Abstract
Sulphur, an essential component for plant as well as animals, is present in soils in both organic and inorganic forms, with organic form particularly sulphate esters and carbon-bonded sulphur contributing ~75–90% of the total. The major sources of sulphur in soils are sulphur-containing minerals, plant and elemental residue and external addition including atmospheric deposition. Sulphur deficiency in plants results in poor nitrogen metabolism thus protein biosynthesis, chlorosis, low oil percentage and ultimately low yield. The conversion of organic sulphur in organic matter to inorganic form and vice versa is dominantly a microbiological process. In well-aerated soil, organic sulphur is mineralized to sulphate and taken up by plants. Concurrently inorganic sulphur is immobilized to organic form and incorporated in microbial tissue. The rate of these processes obviously depends on soil reaction, temperature, moisture and addition of crop residue and many other factors that ultimately affect the activity of microorganism. Several enzymes in soil, viz. arylsulphatase, play a major role in sulphur mineralization process though very little information is available till now towards the pathway of decomposition. In addition to this process, inorganic sulphur in soil undergoes various oxidation and reduction process, modulated by microorganisms. Various reduced inorganic sulphur compounds are oxidized by a group of bacteria in suitable condition and utilize the energy. The wide range of stable redox states and their interconversion affect sulphur cycle, fate of applied fertilizer and ultimately its availability to plants and microbes. In this chapter we reviewed the sulphur cycle and its transformation by various microbial processes.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Besides carbon and nitrogen which are important constituents of plants, microorganisms are also known to influence the availability of sulphur (S) as well as phosphorus and certain trace elements in soil for absorption by plants. Sulphur is the tenth most abundant and widely distributed element in the nature. Sulphur is an essential element for plant as well as animals and found in nature in combined form, viz. gypsum (CaSO4.2H2O) and pyrite (FeS2), and in elemental form (S0). The sulphur is considered as ‘secondary’ nutrient as only because their requirement by plant is quantitatively less as compared to the primary nutrients. In spite of the essentiality, very less importance was given to S addition in field in the past mainly due to restricted area and crops that response with the fertilizer and contribution through major fertilizer or from natural sources (Tandon 2011; Meena et al. 2013a; Bahadur et al. 2014; Maurya et al. 2014; Jat et al. 2015; Kumar et al. 2015).
The S is added in soil through fertilizer, pesticides, irrigation water and adsorption of SO2 gases from atmosphere. An amount of 5–250 kg/ha/year of sulphur is added in soil through rainfall depending on industrial activity and burning of fossil fuel. Highly weathered soils away from sea and industrial activity are generally prone to sulphur deficiency. In earth, the lithosphere is the major sink of sulphur (24.3 × 1018 kg) followed by the hydrosphere (1.3 × 1018 kg), pedosphere (2.7 × 1014 kg) and atmosphere (4.8 × 109 kg), respectively (Stevenson 1982). Sulphur, with atomic weight 32.064, exists in various oxidation states. This is indicated by the oxidation number in several compounds, viz. sulphides (−2), polysulphide (−1), elemental sulphur (0), thiosulphate [(−2) and (+6)], sulphite (+4) and sulphate (+6) (Rao 1999).
3.2 Major Sources of Sulphur in Soil and Its Various Pools
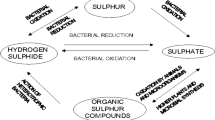
Organic matter is the major source of S in soil in most of the cases. Of the total sulphur present in soil, only 10–15% is in inorganic form (sulphate), and ~75–90% is in organic form. Thus inorganic component of soil sulphur constitutes only a minor portion of the total sulphur content of soils. However, the inorganic sulphur released from mineral in the form of sulphate (SO4 2−) due to weathering is consumed by plants and converted to various organic forms (Fig. 3.1). Upon addition to soil, the bulk of sulphur in the organic form is metabolized by soil microorganisms to make a major part available in an inorganic state (sulphur, sulphates, sulphite, thiosulphate, etc.) for plant nutrition and a small amount converted to humus (Gharmakher et al. 2012).
Sulphur is bound in organic state in proteins of vegetable and animal origin and in the protoplasm of microorganisms in the form of sulphur-containing amino acids (cysteine, cystine, methionine), lipid, proteins, polypeptides, biotin, thiamine, etc. These organic sulphur compounds can broadly be divided in two groups, namely, ester sulphates, which have C-O-SO3 linkages, and carbon-bonded S, which has direct C-S linkages. Other organic forms also exist, but they are of minor importance. Ester sulphates include compounds such as choline sulphate, phenolic sulphates and sulphated polysaccharides. Carbon-bonded S is comprised principally of amino acids such as methionine and cysteine and sulpholipids (Tabatabai and Bremner 1970). Ester sulphate mineralized faster than C-bonded S and acts as readily available S stores for plant and microbes (Kovar and Grant 2011).
In temperate condition even more than 95% of the total sulphur may present as organic form in soil (Stevenson 1982). Inorganic sulphur also present in appreciable amount in arid or semiarid region. Minerals that supply sulphur to soil are mainly pyrites (FeS2), sphalerite (ZnS), chalcopyrite (CuFeS2), gypsum, epsomite (MgSO4,6H2O), etc. Sulphur in soil is found more in fine texture soil as compare to coarse texture sandy soil and in subsurface soil as compare to surface due to difference in distribution of organic carbon. Sulphur released from mineral in the form of sulphate (SO4 2−) due to weathering is consumed by plants and converted to various organic forms (Fig. 3.1).
3.3 Functions of Sulphur
Concentration of sulphur in healthy plants ranges from 0.1 to 0.4% on dry weight basis. Sulphur has several important functions in plant metabolism such as synthesis of glucosides and glucosinolates (in mustard oils) and activation of enzymes and sulphydryl (-SH) linkages that are the source of pungency in oils and is also involved in formation of chlorophyll. Ferredoxin, being a component of Fe-S cluster protein, plays an important role in photosynthetic electron transport system. Sulphur is required for the synthesis of sulphur-containing amino acids methionine (21%), cysteine (26%) and cystine (27%), which are essential components of protein. Approximately 90% of plant sulphur is present in these amino acids (Tandon and Messick 2002). It is also needed for the synthesis of metabolites such as coenzyme A, biotin, thiamin or vitamin B and glutathione. The sulphur requirement of oilseed crops was found to be the highest followed by pulses and cereals. Therefore, sulphur deficiency results in low photosynthetic activity, growth retardation, yellowing of young leaves and ultimately poor yield. Sulphur deficiency also retards nitrogen fixation as both are constituent of protein. Thus the desired N:S ratio is necessary for optimum N metabolism. The optimum N:S ratio should be maintained for obtaining good yield. The desired N:S ratio for legumes has been identified as 15–16:1 and 11–12:1 for cereals (Pasricha and Sarkar 2002).
