Abstract
Medicinal plants are used by 80% of the world population for their primary health care. The medicinal value of plants is primarily attributed to the secondary metabolite content such as terpenoids, alkaloids, and phenolics. These compounds play a crucial role in plant defense, are merchandised valued for their therapeutic applications and ecological role, and are also used as flavoring agents. Arbuscular mycorrhizal fungi (AMF) or Glomeromycota is known to form a symbiotic relationship with many terrestrial plants. AM fungi–plant consortium enhanced the production of plant terpenoids, alkaloids, and phenolics, which are valuable to human health. The potential role of arbuscular mycorrhiza (AM) symbiosis in amplification of the secondary metabolite content has attained enormous recognition for sustainable cultivation of medicinally important crops. AMF–plant symbiosis not only improves the growth and nutrients but also exerts a synergistic effect on accumulation of bioactive compounds with medicinal importance. Current studies have also recognized AM-mediated modulation of morphology, biochemistry, and gene expression in medicinal as well as in the industrial important plants. This chapter provides an appraisal on contemporary finding in the area of AMF investigation with a marked emphasis on the yield of pharmaceutically important plant-derived secondary metabolites.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The use of medicinal plants as therapeutic agents against various diseases has been notable for thousands of years (Gopal 2001). The World Health Organization (WHO) appraises that more than 80% of global population utilize plants and plant products for their primary health care (Cordell 1995). There is a huge demand for traditional herbal medicine which has increased several folds globally during the recent past. The primary reason behind this boon is thought to be the introduction of traditional knowledge-based drugs like Taxol (anticancer), Artemisinin (antimalarial), Forskolin (antihypertensive) (Ghisalberti 1993), and many others in the Western market.
Fransworth et al. (1985) estimated that at least 119 compounds derived from 90 plant species are considerably significant drugs currently practiced in one or more countries and 77% of these are obtained mostly from botanicals used in traditional medicine. The traditional Chinese medicine (TCM), the Indian system of medicine (ISM), the Japanese Kampo, and African folklore are still practiced by major population of Asia and Africa. The botanical-based treatments have remained a major part of TCM, and even today it comprises approximately 50% of total drugs used in China. Of the 5500 medicinal plants used in TCM, about 300–500 are commonly used in day-to-day prescriptions (Corizier 1974). One such drug that has been used in China for the past hundreds of years is Ephedra sinica (Ma huang), the active principle of which is a potent sympathomimetic amine, ephedrine. This medicine in the form of various salts is now used in Western medicine to treat bronchial asthma.
The Indian system of medicine (ISM), mainly constituting of Ayurveda, Siddha, and Unani, is considered as one of the traditional system of medicine with thoroughly documented plant-based therapeutics. In India and abroad also, Ayurveda is being practiced by a vast population. In ISM, the remedies are primarily based on plants and plant products that usually work on the human system to boost immunity, resistance, and strength in order to cure the diseases (Swami Tirtha 1998). Some of the classical examples are as follows: (a) reserpine, an indole alkaloid as a potent antihypertensive and tranquilizing agent developed from the Indian medicinal plant Rauwolfia serpentina (Warrier et al. 1996), and (b) forskolin, a highly oxygenated labdane diterpenoid from the roots of Indian medicinal plant Coleus forskohlii exhibiting potent antihypertensive, antithrombotic, and positive ionotropic properties (Rastogi and Meharotra 1990). The Kampo system of medicine is practiced by a considerable population in Japan, where about 70% of physicians prescribe one or another Kampo formulations (Ishibashi 2002). Some of the bioactive isolates from the plants used in Japanese Kampo are baicalein (from Scutellaria baicalensis) (Huang 1999), glycyrrhizin (from Glycyrrhiza uralensis) (Tang and Eisenbrand 1992), and saikosaponin I (from Bupleurum falcatum) (Shibata et al. 1923).
In most of the African countries, up to 80% of population depends entirely on botanicals for therapeutics (Suzuki 2002; David 2000), many of which have been documented in the form of an African pharmacopeia issued by a scientific technical research commission of the organization of African Unity in 1984. A few promising drugs developed from African medicinal plants include (a) caffeine from the coffee tree Coffea arabica (from the highlands of southwest Ethiopia), a purine alkaloid exhibiting potent CNS activity and positive inotropic activity and activates lipolysis, and (b) ouabain (γ-strophanthin) isolated from Strophanthus gratus (from tropical West Africa) – a cardiac glycoside used to treat heart problems in acquits cardiac insufficiency (Hostettmann et al. 2000).
Due to the devoted work from the researchers, good proportion of promising drugs, numerous therapeutic leads, and many novel pharmacologically potent drugs have been developed from botanicals (Fig. 28.1) (Phillipson 1999). E. Merck, in the year 1826, manufactured an analgesic morphine, which was derived from opium poppy on commercial level, which was marked as the dawn of commercialization of plant-based drugs (Galbley and Thiericke 1999). Reserpine, the chief constituent from the roots of the Indian medicinal plant R. serpentina, introduced by CIBA (USA) in 1953 is another classical example of plant-derived drug. The following (Fig. 28.1) are the examples of plant-derived drugs which are under commercialization.
The widespread skepticism on synthetic medicines and worry of their usage due to adverse side effects such as toxicity, teratogenicity, and carcinogenicity which steered to the noticeable change in the status of herbal drugs as alternative medicine. The recent boon in the demand for herbal drugs is attributed to the introduction of promising plant-based drugs, viz., (1) Taxol (anticancer), (2) artemisinin (anti-malarial), (3) forskolin (antihypertensive), etc. in the Western market. The present annual global market for herbal medicine is 62.0 billion US dollars. However, it is very sad to note the Indian share amounts to a meager 1%.
2 Plant Secondary Metabolites
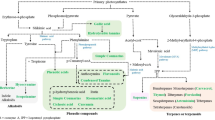
According to Verpoorte (1999): “Secondary metabolites are compounds which act as a defensive role in the interaction of the organism with its environment for survival in the ecosystem and are restricted to particular taxonomic group”. Three major secondary metabolites present in plants such as triterpenoids, alkaloids and phenolics.
2.1 Terpenoids
Terpenoids or isoprenoids are the largest class of secondary metabolites represented by nearly 27,000 compounds. Triterpenoids are classified on the basis of number of isoprenoid units present in any terpenoidal compound. For example, the monoterpenes are made up of two isoprenoid units (citronellal in lemon and menthol from peppermint), sesquiterpenes are made up of three isoprenoid units (zingiberene and artemisinin), diterpenes are made up of four isoprenoid units (gibberellic acid), triterpenes are made up of six isoprenoid units (ursolic, oleanolic, and betulinic acid), and tetraterpenes are made up of eight isoprenoid units (lanosterol, stigmasterols, diosgenin, lupeol) (Gershenzon and Kreis 1999).
2.2 Alkaloids
The alkaloids contain nitrogen besides carbon, oxygen, and hydrogen, commonly as part of a cyclic system, and are numerous among plants with diverse pharmacological properties depending on the specific alkaloid structure. The well-known examples of potent alkaloids are reserpine, ajmaline, vincristine, colchicines, mescaline, nicotine, cocaine, and morphine. Alkaloid biosynthesis in plants is from amino acids, terpenes, and aromatic compounds (Herbert 2001).
2.3 Plant Phenolics
Phenolic compounds are comprised of carbon, hydrogen, and oxygen atoms arranged as an aromatic ring having a hydroxyl substituent. Among plant polyphenols, the flavonoids form the largest group followed by phenolic quinones, lignans, xanthones, coumarins, and monocyclic phenols (Croteau et al. 2000).
