Abstract
Three-dimensional (3D) printing (rapid prototyping or additive manufacturing) technologies have received significant attention in various fields over the past several decades. Tissue engineering applications of 3D bioprinting, in particular, have attracted the attention of many researchers. 3D scaffolds produced by the 3D bioprinting of biomaterials (bio-inks) enable the regeneration and restoration of various tissues and organs. These 3D bioprinting techniques are useful for fabricating scaffolds for biomedical and regenerative medicine and tissue engineering applications, permitting rapid manufacture with high-precision and control over size, porosity, and shape. In this review, we introduce a variety of tissue engineering applications to create bones, vascular, skin, cartilage, and neural structures using a variety of 3D bioprinting techniques.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In recent decades, regenerative medicine and tissue engineering research has been directed toward the regeneration, replacement, or restoration of injured functional living tissues and organs, such as bone, vascular, skin, neural, and cartilage [2, 22, 37, 40, 87]. These tissue engineering applications require the insights of researchers from a number of fields, as well as specialized knowledge of biomaterials, cell biology, biocompatibility, imaging, and the characterization of scaffold surfaces [27, 64]. One of the most important aspects of tissue engineering is the fabrication of porous three-dimensional (3D) scaffolds that provide the appropriate environment for regenerating tissues and organs. 3D scaffolds for use in tissue engineering field are fabricated using a various manufacturing methods and biomaterials [41, 79]. Several important characteristics must be considered in these applications [11]: First and most importantly, a tissue engineering scaffold must be biocompatible. Second, fabricated 3D scaffolds should be biodegradable or bioabsorbable so that tissue ultimately replaces the scaffold. Third, the ideal scaffold should have mechanical properties consistent with the tissue to be implanted. Fourth and finally, the 3D scaffold should be readily manufacturable in a variety of shapes and sizes. Several methods have been developed for fabricating 3D scaffolds using synthetic and natural polymers, including gas foaming, phase separation, electrospinning, and melt molding [20, 40, 55, 65]. These scaffold fabrication methods cannot precisely control the pore size, shape of the scaffold, or the internal channel configuration within the scaffold. Moreover, there are limits on the ability to fabricate scaffolds using cells.
In recent years, 3D bioprinting methods that can readily control the size and shape of a 3D scaffold and the capacity to produce scaffolds together with cells have attracted attention [19, 21]. In this review, we describe 3D bioprinting and its use in adjusting the shape and size of a 3D scaffold. 3D bioprinting was first introduced by Charles W. Hull in 1986 [45]. 3D bioprinting is the process of making 3D solid or gelation objects using 3D modeling software based on computer aided design (CAD) or computer tomography (CT) scan images [7]. 3D bioprinting refers to the technique associated with creating a 3D structure using metals, ceramics, synthetics, or natural polymers [73]. Typically, 3D bioprinting equipment consists of an X-, Y-, Z-axis drive machine, computers, 3D modeling software, and bioprinting materials. After creating a design using CAD or CT images, the 3D bioprinting equipment connected to a computer creates a 3D scaffold structure [91]. 3D bioprinting methods typically consist of stereolithography (SLA) [70], digital light processing (DLP) [25], multi jet modeling (MJM) [89], fused deposition modeling (FDM) [86], selective laser sintering (SLS) [48], and laminated object manufacturing (LOM) [1] components. The SLA and DLP methods use liquid photopolymer resins and ultraviolet (UV) lasers to create 3D structures [25, 70]. The MJM method involves feeding materials through a small diameter nozzle in a material injection process that operates in a manner similar to typical inkjet printers [89]. MJM is an inkjet bioprinting process that uses print head technologies to deposit photo-curable plastic resins or casting wax materials in a layer-by-layer method. The FDM 3D bioprinting method is a common and simple method of using thermoplastic filaments as a bioprinting material [86]. Filaments are melted in the head of a 3D printer by heating and then used to create 3D structures. The SLS bioprinting technology fuses small particles, such as polymers and ceramics, by heating using a high-power laser to form a 3D structure [48]. LOM is a method of creating 3D models by stacking layers of defined sheet materials, such as polymers, plastics, and metals [1]. These methods are used in a variety of fields, including architectural modeling, art, lightweight machinery, as well as in 3D biomaterials used in tissue engineering.
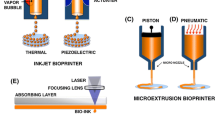
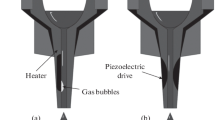
Among the 3D bioprinting methods available SLA and DLP laser-based bioprinting methods, inkjet bioprinting, and the FDM bioprinting of 3D structure used in tissue engineering field have attracted the most attention from researchers. Figure 2.1 illustrates the various 3D bioprinting methods used for tissue engineering applications. 3D bioprinting of biomaterials is a new technology aimed at developing new organs and tissues. 3D bioprinting technologies can adjust the size and shape of a 3D scaffold to control cell proliferation, differentiation, and attachment within the 3D construct. This review introduces a various of tissue engineering field to produce bone, vascular, skin, neuron, and cartilage tissue using 3D bioprinting methods. Table 2.1 summarizes the 3D bioprinting technologies that have been used to produce bio-inks (biomaterials) and cell types.
2 Bone Tissue Engineering Using 3D Bioprinting
Bone has a highly specialized organic–inorganic structure that can be classified as a micro- and nano-composite structure that is maintained adjacent to complex cellular components [9]. Researchers have investigated efficient approaches to replacing lost or defective bones and developing good bone substitutes for a very long time [61]. The 3D artificial bone scaffold is used in the clinic for bone regeneration. Bone displays excellent self-healing capabilities if defects are small; however, large-scale bone losses or defects cannot be completely healed by the body’s innate regeneration systems [82]. In such cases, surgery is needed to replace or repair lost or defective bone. Researchers have attempted to manufacture bone substitutes and to develop restoration methods ([24, 78, 92, 93]). Bones may be classified into two types of structures: cancellous bone (inner part of the bone), which has a spongy structure with a porosity of 50–90%; and cortical bone, which forms a dense outer layer with a porosity of less than 10%. Differences between the internal and external structures of bone require careful consideration for scaffold design for use in bone regeneration. Scaffolds can be used to deliver biomolecules, such as TGF-beta, BMP, IGF, FGF, or VEGF, to promote bone regeneration [49]. Recently, researchers have been interested in 3D bioprinting technologies that can create structures in one step using a various of biomaterials [10, 60].