Sulphur deficiency is reported from larger areas all over the world soils and in many crops too. The reason being the nutrient management strategies mainly depended on application of NPK fertilizers, ignoring the replenishment of other nutrients through fertilizers or organic sources (Sahrawat et al. 2009). Apart from that, progressively higher removal of sulphur owing to high production level led to appearance of sulphur deficiency (Tandon 2011). The availability of sulphur for plant uptake largely depends on the dynamic sulphur cycle and the rate of conversion of organic sulphur to inorganic sulphate which is plant usable form. Such conversion in soil is typically termed as mineralization, and it is strictly a microbial process. Especially in the rhizosphere zone, microbes play a vital role in converting the organic sulphur in to plant available inorganic form. This chapter will consider the microbial processes that influence sulphur cycling in the soil and will summarize what is known about the organisms that catalyse these processes.
3.4 Cycling of Sulphur in Soils
Cycling of sulphur is similar to that of nitrogen. Transformation/cycling of sulphur between organic and elemental states and between oxidized and reduced states is brought about by various microorganisms, specially bacteria. Thus the conversion of organically bound sulphur to the inorganic state by microorganisms is termed as mineralization of sulphur. The sulphur/sulphate, thus released, is either absorbed by the plants or escapes to the atmosphere in the form of oxides (Ahmad et al. 2016; Meena et al. 2016a, b; Parewa et al. 2014; Prakash and Verma 2016; Jaiswal et al. 2016; Jha and Subramanian 2016; Kumar et al. 2016a, b). In the absence of oxygen, certain microorganisms produce hydrogen sulphide from organic sulphur substrates especially in waterlogged soils. Chemical and spectroscopic studies have shown that in agricultural soils, most of the soil sulphur (~ 95%) is present as sulphate esters or as carbon-bonded sulphur (sulphonates or amino acid sulphur), rather than inorganic sulphate (Kertesz and Mirleau 2004; Wang et al. 2006).
Plant sulphur nutrition depends primarily on the uptake of inorganic sulphate. However, recent research has demonstrated that the sulphate ester and sulphonate pools of soil sulphur are also plant-bioavailable, probably due to interconversion of carbon-bonded sulphur and sulphate ester sulphur to inorganic sulphate by soil microbes. In addition to this mineralization of bound forms of sulphur, soil microbes are also responsible for the rapid immobilization of sulphate, first to sulphate esters and subsequently to carbon-bound sulphur. The rate of sulphur cycling depends on the microbial community present, and on its metabolic activity, though it is not yet known if specific microbial species or genera control this process (Kertesz and Mirleau 2004). The genes involved in the mobilization of sulphonate and sulphate ester sulphur by one common rhizosphere bacterium, Pseudomonas putida, have also been investigated by Kertesz and Mirleau (2004). Mutants of this species that are unable to transform sulphate esters show reduced survival in the soil, indicating that sulphate esters are important for bacterial S nutrition in this environment. P. putida S-313 mutants that cannot metabolize sulphonate-sulphur do not promote the growth of tomato plants as the wild-type strain does, suggesting that the ability to mobilize bound sulphur for plant nutrition is an important role of this species (Fig. 3.1).
Thus the sulphur pools in soils are dynamic in nature. Inorganic sulphur compounds are immobilized to organic sulphur, different organic forms interconverted, and immobilized sulphur is simultaneously mineralized to yield plant available inorganic sulphur. Most of the processes are linked to the microbial biomass present in the soils. Especially in the rhizosphere, microbes play a vital role in allowing plants to access soil organosulphur (Priyadharsini and Muthukumar 2016; Kumar et al. 2017; Meena et al. 2015a, b, f; Raghavendra et al. 2016; Zahedi 2016; Dominguez-Nunez et al. 2016; Dotaniya et al. 2016).
3.5 Sulphur Transformations in Soil
The various transformations of sulphur in the biosphere can be summed up as a cyclic reaction involving (i) decomposition of organic sulphur compounds into subunits which are, in turn, converted into inorganic compounds through a process of mineralization; (ii) assimilation of sulphur into the protoplasm of microorganisms, a process referred to as immobilization; (iii) oxidation of inorganic sulphur compounds into elemental sulphur; and (iv) reduction of sulphate. Both aerobic and anaerobic microorganisms take part in organic S formation, though only 1–3% of microbial biomass is composed of S (Strick and Nakas 1984; Chapman 1987). The short life cycles of microorganisms, however, result in rapid turnover and S recycling (Smith and Paul 1990). Microbial biomass has been described as the most active and readily available form of soil organic S, and much of the mineralized S seen in short-term incubation experiments may originate from microbial biomass (McLaren et al. 1985; Gharmakher et al. 2012). Various transformations of the sulphur in soil result mainly due to microbial activity, although some biogeochemical transformations (Lamers et al. 2012) are also possible (e.g. oxidation of iron sulphide). The major types of transformations involved in the cycling of sulphur which are mineralization, immobilization, oxidation and reduction are briefly described below.
3.5.1 Mineralization of Soil Sulphur
Sulphur is taken up by the plant root system largely as the sulphate ion although several amino acids may be assimilated without prior degradation. Since agricultural crops and other vegetation require for growth the sulphate found in their rooting medium, the mineralization of organic sulphur plays an important part in the microbiological reactions required for higher life. The breakdown and/or decomposition of large organic sulphur compounds to smaller units and their conversion into inorganic compounds (sulphates) by the microorganisms. The rate of sulphur mineralization is about 1.0–10.0 percent/year. A diverse group of organic compounds containing sulphur are presented as substrates to the microflora. The elements occur in plant, animal and microbial proteins; in the amino acids, cystine and methionine; and in the B vitamins, thiamine, biotin and thioctic acid. It is also found in the tissues and excretory products of animals as free sulphate, as taurine and, to some extent, as thiosulphate and thiocyanate (Rajvaidya and Markandey 2006).
Upon the addition of plant or animal remains to soil, the sulphur contained therein is mineralized due to microbial activities in soils. A portion of the inorganic products so released is utilized by the microflora for cell synthesis, and the remainder is escaped into the environment. Aerobically, the terminal, inorganic product is sulphate, while in the absence of atmospheric O2, particularly during the putrefaction of proteinaceous matter, H2S and the odoriferous mercaptans are accumulated in soils. Many soil bacteria have the ability to form H2S from partially degraded proteins, and as such it is likely that sulphides are among the major inorganic substances released during the decomposition of proteinaceous substrates (Jez 2008).
The sulphur in cystine and cysteine is recovered quantitatively as sulphate when either of these amino acids is applied to well-aerated soils. The conversion is rapid because many microorganisms attack the two compounds. The decomposition may proceed by any one of several known mechanisms. In soil, cystine can be formed by a chemical oxidation of added cysteine. The sulphur of the molecule in turn is oxidized to sulphate (Solomon et al. 2010) with cystine disulphoxide and possibly cysteine sulphinic acid as intermediates, a reaction sequence not involving H2S (Rawat et al. 2016; Yasin et al. 2016; Meena et al. 2015e, 2016c; Saha et al. 2016a; Yadav and Sidhu 2016; Meena et al. 2016d; Teotia et al. 2016; Bahadur et al. 2016b; Das and Pradhan 2016).