2.4 Transport, Storage, and Turnover of Plant Secondary Metabolites
The biosynthesis of secondary metabolites is restricted in plants to various compartments, and their accumulation, storage, and release occur in highly specialized organs or tissues such as in glands, bark, roots, stem, and flowers (Croteau et al. 2000). In the vacuole the high polar water-soluble compounds are generally accumulated (Wink 1999; Boller and Wiemken 1986), whereas low polar compounds are restricted in other parts of plants such as resin ducts, lactifers, glandular hairs, trichomes, thylakoid membranes, or the cuticle and defend plants from abiotic and biotic stress (Wiermann 1981). In the peppermint monoterpenes, essential oils are accumulated in glandular trichomes during the development of leaves (McGarvey and Croteau 1995).
3 Biotic and Abiotic Factors Influencing Accumulation of Secondary Metabolites
In order to become a key player at the global level and to provide efficacious herbal remedy to the society, it is absolutely necessary to consider certain regulatory aspects. The primary aspect is the identification of high bioactive secondary metabolite-producing plants. The collection of secondary metabolites in a plant species depends principally on (1) soil nature, (2) climatic condition, and (3) altitude. Microbes also play a pivotal role in accumulation of secondary metabolites in herbal plants. Some important microbes such as plant growth-promoting rhizobacteria (PGPR) and mycorrhiza influence the bioactive phytochemical accumulation in herbal plants. Detailed investigation in this aspect will help in identifying the high-yielding plant source as well as will be useful in optimizing the parameters in which the medicinal plants may be cultivated under stringent and unfavorable conditions. Arbuscular mycorrhizal fungi (AM fungi) exist in mutually beneficial symbiotic coexistence with the higher land plants (Smith and Read 1997). Clark and Zeto (2000) and Augé (2001) have indicated that AMF improves plant nutrient assimilation specially phosphorus along with nitrogen, zinc, iron, and copper as well as water relations. Furthermore, AMF ameliorate cultivation condition and abiotic stresses (Jeffries et al. 2003) through high underground root biomass and improved absorption of soil nutrients and secretion of different enzymes by AMF colonized roots and/or hyphae (Marschner 1995; Smith and Read 1997).
3.1 Arbuscular Mycorrhizal Fungi (AMF)
AMF is symbiotic fungi that form a beneficial relation with the roots of a diverse group of terrestrial plants (Fig. 28.1). The AM fungi are classified under the order Glomales under the Zygomycota (Redecker et al. 2000), but currently it is moved to a new phylum, the Glomeromycota (Schüßler et al. 2001). These fungi are widespread soil-borne fungi, whose origin and occurrence have been established more than 450 million years (Redecker et al. 2000). AMF association with higher plants can be seen in temperate, tropical, and arctic regions but was found to be absent in waterlogged conditions (Smith and Read 1997). These fungi have a synergistic effect on plant growth performance in comparison with any microbes by an increase in biomass of the root system of the plant and also by enhancing the root surface area for absorption of minerals and water (Leake et al. 2004). Thus, it can be inferred that arbuscular mycorrhizal (AM) symbiosis with terrestrial plants is of significant utility in forest ecology, land reformation, improved growth, and better yield of plants in low-input systems (Sieverding et al. 1991).
3.2 Agronomic and Ecological Roles of AMF
The economically and medicinally important crops are known to be colonized by AMF (Sieverding et al. 1991). AMF improves the phosphorus nutrition by increasing the root biomass and surface area and by improving the P uptake (Koide 1991). AMF act as phosphate solubilizers by producing organic acids that increased the availability of insoluble mineral phosphates (Lapeyrie 1988) and enhanced the phosphorus uptake by host plants. Moreover, AMF also improves the uptake of macronutrients and micronutrients (Clark and Zeto 2000; Smith et al. 2004). Marschner (1998) and Hodge and Campbell (2001) on their studies on mycorrhiza established that the better plant nutrition is dependent on (i) enhanced root surface via extra-radical hyphae that extend beyond root depletion zone, (ii) decomposition of organic substances, and (iii) enhanced microbial consortium in the rhizosphere zone.
Contemporary studies reveal that AMF is an ideal tool for sustainable agriculture, landscape resurrection, and horticulture via decomposition of organic material, seedling establishment, enhanced pathogenic resistance, herbivore tolerance, soil stability, heavy metal detoxification, water stresses/cold temperature resistance, and reducing desertification (Jeffries et al. 2003; Hart and Trevors 2005). The function of AMF to their hosts in nutrient suppressive soil (less P) environment is high whereas it is less under P-sufficient conditions (Koide and Schreiner 1992), and plant growth rates can be reduced by AM colonization when available P is present (Peng et al. 1993).
3.3 Mycorrhizal Association with Medicinal Plants
Inoculation of crop plants with AMF is known to enhance growth, nutritional, and secondary metabolite contents of many medicinally and industrially significant crops which is attributed to enhanced uptake of nutrients, production of growth-promoting factors, biotic and abiotic stress tolerance, and synergistic interaction with PGPR such as nitrogen-fixing rhizobacteria and PSB (Bagyaraj and Varma 1995; Rajan et al. 2000). Enhanced mineral nutrition, i.e., N, P, Fe, Cu, Zn, and B, helps in the synthesis of chlorophyll thus enhancing photosynthetic rate (Bian et al. 2001; Feng et al. 2002; Toussaint 2007). In addition, AMF inoculation on medicinal plants is responsible for reduction in the intensity of disease caused by bacterial and fungal pathogens by morphological modulations such as thickening of the cell wall, stronger vascular bundles, etc. Moreover AMF inoculation leads to physiological alterations in host such as enhanced levels of P, phenolics, sulfur-containing amino acids, etc. (Boby and Bagyaraj 2003).
AM fungi that have a symbiotic relationship with medicinal plants belong to several families of angiosperms and gymnosperms. AM fungi are associated with Adhatoda vasica and Datura somnifera (Wei and Wang 1989). Several authors reported symbiotic relation of AMF with plants that belong to Araliaceae such as Panax ginseng (Zhang et al. 1990; Xing et al. 2000, 2003; Li 2003a; Cho et al. 2009; Ren et al. 2007; Zhang et al. 2011). There were many plants that belong to Labiatae family having association with AMF such as Salvia miltiorrhiza (He et al. 2009a, b; Wang and He 2009; Ma et al. 2009; He et al. 2010; Meng and He 2011), Schizonepeta tenuifolia (Wei and Wang 1991), Bupleurum scorzonerifolium (Teng and He 2005), Pogostemon cablin (Arpana et al. 2008), Coleus forskohlii (Sailo and Bagyaraj 2005), Salvia officinalis (Nell et al. 2009), Mentha arvensis (Gupta et al. 2002; Karagiannidis et al. 2011), Ocimum basilicum (Copetta et al. 2006; Toussaint et al. 2007; Lee and Scagel 2009; Prasad et al. 2011; Rasouli-Sadaghianil et al. 2010), and Origanum sp. (Khaosaad et al. 2006; Morone Fortunato and Avato 2008; Karagiannidis et al. 2011). Mycorrhization was also observed in plants that belong to Umbelliferae such as Anethum graveolens, Trachyspermum ammi (Kapoor et al. 2002a), Coriandrum sativum (Kapoor et al. 2002b; Farahani et al. 2008), Foeniculum vulgare (Kapoor et al. 2004), and Angelica dahurica (Cao and Zhao 2007; Zhao et al. 2009; Zhao and He 2011). AMF was reported in several plants that belong to Liliaceae such as Gloriosa superba L. (Yadav et al. 2013; Pandey et al. 2014), Aloe barbadensis (Gong et al. 2002; Mamta et al. 2012; Pandey and Banik 2009; Burni et al. 2013), Ophiopogon japonicus (Pan et al. 2008), Paris polyphylla var. (Zhou et al. 2009, 2010), and Allium sativum (Borde et al. 2009). Several authors reported mycorrhizal plants belonging to Leguminosae such as Pueraria lobata, Astragalus membranaceus, Glycyrrhiza inflata, Castanospermum australe, and Prosopis laevigata (Wang et al. 2006; Liu and He 2008, 2009; He et al. 2009b; Liu et al. 2007; Abu-Zeyad et al. 1999; Rojas-Andrade et al. 2003). AMF reported in plants belong to the Asteraceae family (Binet et al. 2011; Asrar and Elhindi 2010; Jurkiewicz et al. 2010; Araim et al. 2009; Kapoor et al. 2007; Rapparini et al. 2008; Chaudhary et al. 2008; Awasthi et al. 2011; Huang et al. 2011; Guo et al. 2006; Zhang et al. 2010, 2011; Asrar and Elhindi 2010). AMF is also reported in Forsythia suspense, Taxus chinensis var., Pinellia ternata, Catharanthus roseus, Valeriana officinalis, Atractylodes macrocephala, Camptotheca acuminate, Coix lacryma-jobi var., Ginkgo biloba, and Gentiana manshurica (Wang et al. 1998, 2010; Qi et al. 2002, 2003; Zhang et al. 2004; Wu and Wei 2008; Li 2003b; Huang et al. 2003; Zhao et al. 2006, 2007; Yu et al. 2010; Lu and He 2005, 2008; Lu et al. 2008a, b, 2011; Ren et al. 2008; Chen et al. 2009a, b, 2010; Guo et al. 2010; Shen et al. 2011; Zubek et al. 2012; Rosa-Mera et al. 2011; Nell et al. 2010; Geneva et al. 2010) (Fig. 28.2 and Table 28.1).