This chapter examines bone tissue engineering field based on various 3D bioprinting techniques. Rath et al. performed preliminary in vitro tests to evaluate the effects of dynamic versus static 3D culture conditions during the seeding of osteogenic cells (bone marrow-derived stromal cells and osteoblasts) onto biphasic calcium phosphate (CaP) 3D scaffolds under osteoinductive and basal culture conditions [71]. Dong et al. integrated poly(ε-caprolactone) (PCL) and chitosan thermogels to form a hybrid 3D scaffold for bone regeneration [17]. The 3D PCL scaffolds were fabricated by FDM bioprinting method. The in vitro study shows that the hybrid 3D scaffold could enhance cell proliferation and improve the osteogenesis of rabbit bone marrow mesenchymal stem cells (MSCs). They hypothesized that the PCL/chitosan 3D scaffolds could improve osteoinductivity, cell seeding efficacy, and provide excellent mechanical properties compared to the PCL or chitosan-thermogel 3D scaffold alone. Corcione et al. developed a solvent-free process for producing a hydroxyapatite and poly(lactic acid) composite material suitable for 3D bioprinting processes (using the FDM method) to realize customized scaffolds for bone tissue engineering [14]. In their study, a clinical image of maxillary sinus obtained by cone beam computer tomography were converted into a suitable format and successfully used to fabricate a 3D maxillary sinus model using 3D bioprinting of the composite material. Wang et al. explained that a cell-laden collagen/alginate scaffold could be supplemented with bioglass particles, a well-fabricated, porous, hard material used to fabricate bone replacement scaffolds, to increase the material stiffness and stimulate cell growth and mineralization [90].
3 Neural Tissue Engineering Using 3D Bioprinting
More than a billion people around the world are thought to suffer from nervous system disorders [5]. Chronic degenerative diseases or traumatic injury of the nervous system affect central nervous system (CNS) function. Neurodegenerative diseases due to aging are also becoming increasingly important. Despite many studies, treatments that can fully restore neuronal function are not yet available, and our molecular understanding of pathogenic mechanisms is limited. These limitations arise from the lack of models suitable for simulating complex environments in vivo. 2D cultures are primarily used for their cost-effectiveness, ease of handling, and applicability to various cell types; however, 2D cultures are not able to support the cell–cell and cell–extracellular matrix (ECM) interactions present in vivo [33, 84, 96, 97]. By contrast, 3D tissue engineering is thought to provide a more human-like environment for cells. A variety of 3D tissue engineering systems have been studied for their ability to integrate multiple cell types and create complex neural tissue organization structures [23, 36, 38, 83]. 3D bioprinting can accurately mimic the complexity of our bodies using precisely placed cells and biomaterials based on a desired design [62]. This chapter reviews research into 3D neural tissue models prepared using 3D bioprinting technologies.
Xu et al. used fibrin as a bio-ink to create 3D cellular structures. Whole-cell patch-clamp recordings and immunostaining analysis showed that embryonic hippocampal and cortical neurons maintained their functions and basic cellular properties, including normal, healthy neuronal phenotypes and electrophysiological characteristics, after being printed through thermal inkjet nozzles [95]. Ilkhanizadeh et al. used an inkjet bioprinting system to print biologically active macromolecules onto poly(acrylamide)-based hydrogels that were subsequently seeded with primary fetal neural stem cells (NSCs). They found that the printed macromolecules remained biologically active when printed onto poly(acrylamide)-based hydrogels and influenced the differentiation of multipotent primary fetal NSCs in an efficient and spatially well-controlled manner [32]. Lee et al. reported a tissue engineering scaffold for bioprinting murine NSCs, VEGF-releasing fibrin gels and collagen hydrogels in the construction of an artificial neural tissue. They confirmed the morphological changes displayed by the printed murine NSCs embedded in the collagen and its migration toward the VEGF-releasing fibrin gel. The murine NSCs showed high viability (92.9%) after bioprinting, a viability equivalent to that of manually plated cells. Murine NSCs printed within 1 mm from the border of a VEGF-releasing fibrin gel displayed growth factor-induced morphological changes. The cells printed within this range migrated toward the VEGF-releasing fibrin gel, with a total migration distance of 102 ± 76 μm after 3 days of culture. The results show that 3D bioprinting of VEG-releasing fibrin gel supported sustained release of the growth factor in the collagen scaffold [52]. Owens et al. printed fully biological grafts composed exclusively of cells and cell secreted material. They printed grafts in a rat sciatic nerve injury model of both motor and sensory function. In particular, they compared the regenerative capacity of the 3D bioprinted grafts with that grafts composed of hollow collagen tubes or autologous grafts by measuring the compound action potential (for motor function) and the change in the mean arterial blood pressure as a consequence of electrically eliciting the somatic pressor reflex [66]. Hsieh et al. used a thermo-responsive hydrogel as a bio-ink and 3D bioprinted NSCs. The stiffness of the hydrogel could be easily fine-tuned based on the solid content of the dispersion. The NSCs in the PCL/poly-DL-lactide (PDLLA) hydrogels displayed differentiation and excellent proliferation but not in the PCL/poly-L-lactide (PLLA) hydrogels. Moreover, the NSCs-laden PCL/PDLLA hydrogels injected into a zebrafish embryo neural injury model rescued the function of the impaired nervous system; however, the NSCs-laden PCL/PLLA hydrogels only displayed minor repair effects in the neural injury model. The function of an adult zebrafish with traumatic brain injury was rescued after implanting the 3D bioprinted NSCs-laden PCL/PDLLA constructs [28]. Gu et al. produced neural tissue by 3D bioprinting human NSCs that were supporting neuroglia and differentiated in situ into functional neurons. The bio-ink incorporated novel clinically relevant polysaccharide-based biomaterials comprising agarose, alginate and carboxymethyl chitosan. Differentiated neurons formed synaptic contacts, established networks, were spontaneously active, showed a bicuculline-induced increased calcium response, and predominantly expressed gamma-aminobutyric acid [19, 21].