Alternatively, fungi such as Microsporum gypseum convert cysteine sulphur to sulphate by a mechanism possibly involving the consecutive formation of cystine, sulphenic and sulphinic acids, sulphite and sulphate (Stahl et al. 1949). In the process of ammonification of organic nitrogen, the extent of mineral sulphur formation is influenced by the sulphur content and the C:S ratio of the decomposing substrate. Sulphate accumulates only when the sulphur level in the organic matter exceeds the microbial needs. Thus, it is likely that the percentage of sulphur mineralized per annum is similar to the figure for nitrogen mineralization, i.e. 1–3% of the total supply in soils of the humid-temperate zone. It is also likely that environmental factors that govern microbial growth in general would affect the rate of sulphur mineralization in soils.
3.5.2 Immobilization of Sulphur
Immobilization of sulphur represents the microbial conversion of inorganic sulphur compounds to organic sulphur compounds. The major sulphur-containing compounds are sulphate, hyposulfite, sulphoxylate, thiosulphate, persulphate, sulphide, elemental sulphur, sulphite, tetrathionate and thiocyanate among the inorganic substances and cysteine, cystine, methionine, taurine and undecomposed proteins of the organic group. Sulphate immobilization is a reductive process and is performed by both aerobic and anaerobic chemotrophs and phototrophs. However, certain anaerobic microbes (e.g. the phototrophic green S bacteria) are only capable of sulphide immobilization for their needs which requires less energy than sulphate assimilation (Bauld 1986).
The C:S ratio of microbial tissue is in the range 57–85 in bacteria and 180–230 for fungus. The sulphur content of most microorganisms lies between 0.1 and 1.0% of the dry weight, and the most conspicuous cellular constituents containing the element are the amino acids, cystine and methionine. Immobilized sulphur is assimilated into organic matter generally by covalent bonding (Strickland et al. 1987). Sulphate added to soil can be adsorbed quickly or transformed to low molecular weight organic S compounds (Jez 2008), especially ester sulphates as fulvic acids (Saggar et al. 1981), which later can be polymerized to larger insoluble organic compounds (Strickland et al. 1986). Sulphur, immobilized by microbes, can be estimated by measuring inorganic sulphate released in chloroform fumigation technique. Though less quantity of sulphur is actually sequestered in microbial biomass, the fraction is extremely labile (Balota et al. 2003) and an important indicator of plant availability.
The addition of starch to a sulphur-poor soil depresses crop yields, but the reduction in yield is prevented if sulphates are applied. The detrimental effect is probably a result of microbial utilization of the available sulphur during the decomposition of the starch, leading to an immobilization of the nutrient. The critical C:S ratio in carbonaceous materials above which immobilization is dominant to mineralization is reported to be approximately 50:1. Critical C:S ratio of the substrate, diversity within microbes present in soil and environmental factors, viz. temperature, moisture, organic matter, atmospheric deposition inputs and other factors, influence immobilization rates.
3.5.3 Oxidation of Inorganic Sulphur
Oxidation of elemental sulphur and inorganic sulphur compounds (such as H2S, sulphite and thiosulphate) to sulphate (SO4) is brought about by chemoautotrophic and photosynthetic bacteria. When plant and animal proteins are degraded, the sulphur is released from the amino acids and accumulates in the soil which is then oxidized to sulphates in the presence of oxygen and under anaerobic condition (waterlogged soils); organic sulphur is decomposed to produce hydrogen sulphide (H2S). H2S can also accumulate during the reduction of sulphates under anaerobic conditions which is further oxidized to sulphates if aerobic conditions prevail in soils (Behera et al. 2014).
-
(a)
$$ 2\mathrm{S}+3{\mathrm{O}}_2+2{\mathrm{H}}_2\mathrm{O}\to 2{\mathrm{H}}_2{\mathrm{SO}}_4\overset{\mathrm{Ionization}}{\to }2{\mathrm{H}}^{+}+{\mathrm{SO}}_4\left(\mathrm{Aerobic}\right) $$
-
(b)
$$ {\mathrm{CO}}_2+2{\mathrm{H}}_2\mathrm{S}\overset{\mathrm{Light}}{\to}\left({\mathrm{CH}}_2\mathrm{O}\right)+{\mathrm{H}}_2\mathrm{O}+2\mathrm{S} $$
Or
3.5.3.1 Bacteria of Genus Thiobacillus
The members of genus Thiobacillus (obligate chemolithotrophic, non-photosynthetic), e.g. T. ferrooxidans and T. thiooxidans, are the main organisms involved in the oxidation of elemental sulphur to sulphates. These are aerobic, nonfilamentous, chemosynthetic autotrophs.
3.5.3.2 Green and Purple Sulphur Bacteria
Green and purple bacteria (photolithotrophs) of genera Chlorobium, Chromatium and Rhodopseudomonas are also reported to oxidize sulphur in aquatic environment (Madigan and Martinko 2006). They are classified within the families Thiorhodaceae and Chlorobacteriaceae. The green and purple bacteria, developed anaerobically, meet their energy requirements from light, with carbon dioxide as their only source of carbon, and oxidizing reduced sulphur materials. They are most commonly found in the bottom of water body containing sulphur material.
3.5.3.3 Colourless Filamentous Sulphur Bacteria
These bacteria of species Thiothrix, Beggiatoa, Thiospirillopsis and Thioploca are found in sulphide-containing waters and oxidize sulphide to sulphate with accumulation of elemental sulphur in cells (Starkey 1950). Besides, heterotrophic bacteria (Bacillus, Pseudomonas and Arthrobacter) and fungi (Aspergillus, Penicillium) and some actinomycetes are also reported to oxidize sulphur compounds.
Sulphuric acid produced during oxidation of sulphur and H2S is of great significance in reducing the pH of alkaline soils and in controlling potato scab and rot diseases caused by Streptomyces bacteria. The formation of sulphate/sulphuric acid is beneficial in agriculture in different ways: (i) as it is the anion of strong mineral acid (H2SO4), it can render alkali soils fit for cultivation by correcting soil pH and (ii) solubilize inorganic salts containing plant nutrients and thereby increase the level of soluble phosphate, potassium, calcium, magnesium, etc. for plant nutrition (Chien et al. 2011; Karimizarchi et al. 2014).
3.5.4 Reduction of Sulphate
Sulphate in soil is taken up by plants and microorganisms and assimilated into proteins. This is known as ‘assimilatory sulphate reduction’. Sulphate can also be reduced to hydrogen sulphide (H2S) by sulphate-reducing bacteria (e.g. Desulfovibrio and Desulfotomaculum) and may render the availability of sulphur for plant nutrition. Such conversion of sulphate to H2S is termed as ‘dissimilatory sulphate reduction’ which is not at all desirable from soil fertility and agricultural productivity view point. The favourable environment for dissimilatory sulphate reduction is alkaline and anaerobic condition of soil.
For example, calcium sulphate is attacked under anaerobic condition by the members of the genus Desulfovibrio and Desulfotomaculum to release H2S.
Hydrogen sulphide produced by the reduction of sulphate and decomposition of sulphur-containing amino acids is further oxidized by some species of green and purple phototrophic bacteria (e.g. Chlorobium, Chromatium) to release elemental sulphur.