3.4 Synergistic Effects of AMF on Secondary Metabolites Accumulation in Medicinal Plants
There is alteration in the secondary metabolite accumulation due to chemical and biological events taking place during the AMF–host interaction (Akiyama and Hayashi 2002; Allen et al. 1982; Barrios 2007; Cai et al. 2008). AMF is known to enhance contents of secondary metabolites such as terpenoids, alkaloids, and phenolics in many economically and industrially significant crops such as production of flavonoids, cyclohexanone derivatives and apocarotenoids, phytoalexins, phenolic compounds, triterpenoids, and glucosinolates in herbal, and medicinal important plants colonized by AMF has been reported (Gianinazzi et al. 2010; Hadwiger et al. 1986; Harris et al. 2001; Harrison, 1999; Heet al. 2009c; Huang et al. 2004; Janardhan and Abdul-Khaliq 1995, Jie et al. 2007; Loomis and Corteau 1972; Maier et al. 1999; Paterson and Simmonds 2003; Shah et al. 1980; Silva et al. 2008; Singh et al. 2013; Smith and Read 2008; Szakiel and Paczkoski 2011a, b; Toussaint et al. 2004; Volpin et al. 1994; Wink 1997; Wu et al. 2010; Xiao et al. 2011; Yang et al. 2008; Zeng et al. 2007; Zubek et al. 2010, 2013).
3.4.1 Plant Terpenoid Accumulation Associated with AMF
The influence of mycorrhization on the terpenoid production in different plants has been presented in Table 28.2. The enhancements in triterpenoids are both qualitative and quantitative due to modifications in morphology of various aromatic plants due to (atractylol, anethole, thymol, artemisinin, stevioside, rebaudioside A, β-caryophyllene, p-cymene, geraniol, patchoulol, valerenic acid, and glycyrrhizic acid) in AMF-colonized plants (Kapoor et al. 2007; Mandal et al. 2013, 2015; Morone Fortunato and Avato 2008; Khaosaad et al. 2006; Lu et al. 2011; Farahani et al. 2008; Wei and Wang 1991; Arpana et al. 2008; Geneva et al. 2010; Liu et al. 2007; Jurkiewicz et al. 2010). Furthermore many researchers reported that increase in terpenoids (thymol, geraniol, anethol, forskolin) in aromatic and medicinal plants is due to P availability (Torelli et al. 2000; Kapoor et al. 2002a; Strack et al. 2003; Krishna et al. 2005; Sailo and Bagyaraj 2005; Bagheri et al. 2014; Guo et al. 2006; Zhang et al. 2010, 2011) and transcription of gene responsible for terpenoid biosynthetic pathways (Floß et al. 2008; Mandal et al. 2014, 2015). Adams et al. (2004) investigated that composition of essential oil (EO) levels in Vetiver roots is altered in the presence of unidentified bacteria PGPR, and AMF were suggested to be involved in the modulation of essential oil (EO) accumulation. Copetta et al. (2006) and Freitas et al. (2004) reported that AM fungal root colonization enhances the essential oil content (linalool and geraniol, menthol, menthone, carvone, and pulegone) in Ocimum basilicum and Mentha arvensis, respectively. Moreover, many authors reported increase in terpenoids such as menthol, menthone, carvone, pulegone, carvacrol, and thymol in aromatic plants (Gupta et al. 2002; Karagiannidis et al. 2011; Khaosaad et al. 2006; Morone Fortunato and Avato 2008; Rasouli Sadaghianil et al. 2010; Chaudhary et al. 2008). Moreover, studies on anatomical and pharmacognosy suggested that enhanced production of essential oils was due to improvement in the number of glandular hairs in leaves in AM-inoculated plants (Guerrieri et al. 2004). Furthermore, plant growth regulator levels are also known to be amended in both arbuscule-containing cells and whole tissues of mycorrhizal plants (Allen et al. 1980).
3.4.2 Plant Alkaloid Accumulation Associated with AMF
The effect of arbuscular mycorrhizae (AM) on accumulation and biosynthesis of alkaloids has been attributed to mycorrhization in various medicinal plants such as Castanospermum australe (Abu-Zeyad et al. 1999), Catharanthus roseus (Rosa-Mera et al. 2011; Andrade et al. 2013), Datura stramonium (Wei and Wang (1989), Phellodendron amurense (Fan et al. 2006), and Camptotheca acuminate (Zhao et al. 2006; Yu et al. 2010). Liu et al. (2007) investigated that enhanced production of alkaloid in medicinal plants are linked with the higher biomass production in AM-inoculated plants which are usually connected to the nutritional benefits of mycorrhization. AM fungi directly controlled the expression of different genes associated with the plant defense system, with consequences for whole-plant fitness and its response to biotic stresses (Pozo and Azco’n-Aguilar 2007; Fan et al. 2006; Zhou and Fan 2007). It was reported in many medicinal plants that mycorrhization enhances alkaloid contents such as ephedrine, camptothecin, and alliin (Guo et al. 2010; Zhao et al. 2006; Yu et al. 2010; Borde et al. 2009). Studies performed by El-Sayed and Verpoorte (2007) and Roepke et al. (2010) reported the improved production of several pharmacologically important monoterpene indole alkaloids (MIAs), such as vinblastine, vincristine, ajmalicine, vindoline, catharanthine, and serpentine in Catharanthus roseus and pyridine alkaloids (PAs) in Nicotiana species.
3.4.3 Plant Phenolic Accumulation Associated with AMF
The effect of AM on the production of phenolics in various plants has been summarized in Table 28.2. The enhancements of phenolics such as anthraquinone glycosides and flavanoids are both qualitative and quantitative (Araim et al. 2009; Silva et al. 2014; Rosa-Mera et al. 2011; Bagheri et al. 2014; De Sousa et al. 2013; He et al. 2009a, b, c; Meng and He 2011; Zhao et al. 2009; Zhao and He 2011; Nell et al. 2009). It was observed by many researchers that the enhancement of phenolics in medicinal plants was due to increase in the biomass of plants due to better nutrition and expression of genes related to the defense system of plants. Jurkiewicz et al. (2010) reported increase in the caffeic and chlorogenic acid in Arnica Montana. Furthermore there was improved production of phenolics in Viola tricolor (Zubek et al. 2015), Salvia spp. (Yang et al. 2017), and Aloe vera (Pandey and Banik 2009; Mamta et al. 2012). There were significant increases in the content of rosmarinic and caffeic acids (Jugran et al. 2015; Zubek et al. 2015; Jurkiewicz et al. 2010; Toussaint et al. 2007; Lee and Scagel 2009) and diobulbinone (Lu et al. 2015).