4 Vascular Tissue Engineering Using 3D Bioprinting
3D tissue engineering to mimic human body functions has been pursued for a long time. As the thickness of a 3D tissue increases, blood vessels become essential components. In a 2D cell culture having a cell population thickness of approximately 20~30 μm, nutrients and oxygen readily diffuse. However, when the 3D tissue thickness exceeds 100 μm, it is difficult for oxygen and nutrients to diffuse to every corner of the tissue [13]. Therefore, vascular tissue guided into the 3D tissue serves to supply nutrients, oxygen and remove waste products. New blood vessel formation in highly vascularized tissues (e.g., liver, kidney, lung, spleen, heart, pancreas, or thyroid) is essential [74, 75]. Thus, there is general consensus that the ability to reconstruct complex vascular networks is crucial to 3D tissue engineering [53]. However, this issue remains a major stumbling block to efforts to create 3D engineering structures with the volume and complexity of human organs [50, 51, 67]. 3D bioprinting technologies that create objects of a desired shape using a variety of bio-inks and cell types have emerged as attractive approaches to designing small-diameter vessels. The advantages of 3D bioprinting method are that researchers can produce heterocellular tissue constructs that readily control the cell density. This provides researchers with finer tools for addressing angiogenic problems in 3D tissue engineering [57]. These techniques can create biomimetic microenvironments in 3D tissues and produce vessels with ideal functions and structures. This chapter introduces research using 3D bioprinting technologies to form blood vessels in 3D tissues.
Cui et al. fabricated fibrin micro-channels using an inkjet-based bioprinting method. When bioprinting human microvascular endothelial cells (HMVEC) laden fibrin hydrogel, they confirmed that the cells aligned themselves within the fibrin channels and proliferated to form confluent linings. A 3D tubular structure was obtained from the printed patterns. They concluded that simultaneously bioprinting both the cells and scaffold promoted HMVEC proliferation and microvasculature formation [16]. Norotte et al. printed small-diameter multi-layered tubular vascular grafts that were readily perfused for further maturation. Agarose was used as a bio-ink to print smooth muscle cells and fibroblasts, aggregated into discrete units, either multicellular spheroids or cylinders of a controlled diameter (300–500 μm). The post-bioprinting fusion of the discrete units resulted in single- and double-layered small diameter vascular tubes [63]. Wu et al. used an omnidirectional bioprinting method to fabricate 3D microvascular networks embedded within a pluronic F127 hydrogel scaffold. Using this method, they fabricated 3D microvascular networks using a hierarchical, 3-generation branching topology to form microchannels of diameter 200–600 μm, in which two large parent channels were subdivided into many smaller microchannels [94]. Kolesky et al. reported a new 3D bioprinting method for fabricating 3D tissue constructs replete with vasculature, ECM and multiple types of cells. They confirmed the printability of these structures using two materials, a silicone elastomer and pluronic F127, and confirmed cell viability using a cell-laden gelatin methacryloyl (GelMA) hydrogel. They found that human neonatal dermal fibroblasts and human umbilical vein endothelial cells (HUVEC) proliferated over time [44]. Jia et al. [35] developed perfusable vascular structures with highly ordered arrangements in a single-step process. 4-arm poly(ethylene glycol)-tetra acrylate (PEGTA), GelMA, and alginate were used in combination with a multilayered coaxial extrusion printing system to achieve direct 3D bioprinting. The rheological properties of the bio-ink and the mechanical strengths of the resulting constructs were tuned by introducing PEGTA, which facilitated the precise deposition of complex multilayered 3D perfusable hollow tubes. This blend bio-ink also displayed favorable biological characteristics that supported the proliferation and spread of encapsulated endothelial and stem cells within the 3D bioprinted constructs, leading to the highly organized, formation of biologically relevant, perfusable vessels [35]. In other approaches, perfusable systems and 3D bioprinting have been integrated to achieve 3D tissue vascularization. Pimentel et al. [69] fabricated thick (1 cm) and densely populated tissue constructs using a 3D 4-arm branch network with stiffness comparable to that of soft tissues. This construct could be directly perfused on a fluidic platform over long periods of time (>14 days). They used poly(lactic acid) (PLA) as the support structure and poly(vinyl alcohol) (PVA) as the water-soluble main material. The PLA was selectively removed, and the PVA structure was used to create a artificial 3D vascular network within the ECM that mimicked the stiffness of the liver and encapsulated hepatocellular carcinoma (HepG2) cells. These hybrid constructs were directly perfused with medium to induce the proliferation and formation of HepG2 spheroids. In this study, the highest spheroid density was obtained with perfusion, but overall, the tissue construct displayed two distinct zones: one with a high cell death rate and almost no cell division and one of rapid proliferation. The model, therefore, simulated tissue gradients within necrotic tumor regions [69].
5 Skin Tissue Engineering Using 3D Bioprinting
In human body, the skin is the largest organ and protects other tissues from external stimuli. Skin damage leading to infections, or other genetic or physical ailments, can produce chronic ulcers. Skin injuries can expose other tissues to the external environment, including bacteria and viruses. Skin loss can disrupt body temperature regulation. Pathological components of normal skin flora can proliferate in the presence of a broken skin barrier. Skin loss in humans can lead to death in severe cases [12]. Thus, skin damage is a major problem with far-reaching effects on other tissues. Autologous grafts obtained directly from the patient are often used to avoid immune rejection and restore skin function and wound healing after skin damage. Unfortunately, skin damage wounds over large areas or with a significant depth are not adequately healed using autologous grafts [3, 54, 76]. For this reason, there is a need to produce artificial skin substitutes using novel approaches to skin regeneration [34, 58]. These studies have developed sophisticated skin substitutes that interact with human tissues after in vitro maturation and transplantation [56, 77, 85]. It has been difficult to mimic the skin of a person while accommodating nerve endings, capillaries, multi-layered 3D structures, and the numerous derivative structures, such as sebaceous glands, sweat glands, and hair follicles. Complex skin structures require accurate signaling systems. Without such systems, the skin structure is lost [80]. In this respect, 3D bioprinting is a very attractive method that enables the construction of human skin structure mimics using the spatio-temporal patterning of various bio-inks and cells. This chapter introduces research into human skin models using 3D bioprinting technologies.