The predominant sulphate-reducing bacterial genera in soil are Desulfovibrio, Desulfotomaculum and Desulfomonas (all obligate anaerobes) although Desulfovibrio desulfuricans are most ubiquitous in soils. It is a non-spore forming, obligate anaerobe that reduces sulphates at rapid rate in waterlogged/flooded soils, while species of Desulfotomaculum are spore-forming, thermophilic, obligate anaerobes that reduce sulphates in dry land soils. All these sulphate-reducing bacteria excrete an enzyme called ‘desulphurases’ or ‘bisulphate reductase’ which is responsible for reduction of sulphur. The rate of sulphate reduction in nature is accentuated with increasing water levels (flooding), organic matter content and increased temperature.
3.6 Enzyme Reactions in Soil Involving Sulphur Compounds
A. Sulphatases
Since much of the soil organic sulphur is present as sulphate esters, aryl and alkylsulphatase enzymes are thought to play a key role in sulphur mineralization. The overall reaction can be written as
Sulphatases are classified according to the nature of substrate over which it works and mainly categorized into arylsulphatases, alkylsulphatases, glucosulphatases, mycosulphatases (Roy and Trudinger 1970), etc. Arylsulphatases or phenol sulphatases are most widely distributed (Wyszkowska et al. 2016) among other sulphatase enzymes and found in soils of cultivable land, forest, sediment, marshes, etc. (Fitzgerald 1978). The major sources of this enzyme in soils are bacteria and fungi. Arylsulphatases, first reported by Tabatabai and Bremner (1970), are assayed in soil by measuring the amount of p-nitrophenol released from p-nitrophenyl sulphate added to soil and incubating for 1–2 h.
Cysteine and methionine, the two major sulphur amino acids, also undergo enzyme-catalysed transformations in soil. First, however, oxidation of cysteine to cystine (the disulphide form of the amino acid) rapidly occurs in soil as this reaction can be catalysed by trace amounts of a number of metal ions. An enzyme called cystathionine lyase acts upon cystine to form a disulphide called thiocysteine. Thiocysteine can then react with a free sulfhydryl group to form hydrogen sulphide (H2S).

Inspection of the chemical structures of cysteine and thiocysteine illustrates how the hydrogen sulphide may be formed during the sequence of reactions as follows:
In environments that are neither highly aerobic nor anaerobic, both cysteine and cystine (which contains a free sulphydryl) may be present. Field experiments have shown that losses of hydrogen sulphide are more likely to occur during the initial period waterlogging than after a more strongly anaerobic condition has developed.
The activity of arylsulphatase varies according to soil type, soil depth, organic matter content, season and climate. Maximum activity of this enzyme is observed in surface soils with optimum pH 5.5–6.2 (Tabatabai and Bremner 1970). Factors affecting microbial biomass are also known to influence the activity of the enzyme. Arylsulphatase activity in soil is significantly correlated with clay content, moisture percentage, organic carbon, nitrogen content, etc. The different types of vegetation and their rhizospheric effect have also significant influence on arylsulphatase activity in soils. Repeated application of S0 fertilizer in soil declines the enzyme activity due to decline in microbial population and inhibitory effect of large quantities of SO4 2− in this fertilized soil (Gupta et al. 1988). The enzyme activity is measured by pretreating the soils with toluene followed by incubation with buffered S2O3 2− and CN− solution, and the SCN− thus produced is measured calorimetrically (Saha et al. 2016b; Verma et al. 2014; Verma et al. 2015b; Meena et al. 2013c, 2014a, 2016e; Singh et al. 2015; Bahadur et al. 2016a; Masood and Bano 2016).
B. Rhodanese
One other enzyme involved in the sulphur cycle has been detected and characterized in soil. This enzyme is called rhodanese (thiosulphate cyanide sulfotransferase) and belongs to the transferase class of enzymes. It catalyses the formation of thiocyanate from thiosulphate and cyanide according to the following reaction.
The enzyme is found in animal, plant tissue, bacteria and soils. Rhodanese activity is found in a large number and variety of soils. Both thiosulphate and tetrathionate are formed as intermediates during the oxidation of elemental sulphur to sulphate, and the rhodanese-catalysed reaction may be involved in the further metabolism of these compounds in soil.
3.7 Groups of Microorganisms Involved in Sulphur Transformation
Efficient microbes play important roles in releasing S from elemental S and sulphide minerals in the earth surface to soil. Only from sulphate minerals, S becomes readily available in soil and to plants, since plants take up sulphur only as SO4 = form preferentially. Bacteria, archaea as well as fungi are involved in the oxidation of sulphur; however, the major role is played by the bacteria, Thiobacillus sp. Among the archaea, aerobic oxidation of sulphur is restricted to the members of the Sulfolobales only (Setter et al. 1990). Fungi like Alternaria tenuis, Aureobasidium pullulans and Epicoccum nigrum and a range of Penicillium sp., Scolecobasidium constrictum, Myrothesium circutum and Aspergillus sp. are reported to be involved in the oxidation of elemental S and thiosulphate (Wainwright 1978; Shinde et al. 1996). Bacteria involved in the oxidation of sulphur can be broadly classified into three groups as chemolithoautotrophs, chemolithoheterotrophs and chemolithomesotrophs (Aragono 1991; Vidyalakshmi et al. 2009).
3.7.1 Chemolithoautotrophs
These bacteria obtain energy from oxidation of sulphur and carbon from carbon dioxide for their growth and development. The examples are Thiobacillus thioparus, T. neapolitanus, T. denitrificans, T. thiooxidans, T. ferrooxidans, T. halophilus and some species of Thiomicrospira.
3.7.2 Chemolithoheterotrophs
These bacteria obtain energy from oxidation of sulphur and carbon from organic molecules for their growth and development. The examples are Thiobacillus novellus, T. acidophilus, T. aquaesulis, Paracoccus denitrificans, P. versutus, Xanthobacter tagetidis, Thiosphaera pantotroph and Thiomicrospira thasirae (Prasad and Shivay 2016).
3.7.3 Chemolithomesotrophs
These bacteria obtain energy from oxidation of sulphur and carbon from inorganic as well as organic molecules for their growth and development. The examples include Thiobacillus denitrificans and T. ferrooxidans. There are a number of enzymes involved in sulphur oxidation. These include thiosulphate dehydrogenase, tetrathionate hydrolase, trithionate hydrolase and sulphur oxygenase (Friedrich et al. 2001; Keppler et al. 2000). On the contrary, sulphates are reduced to H2S by S-reducing organisms under anaerobic conditions such as those obtained in lowland rice paddies. H2S is responsible for the bad odour from paddy fields. Sulphate-reducing bacteria reduce sulphate to obtain energy. Sixty genera containing 220 species of sulphate-reducing bacteria are known (Barton and Fauque 2009). The largest group (about 23 genera) includes Desulfobacterales, Desulfovibrionales and Syntrophobacterales (Muzer and Stams 2008). The second largest group includes genera Desulfotomaculum, Desulfosporomusa and Desulfosporosinus (Prasad and Shivay 2016).