Structure of Secondary Metabolites Affected by AM




A. Triterpene








B. Alkaloids



C. Flavanoids and Phenolic Compounds
4 Conclusion
Now it is well-established that symbiotic association between medicinal plants and AM fungi can be exploited for the enhanced production of plant secondary metabolites. These secondary metabolites not only play an important role in uplifting the defense system of plants but also can be used for curing human ailments. In production of herb-based materials, there is a need to implement mycorrhizal technology to explore the role of AM fungi in the cultivation of medicinal plants. Thus future research priorities should emphasize on (1) isolation, selection, and screening of promising and effective AM fungal strains which can be adapted to natural habitat where medicinal and aromatic plants have to be grown, (2) strategies for the production of seedlings of medicinal and aromatic plants with mycorrhiza, (3) intensive cultivation of medicinal plants and the production of medicinally important phytochemicals as well as plant parts rich in medicinally important compounds, and (4) strategies by which AM fungi modulate the contents of bioactive principles in medicinal plants.
References
Abu-Zeyad R, Khan AG, Khoo C (1999) Occurrence of arbuscular mycorrhiza in Castanospermum australe A. Cunn. & C. Fraser and effects on growth and production of castanospermine. Mycorrhiza 9:111–117
Adams RP, Habte M, Park S, Dafforn MR (2004) Preliminary comparison of vetiver root essential oils from cleansed (bacteria-and fungus-free) versus non-cleansed (normal) vetiver plants. Biochem Syst Ecol 32(12):1137–1144
Akiyama K, Hayashi H (2002) Arbuscular mycorrhizal fungus-promoted accumulation of two new triterpenoids in cucumber roots. Biosci Biotechnol Biochem 66(4):762–769
Allen MF, Moore TS, Christensen M (1980) Phytohormone changes in Bouteloua gracilis infected by vesicular-arbuscular mycorrhizae. I. Cytokinin increases in the host plant. Can J Bot 58:371–374
Allen MF, Moore TS, Christensen M (1982) Phytohormone changes in Bouteloua gracilis infected by vesicular-arbuscular mycorrhizae. II. Altered levels of gibberellin-like substances and abscisic acid in the host plant. Can J Bot 60:468–471
Andrade SAL, Malik S, Sawaya ACHF, Bottcher A, Mazzafera P (2013) Association with arbuscular mycorrhizal fungi influences alkaloid synthesis and accumulation in Catharanthus roseus and Nicotiana tabacum plants. Acta Physiol Plant 35(3):867–880
Araim G, Saleem A, Arnason JT, Charest AC (2009) Root colonization by an arbuscular mycorrhizal (AM) fungus increases growth and secondary metabolism of purple coneflower, Echinacea purpurea (L.) Moench. J Agric Food Chem 57:2255–2258
Arpana J, Bagyaraj DJ, Prakasa Rao EVS, Parameswaran TN, Abdul Rahiman BA (2008) Symbiotic response of patchouli [Pogostemon cablin (Blanco) Benth.] to different arbuscular mycorrhizal fungi. Adv Environ Biol 2(1):20–24
Asrar AWA, Elhindi KM (2010) Elhindi. Alleviation of drought stress of marigold (Tagetes erecta) plants by using arbuscular mycorrhizal fungi. Saudi J Biol Sci 18(1):93–98
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11(1):3–42
Awasthi A, Bharti N, Nair N, Singh R, Shukla AK, Gupta MM, Darokar MP, Kalra A (2011) Synergistic effect of Glomus mosseae and nitrogen fixing Bacillus subtilis strain Daz26 on artemisinin content in Artemisia annua L. Appl Soil Ecol 49:125–130
Bagheri S, Ebrahimi MA, Davazdahemami S, Minooyi J (2014) Terpenoids and phenolic compounds production of mint genotypes in response to mycorrhizal bio-elicitors. Tech J Eng Appl Sci 4:339–348
Bagyaraj D J, Varma A (1995) Interaction between arbuscular mycorrhizal fungi and plants. In Advances in Microbial Ecology Springer, Boston pp 119–142
Barrios E (2007) Soil biota, ecosystem services and land productivity. Ecol Econ 64:269–285
Bian XJ, Hu L, Li XL, Zhang FS (2001) Effect of VA mycorrhiza on the turfgrass quality and mineral nutrient uptakes. Acta Pratacul Sin 10(3):42–46
Binet MN, Van Tuinen D, Deprêtre N, Koszela N, Chambon C, Gianinazzi S (2011) Arbuscular mycorrhizal fungi associated with Artemisia umbelliformis Lam, an endangered aromatic species in Southern French Alps, influence plant P and essential oil contents. Mycorrhiza 21:523–535
Boby VU, Bagyaraj DJ (2003) Biological control of root-rot of Coleus forskohlii Briq. using microbial inoculants. World J Microbiol Biotechnol 19(2):175–180
Boller T, Wiemken A (1986) Dynamics of vacuolar compartmentation. Annu Rev Plant Physiol 37(1):137–164
Borde M, Dudhane M, Jite PK (2009) Role bioinoculant (AM fungi) increasing in growth, flavor content and yield in Allium sativum L. under field condition. Not Bot Hortic Agrobot Cluj 37(2):124–128
Cai BY, Jie WG, Ge JP, Yan XF (2008) Molecular detection of the arbuscular mycorrhizal fungi in the rhizosphere of Phellodendron amurense. Mycosystema 27(6):884–893
Cao DX, Zhao JL (2007) The investigation of arbuscular mycorrhizal fungi and soil factors from the rhizospere of medicinal plant Angelica dahurica. Acta Agric Boreali-Sin 22:47–50
Copetta A, Lingua G, Berta G (2006) Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza 16:485–494
Chaudhary V, Kapoor R, Bhatnagar AK (2008) Effectiveness of two arbuscular mycorrhizal fungi on concentrations of essential oil and artemisinin in three accessions of Artemisia annua L. Appl Soil Ecol 40:174–181
Chen LT, Guo QS, Liu ZY (2009a) Colonization pattern and dynamic change of arbuscular mycorrhizal fungi in Pinellia ternate. Guizhou Agric Sci 37(2):37–39
Chen LT, Liu ZY, Guo QS, Zhu GS (2009b) Advances in studies on arbuscular mycorrhizas in medicinal plants. Chin Tradit Herb Drugs 40(1):156–160
Chen LT, Guo QS, Liu ZY (2010) Arbuscular mycorrhiza of cultivated and wild Pinellia ternate. Chin J Chin Mater Med 35(4):405–410
Cho EJ, Lee DJ, Wee CD, Kim HL, Cheong YH, Cho JS, Sohn BK (2009) Effects of AM FUNGI inoculation on growth of Panax ginseng C.A. Meyer seedlings and on soil structures in mycorrhizosphere. Sci Hortic 122(4):633–637
Clark RB, Zeto SK (2000) Mineral acquisition by arbuscular mycorrhizal plants. J Plant Nutr 23(7):867–902
Cordell GA (1995) Changing strategies in natural products chemistry. Phytochem 40(6):1585–1612
Corizier R (1974) In: Kleinman VS et al. (eds) Medicine in Chinese Culture, Department of Health, Education and Welfare Publications, pp 26
Croteau R, Kutchan TM, Lewis NG (2000) Natural products (secondary metabolites). Biochem Mol Biol 24:1250–1319
David S (2000) The history of WWII medicine. http://home.att.net/~steinert/wwii.htm
De la Rosa-Mera CJ, Ferrera-Cerrato R, Alarcón A, de Jesús Sánchez-Colín M, Muñoz-Muñiz OD (2011) Arbuscular mycorrhizal fungi and potassium bicarbonate enhance the foliar content of the vinblastine alkaloid in Catharanthus roseus. Plant Soil 349(1–2):367–376
