitin protein ligase A (UBE2A), an important AD target, functionally involved in clearing toxic amyloid peptides from AD brain. This chapter focuses on the functional relationship of circRNA with AD and interplay of miRNA-mRNA-mediated genetic regulatory networks. Our conceptual understanding also suggests that circRNA can be considered as a potential biomarker and therapeutic target in AD diagnosis and treatment.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
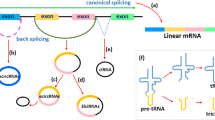
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder and the most common cause of dementia in aging population. AD is clinically apparent as the insidious impairment of higher intellectual functions that ultimately leads to death from complete brain failure. There are regions of the brain that are specifically affected sites of neuropathology in Alzheimer’s disease, and they include the hippocampus, the amygdala, the temporal cortex, and the frontal cortex. Complex multifactorial interactions among genetic, epigenetic, and environmental components are responsible for causation of AD. Recent research has come up with an interesting entity of RNA, an endogenous noncoding circular RNA (circRNA) abundantly expressed in eukaryotes, which has important functions in many disease-associated gene regulations including AD. These structures have the 3′ and 5′ ends joined together by covalent bonds giving a circular appearance which is unlike linear RNA. These molecules which are evolutionarily conserved had been dismissed as a rare, exotic RNA species for decades.
2 circRNAs in Neural Development
circRNAs are enriched in the brain. These neural circRNAs are derived from synaptic genes named Dscam and Homer1 or from genes with important functions in early neural development, such as genes involved in axon guidance, Wnt and TGF-β signaling [1, 2]. These are localized mostly in neuronal cell bodies and neuropil [2] and found to be highly enriched in hippocampal synaptosomes. The developmental role of expression of circRNAs in the brain is determined by profiling the circRNA population in the hippocampus over several stages: embryonic (E18), early postnatal (P1), postnatal at the beginning of synapse formation (P10), and late postnatal hippocampus following the establishment of mature neural circuits (P30) [2]. The existence of circRNA in vivo as well as in primary neuron cultures and cell lines suggests a diverse distribution of circRNAs within neurons. This also cites an important role for circRNAs in neuronal development and plasticity [2, 3].
circRNAs are flanked by introns. Neuronal genes usually have long (> 10 kb) introns which are highly conserved in evolution. So the circRNAs flanked by long introns are mostly evolutionarily conserved [4]. Alternative explanation for conserved circRNA could be that introns in these genes are long for other reasons and that circRNA is produced due to recursive splicing, i.e., circRNAs are produced as by-product of the complex splicing [5].
The function of circRNA in mammalian brain remains to be defined. Since circRNAs are highly stable, they could serve as topologically complex platforms for protein or RNA transportation. The prominence of stable circRNAs in the synapse provides both the stability and flexibility of neuronal networks which are vital to all behavior, including learning and memory. Future functional research should be directed in understanding the effect of genetic perturbation of specific circRNAs followed by phenotypic examination which will address circRNA function in the nervous system in respect to molecular memory.
3 Role of circRNA in Alzheimer’s Disease
circRNAs tend to accumulate during normal process of brain aging and thus make susceptible to age-related neurodegenerative diseases like AD. This disease is the most common cause of dementia in elderly population characterized by the presence of neurotoxic senile amyloid plaques, hyper-phosphorylated tau tangles, massive neuron death, and neuro-inflammation. According to the amyloid hypothesis, accumulation of Aβ in the brain is central to AD pathogenesis [6]. Amyloid is a general term for protein fragments of albuminoid proteinaceous material that the body produces normally. β-amyloid is a 36–43 amino acid peptide fragment clipped from amyloid precursor protein (APP). Most cases of Alzheimer’s belong to the late-onset category, which occurs after age 60. The reasons of late-onset Alzheimer’s are not yet completely elucidated, but they include a combination of genetic, environmental, and lifestyle factors that have an important influence on disease susceptibility of a person. The single-gene mutations are mostly directly responsible for early-onset Alzheimer’s disease but do not seem to be involved in late-onset Alzheimer’s, and thus a specific gene mutation does not cause the late onset of the disease complex multifactorial interactions among genetic, epigenetic, and environmental components which is responsible for causation of AD. Familial AD is characterized by the genetic mutations involved in Aβ peptide biogenesis which consists of four well-studied AD genes, the APP, PS1, PS2, and APOE. These genes exhibit mutations that enhance the relative rate of generation of Aβ42, the longer form of the peptide that is much more prone to oligomerization and fibrillation than Aβ40 [7].The spatial and temporal patterns of senile plaques consisting of fibrillar Aβ do not equate very well with the degree of dementia in AD, and thus the traditional amyloid hypothesis remains debatable. In contrast, cognitive malfunctioning displays a profound relationship with most common type of sporadic AD which remains largely unknown. circRNA expressed in the human brain might play a causative role in AD and other neurodegenerative conditions. Interestingly, circRNA could emerge as a potential therapeutic target in AD diagnosis and treatment.