Bacteria capable of oxidizing organic sulphur compounds could be either aerobic or anaerobic. Their morphology varies from nonfilamentous (Thiobacillus) to filamentous forms (Beggiatoa, Thiothrix and Thioploca). Several fungi and actinomycetes have also been reported to be sulphur oxidizers (Aspergillus, Penicillium, Microsporum). Among these microorganisms, Thiobacillus deserves special mention as it produces sulphuric acid when elemental sulphur is added to soil with the result that the pH of soil may fall as low as 2.0 after prolonged incubation with the bacterium. The possible role of Thiobacillus in controlling plant diseases in sulphur-amended soils has been demonstrated with regard to potato scab caused by Streptomyces scabies and the rot of sweet potatoes caused by S. ipomoea. Under acidic soil conditions (below pH 5.0), inoculation of soil with Thiobacillus after addition of sulphur effectively minimizes losses of sulphur in soils. The application of sulphur coupled with Thiobacillus inoculation has also the potentiality of rendering alkali soils fit for cultivation of crops. The formation of H2SO4 in soil following additions of elemental sulphur augments nutrient mobilization by increasing the level of soluble phosphate, potassium, calcium, manganese, aluminium and magnesium (Chien et al. 2011; Karimizarchi et al. 2014). In fact, manganese deficiency in soils can be corrected by sulphur applications.
Sulphate-reducing bacteria, i.e. those bacteria which reduce inorganic sulphate into hydrogen sulphide, may diminish the availability of sulphur for plant nutrition and thus influence agricultural production. Desulfovibrio desulfuricans is a species belonging to this class of bacteria which is an obligate anaerobe capable of producing hydrogen sulphide at a rapid rate. Other species of Desulfovibrio are also active in inorganic sulphate reduction, but the exact pathway is not yet clearly understood.
3.8 Role of Mycorrhizal Association in Plant Sulphur Supply
Several fungi in soil are capable of mineralizing S from sulphate esters (Klose et al. 1999). In contrast, an exclusively bacterial multicomponent monooxygenase enzyme complex is necessary to mobilize sulfonates, the dominant organo-S source in soil (Vermeij et al. 1999; Kertesz and Mirleau 2004). In fact, soil S cycling may involve complex interactions between several free-living and symbiotic root-associated microbial populations. Arbuscular mycorrhizal (AM) fungi form symbiosis with 80% of land plant species which depend upon them for growth (Wang and Qiu 2006). AM fungal symbiosis is characterized by fungal penetration of root cortical cells forming microscopic branched structures called arbuscules that increase efficiency of plant-fungus metabolite exchange. Extraradicular AM hyphae provide surfaces for functional bacterial populations to colonize. A number of studies have reported interactions between AM fungi and phosphorus (P) and nitrogen (N) mobilizing bacteria (Hodge and Storer 2015). Like S, both N and P exist predominantly inaccessible to plants which rely on interactions with mycorrhizal fungi and associated microbes to facilitate their mobilization (Richardson et al. 2009). The rhizosphere is regarded as a hot spot for microbial activity, and recent studies indicate that this is also the case for the mycorrhizosphere where bacteria may attach to the fungal hyphae capable of mobilizing organo-S (Sharma et al. 2016; Verma et al. 2015a; Meena et al. 2013b, 2015c; Shrivastava et al. 2016; Velazquez et al. 2016). While current evidence is not showing sulphatase and sulphonate activity in arbuscular mycorrhiza, their effect on the expression of plant host sulphate transporters is documented (Gahan and Schmalenberger 2014).
3.9 Sulphur Management for Sustainable Crop Production
Sulphur has become more important as a limiting nutrient in crop production in recent years for several reasons. These include higher crop yields that require more S, less S impurities in modern fertilizers, less use of S-containing pesticides, reduced industrial S emissions to the atmosphere and a greater awareness of S needs. The crop’s need for S is closely associated with N. The relationship between S and N is not surprising since both are components of protein and are involved in chlorophyll formation. They are also linked by the role of S in the conversion of nitrate to amino acids. Crops having high N need will usually also have high S needs. The majority of S in most soils is contained in organic matter. Organic S must be mineralized to the inorganic sulphate anion before it can be taken up by crops. Organic matter decomposition and the resulting S release are affected by temperature and moisture, and generally conditions that favour crop growth also favour mineralization and release of S, although this may be less likely with cool season crops. Sulphate, like most anions, is somewhat mobile in soils and therefore subject to leaching. Soil conditions where S is most likely to be deficient are low organic matter levels, coarse (sandy) texture with good drainage and high rainfall conditions. But, these are generalizations and S can be deficient under other conditions as well. Several factors should be taken into account when making S fertilization decisions. Among these are crop and yield goal, soil and plant analysis, organic matter content, soil texture and contribution from other sources such as irrigation water and manure. High-yielding forage crops such as alfalfa and hybrid Bermuda grass remove more S than most grain crops and tend to be relatively responsive. Soil test S is usually a measure of sulphate-S, and as with nitrate-N samples should be taken deeper than normal (0–2 ft) because of sulphate mobility in the soil. Soils containing less than 2% organic matter are most commonly S deficient; however, deficiencies do occur in soils with higher organic matter.
Coarse-textured soils are more apt to need S, but finer-textured soils can also be deficient. Sulphur content of irrigation water should be determined since in some cases it can deliver significant amounts of S. There are several S fertilizer sources available. Most soluble S fertilizer contains sulphate, but others such as bisulphites, thiosulphates and polysulphides are also available. The most common insoluble S fertilizer is elemental S, which must be oxidized to sulphate before plants can use it. This is a biological process and is affected by temperature, moisture, aeration and particle size. This process also produces acidity, and elemental S can be used in some instances specifically to acidify soils. Sulphur is an important component of complete and balanced crop nutrition and has justifiably gained more attention in recent years (Sindhu et al. 2016; Meena et al. 2014b, 2015d; Singh et al. 2016). Several factors should be considered to make the best decision regarding S need and fertilization.
3.10 Concluding Remark and Future Prospective
Wide application of sulphur-free fertilizer costs spreading of sulphur deficiency in agriculture soils of humid and semi-humid region, and it has become a deterrent towards achieving optimum production. Plants can able to synthesize sulphur-containing amino acids only in the presence of sufficient amount sulphur in available form in soil. It has been elucidated that there is an active interconversion of organic and inorganic sulphur forms in the soil, controlled largely by the group of microorganism, and this cycle determines the sulphur nutrition of plants and others. The future study should look upon composition of soil microbial communities responsible for sulphur transformation, detection of changes in their activity in different microclimate and the detail pathway of the same.