El-Sayed M, Verpoorte R (2007) Catharanthus terpenoid indole alkaloids: biosynthesis and regulation. Phytochem Rev 6(2–3):277–305
Fan JH, Yang GT, Mu LQ, Zhou JH (2006) Effect of AM fungi on the content of berberine, jatrorrhizine and palmatine of Phellodendron amurense seedings. Prot For Sci Technol 5:24–26
Farahani HA, Lebaschi MH, Hamidi A (2008) Effects of arbuscular mycorrhizal fungi, phosphorus and water stress on quantity and quality characteristics of coriander. J Adv Nat Appl Sci 2(2):55–59
Feng G, Zhang F, Li X, Tian C, Tang C, Rengel Z (2002) Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza 12(4):185–190
Floß DS, Hause B, Lange PR, Küster H, Strack D, Walter MH (2008) Knock-down of the MEP pathway isogene 1-deoxy-d-xylulose 5-phosphate synthase 2 inhibits formation of arbuscular mycorrhiza-induced apocarotenoids, and abolishes normal expression of mycorrhiza-specific plant marker genes. Plant J 56(1):86–100
Fransworth NR, Akerele O, Bingel AS, Soejarto DD, Guo Z (1985) Drugs from medicinal plants. Bull WHO 63:965–981
Freitas MSM, Martins MA, Vieira IJC (2004) Yield and quality of essential oils of Mentha arvensis in response to inoculation with arbuscular mycorrhizal fungi. Pesq Agropec Bras 39(9):887–894
Galbley S, Thiericke R (1999) Drug Discovery FROM Nature. Springer, Berlin
Geneva MP, Stancheva IV, Boychinova MM, Mincheva NH, Yonova PA (2010) Effects of foliar fertilization and arbuscular mycorrhizal colonization on Salvia officinalis L. growth, antioxidant capacity, and essential oil composition. J Sci Food Agric 90:696–702
Gershenzon J, Kreis W (1999) Biochemistry of terpenoids: monoterpenes, sesquiterpenes, diterpenes, sterols, cardiac glycosides and steroid saponins. Biochem Plant Sec Met 2:222–299
Ghisalberti EL (1993) Detection and isolation of bioactive natural products. In: Colegate SM, Molyneux RJ (eds) Bioactive natural products: detection, isolation, and structural determination. CRC Press, Boca Raton, pp 9–57
Gianinazzi S, Gollotte A, Binet MN, Tuinen DV, Redecker D, Wipf D (2010) Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20:519–530
Gong MQ, Wang FZ, Chen Y (2002) Study on application of arbuscular-mycorrhizas in growing seedling of Aloe vera. J Chin Med Mater 25(1):1–3
Gopal RM (2001) J Med Aromat Plant Sci, 22/4A & 23/1A, 572
Guerrieri E, Lingua G, Digilio MC, Massa N, Berta G (2004) Do interactions between plant roots and the rhizosphere affect parasitoid behaviour? Ecol Entomol 29(6):753–756
Guo LP, Wang HG, Hang LQ (2006) Effects of arbuscular mycorrhizae on growth and essential oil of Atractylodes lancea. Chin J Chin Mater Med 31(8):1491–1495
Guo QS, Chen LT, Liu ZY (2010) Study on influence of arbuscular mycorrhizal fungi on Pinellia ternata yield and chemical composition. Chin J Chin Med 35(3):333–338
Gupta ML, Prasad A, Ram M, Kumar S (2002) Effect of the vesicular-arbuscular mycorrhizal (VAM) fungus Glomus fasciculatum on the essential oil yield related characters and nutrient acquisition in the crops of different cultivars of menthol mint (Mentha arvensis) under field conditions. Bioresour Technol 81(1):77–79
Hadwiger A, Neimann H, Kaebisch A, Bauer H, Tamura T (1986) Appropriate glucosylation of the FMS gene product is a prerequisite for its transforming potency. EMBO J 5:689–694
Harris JC, Cottrell S, Plummer S, Lloyd D (2001) Antimicrobial properties of Allium sativum (garlic). Appl Microbiol Biotechnol 57:282–286
Harrison M (1999) Molecular and cellular aspects of the arbuscular mycorrhizal symbiosis. Annu Rev Plant Biol 50:361–389
Hart MM, Trevors JT (2005) Microbe management: application of mycorrhyzal fungi in sustainable agriculture. Front Ecol Environ 310:533–539
Herbert RB (2001) The biosynthesis of plant alkaloids and nitrogenous microbial metabolites. Nat Prod Rep 18(1):50–65
He XL, Li J, Gao AX, Zhao LL, Zao JL (2009a) Effects of different host plants on the development of AM fungi in the rhizospere of Salvia miltiorrhiza. J Hebei Univ 29(5):533–537
He XL, Li J, He C (2009b) Effects of AM fungi on the chemical components of Salvia miltiorrhiza Bge. Chin Agric Sci Bull 25(14):182–185
He XL, Liu T, Zhao LL (2009c) Effects of inoculating AM fungi on physiological characters an nutritional components of Astragalus membranaceus under different N application levels. Chin J Appl Ecol 20(9):2118–2122
He XL, Wang LY, Ma J, Zhao LL (2010) AM fungal diversity in the rhizosphere of Salvia miltiorrhiza in Anguo city of Hebei province. Biodivers Sci 18(2):187–194
Hodge A, Campbell CDFAH (2001) An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413:297–299
Hostettmann K, Marston A, Ndjoko K, Wolfender JL (2000) The potential of African plants as a source of drugs. Curr Org Chem 4(10):973–1010
Huang KC (1999) The pharmacology of Chinese herbs: a brief history of Chinese medicine, 2nd edn. CRC Press, Boca Raton, pp 1–16
Huang YF, Li HH, Chen HY, Li Y (2003) Preliminary study on the mycorrhiza inoculation on the seeding of camptotheca acuminate. Guangdong For Sci Technol 19(1):40–42
Huang LQ, Chen ML, Xiao PG (2004) The modern biological basis and model hypothesis on the research of genuineness of Chinese herbal medicine. Chin J Chin Mater Med 29(6):494–496
Huang JH, Tan JF, Jie HK, Zeng RS (2011) Effects of invoculating arbuscular mycorrhizal fungi on Artemisia annua growth and its officinal components. Chin J Appl Ecol 22(6):1443–1449
Ishibashi A (2002) In: Complementary and alternative medicine in Japan, SY19-4
Janardhanan KK, Abdul-Khaliq K (1995) Influence of vesicular arbuscular mycorrhizal fungi on growth and productivity of German chamomile in alkaline usar soil. In: Adholeya A, Singh S (eds) Mycorrhizae: biofertilizers for the future. Tata Energy Research Institute, New Delhi, pp 410–412
Jeffries P, Gianinazzi S, Peretto S, Turnau K, Barea JM (2003) The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol Fertil Soils 37:1–16
Jie WG, Cai BY, Ge JP, Yan XF (2007) Identification of arbuscular mycorrhizal fungi of Phellodendren amurense Rupr. Biotechnology 17(6):32–35
Jugran AK, Bahukhandi A, Dhyani P, Bhatt ID, Rawal RS, Nandi SK, Palni LMS (2015) The effect of inoculation with mycorrhiza: AM on growth, phenolics, tannins, phenolic composition and antioxidant activity in Valeriana jatamansi Jones. J Soil Sci Plant Nutr 15(4):1036–1049
Jurkiewicz A, Ryszka P, Anielska T, Waligórski P, Białońska D, Góralska K, Michael MT, Turnau K (2010) Optimization of culture conditions of Arnica montana L.: effects of mycorrhizal fungi and competing plants. Mycorrhiza 20:293–306
Kapoor R, Giri B, Mukerji KG (2002a) Glomus macrocarpum: a potential bioinoculant to improve essential oil quality and concentration in Dill (Anethum graveolens L.) and Carum (Trachyspermum ammi (Linn.) Sprague). World J Microbiol Biotechnol 18(5):459–463