Koch et al. fabricate a skin model containing dermis and epidermis layers using 3D laser-based bioprinting system. They used an alginate hydrogel as a bio-ink and printed fibroblast, keratinocyte, and hMSC. They then evaluated the influence of the laser-based bioprinting system on the cellular proliferation, survival rate, and apoptotic activity. Modifications of the cell surface markers and DNA damage were assessed and statistically evaluated over several days. The cells survived the transfer procedure with a viability exceeding 98%. All cell types tested maintained their proliferation ability after 3D bioprinting [43]. A collagen bio-ink, keratinocytes, and fibroblasts were printed to form a simple skin structure. The 3D bioprinted cell constructs were assessed after different culture times using immunohistological methods. The presence of cell–cell channels, which indicated tissue formation, was investigated in the vital 3D structures [42]. Michael et al. fabricated a skin substitute using 3D laser-based bioprinting. The skin substitutes were created using fibroblasts and keratinocytes. These 3D structures were subsequently tested in vivo. The bioprinted keratinocytes formed a multi-layered epidermis with initial differentiation and stratum corneum after 11 days of culture. Their proliferation was mainly detected in the suprabasal layers. E-cadherin, an indicator of adherens junctions and, therefore, tissue formation, was found in the epidermis in vivo as well as in vitro. In mice, some blood vessels were found to grow from the wound edges and the wound bed in the bioprinted direction of the cells [59]. Lee et al. demonstrated the potential utility of 3D bioprinting in tissue engineering using skin model as a prototypical human example. They printed collagen as a bio-ink, and fibroblasts and keratinocytes were used as constituent cells to form the dermis and epidermis. Immunohistological characterization revealed that the 3D bioprinted skin tissue was biologically and morphologically representative of human skin tissue in vivo. Compared to traditional methods of tissue engineering for skin, 3D bioprinting offers several advantages in terms of form retention and shape, reproducibility, flexibility, and a high culture throughput [50, 51]. Cubo et al. printed bilayer skin using fibrin bio-inks containing human primary keratinocytes and fibroblasts and human plasma. The structure and function of the 3D bioprinted skin was analyzed using immunohistochemical methods, both in 3D cultures in vitro and after long-term transplantation into immunodeficient mice. In both cases, the regenerated skin was very similar to human skin and, furthermore, was indistinguishable from the bilayered dermo-epidermal equivalents that were handmade in laboratories [26]. Nanoparticles have recently emerged as a transdermal delivery system. Their surface properties and size determine their efficacy and efficiency in penetrating the skin tissue. Hou et al. utilized 3D bioprinting technologies to generate a simplified artificial skin model useful for rapidly screening nanoparticles for their transdermal penetration capacity. A collagen hydrogel was used as a bio-ink, and fibroblasts were printed into the structure. The effectiveness of this platform was evaluated by using a 3D scaffold using one layer of fibroblasts sandwiched between two layers of a collagen hydrogel to screen silica nanoparticles with different surface charges for their penetration ability. Positively charged nanoparticles demonstrated deeper penetration, consistent with observations from previous studies involving living skin tissue [15].
6 Cartilage Tissue Engineering Using 3D Bioprinting
Cartilage, which is only a few millimeters thick, prevents friction between joints and endures extreme load stresses during limb movements. The cartilage defects due to aging, degenerative diseases, trauma or other several factors inevitably lead to arthralgia and chronic disorders [4, 31]. Despite numerous attempts, artificial cartilage that can fully mimic the composition of the tissue, ECM, and mechanical properties has not yet been developed [30]. 3D bioprinting, which can fabricate products of a desired shape using various materials and cells, presents a great opportunity in cartilage tissue engineering. This chapter discusses research into cartilage tissue engineering using 3D bioprinting.
Kundu et al. fabricated cell-printed 3D scaffolds using the PCL and chondrocyte- encapsulated alginate hydrogel. Cell-based biochemical in vitro assays were performed to measure the DNA, total collagen content, and glycosaminoglycans (GAGs) in the different alginate/PCL gel constructs. Alginate/PCL gels containing transforming growth factor-β (TGF-β) displayed greater ECM formation. The cell-printed 3D alginate/PCL gel scaffolds were implanted in the dorsal subcutaneous spaces of female nude mice. Immunohistochemical analyses revealed enhanced cartilage tissue and collagen type II fibril formation in the alginate/PCL gel (TGF- β) hybrid scaffold after 4 weeks [47]. Kesti et al. developed a cartilage-specific bio-ink for use in 3D bioprinting applications based on a blend of alginate and gellan mixed with commercially available BioCartilage particles. They imaged bioprinted scaffolds using magnetic resonance imaging (MRI) to compare the 3D shapes with the original model, and evaluated the utility of MRI in detecting changes in the water relaxation times as they related to ECM production in tissue-engineered grafts. To evaluate cartilage formation, cell-laden Bioink and Bioink/BioCartilage disks were cultured for 8 weeks in vitro with and without TGF-β3 supplementation. All of the characteristics of the 3D bioprinted scaffolds were superior to those of native articular cartilage [39]. Ren et al. used collagen hydrogel as a bioink and fabricated 3D cartilage constructs. The chondrocyte density gradient indicated a zonal distribution throughout the ECM. They evaluated the effect of the chondrocyte density gradient on the formation of the regional distribution of ECM in the bioprinted 3D structure. The ECM production was positively correlated with the cell density during the early stages of culture, and the biosynthetic abilities of chondrocytes were affected by both the cell density and the cell distribution in the bioprinted 3D structure [72].
7 Cancer Model Using 3D Bioprinting
Cancer is a leading cause of disease and death throughout the world. Despite advances in cancer treatment, many challenges remain, and the characteristics of the tumor microenvironment must be considered [6, 68]. Cancer research commonly relies on 2D cultures and animal models; however, it is difficult to imitate 3D human tissues in 2D cultures, and animal model results cannot necessarily be extrapolated to the human response [8, 18, 46, 88, 96, 97]. These problems may be addressed by furthering our understanding of complex cancers tissue using 3D tumor models that can mimic the microenvironment of native cancer. This chapter introduces research into bioprinted 3D cancer models.
Snyder et al. fabricated a 3D liver cancer model and studied the radiation protection functionalities and drug conversion of living liver tissue analogs. They used matrigel as a bio-ink onto which were printed human hepatic carcinoma cells. Cell-laden bioprinted matrigel and microfluidic chips were used to evaluate the radiation shielding properties of the liver cells using the amifostine pro-drug. The benefits of radiation protection and the conversion of pro-drug by multiple cell types were best realized using a dual-tissue model [81]. Huang et al. created a in vitro 3D micro-chip in a hydrogel using 3D projection bioprinting. The micro-chip featured a honeycomb branched structure that mimicked a 3D vascular morphology useful for monitoring, and analyzing differences in the behaviors of cancer cell lines (Hela) versus normal cells (fibroblasts). Fibroblasts exhibited greater morphological changes due to channel width than HeLa cell lines; however, the channel width had a limited influence on fibroblasts migration, whereas HeLa cells migration increased as the channel width decreased [29]. Zhao et al. reported a HeLa cells laden 3D bioprinting alginate/fibrinogen/gelatin hydrogels to construct in vitro cervical tumor models. Cell viability was exceeding 90% using the defined bioprinting technology. Comparisons between the 2D and 3D culture models revealed that the HeLa cells showed a higher proliferation rate in the bioprinted 3D culture model and tended to form HeLa cells spheroids, whereas they formed monolayer cell sheets in the 2D culture system. HeLa cells in the bioprinted 3D models also displayed higher chemoresistance and higher matrix metalloproteinase (MMP) expression than those in the 2D cultures [98].