References
Ahmad M, Nadeem SM, Naveed M, Zahir ZA (2016) Potassium-solubilizing bacteria and their application in agriculture. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 293–313. https://doi.org/10.1007/978-81-322-2776-2_21
Aragono M (1991) Aerobic chemolithoautotrophic bacteria. In: Christjansson JK (ed) Thermophilic bacteria. CRC Press, Boca Raton, pp 7–103
Bahadur I, Meena VS, Kumar S (2014) Importance and application of potassic biofertilizer in Indian agriculture. Int Res J Biol Sci 3:80–85
Bahadur I, Maurya BR, Kumar A, Meena VS, Raghuwanshi R (2016a) Towards the soil sustainability and potassium-solubilizing microorganisms. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 225–266. https://doi.org/10.1007/978-81-322-2776-2_18
Bahadur I, Maurya BR, Meena VS, Saha M, Kumar A, Aeron A (2016b) Mineral release dynamics of tricalcium phosphate and waste muscovite by mineral-solubilizing rhizobacteria isolated from indo-gangetic plain of India. Geomicrobiol J 34(5):456–466. https://doi.org/10.1080/01490451.2016.1219431
Balota EL, Filho AC, Andrade DS, Dick RP (2003) Microbial biomass in soils under different tillage and crop rotation systems. Biol Fert Soils 38(1):15–20
Barton LL, Fauque AJ (2009) Biochemistry, physiology and biotechnology of sulphate reducing bacteria. Adv Appl Microbiol 68:41–98
Bauld J (1986) Transformation of sulphur species by phototrophic and chemotrophic microbes. In: The importance of chemical “speciation” in environmental processes. Springer, Berlin/Heidelberg, pp 255–274
Behera BC, Mishra RR, Dutta SK, Thatoi HN (2014) Sulphur oxidizing bacteria in mangrove ecosystem: a review. African J Biotech 13(29):2897–2907
Chapman SJ (1987) Microbial sulphur in some Scottish soils. Soil Biol Biochem 19:301–305
Chien SH, Gearhart MM, Villagarcía S (2011) Comparison of ammonium sulphate with other nitrogen and sulphur fertilizers in increasing crop production and minimizing environmental impact: a review. Soil Sci 176(7):327–335
Das I, Pradhan M (2016) Potassium-solubilizing microorganisms and their role in enhancing soil fertility and health. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 281–291. https://doi.org/10.1007/978-81-322-2776-2_20
Dominguez-Nunez JA, Benito B, Berrocal-Lobo M, Albanesi A (2016) Mycorrhizal fungi: role in the solubilization of potassium. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 77–98. https://doi.org/10.1007/978-81-322-2776-2_6
Dotaniya ML, Meena VD, Basak BB, Meena RS (2016) Potassium uptake by crops as well as microorganisms. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 267–280. https://doi.org/10.1007/978-81-322-2776-2_19
Fitzgerald JW (1978) Naturally occurring organo sulphur compound in soil. In: Nriagu JO (ed) Sulphur in the environment. Ecological impacts, Part II. Wiley, New York, pp 391–443
Friedrich CG, Rother D, Bardischewsky F, Quentmeier A, Fischer J (2001) Oxidation of reduced inorganic compounds by bacteria: emergence of a common mechanism. Appl Environ Microbiol 67(7):2873–2882
Gahan J, Schmalenberger A (2014) The role of bacteria and mycorrhiza in plant sulphur supply. Front Plant Sci 5:723. https://doi.org/10.3389/fpls.2014.00723
Gharmakher NH, Piutti S, Machet JM, Benizri E, Recous S (2012) Mineralization-immobilization of sulphur in a soil during decomposition of plant residues of varied chemical composition and S content. Plant Soil 360(1):391–404
Gupta VVSR, Lawrence JR, Germida JJ (1988) Impact of elemental sulphur fertilization on agricultural soils. I. Effects on microbial biomass and enzyme activities. Can J Soil Sci 68:463–473
Hodge A, Storer K (2015) Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant Soil 386(1–2):1–9. https://doi.org/10.1007/s11104-014-2162-1
Jaiswal DK, Verma JP, Prakash S, Meena VS, Meena RS (2016) Potassium as an important plant nutrient in sustainable agriculture: a state of the art. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 21–29. https://doi.org/10.1007/978-81-322-2776-2_2
Jat LK, Singh YV, Meena SK, Meena SK, Parihar M, Jatav HS, Meena RK, Meena VS (2015) Does integrated nutrient management enhance agricultural productivity? J Pure Appl Microbiol 9(2):1211–1221
Jez J (2008) Sulphur: a missing link between soils, crops, and nutrition. American Society of Agronomy, ASA-CSSA-SSSA, Madison, p 323
Jha Y, Subramanian RB (2016) Regulation of plant physiology and antioxidant enzymes for alleviating salinity stress by potassium-mobilizing bacteria. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 149–162. https://doi.org/10.1007/978-81-322-2776-2_11
Karimizarchi M, Aminuddin H, Khanif MY, Radziah O (2014) Elemental sulphur application effects on nutrient availability and sweet maize (Zea mays L.) response in a high pH soil of Malaysia. Mal J Soil Sci 18:75–86
Keppler U, Bennet B, Rethmeier J, Schwarz G, Deutzmann R, McEwan AG, Dahl C (2000) Sulfit: cytochrome c oxidoreductase from Thiobacillus novellus, purification, characterization and molecular biology of a heterodimeric member of sulfite oxidase family. J Biol Chem 275(18):13202–13212
Kertesz MA, Mirleau P (2004) The role of soil microbes in plant sulphur nutrition. J Exp Bot 55(404):1939–1945
Klose S, Moore JM, Tabatabai MA (1999) Arylsulphatase activity of microbial biomass in soils as affected by cropping systems. Biol Fertil Soils 29:46–54. https://doi.org/10.1007/s003740050523
Kovar JL, Grant CA (2011) Nutrient cycling in soils: sulphur. Publications from USDA-ARS/UNL Faculty, Paper 1383
Kumar A, Bahadur I, Maurya BR, Raghuwanshi R, Meena VS, Singh DK, Dixit J (2015) Does a plant growth-promoting rhizobacteria enhance agricultural sustainability? J Pure Appl Microbiol 9:715–724
Kumar A, Meena R, Meena VS, Bisht JK, Pattanayak A (2016a) Towards the stress management and environmental sustainability. J Clean Prod 137:821–822
Kumar A, Patel JS, Bahadur I, Meena VS (2016b) The molecular mechanisms of KSMs for enhancement of crop production under organic farming. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 61–75. https://doi.org/10.1007/978-81-322-2776-2_5
Kumar A, Maurya BR, Raghuwanshi R, Meena VS, Islam MT (2017) Co-inoculation with Enterobacter and Rhizobacteria on yield and nutrient uptake by wheat (Triticum aestivum L.) in the alluvial soil under indo-gangetic plain of India. J Plant Growth Regul 36(3):608–617. https://doi.org/10.1007/s00344-016-9663-5
Lamers LPM, van Diggelen JMH, den Camp HJMO, Visser EJW, Lucassen ECHET, Vile MA, Jetten MSM, Smolders AJP, Roelofs JGM (2012) Microbial transformations of nitrogen, sulphur, and iron dictate vegetation composition in wetlands: a review. Front Microbiol 3:156. https://doi.org/10.3389/fmicb.2012.00156