Kapoor R, Giri B, Mukerji KG (2002b) Mycorrhization of coriander (Coriandrum sativum L.) to enhance the concentration and quality of essential oil. J Sci Food Agric 82:339–342
Kapoor R, Giri B, Mukerji KG (2004) Improved growth and essential oil yield and quality in Foeniculum vulgare mill on mycorrhizal inoculation supplemented with P-fertilizer. Bioresour Technol 93:307–311
Kapoor R, Chaudhary V, Bhatnagar AK (2007) Effects of arbuscular mycorrhiza and phosphorus application on artemisinin concentration in Artemisia annua L. Mycorrhiza 17:581–587
Karagiannidis N, Thomidisa T, Lazari D, Filotheou EP, Karagiannidou C (2011) Effect of three Greek arbuscular mycorrhizal fungi in improving the growth, nutrient concentration, and production of essential oils of oregano and mint plants. Sci Hortic 129:329–334
Khaosaad T, Vierheilig H, Nell M, Zitterl-Eglseer K, Novak J (2006) Arbuscular mycorrhiza alter the concentration of essential oils in oregano (Origanum sp., Lamiaceae). Mycorrhiza 16:443–446
Koide RT (1991) Nutrient supply, nutrient demand and plant response to mycorrhizal infection. New Phytol 117:365–386
Koide RT, Schreiner RP (1992) Regulation of the vesicular-arbuscular mycorrhizal symbiosis. Annu Rev Plant Physiol Plant Mol Biol 43:557–581
Krishna H, Singh S, Sharma RR, Khawale RN, Grover M, Patel VB (2005) Biochemical changes in micropropagated grape (Vitis vinifera L.) plantlets due to arbuscular-mycorrhizal fungi (AMF) inoculation during ex vitro acclimatization. Sci Hort 106(4):554–567
Lapeyrie F (1988) Oxalate synthesis from soil bicarbonate by fungus Paxillus involutus. Plant Soil 110:3–8
Leake J, Johnson D, Donnelly D, Muckle G, Boddy L, Read (2004) Networks of power and influence: the role of mycorrhizal mycelium in controlling plant communities and agroecosystem functioning. Can J Bot 82:1016–1045
Lee J, Scagel CF (2009) Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem 115(2):650–656
Li CX (2003a) Effect of vesicular-arbuscular mycorrhizal fungi on production of Ginseng. J Chin Med Mater 26(7):475–476
Li CX (2003b) Effects of infecting vesicular-arbuscular mycorrhiza on growth and development of Coix Lachryma-jobi L. J Shanxi Agric Univ 23(4):351–353
Liu T, He XL (2008) Research on the formation course of arbuscular mycorrhizae from Astragalus membranaceus (Fisch.) Bunge seedlings. J Hebei For Orc Res 23(3):311–314
Liu SL, He XL (2009) Effects of AM fungi on growth of Glycyrrhiza inflata Bat under water stress. J Nucl Agric Sci 23(4):692–696
Liu JN, Wu LJ, Wei SL, Xiao X, Su CX, Jiang P, Song ZB, Wang T, Yu ZL (2007) Effects of arbuscular mycorrhizal fungi on the growth, nutrient uptake and glycyrrhizin production of licorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul 52(1):29–39
Loomis WD, Corteau R (1972) Essential oil biosynthesis. Rec Adv Phytochem 6:147–185
Lu YQ, He XL (2005) Effects of AM fungi on the chemical composition and growth amount of Atractylodes macrocephala koidz seedling on different N levels. J Hebei Univ 25(6):650–653
Lu YQ, He XL (2008) Effects of AM fungi on photosynthetic pigment of Atractylodes macrocephala under different nitrogen levels. Acta Agric Bor Occi Sin 17(4):314–321
Lu YQ, Cui Y, He XL (2008a) Effects of AM fungi on biomass and nitrogen content of Atractylodes macrocephala under different nitrogen levels. J Henan Agric Sci 4:94–96
Lu YQ, He XL, Li LZ (2008b) Effects of AM fungi on leaf protective enzymes of Atractrlodes macrocephala under different nitrogen levels. Hubei Agric Sci 47(6):659–660
Lu YQ, Wang DX, Lu XL, Li LM, Li Y, He XL (2011) Effects of AM fungi on physiological character and nutritional component of Atractylodes macrocephala under different N levels. Acta Bot Bor Occi Sin 31(2):351–356
Lu FC, Lee CY, Wang CL (2015) The influence of arbuscular mycorrhizal fungi inoculation on yam (Dioscorea spp.) tuber weights and secondary metabolite content. Peer J 3:e1266
Ma J, He XL, Jiang ZM, Wang LY (2009) Influence of soil factors on arbuscular mycorrhizal fungal colonization of Salvia miltiorrhiza. Acta Agric Bor Occi Sin 18(5):194–198
Maier W, Schmidt J, Wray V, Walter MH, Strack D (1999) The arbuscular mycorrhizal fungus, Glomus intraradices, induces the accumulation of cyclohexenone derivatives in tobacco roots. Planta 207:620–623
Mamta G, Rahi P, Pathania V, Gulati A, Singh B, Bhanwra RK, Tewari R (2012) Comparative efficiency of phosphate-solubilizing bacteria under greenhouse conditions for promoting growth and aloin-A content of Aloe barbadensis. Arch Agron Soil Sci 58(4):437–449
Mandal S, Evelin H, Giri B, Singh VP, Kapoor R (2013) Arbuscular mycorrhiza enhances the production of stevioside and rebaudioside-A in Stevia rebaudiana via nutritional and non-nutritional mechanisms. Appl Soil Ecol 72:187–194
Mandal A, Mandal S, Park MH (2014) Genome-wide analyses and functional classification of proline repeat-rich proteins: potential role of eIF5A in eukaryotic evolution. PLoS One 9(11):e111800
Mandal S, Upadhyay S, Singh VP, Kapoor R (2015) Enhanced production of steviol glycosides in mycorrhizal plants: a concerted effect of arbuscular mycorrhizal symbiosis on transcription of biosynthetic genes. Plant Physiol Biochem 89:100–106
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Elsevier Science, San Diego
Marschner H (1998) Mineral nutrition of higher plants. Academic, London
McGarvey DJ, Croteau R (1995) Terpenoid metabolism. Plant Cell 7(7):1015
Meng JJ, He XL (2011) Effects of AM fungi on growth and nutritional contents of Salvia miltiorrhiza Bge. under drought stress. J Agric Univ Hebei 34(1):51–61
Morone Fortunato I, Avato P (2008) Plant development and synthesis of essential oils in micropropagated and mycorrhiza inoculated plants of Origanum vulgare L. ssp. hirtum (Link) Ietswaart. Plant Cell Tissue Organ 93:139–149
Nell M, Vötsch M, Vierheilig H, Steinkellner S, Zitterl-Eglseer K, Franz C, Novak J (2009) Effect of phosphorus uptake on growth and secondary metabolites of garden (Salvia officinalis L.). J Sci Food Agric 89:1090–1096
Nell M, Wawrosh C, Steinkellner S, Vierheilig H, Kopp B, Lössl A, Franz C, Novak J, Zitterl-Eglseer K (2010) Root solonization by symbiotic arbuscular mycorrhizal fungi increases sesquiterpenic acid concentrations in Valeriana of ficinalis L. Planta Med 76:393–398
Pan PL, Chen DQ, Chen YT, Zhou FM (2008) The research on the sift and germinate of AM FUNGI spore of Ophiopogon japonicas. Mod Chin Med 10(10):13–14
Pandey DK, Banik RM (2009) The influence of dual inoculation with Glomus mossae and Azotobacter on growth and barbaloin content of Aloe vera. Am Eu J Sust Agric 3(4):703–714