Although circRNA has been reported in many diseases, their role in Alzheimer’s disease remains unclear. Interestingly, evolutionarily conserved microRNA-7 which is highly abundant in human brain is associated with a circRNA for miRNA-7 (ciRS-7, also known as CDR1as). ciRS-7 contains multiple, tandem anti-miRNA-7 sequences that thereby act as an endogenous, anticomplementary miRNA “sponge” to adsorb and hence quench normal miRNA-7 functions [8, 9]. In the hippocampal CA1 region of sporadic AD patients, miR-7 circRNA system is dysregulated which is confirmed by Northern blot hybridization techniques and the circularity-sensitive circRNA probe RNase R [8]. Downregulation of ciRS-7 and ciRS-7 “sponging activities” might increase endogenous miRNA-7 levels in AD [10]. The elevated miRNA-7, due to inhibition in ciRS-7 “sponging” effects, can downregulate AD-associated targets like ubiquitin protein ligase, UBE2A, and an autophagic, phagocytic protein essential in the clearance of amyloid peptides in AD brain [11, 12]. Such miRNA-mRNA regulatory systems may represent another crucial aspect of epigenetic control over gene expression in health and disease. Inhibition of “miRNA sponging systems” and increase of specific inducible miRNAs might be a reason for downregulation of important genes related to sporadic AD brain [8, 13].
4 circRNA in Other Neuropathies
circRNA and Parkinson’s disease
Parkinson’s disease (PD) is a neurodegenerative disorder that affects mainly dopamine-producing neurons in a specific area of the brain, substantia nigra pars compacta. CDR1as downregulates miR-7 [14]. It has already been reported that miR-7 can inhibit the expression of α-synuclein, a crucial constituent of Lewy bodies in the PD brain. α-Synuclein protein is expressed highly in the diseased brain and considered as hallmark feature in PD pathogenesis. Moreover, downregulation of α-synuclein by miR-7 protects cells against oxidative stress [15]. miRNA-7 can provide protection against neuron death caused by 1-methyl-4-phenylpyridinium (MPP+) by targeting the nuclear factor (NF)-κB signaling pathway [14, 16].
circRNA and Neoplasms
High expression of CDR1as is evident in the brain cerebrum. CDR1as is highly expressed in neuroblastomas and astrocytoma [17]. miR-7 was found to be downregulated in astrocytoma and neuroblastoma compared to other brain tissue. Another study indicated that miR-7 could suppress EGFR expression in a glioblastoma cell line and downregulate IRS-1 and IRS-2 expression by repressing protein kinase B [18]. CDR1as acts as negative regulator of miR-7 [9, 14]. These evidences indicate possible role of circRNAs in the pathophysiology of nervous system neoplasms.
circRNA and Neuro-inflammation
Virus-associated miRNA binding sites are present in some circRNA which plays vital role in immunoregulation as, for instance, hsa-circRNA 2149 contains 13 unique, head-to-tail spanning reads. Hsa-circRNA 2149 is present in CD19+ leukocytes but not in CD341 leukocytes or neutrophils. Another circRNA, circRNA100783, has implication in chronic CD28-associated CD8(+)T cell aging which could be utilized as an important biomarker for this disease [14, 19]. circRNA from SRY can repress miR-138 activity. miR-138 downregulates runt-related transcription factor 3 (RUNX3) which plays an essential role in the regulation of T helper cells [14, 20]. These studies provide indication of association of circRNA with neuro-inflammation.