Madigan MT, Martinko JM (2006) Brock biology of microorganisms. Pearson Prentice Hall, Upper Saddle River, p 1056
Masood S, Bano A (2016) Mechanism of potassium solubilization in the agricultural soils by the help of soil microorganisms. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 137–147. https://doi.org/10.1007/978-81-322-2776-2_10
Maurya BR, Meena VS, Meena OP (2014) Influence of Inceptisol and Alfisol’s potassium solubilizing bacteria (KSB) isolates on release of K from waste mica. Vegetos 27:181–187
McLaren RG, Keer JJ, Swift RW (1985) Sulphur transformations in soils using sulphur-35 labelling. Soil Biol Biochem 17:73–79
Meena OP, Maurya BR, Meena VS (2013a) Influence of K-solubilizing bacteria on release of potassium from waste mica. Agric Sust Dev 1:53–56
Meena VS, Maurya BR, Bohra JS, Verma R, Meena MD (2013b) Effect of concentrate manure and nutrient levels on enzymatic activities and microbial population under submerged rice in alluvium soil of Varanasi. Crop Res 45(1,2 & 3):6–12
Meena VS, Maurya BR, Verma R, Meena RS, Jatav GK, Meena SK, Meena SK (2013c) Soil microbial population and selected enzyme activities as influenced by concentrate manure and inorganic fertilizer in alluvium soil of Varanasi. Bioscan 8(3):931–935
Meena VS, Maurya BR, Bahadur I (2014a) Potassium solubilization by bacterial strain in waste mica. Bang J Bot 43:235–237
Meena VS, Maurya BR, Verma JP (2014b) Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol Res 169:337–347
Meena RS, Meena VS, Meena SK, Verma JP (2015a) The needs of healthy soils for a healthy world. J Clean Prod 102:560–561
Meena RS, Meena VS, Meena SK, Verma JP (2015b) Towards the plant stress mitigate the agricultural productivity: a book review. J Clean Prod 102:552–553
Meena VS, Maurya BR, Meena RS (2015c) Residual impact of wellgrow formulation and NPK on growth and yield of wheat (Triticum aestivum L.). Bangladesh J Bot 44(1):143–146
Meena VS, Maurya BR, Verma JP, Aeron A, Kumar A, Kim K, Bajpai VK (2015d) Potassium solubilizing rhizobacteria (KSR): isolation, identification, and K-release dynamics from waste mica. Ecol Eng 81:340–347
Meena VS, Meena SK, Verma JP, Meena RS, Ghosh BN (2015e) The needs of nutrient use efficiency for sustainable agriculture. J Clean Prod 102:562–563. https://doi.org/10.1016/j.jclepro.2015.04.044
Meena VS, Verma JP, Meena SK (2015f) Towards the current scenario of nutrient use efficiency in crop species. J Clean Prod 102:556–557. https://doi.org/10.1016/j.jclepro.2015.04.030
Meena RK, Singh RK, Singh NP, Meena SK, Meena VS (2016a) Isolation of low temperature surviving plant growth-promoting rhizobacteria (PGPR) from pea (Pisum sativum L.) and documentation of their plant growth promoting traits. Biocatal Agric Biotechnol 4:806–811
Meena RS, Bohra JS, Singh SP, Meena VS, Verma JP, Verma SK, Sihag SK (2016b) Towards the prime response of manure to enhance nutrient use efficiency and soil sustainability a current need: a book review. J Clean Prod 112(1):1258–1260
Meena SK, Rakshit A, Meena VS (2016c) Effect of seed bio-priming and N doses under varied soil type on nitrogen use efficiency (NUE) of wheat (Triticum aestivum L.) under greenhouse conditions. Biocatal Agric Biotechnol 6:68–75
Meena VS, Bahadur I, Maurya BR, Kumar A, Meena RK, Meena SK, Verma JP (2016d) Potassium-solubilizing microorganism in evergreen agriculture: an overview. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 1–20. https://doi.org/10.1007/978-81-322-2776-2_1
Meena VS, Meena SK, Bisht JK, Pattanayak A (2016e) Conservation agricultural practices in sustainable food production. J Clean Prod 137:690–691
Muzer G, Stams AJ (2008) The ecology of biotechnology of sulphate-reducing bacteria. Nat Rev Micriobiol 6:441–454
Parewa HP, Yadav J, Rakshit A, Meena VS, Karthikeyan N (2014) Plant growth promoting rhizobacteria enhance growth and nutrient uptake of crops. Agric Sustain Dev 2(2):101–116
Pasricha NS, Sarkar AK (2002) Secondary nutrients. In: Sekhon GS, Chhonkar PK, Das DK, Goswami NN, Narayanasamy G, Poonia SR, Rattan RK, Sehgal J (eds) Fundamentals of soil science. Indian Society of Soil Science, New Delhi, pp 381–389
Prakash S, Verma JP (2016) Global perspective of potash for fertilizer production. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 327–331. https://doi.org/10.1007/978-81-322-2776-2_23
Prasad R, Shivay YS (2016) Sulphur in soil, plant and human nutrition. Proc Natl Acad Sci India Sect B Biol Sci:1–6. https://doi.org/10.1007/s40011-016-0769-0
Priyadharsini P, Muthukumar T (2016) Interactions between arbuscular mycorrhizal fungi and potassium-solubilizing microorganisms on agricultural productivity. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 111–125. https://doi.org/10.1007/978-81-322-2776-2_8
Raghavendra MP, Nayaka NC, Nuthan BR (2016) Role of rhizosphere microflora in potassium solubilization. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 43–59. https://doi.org/10.1007/978-81-322-2776-2_4
Rajvaidya N, Markandey DK (2006) Genetical and biochemical applications of microbiology. Microbial genetics. APH Publishing, New Delhi, p 345
Rao CNR (1999) Understanding chemistry. University Press (India) Ltd., Hyderabad
Rawat J, Sanwal P, Saxena J (2016) Potassium and its role in sustainable agriculture. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 235–253. https://doi.org/10.1007/978-81-322-2776-2_17
Richardson AE, Barea JM, Mcneill AM, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339. https://doi.org/10.1007/s11104009-9895-2
Roy AB, Trudinger PA (1970) The biochemistry of inorganic compounds of sulphur. Cambridge University Press, Cambridge, p 399
Saggar S, Bettany JR, Stewart JWB (1981) Measurement of microbial sulphur in soil. Soil Biol Biochem 13:493–498
Saha M, Maurya BR, Bahadur I, Kumar A, Meena VS (2016a) Can potassium-solubilising bacteria mitigate the potassium problems in India? In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 127–136. https://doi.org/10.1007/978-81-322-2776-2_9
Saha M, Maurya BR, Meena VS, Bahadur I, Kumar A (2016b) Identification and characterization of potassium solubilizing bacteria (KSB) from indo-Gangetic Plains of India. Biocatal Agric Biotechnol 7:202–209
Sahrawat KL, Murthy KVS, Wani SP (2009) Comparative evaluation of ca chloride and ca phosphate for extractable sulphur in soils with a wide range in pH. J Plant Nutr Soil Sci 172:404–407