Pandey DK, Banik RM, Dey A, Panwar J (2014) Improved growth and colchicines concentration in Gloriosa superb in mycorrhizal inoculation supplemented with phosphorus – fertilizer. Afr J Tradit Complement Altern Med 11(2):439–446
Peng S, Eissenstat DM, Graham JH, Williams K, Hodge NC (1993) Growth depression in mycorrhizal citrus at high-phosphorus supply: analysis of carbon costs. Plant Physiol 101:1063–1071
Petersen M, Simmonds MSJ (2003) Rosmarinic acid. Phytochemistry 62:121–125
Phillipson JD (1999) New drugs from nature—It could be yew. Phytother Res 13(1):2–8
Pozo MJ, Azcón-Aguilar C (2007) Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol 10(4):393–398
Prasad A, Kumar S, Khaliq A, Pandey A (2011) Heavy metals and arbuscular mycorrhizal (AM) fungi can alter the yield and chemical composition of volatile oil of sweet basil (Ocimum basilicum L.). Biol Fertil Soils 47(8):853–861
Qi GH, Zhang LP, Yang WL, Lu XR, Li CL (2002) Effects of arbuscular mycorrhizal fungi on growth and disease resistance of replanted ginkgo (Ginkgo biloba L.) seedlings. J Hebei For Orch Res 17(1):58–61
Qi GH, Zhang LP, Yang WL, Lv GY (2003) The effects of abruscular mycorrhiza fungi on ginkgo (Ginkgo biloba L.) in the field. Hebei Fruits 19(1):40–42
Rajan SK, Reddy BJD, Bagyaraj DJ (2000) Screening of arbuscular mycorrhizal fungi for their symbiotic efficiency with Tectona grandis. Forest Ecol Manag 126(2):91–95
Rapparini F, Llusia J, Penuelas J (2008) Effect of arbuscular mycorrhizal (AM) colonization on terpene emission and content of Artemisia annua L. Plant Biol 10:108–122
Rasouli-Sadaghianil MH, Hassani A, Barin M, Danesh YR, Sefidkon F (2010) Effects of arbuscular mycorrhizal (AM) fungi on growth, essential oil production and nutrients uptake in basil. J Med Plant Res 4(21):2222–2228
Rastogi PR, Meharotra BN (1990) (eds) In: Compendium of Indian medicinal plants, publications and information directorate, CSIR, New Delhi, vol.l, p 339
Redecker D, Morton JB, Bruns TD (2000) Ancestral lineages of arbuscular mycorrhizal fungi (Glomales). Mol Phylogenet Evol 14:276–284
Ren JH, Liu RX, Li YL (2007) Study on arbuscular mycorrhizae of Panax notoginseng. Microbiology 34(2):224–227
Ren JH, Zhang JF, Liu RX, Li YQ (2008) Study on arbuscular mycorrhizae in Taxus chinensis var. mairei. Acta Bot Bor Occi Sin 28(7):1468–1473
Roepke J, Salim V, Wu M, Thamm AM, Murata J, Ploss K, Wilhelm B, De Luca V (2010) Vinca drug components accumulate exclusively in leaf exudates of Madagascar periwinkle. Proc Natl Acad Sci 107(34):15287–15292
Rojas-Andrade R, Cerda-GarcÍa-Rojas CM, FrÍas-Hernández JT, Dendooven L, Olalde-Portugal V, Ramos-Valdivia AC (2003) Changes in the concentration of trigonelline in a semi-arid leguminous plant (Prosopis laevigata) induced by an arbuscular mycorrhizal fungus during the presymbiotic phase. Mycorrhiza 13:49–52
Sailo GS, Bagyaraj DJ (2005) Influence of different AM-fungi on the growth, nutrition and forskolin content of Coleus forskohlii. Mycol Res 109(7):795–798
Schüßler A, Schwarzott D, Walker C (2001) A new fungal phylum, the Glomeromycota, phylogeny and evolution. Mycol Res 105:1413–1421
Singh R, Soni SK, Kalra A (2013) Synergy between Glomus fasciculatum and a beneficial Pseudomonas in reducing root diseases and improving yield and forskolin content in Coleus forskohlii Briq. under organic field conditions. Mycorrhiza 23:35–44
Shah V, Bhat SV, Bajwa BS, Domacur H, De SNJ (1980) The occurrence of forskolin in Labiatae. Planta Med 39:183–185
Shen XL, Guo QS, Liu ZY, Zhu GS, Liu YX (2011) Colonization progress of arbuscular mycorrhizae on tissue-cultured plantlets of Pinellia ternata. Chin J Chin Mater Med 36:93–96
Shibata K, Iwata S, Nakamura M (1923) Baicalin, a new flavone-glucuronic acid compound from the roots of Scutellaria baicalensis. Acta Phytochim 1:105–139
Sieverding E, Friedrichsen J, Suden W (1991) Vesicular-arbuscular mycorrhiza management in tropical agrosystems. Sonderpublikation der GTZ (Germany)
Silva MFD, Pescador R, Rebelo RA, Stürmer SL (2008) The effect of arbuscular mycorrhizal fungal isolates on the development and oleoresin production of micropropagated Zingiber officinale. Braz J Plant Physiol 20(2):119–130
Silva FA, Ferreira MR, Soares LA, Sampaio EV, Maia LC (2014) Arbuscular mycorrhizal fungi increase gallic acid production in leaves of field grown Libidibia ferrea (Mart. ex Tul.) LP Queiroz. J Med Plant Res 8(36):1110–1115
Smith SE, Read DJ (1997) Mycorrhizal symbiosis. Academic, London
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, London
Smith SE, Smith FA, Jakobsen I (2004) Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol 162:511–524
de Sousa OM, da Silva Campos MA, de Albuquerque UP, da Silva FSB (2013) Arbuscular mycorrhizal fungi (AMF) affects biomolecules content in Myracrodruon urundeuva seedlings. Ind Crop Prod 50:244–247
Strack D, Fester T, Hause B, Schliemann W, Walter MH (2003) Arbuscular mycorrhiza: biological, chemical, and molecular aspects. J Chem Ecol 29(9):1955–1979
Suzuki Y (2002, May 29) In Current status and future directions of alternative medicine, WHO, Geneva, Switzerland
Swami Tirtha SS (1998) In: Uniyal RC et al (eds) Ayurvedic Encyclopedia, Natural Secrets to Healing, Prevention, and Longevity: history of Ayurvedic Tree, 1st edn. New Delhi, Sai Satguru Publications
Szakiel A, Pączkowski H (2011a) Influence of environmental biotic factors on the content of saponins in plants. Phytochem Rev 10:493–502
Szakiel A, Pączkowski H (2011b) Influence of environmental abiotic factors on the content of saponins in plants. Phytochem Rev 10:471–491
Burni T, Saadia N, Tabassum Y, Sakina B (2013) Arblscular mycorrhizal stuidies in “Aloe vera (l). burm. f.” biologically active and potential medicinal plant. Wudpecker J Agric Res 2(1):039–042
Tang W, Eisenbrand G (1992) In: Chinese drugs of plant origin, SpingerVerlag, Berlin, p 127
Teng HR, He XL (2005) Effects of different AM fungi and N levels on the flavonoid content of Bupleuruin scorzonerifolium Willd. J Shanxi Agric Sci 4:53–54
Torelli A, Trotta A, Acerbi L, Arcidiacono G, Berta G, Branca C (2000) IAA and ZR content in leek (Allium porrum L.), as influenced by P nutrition and arbuscular mycorrhizae, in relation to plant development. Plant Soil 226(1):29–35
Toussaint JP (2007) Investigating physiological changes in the aeria parts of AM plants: what do we know and where should we be heading? Mycorrhiza 17:349–353
Toussaint JP, St-Arnaud M, Charest C (2004) Nitrogen transfer and assimilation between the arbuscular mycorrhizal fungus Glomus intraradices Schenck & Smith and Ri T-DNA roots of Daucus carota L. in an in vitro compartmented system. Can J Microbiol 50:251–260
Toussaint JP, Smith FA, Smith SE (2007) Arbuscular mycorrhizal fungi can induce the production of phytochemicals in sweet basil irrespective of phosphorus nutrition. Mycorrhiza 17:291–297