circRNA and Prion Diseases
Progressive neurodegeneration is evident in Prion diseases with neuronal loss and a failure to induce inflammatory response. These diseases include Creutzfeldt–Jakob disease (CJD), Gerstmann-Straussler syndrome (GSS), and also fatal familial insomnia. Prion diseases are mainly caused by alteration of a normal cell-surface glycoprotein (PrPC) into a modified isoform (PrPSc) that renders infectious nature to PrP in the absence of nucleic acid. Reports show PrPC overexpression induces CDR1as expression [21]. Thus, CDR1as might have some implication in the etiology of prion diseases.
5 circRNA Detection in the Brain
Numerous strategies are employed to detect genome-wide circRNA expression over the past few years, but little is known to find out the accuracy of these approaches. Experimental and bioinformatic tools along with accurate statistical approaches can help address these objectives of circRNA detection. Recently, scientists have shown that circular RNA is associated with brain functions. When CDR1as, an RNA molecule highly expressed in human and mouse brain, was deleted from the genome of mice, the animal brain failed to retain important information and disregard the unnecessary ones like in other mental disorders [22]. Copious circRNAs are highly abundant in mammalian brain expression with conserved expression. The well-known CDR1as is strongly bound by miR-7 and miR-671 in the human and mouse brain. Expression of these two microRNAs was posttranscriptionally dysregulated in all brain regions. Early genes such as Fos, a direct miR-7 target, were enhanced in CDR1as-deficient brains, indicating a possible molecular link to the behavioral phenotype [22].
The most popular technology microarray was a preferred way for global RNA expression analysis before the arrival of next-generation sequencing (NGS), but it was not convenient to screen circRNAs or its expression from linear counterparts. The high-throughput NGS technology has provided a competent way to detect circRNAs. Many software packages came to the rescue to decipher circRNAs from RNA-Seq data. Common RNA-seq protocols have limitations as it may introduce technical artifacts that can result in wrong identification of circRNA isoforms as exonucleases might act upon some circRNA and inhibit their expression [1, 23]. In mouse, deep sequencing of multiple organs reveals significantly greater fraction of circRNA junctional reads. Similar reports were found in human tissues [3, 24]. circRNAs show different patterns of expression respective to brain areas which include the striatum, prefrontal cortex, olfactory cortex, cerebellum, and hippocampus. A gene ontology analysis of the transcripts generating circRNAs reveals synaptic genes encoding pre- and postsynaptic functional groups are fortified as circRNA host genes and thus provide an important reasoning for the abundance of circRNA in the brain. Along with that, many circRNAs are highly distributed in synaptic fractions and synaptosomes [24]. Rare circRNA localization in cell body and dendrites of cultured hippocampal neurons and hippocampal slices can be targeted by high-resolution in situ hybridization technique [24].
6 Conclusion
circRNA function and their relationships with Alzheimer’s disease and other neuropathies remain to be fully elucidated. circRNAs are usually abundant and found to be stable in vivo, which might attribute to their importance in molecular diagnostics. Until then, the role of circRNA in gene regulation may be utilized as imperative treatment option. Importantly, the potential role of circRNAs as miRNA sponges can be utilized as an innovative approach to regulate gene expression. Further research on circRNA will enhance our understanding in relation to neuropathies like AD and lead to new diagnostic biomarkers and promising therapeutic options.