Setter KO, Fiala G, Huber G, Huber H, Segerer A (1990) Hyperthermophilic microorganisms. FEMS Microbiol Rev 75:117–124
Sharma A, Shankhdhar D, Shankhdhar SC (2016) Potassium-solubilizing microorganisms: mechanism and their role in potassium solubilization and uptake. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 203–219. https://doi.org/10.1007/978-81-322-2776-2_15
Shinde DB, Patil PL, Patil BR (1996) Potential use of sulphur oxidizing microorganisms as soil inoculants. Crop Res 11:291–295
Shrivastava M, Srivastava PC, D’Souza SF (2016) KSM soil diversity and mineral solubilization, in relation to crop production and molecular mechanism. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 221–234. https://doi.org/10.1007/978-81-322-2776-2_16
Sindhu SS, Parmar P, Phour M, Sehrawat A (2016) Potassium-solubilizing microorganisms (KSMs) and its effect on plant growth improvement. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 171–185. https://doi.org/10.1007/978-81-322-2776-2_13
Singh NP, Singh RK, Meena VS, Meena RK (2015) Can we use maize (Zea mays) rhizobacteria as plant growth promoter? Vegetos 28(1):86–99. https://doi.org/10.5958/2229-4473.2015.00012.9
Singh M, Dotaniya ML, Mishra A, Dotaniya CK, Regar KL, Lata M (2016) Role of biofertilizers in conservation agriculture. In: Bisht JK, Meena VS, Mishra PK, Pattanayak A (eds) Conservation agriculture: an approach to combat climate change in Indian Himalaya. Springer, Singapore, pp 113–134. https://doi.org/10.1007/978-981-10-2558-7_4
Smith JL, Paul EA (1990) The significance of soil microbial biomass estimations. In: Bollagand JM, Stotzky G (eds) Soil biochemistry. Marcel Dekker, Inc, New York, pp 357–396
Solomon D, Lehmann J, de Zarruk KK, Dathe J, Kinyangi J, Liang B, Machado S (2010) Speciation and long- and short-term molecular level dynamics of soil organic sulphur studied by X-ray absorption near-edge structure spectroscopy. J Environ Qual. https://doi.org/10.2134/jeq2010.0061
Stahl WH, McQue B, Mandels GR, Siu RGH (1949) Studies on the microbiological degradation of wool. I. Sulfur metabolism. Arch Biochem 20:422–432
Starkey RL (1950) Relation of microorganisms to transformation of sulphur in soils. Soil Sci 70:55
Stevenson FJ (1982) Humus chemistry. Wiley, New York
Strick JE, Nakas JP (1984) Calibration of a microbial sulphur technique for use in forest soils. Soil Biol Biochem 16:289–291
Strickland TC, Fitzgerald JW, Swank WT (1986) In situ measurements of sulphate incorporation into forest floor and soil organic matter. Can J For Res 16:549–553
Strickland TC, Fitzgerald JW, Ash JT, Swank WT (1987) Organic sulphur transformations and sulphur pool sizes in soil and litter from a southern appalachian hardwood forest. Soil Sci 143(6):453–458
Tabatabai MA, Bremner JM (1970) Factors affecting soil arylsulphatase activity. Soil Sci Soc Am Proc 34:427–429
Tandon HLS (2011) Sulphur in soils, crops and fertilizers. Fertilizer Development and Consultation Organization (FDCO), New Delhi
Tandon HLS, Messick DL (2002) Practical sulphur guide. The Sulphur Institute, Washington, DC, p 20
Teotia P, Kumar V, Kumar M, Shrivastava N, Varma A (2016) Rhizosphere microbes: potassium solubilization and crop productivity-present and future aspects. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 315–325. https://doi.org/10.1007/978-81-322-2776-2_22
Velazquez E, Silva LR, Ramírez-Bahena MH, Peix A (2016) Diversity of potassium-solubilizing microorganisms and their interactions with plants. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 99–110. https://doi.org/10.1007/978-81-322-2776-2_7
Verma R, Maurya BR, Meena VS (2014) Integrated effect of bio-organics with chemical fertilizer on growth, yield and quality of cabbage (Brassica oleracea var capitata). Ind J Agric Sci 84(8):914–919
Verma JP, Jaiswa DK, Meena VS, Meena RS (2015a) Current need of organic farming for enhancing sustainable agriculture. J Clean Prod 102:545–547
Verma JP, Jaiswal DK, Meena VS, Kumar A, Meena RS (2015b) Issues and challenges about sustainable agriculture production for management of natural resources to sustain soil fertility and health. J Clean Prod 107:793–794
Vermeij P, Wietek C, Kahnert A, Wüest T, Kertesz MA (1999) Genetic organization of sulphur-controlled aryl desulphonation in Pseudomonas putida S-313. Mol Microbiol 32:913–926. https://doi.org/10.1046/j.1365-2958.1999.01398.x
Vidyalakshmi R, Paranthaman R, Bhakyaraj R (2009) Sulphur oxidizing bacteria and pulse nutrition—a review. World J Agric Sci 5(3):270–l278
Wainwright M (1978) A modified sulphur medium for the isolation of sulphur oxidizing fungi. Plant Soil 49(1):191–193
Wang B, Qiu YL (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363. https://doi.org/10.1007/s00572-0050033-6
Wang J, Solomon D, Lehmann J, Zhang X, Amelung W (2006) Soil organic sulphur forms and dynamics in the great plains of North America as influenced by long-term cultivation and climate. Geoderma 133:160–172
Wyszkowska J, Wieczorek K, Kucharski J (2016) Resistance of arylsulphatase to contamination of soil by heavy metals. Pol J Environ Stud 25(1):365–375
Yadav BK, Sidhu AS (2016) Dynamics of potassium and their bioavailability for plant nutrition. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 187–201. https://doi.org/10.1007/978-81-322-2776-2_14
Yasin M, Munir I, Faisal M (2016) Can Bacillus spp. enhance K+ uptake in crop species. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 163–170. https://doi.org/10.1007/978-81-322-2776-2_12
Zahedi H (2016) Growth-promoting effect of potassium-solubilizing microorganisms on some crop species. In: Meena VS, Maurya BR, Verma JP, Meena RS (eds) Potassium solubilizing microorganisms for sustainable agriculture. Springer, New Delhi, pp 31–42. https://doi.org/10.1007/978-81-322-2776-2_3
Acknowledgement
Authors are grateful to Prof. Biswapati Mandal, Dept. of Soil Science and Agricultural Chemistry, Bidhan Chandra Krishi Viswavidyalaya, Mohanpur, Nadia, West Bengal-741252, India, for his guidance in critically reviewing the manuscript.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Saha, B., Saha, S., Roy, P.D., Padhan, D., Pati, S., Hazra, G.C. (2018). Microbial Transformation of Sulphur: An Approach to Combat the Sulphur Deficiencies in Agricultural Soils. In: Meena, V. (eds) Role of Rhizospheric Microbes in Soil. Springer, Singapore. https://doi.org/10.1007/978-981-13-0044-8_3
Download citation
DOI: https://doi.org/10.1007/978-981-13-0044-8_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-0043-1
Online ISBN: 978-981-13-0044-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)