8 Challenges and Future Directions
Thus far, a variety of 3D bioprinting technologies have been used to study tissue engineering applications intended to mimic a variety of tissues and organs. 3D bioprinting has paved the way combining biomaterials, imaging, modeling, and computational technologies in the biomedical and tissue engineering field. The 3D bioprinting technologies permits adjustments to the shape, porosity, and size of the 3D scaffolds, attracting much attention in the tissue engineering field. Several challenges remain, for example the development of biomaterials (bio-inks) for use in bioprinting tissues or organs. Conventional 3D bioprinting focuses on 3D structure creation without cells, whereas recent 3D bioprinting technologies have quickly and accurately produced 3D structures using cells in one step. 3D bioprinting with cells requires materials with excellent biocompatibility and cell affinity. For this reason, the development of bio-inks is very important for bioprinting with cells. In the future, it will be necessary to develop new biomaterials and increase the precision of bioprinting equipment to quickly create accurate 3D structures.
References
Ahn D, Kweon JH, Lee S (2012) Quantification of surface roughness of parts processed by laminated object manufacturing. J Mater Proc Technol 212:339–346. https://doi.org/10.1016/j.jmatprotec.2011.08.013
Amini AR, Laurencin CT, Nukavarapu SP (2012) Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40(5):363–408. https://doi.org/10.1615/CritRevBiomedEng.v40,i5.10
Andreassi A, Bilenchi R, Biagioli M, D’Aniello C (2005) Classification and pathophysiology of skin grafts. Clin Dermatol 23:332–337. https://doi.org/10.1016/j.clindermatol.2004.07.024
Arkel RV, Amis A (2013) Basics of orthopaedic biomechanics. Orthopaedics and Trauma 27(2):67–75. https://doi.org/10.1016/J.mporth.2013.01.003
Benam KH, Dauth S, Hassell B, Herland A, Jain A, Jang KJ, Karalis K, Kim HJ, MacQueen L, Mahmoodian R, Musah S, Torisawa Y, Meer AD, Villenave R, Yadid M, Parker KK, Ingber DE (2015) Engineered in vitro disease models. Annu Rev Pathol Mech Dis 10:195–262. https://doi.org/10.1146/annurev-pathol-012414-040418
Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, Sboner A, Esgueva R, Pflueger D, Sougnez C, Onofrio R, Carter SL, Park K, Habegger L, Ambrogio L, Fennell T, Parkin M, Saksena G, Voet D, Ramos AH, Pugh TJ, Wilkinson J, Fisher S, Winckler W, Mahan S, Ardlie K, Baldwin J, Simons JW, Kitabayashi N, MacDonald TY, Kantoff PW, Chin L, Gabriel SB, Gerstein MB, Golub TR, Meyerson M, Tewari A, Lander ES, Getz G, Rubin MA, Garraway LA (2011) The genomic complexity of primary human prostate cancer. Nature 470:214–220. https://doi.org/10.1038/nature09744
Bikas H, Stavropoulos P, Chryssolouris G (2016) Additive manufacturing methods and modeling approaches: a critical review. Int J Adv Manuf Technol 83:389–405. https://doi.org/10.1007/s00170-015-7576-2
Bildziukevich U, Rárová L, Šaman D, Wimmer Z (2018) Picolyl amides of betulinic acid as antitumor agents causing tumor cell apoptosis. Eur J Med Chem 145:41–50. https://doi.org/10.1016/j.ejmech.2017.12.096
Bose S, Roy M, Bandyopadhyay A (2012) Recent advances in bone tissue engineering scaffolds. Trends Biotechnol 30(10):546–554. https://doi.org/10.1016/j.tibtech.2012.07.005
Bose S, Vahabzadeh S, Bandyopadhyay A (2013) Bone tissue engineering using 3D printing. Mater Today 16(12):496–504. https://doi.org/10.1016/j.mattod.2013.11.017
Chan BP, Leong KW (2008) Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J 17:S467–S479. https://doi.org/10.1007/s00586-008-0745-3
Church D, Elsayed S, Reid O, Winston B, Lindsay R (2006) Burn wound infections. Clin Microbiol Rev 19(2):403–434. https://doi.org/10.1128/CMR.19.2.403–434.2006
Colton CK (1995) Implantable biohybrid artificial organs. Cell Transplant 4(4):415–436. https://doi.org/10.1016/0963-6897(95)00025-S
Corcione CE, Gervaso F, Scalera F, Montagna F, Maiullaro T, Sannino A, Maffezzoli A (2017) 3D printing of hydroxyapatite polymer-based composites for bone tissue engineering. J Polym Eng 37(8):741–746. https://doi.org/10.1515/polyeng-2016-0194
Cubo N, Garcia M, Cañizo JF, Velasco D, Jorcano JL (2017) 3D biopriting of functional human skin: production and in vivo analysis. Biofabrication 9:015006. https://doi.org/10.1088/1758-5090/9/1/015006
Cui X, boland T (2009) Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 30:6221–6227. https://doi.org/10.1016/j.biomaterials.2009.07.056
Dong L, Wang SJ, Zhao XR, Zhu YF, Yu JK (2017) 3D-printed poly(ε-caprolactone) scaffold integrated with cell-laden chitosan hydrogels for bone tissue engineering. Sci Rep 7:13412. https://doi.org/10.1038/s41598-017-13838-7
Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R (2006) Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. PNAS 103(16):6315–6320. https://doi.org/10.1073/pnas.060175510
Gu BK, Choi DJ, Park SJ, Kim MS, Kang CM, Kim CH (2016a) 3-dimensional bioprinting for tissue engineering applications. Biomater. Res. 20:12. https://doi.org/10.1186/s40824-016-0058-2
Gu BK, Park SJ, Kim MS, Kang CM, Kim JI, Kim CH (2013) Fabrication of sonicated chitosan nanofiber mat with enlarged porosity for use as hemostatic materials. Carbohyd Polym 97:65–73. https://doi.org/10.1016/j.carbpol.2013.04.060
Gu Q, Tomaskovic-Crook E, Lozano R, Chen Y, Kapsa RM, Zhou Q, Wallace GG, Crook JM (2016b) Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Adv Healthc Mater 5:1429–1438. https://doi.org/10.1002/adhm.201600095
Gu X, Ding F, Williams DF (2014) Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 35(24):6143–6156. https://doi.org/10.1016/j.biomaterials.2014.04.064