References
Veno MT, Hansen TB, Veno ST et al (2015) Spatio-temporal regulation of circular RNA expression during porcine embryonic brain development. Genome Biol 16:245
You X, Vlatkovic I, Babic A et al (2015) Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci 18(4):603–610
Rybak-Wolf A, Stottmeister C, Glazar P et al (2015) Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell 58(5):870–885
Ivanov A, Memczak S, Wyler E et al (2015) Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep 10(2):170–177; Jeck WR, Sorrentino JA, Wang K et al (2013) Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19(2):141–157
Burnette JM, Miyamoto-Sato E, Schaub MA et al (2005) Subdivision of large introns in Drosophila by recursive splicing at nonexonic elements. Genetics 170(2):661–674
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580):353–356
Bertram L, Tanzi RE (2008) Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat Rev Neurosci 9(10):768–778
Lukiw WJ (2013) Circular RNA (circRNA) in Alzheimer’s disease (AD). Front Genet 4:307
Hansen TB, Jensen TI, Clausen BH et al (2013) Natural RNA circles function as efficient microRNA sponges. Nature 495(7441):384–388
Cogswell JP, Ward J, Taylor IA et al (2008) Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J Alzheimers Dis 14(1):27–41
Bingol B, Sheng M (2011) Deconstruction for reconstruction: the role of proteolysis in neural plasticity and disease. Neuron 69(1):22–32
Lonskaya I, Shekoyan AR, Hebron ML et al (2013) Diminished parkin solubility and co-localization with intraneuronal amyloid-beta are associated with autophagic defects in Alzheimer’s disease. J Alzheimers Dis 33(1):231–247
Ginsberg SD, Alldred MJ, Che S (2012) Gene expression levels assessed by CA1 pyramidal neuron and regional hippocampal dissections in Alzheimer’s disease. Neurobiol Dis 45(1):99–107
Shao Y, Chen Y (2016) Roles of circular RNAs in neurologic disease. Front Mol Neurosci 9:25
Junn E, Lee KW, Jeong BS et al (2009) Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci USA 106(31):13052–13057
Choi DC, Chae YJ, Kabaria S et al (2014) MicroRNA-7 protects against 1-methyl-4-phenylpyridinium-induced cell death by targeting RelA. J Neurosci 34(38):12725–12737
Chen YT, Rettig WJ, Yenamandra AK et al (1990) Cerebellar degeneration-related antigen: a highly conserved neuroectodermal marker mapped to chromosomes X in human and mouse. Proc Natl Acad Sci USA 87(8):3077–3081; Dropcho EJ, Chen YT, Posner JB et al (1987) Cloning of a brain protein identified by autoantibodies from a patient with paraneoplastic cerebellar degeneration. Proc Natl Acad Sci USA 84(13):4552–4556
Liu Z, Jiang Z, Huang J et al (2014) miR-7 inhibits glioblastoma growth by simultaneously interfering with the PI3K/ATK and Raf/MEK/ERK pathways. Int J Oncol 44(5):1571–1580
Wang YH, Yu XH, Luo SS et al (2015) Comprehensive circular RNA profiling reveals that circular RNA100783 is involved in chronic CD28-associated CD8(+)T cell ageing. Immun Ageing 12:17
Fu D, Yu W, Li M et al (2015) MicroRNA-138 regulates the balance of Th1/Th2 via targeting RUNX3 in psoriasis. Immunol Lett 166(1):55–62
Satoh J, Obayashi S, Misawa T et al (2009) Protein microarray analysis identifies human cellular prion protein interactors. Neuropathol Appl Neurobiol 35(1):16–35; Satoh J, Yamamura T (2004) Gene expression profile following stable expression of the cellular prion protein. Cell Mol Neurobiol 24(6):793–814
Piwecka M, Glazar P, Hernandez-Miranda LR et al (2017) Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science 357(6357)
Guo JU, Agarwal V, Guo H et al (2014) Expanded identification and characterization of mammalian circular RNAs. Genome Biol 15(7):409
Chen W, Schuman E (2016) Circular RNAs in brain and other tissues: a functional enigma. Trends Neurosci 39(9):597–604
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Akhter, R. (2018). Circular RNA and Alzheimer’s Disease. In: Xiao, J. (eds) Circular RNAs. Advances in Experimental Medicine and Biology, vol 1087. Springer, Singapore. https://doi.org/10.1007/978-981-13-1426-1_19
Download citation
DOI: https://doi.org/10.1007/978-981-13-1426-1_19
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1425-4
Online ISBN: 978-981-13-1426-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)