Verpoorte R (1999) Secondary metabolism. In: Verpoorte R, Alfermann AW (eds) Metabolic engineering of plant secondary metabolism. Klumer Academic Publishers, Dordrecht, pp 1–29
Volpin H, Elkind Y, Okon Y, Kapulnik Y (1994) A vesicular arbuscular mycorrhizal fungus (Glomus intraradices) induces a defense response in alfalfa roots. Plant Physiol 104:683–689
Wang LY, He XL (2009) The resource and spatio-temporal distribution of AM fungi from Salvia miltiorrhiza in Anguo. J Agric Univ Hebei 32(6):73–79
Wang Q, Li HQ, Du YR, Li Y, Li HW (1998) Isolation and identification of VA mycorrhizal fungi on Radix gentianae. Biotechnology 8(2):19–22
Wang Q, He XL, Chen TS, Dou WF (2006) Ecological research of arbuscular mycorrhizal fungi in rhizosphere of Puerraria lobata. J Hebei Univ 26(4):420–425
Wang DX, Lu YQ, He XL (2010) Effects of AM fungi on growth and physiological characters of Atractylodes macrocephala under different P-applied levels. Acta Bot Bor Occi Sin 30(1):136–142
Warrier PK, Nambiar VPK, Ramankutti C (1996) (eds), In Indian medicinal plants: a compendium of 500 species, Orient Longmann, Hyderabad, vol 4. p 409
Wei GT, Wang HG (1989) Effects of VA mycorrhizal fungi on growth, nutrient uptake and effective compounds in Chinese medicinal herb Datura stramonium L. Sci Agric Sin 22(5):56–61
Wei GT, Wang HG (1991) Effect of vesicular-arbuscular mycorrhizal fungi on growth, nutrient uptake and synthesis of volatile oil in Schizonepeta tenuifolia Briq. Chin J Chin Mater Med 16(3):139–142
Wiermann R (1981) Secondary plant products and cell and tissue differentiation. In: The biochemistry of plants, vol. 7. Academic, New York, pp 85–116
Wink M (1997) Compartmentation of secondary metabolites and xenobiotics in plant vacuoles. Adv Bot Res 25:141–169
Wink M (1999) Introduction: biochemistry, role and biotechnology of secondary metabolites. In: Wink M (ed) Biochemistry of plant secondary metabolism. Sheffield Academic Press Ltd, Sheffield, pp 1–16
Wu QC, Wei QA (2008) Arbuscular mycorrhizae of Ginkgo biloba and its correlation with soil available phosphorus. J Yangtze Univ 5(3):49–52
Wu QS, Liu W, Zhai HF, Ye XF, Zhao LJ (2010) Influences of AM fungi on growth and root antioxidative enzymes of Trifoliate orange seedlings under salt stress. Acta Agric Univ Jiangxiensis 32(4):759–762
Xing XK, Li Y, Yolande D (2000) Ten species of vAM fungi in five ginseng fields of Jilin province. J Jilin Agric Univ 22(2):41–46
Xing XK, Li Y, Wang Y, Zhang MP (2003) Foundation of dual cultural system of ginseng VA mycorrhiza fungi. J Jilin Agric Univ 25(2):154–157
Xiao WJ, Yang G, Chen ML, Guo LP, Wang M (2011) AM and its application in plant disease prevention of Chinese medicinal herbs cultivation. Chin J Chin Med 36(3):252–257
Yang G, Guo LP, Huang LQ, Chen M (2008) Inoculation methods of AM fungi in medicinal plant. Resour Sci 30(5):778–785
Yang Y, Ou X, Yang G, Xia Y, Chen M, Guo L, Liu D (2017) Arbuscular mycorrhizal fungi regulate the growth and phyto-active compound of Salvia miltiorrhiza seedlings. Appl Sci 7(1):68
Yadav K, Aggarwal A, Singh N (2013) Arbuscular mycorrhizal fungi (AMF) induced acclimatization, growth enhancement and colchicine content of micropropagated Gloriosa superba L. plantlets. Ind Crop Prod 45:88–93
Yu Y, Yu T, Wang Y, Yan XF (2010) Effect of inoculation time on camptothecin content in arbuscular mycorrhizal Camptotheca acuminate seedlings. Chin J Plant Ecol 34(6):687–694
Zeng Y, Guo LP, Hang LQ, Zhou J, Sun YZ (2007) AM and its application in TCM cultivation. World Sci Technol/Moder TCM Mater Med 9(6):83–87
Zhang MC, Jing YJ, Ma J (1990) The changing of microbial ecological types after the improvement of ginseng soil. J Jilin Agric Univ 12(4):42–46
Zhang Y, Xie LY, Xiong BQ, Zeng M, Yu D (2004) Correlation between the growth of arbuscular mycorrhizal fungi in the rhizosphere and the flavonoid content in the root of Ginkgo biloba. Mycosystema 23(1):133–138
Zhang J, Liu DH, Guo LP, Jin H, Zhou J, Yang G (2010) Effects of four AM fungi on growth and essential oil composition in rhizome of Atractylodes lancea. World Sci Technol/Moder TCM Mater Med 12(5):779–782
Zhang J, Liu DH, Guo LP, Jin H, Yang G, Zhou J (2011) Effects of arbuscular mycorrhizae fungi on biomass and essential oil in rhizome of Atractylodes lancea in different temperatures. Chin Tradit Herb Drugs 42(2):372–375
Zhao JL, He XL (2011) Effects of AM fungi on drought resistance and content of chemical components in Angelica dahurica. Acta Agric Bor Occi Sin 20(3):184–189
Zhao X, Wang BW, Yan XF (2006) Effect of arbuscular mycorrhiza on camptothecin content in Camptotheca acuminate seedlings. Acta Ecol Sin 26(4):1057–1062
Zhao PJ, An F, Tang M (2007) Effects of arbuscular mycorrhiza fungi on drought resistance of Forsythia suspense. Acta Bot Bor Occi Sin 27(2):396–399
Zhao JL, Deng HY, He XL (2009) Effects of AM fungi on the quality of trueborn Angelica dahurica from Hebei province. Acta Agric Boreali-Sin 24:299–302
Zhou JH, Fan JH (2007) Effects of AM fungi on the berberine content in Phellodendron chinense seedings. North Hortic 12:25–27
Zhou N, Xia CL, Jiang B, Bai ZC, Liu GN, Ma XK (2009) Arbuscular mycorrhiza in Paris polyphylla var. yunnanensis. Chin J Chin Med 34(14):1768–1772
Zhou N, Zou L, Wang GZ, Jiang B (2010) Primary explore to relation of arbuscular mycorrhizae and its secondary metabolite steroidal saponin in Paris polyphylla. Chin J Exp Tradit Med Formulae 16(16):85–88
Zubek S, Stojakowska A, Anielska T, Turnau K (2010) Arbuscular mycorrhizal fungi alter thymol derivative contents of Inula ensifolia L. Mycorrhiza 20:497–504
Zubek S, Mielcarek S, Turnau K (2012) Hypericin and pseudohypericin concentrations of a valuable medicinal plant Hypericum perforatum L. are enhanced by arbuscular mycorrhizal fungi. Mycorrhiza 22:149–156
Zubek S, Błaszkowski J, Seidler-Łożykowska K, Bąba W, Mleczko P (2013) Arbuscular mycorrhizal fungi abundance, species richness and composition under the monocultures of five medicinal plants. Acta Sci Pol-Hortoru 12:127–141
Zubek S, Rola K, Szewczyk A, Majewska ML, Turnau K (2015) Enhanced concentrations of elements and secondary metabolites in Viola tricolor L. induced by arbuscular mycorrhizal fungi. Plant Soil 390(1–2):129–142
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Pandey, D.K., Kaur, P., Dey, A. (2018). Arbuscular Mycorrhizal Fungi: Effects on Secondary Metabolite Production in Medicinal Plants. In: Gehlot, P., Singh, J. (eds) Fungi and their Role in Sustainable Development: Current Perspectives. Springer, Singapore. https://doi.org/10.1007/978-981-13-0393-7_28
Download citation
DOI: https://doi.org/10.1007/978-981-13-0393-7_28
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-0392-0
Online ISBN: 978-981-13-0393-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)