Haring AP, Sontheimer H, Johnson BN (2017) Microphysiological hman brain and neural systems-on-a-chip: potential alternatives to small animal models and emerging platforms for drug discovery and personalized medicine. Stem Cell Rev Rep 13:381–406. https://doi.org/10.1007/s12015-017-9738-0
Henkel J, Woodruff MA, Epari DR, Steck R, Glatt V, Dickinson IC, Choong PFM, Schuetz MA, Hutmacher DW (2013) Bone regeneration based on tissue engineering conceptions – a 21st century perspective. Bone Resear 1(3):216–248. https://doi.org/10.4248/BR201303002
Holmers LR, Riddick JC (2014) Research summary of an additive manufacturing technology for the fabrication of 3D composites with tailored internal structure. J Minerals, Metals, Materials Society 66(2):270–274. https://doi.org/10.1007/s11837-013-0828-4
Hou X, Liu S, Wang M, Wiraja C, Huang W, Chan P, Tan T, Xu C (2017) Layer-by-layer 3D constructs of fibroblasts in hydrogel for examining transdermal penetration capability of nanoparticles. SLAS Technol 22(4):447–453. https://doi.org/10.1177/2211068216655753
Howard D, Buttery LD, Shakesheff KM, Roberts SJ (2008) Tissue engineering: strategies, stem cells and scaffolds. J Anat 213:66–72. https://doi.org/10.1111/j.1469-7580.2008.00878.x
Hsieh FY, Lin HH, Hsu SH (2015) 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 71:48–57. https://doi.org/10.1016/j.biomaterials.2015.08.028
Huang TQ, Qu X, Liu J, Chen S (2014) 3D printing of biomimetic microstructures for cancer cell migration. Biomed Microdevices 16:127–132. https://doi.org/10.1007/s10544-013-9812-6
Hunziker EB (2001) Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartil 10:432–463. https://doi.org/10.1053/joca.2002.0801
Hutmacher DW (2000) Scaffold in tissue engineering bone and cartilage. Biomaterials 21:2529–2543. https://doi.org/10.1016/B978-008045154-1.50021-6
Ilkhanizadeh S, Teixeira AI, Hermanson O (2007) Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials 28:3936–3943. https://doi.org/10.1016/j.biomaterials.2007.05.01
Imamura Y, Mukohara T, Shimono Y, Funakoshi Y, Chayahara N, Toyoda M, Kiyota N, Takao S, Kono S, Nakatuura T, Minami H (2015) Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Onc Rep 33:1837–1843. https://doi.org/10.3892/or.2015.3767
Jean J, Garcia-Perez ME, Pouliot R (2011) Bioengineered skin: the self-assembly approach. J Tissue Sci Eng S5:001. https://doi.org/10.4172/2157-7552.S5-001
Jia WJ, Gungor-Ozkerim PS, Zhang YS, Yue K, Zhu K, Liu W, Pi Q, Byambaa B, Dokmeci MR, Shin SR, Khademhosseini A (2016) Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 106:58–68. https://doi.org/10.1016/j.biomaterials.2016.07.038
Jo J, Xiao Y, Sun AX, Cukuroglu E, Tran HD, Göke J, Tan ZY, Saw TY, Tan CP, Lokman H, Lee Y, Kim D, Ko HS, Kim SO, Park JH, Cho NJ, Hyde TM, Kleinman JE, Shin JH, Weinberger DR, Tan EK, Je HS, Ng HH (2016) Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell 19:248–257. https://doi.org/10.1016/j.stem.2016.07.005
Jung JP, Bhuiyan DB, Ogle BM (2016) Solid organ fabrication: comparison of decellularization to 3D bioprinting. Biomater. Res. 20:27. https://doi.org/10.1186/s40824-016-0074-2
Kato-Negishi M, Morimoto Y, Onoe H, Takeuchi S (2013) Millimeter-sized neural building blocks for 3D heterogeneous neural network assembly. Adv Healthc Mater 2:1564–1570. https://doi.org/10.1002/adhm.201300052
Kesti M, Eberhardt C, Pagliccia G, Kenkel D, Grande D, Boss A, Wong MZ (2015) Bioprinting complex cartilaginous structures with clinically compliant biomaterials. Adv Funct Mater 25:7406–7417. https://doi.org/10.1002/adfm.201503423
Kim JE, Kim SH, Jung Y (2016) Current status of three-dimensional printing inks for soft tissue regeneration. Tissue Eng. Regen. Med. 13(6):636–646. https://doi.org/10.1007/s13770-016-0125-8
Knight E, Przyborski S (2015) Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J Anat 227:746–756. https://doi.org/10.1111/joa.12257
Koch L, Deiwick A, Schlie S, Michael S, Gruene M, Coger V, Zychlinski D, Schambach A, Reimers K, Vogy PM, Chichkov B (2012) Skin tissue generation by laser cell printing. Biotechnol Bioeng 109(7):1855–1863. https://doi.org/10.1002/bit.24455
Koch L, Kuhn S, Sorg H, Gruene M, Schlie S, Gaebel R, Polchow B, Reimers K, Stoelting S, Ma N, Vogt PM, Steinhoff G, Chichkov B (2010) Laser printing of skin cells and human stem cells. Tissue engineering: part C 16(5):847–185. https://doi.org/10.1089/ten.tec.2009.0397
Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA (2014) 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater 26:3124–3130. https://doi.org/10.1002/adma.201305506
Kruth JP, Leu MC, Nakagawa T (1998) Progress in additive manufacturing and rapid prototyping. ClRP Annals 47(2):525–540. https://doi.org/10.1016/s0007-8506(7)63240-5
Kukowska-Latallo JF, Candido KA, Cao Z, Nigavekar SS, Majoros IJ, Thomas YP, Balogh LP, Khan MK, Baker JR (2005) Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res 65(12):5317–5324. https://doi.org/10.1158/0008-5472.CAN-04-3921
Kundu J, Shim JH, Jang J, Kim SW, Cho DE (2015) An additive manufacturing-based PCL–alginate–chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng Regen Med 9:1286–1297. https://doi.org/10.1002/term.1682
Land WS II, Zhang B, Ziegert J, Davies A (2015) In-situ metrology system for laser powder bed fusion additive process. Procedia Manuf 1:393–403. https://doi.org/10.1016/j.promfg.2015.09.047
Lee K, Silva EA, Mooney DJ (2011) Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 8:153–170. https://doi.org/10.1098/rsif.2010.0223
Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Tran TN, Yoo SS, Dai G, Karande P (2014b) Design and fabrication of human skin by three-dimensional bioprinting. Tissue engineering: part C. 20(6):473–484. https://doi.org/10.1089/ten.tec.2013.0335
Lee VK, Lanzi AM, Ngo H, Yoo SS, Vincent PA, Dai G (2014a) Generation of multi-scale vascular network system within 3D hydrogel using 3D bio-printing technology. Cell Mol Bioeng 7(3):460–472. https://doi.org/10.1007/s12195-014-0340-0
Lee YB, Polio S, Lee W, Dai G, Menon L (2010) Bio-printing of collagen and VEGF-releasing fibrin gel scaffolds for neural stem cell culture. Exp Neurol 223:645–652. https://doi.org/10.1016/j.expneurol.2010.02.014
Leong MF, Toh JKC, Du C, Narayanan K, Lu HF, Lim TC, Wan ACA, Ying JY (2013) Patterned prevascularised tissue constructs by assembly of polyelectrolyte hydrogel fibres. Nat Commun 4:2353. https://doi.org/10.1038/ncomms3353
Loss M, Wedler V, Künzi W, Meuli-Simmen C, Meyer VE (2000) Artifcial skin, split-thickness autograft and cultured autologous keratinocytes combined to treat a severe burn injury of 93% of TBSA. Burns 26:644–652
Ma H, Xue L (2015) Carbon nanotubes reinforced poly(L-lactide) scaffolds fabricated by thermally induced phase separation. Nanotechnology 26:025701. https://doi.org/10.1088/0957-4484/26/2/025701
MacNeil S (2007) Progress and opportunities for tissue-engineered skin. Nature 445:874–880. https://doi.org/10.1038/nature05664
Melchiorri AJ, Fisher JP (2015) Bioprinting of blood vessels, in Essentials 3D Biofabrication Translation. pp 337–350
Metcalfe AD, Ferguson MWJ (2007) J R Soc Interface 4:413–437. https://doi.org/10.1098/rsif.2006.0179
Michael S, Sorg H, Peck CT, Koch L, Deiwick A, Chichkov B, Vogt PM, Reimers K (2013) Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice. PLoS One 8(3):e57741. https://doi.org/10.1371/journal.pone.0057741
Mota RCAG, Silva EO, Lima FF, Menezes LR, Thiele ACS (2016) 3D printed scaffolds as a new perspective for bone tissue regeneration: literature review. Mater Sci App 7:430–452. https://doi.org/10.4236/msa.2016.78039
Mravic M, Péault B, James AW (2014) Current trends in bone tissue engineering. BioMed Resear Inter 865270:1. https://doi.org/10.1155/2014/865270
Ng WL, Wang S, Yeoung WY, Naing MW (2016) Skin bioprinting: impending reality or fantasy? Trends in Biotechnol 34(9):689–699. https://doi.org/10.1016/j.tibtech.2016.04.006
Norotte C, Marga FS, Niklason LE, Forgacs G (2009) Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30:5910–5917. https://doi.org/10.1016/j.biomaterials.2009.06.034
O’Brien FJ (2011) Biomaterials and scaffolds for tissue engineering. Mater Today 14(3):88–95. https://doi.org/10.1016/S1369-7021(11)70058-X
Oh SH, Kang SG, Kim ES, Cho SH, Lee JH (2003) Fabrication and characterization of hydrophilic poly(lactic-co-glycolic acid)/poly(vinyl alcohol) blend cell scaffolds by melt-molding particulate-leaching method. Biomaterials 24:4011–4021. https://doi.org/10.1016/S0142-9612(03)00284-9
Owens CM, Marga F, Forgacs G, Heesch CM (2013) Biofabrication and testing of a fully cellular nerve graft. Biofabrication 5:045007. https://doi.org/10.1088/1758-5082/5/4/045007
Ozbolat IT (2015) Bioprinting scale-up tissue and organ constructs for transplantation. Trends in Biotechnol. 33(7):395–400. https://doi.org/10.1016/j.tibtech.2015.04.005
Palanisamy N, Ateeq B, Sundaram SK, Pflueger D, Ramnarayanan K, Shankar S, Han B, Cao Q, Cao X, Suleman K, Sinha CK, Dhanasekaran SM, Chen YB, Esgueva R, Banerjee S, LaFargue CJ, Siddiqui J, Demichelis F, Moeller P, BismarTA KR, Fullen DR, Johnson TM, Greenson JK, Giordano TJ, Tan P, Tomlins SA, Varambally S, Rubin MA, Maher CA, Chinnaiyan AM (2010) Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nat Med 16(7):793–798. https://doi.org/10.1038/nm.2166
Pimentel CR, Ko SK, Caniglia C, Wolff A, Emnéus J, Keller SS, Dufva M (2017) Three-dimensional fabrication of thick and densely populated soft constructs with complex and actively perfused channel network. Acta Biomater 65:174–184. https://doi.org/10.1016/j.actbio.2017.10.047
Quan Z, Wu A, Keefe M, Qin X, Yu J, Suhr J, Byun JH, Kim BS, Chou TW (2015) Additive manufacturing of multi-directional performs for composites: opportunities and challenges. Mater Today 18(9):503–512. https://doi.org/10.1016/j.mattod.2015.05.001
Rath SN, Strobel LA, Arkudas A, Beier JP, Maier AK, Greil P, Horch RE, Kneser U (2012) Osteoinduction and survival of osteoblasts and bone-marrow stromal cells in 3D biphasic calcium phosphate scaffolds under static and dynamic culture conditions. J Cell Mol Med 16(10):2350–2361. https://doi.org/10.1111/j.1582-4934.2012.01545.x
Ren X, Wang F, Chen C, Gong X, Yin L, Yang L (2016) Engineering zonal cartilage through bioprinting collagen type II hydrogel constructs with biomimetic chondrocyte density gradient. BMC Musculoskelet Disord 17:301. https://doi.org/10.1186/s12891-016-1130-8
Rengier F, Mehndiratta A, Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor HU, Giesel FL (2010) 3D printing based on imaging data: review of medical applications. Int J CARS 5:335–341. https://doi.org/10.1007/s11548-010-0476-x
Richards D, Jia J, Yost M, Markwald R, Mei Y (2016) 3D bioprinting for vascularized tissue fabrication. Annals Biomed Engin 45(1):132–147. https://doi.org/10.1007/s10439-016-1653-z
Santos MI, Reis RL (2010) Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol Biosci 10:12–27. https://doi.org/10.1002/mabi.200900107
Sheridan R (2009) Closure of the excised burn wound: autografts, semipermanent skin substitutes, and permanent skin substitutes. Clin Plastic Surg 36:643–651. https://doi.org/10.1016/j.cps.2009.05.010
Shevchenko RV, James SL, James SE (2010) A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface 7:229–258. https://doi.org/10.1098/rsif.2009.0403
Shin M, Yoshimoto H, Vacanti JP (2004) In vivo bone tissue engineering using mesenchymal stem cells on a novel electrospun nanofibrous scaffold. Tissue Eng 10(1/2):33–41. https://doi.org/10.1089/107632704322791673
Shivalkar S, Singh S (2017) Solid freeform techniques application in bone tissue engineering for scaffold fabrication. Tissue Eng Regen Med 14(3):187–200. https://doi.org/10.1007/s13770-016-0002-5
Singer AJ, Clark RAF (1999) Cutaneous wound healing. N Engl J Med 341(10):738–746. https://doi.org/10.1056/NEJM199909023411006
Snyder JE, Hamid Q, Wang C, Chang R, Emami K, Wu H, Sun W (2011) Bioprinting cell-laden matrigel for radioprotection study of liver by pro-drug conversion in a dual-tissue microfluidic chip. Biofabrication 3:034112. https://doi.org/10.1088/1758-5082/3/3/034112
Stevens MM (2008) Biomaterials for bone tissue engineering. Mater Today 11(5):18–25. https://doi.org/10.1016/S1369-7021(08)70086-5
Tang-Schomer MD, White JD, Tien LW, Schmitt LI, Valentin TM, Graziano DJ, Hopkins AM, Omenetto FG, Haydon PG, Kaplan DL (2014) Bioengineered functional brain-like cortical tissue. PNAS 111(38):13811–13816. https://doi.org/10.1073/pnas.1324214111
Tian XF, Heng BC, Ge Z, Lu K, Rufaihah AJ, Fan VTW, Yeo JF, Cao T (2008) Comparison of osteogenesis of human embryonic stem cells within 2D and 3D culture systems. Scandinavian J Clin Lab Inves 68(1):58–67. https://doi.org/10.1080/00365510701466416
Trottier V, Marceau-Fortier G, Germain L, Vincent C, Fradette J (2008) IFATS collection: using human adipose-derived stem/stromal cells for the production of new skin substitutes. Stem Cells 26:2713–2723. https://doi.org/10.1634/stemcells.2008-0031
Turner BN, Strong R, Gold SA (2014) A review of melt extrusion additive manufacturing processes: I. Process design and modeling. Rapid Prototyp J 20(3):192–204. https://doi.org/10.1108/RPJ-01-2013-0012
Vig K, Chaudhari A, Tripathi S, Dixit S, Sahu R, Pillai S, Dennis VA, Singh SR (2017) Advances in skin regeneration using tissue engineering. Int J Mol Sci 18:789. https://doi.org/10.3390/ijms18040789
Wang F, Tang J, Li P, Si S, Yu H, Yang X, Tao J, Lv Q, Gu M, Yang H, Wang Z (2018) Chloroquine enhances the radiosensitivity of bladder cancer cells by inhibiting autophagy and activating apoptosis. Cell Physiol Biochem 45:54–66. https://doi.org/10.1159/000486222
Wang X, Ao Q, Tian X, Fan J, Wei Y, Hou W, Tong H, Bai S (2016) 3D bioprinting technologies for hard tissue and organ engineering. Materials 9:802. https://doi.org/10.3390/ma9100802
Wang X, Tolba E, Schröder HC, Neufurth M, Feng Q, Diehl-Seifert B, Müller WEG (2014) Effect of bioglass on growth and biomineralization of SaOS-2 cells in hydrogel after 3D cell bioprinting. PLoS One 9(11):e11497. https://doi.org/10.1371/journal.pone.0112497.
Wong KV, Hernandez A (2012) A review of additive manufacturing. Inter Scholar Resear Net 208760:1. https://doi.org/10.5402/2012/208760
Wu H, Lei P, Liu G, Zhang YS, Yang J, Zhang L, Xie J, Niu W, Liu H, Ruan J, Hu Y, Zhang C (2017a) Reconstruction of large-scale defects with a novel hybrid scaffold made from poly(L-lactic acid)/nanohydroxyapatite/alendronate-loaded chitosan microsphere: in vitro and in vivo studies. Sci Rep 7:359. https://doi.org/10.1038/s41598-017-00506-z
Wu T, Yu S, Chen D, Wang Y (2017b) Bionic design, materials and performance of bone tissue scaffolds. Materials 10:1187. https://doi.org/10.3390/ma10101187
Wu W, DeConinck A, Lewis JA (2011) Omnidirectional printing of 3D microvascular networks. Adv Mater 23:H178–H183. https://doi.org/10.1002/adma.201004625
Xu T, Gregory CA, Molnar P, Cui X, Jalota S, Bhaduri SB, Boland T (2006) Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 27:3580–3588. https://doi.org/10.1016/j.biomaterials.2006.01.048
Zhang D, Pekkanen-Mattila M, Shahsavani M, Falk A, Teixeira AI, Herland A (2014b) A 3D Alzheimer’s disease culture model and the induction of P21-activated kinase mediated sensing in iPSC derived neurons. Biomaterials 35:1420–1428. https://doi.org/10.1016/j.biomaterials.2013.11.028
Zhang XD, Chen J, Min Y, Park GB, Shen X, Song SS, Sun YM, Wang H, Long W, Xie J, Gao K, Zhang L, Fan S, Fan F, Jeong U (2014a) Metabolizable Bi2Se3 nanoplates: biodistribution, toxicity, and uses for cancer radiation therapy and imaging. Adv Funct Mater 24:1718–1729. https://doi.org/10.1002/adfm.201302312
Zhao Y, Yao R, Ouyang L, Ding H, Zhang T, Zhang K, Cheng S, Sun W (2014) Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 6:035001. https://doi.org/10.1088/1758-5082/6/3/035001
Acknowledgements
This study was supported by a grant of KIRAMS, funded by Ministry of Science, ICT and Future Planning, South Korea (1711061997/50531-2018) and the Technology Innovation Program (10053595, Development of functionalized hydrogel scaffold based on medical grade biomaterials with 30% or less of molecular weight reduction) funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gu, B.K., Choi, D.J., Park, S.J., Kim, YJ., Kim, CH. (2018). 3D Bioprinting Technologies for Tissue Engineering Applications. In: Chun, H., Park, C., Kwon, I., Khang, G. (eds) Cutting-Edge Enabling Technologies for Regenerative Medicine. Advances in Experimental Medicine and Biology, vol 1078. Springer, Singapore. https://doi.org/10.1007/978-981-13-0950-2_2
Download citation
DOI: https://doi.org/10.1007/978-981-13-0950-2_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-0949-6
Online ISBN: 978-981-13-0950-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)