Abstract
Emerging evidence has demonstrated that almost each person is infected at least one potentially cancer-causing organism; however, only a small proportion of infected individual develops cancer. In this review, to elucidate the potential role of infectious organisms in the development and progression of human cancers, we summarize the previous history and current understandings of infection-associated cancers and highlight the common molecular mechanisms of cancers caused by infectious agents and their potential cofactors, which may bring us to effectively prevent and reduce the infection-associated cancers in the future.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
It has been estimated that over 99% people worldwide are infected with at least one potential cancer-causing organism during whole lifetime and about six million people die each year of cancer [1]. Globally, at least 16% of all cancers are associated with chronic infections, while in the developing countries the proportion could be greater than 25% [1], which is underestimated due to absence of cases of infections acting as cocarcinogens.
Although it has been debated for over two centuries whether cancer is an infectious disease or not, the interval between the first recognition of the virus (tobacco mosaic by Ivanovsky in 1892) and the first proposal that animal virus plays a critical role in some cancer formation (foot-and-mouth virus by Loeffler and Frosch in 1898) was short [2]. After yellow fever as the first human virus identified by Reed in 1901, viruses have been proposed as common causes of cancer 2 years later, which led to establishment of how important and widespread viruses are as human carcinogens and the appearance of concepts of the oncogene and tumor suppressor (like p53) [2]. Several key milestones occur in history of infection-associated cancer as shown in Fig. 1.1. The first tumor virus was discovered by Peyton Rous from Rockefeller Institute in 1911, which he provided the first experimental proof of the malignant avian tumor is dependent on a filterable virus [3, 4]. However, despite Rous went on to confirm that other avian tumors were also transmissible in a similar fashion, there were many arguments against that filtrations could be inadequate to remove all cell-fragments, or no relevance for human cancers. Until the 1930s, two major events further stimulate the notion of infection as a significant cause of cancer—one is that Richard Shope from Rockefeller Institute also reported that papillomaviruses can induce tumors in rabbits [5] and another is that Nobel Prize in 1926 was awarded to Johannes Fibiger, a Danish medical researcher who has demonstrated that a nematode worm causes stomach cancer in laboratory rats, albeit it has been proved later that diet (vitamin A-deficient rat) not nematodes was the crucial factor causing the cancer [6, 7]. Due to Fibiger’s “mistaken” Nobel award, Rous eventually received a Nobel Prize for his viral cancer-related work 40 years later (just 4 years before he died) [8]. In addition, it is also worthy to mention John Bittner’s discoveries of nonchromosomal influence in the incidence of murine mammary tumor [9], which leads to the identifications of the first retroviruses called MMTV and reverse transcriptase later.
In human, Burkitt’s lymphoma (BL) was first described as a sarcoma in African children in 1958 by Denis Burkitt who is a surgeon of Uganda [10]. Three years later, virologist Anthony Epstein occasionally attended Burkitt’s lecture and was intrigued by the possibility of a viral cause and started to prove his speculation based on frozen tumor samples which are kindly provided by Dr. Burkitt [11]. Another 3 years later (in 1964), Epstein, along with colleagues Bert Achong and Yvonne Barr, identified the first human tumor virus named as Epstein-Barr virus (EBV) from Burkitt lymphoma cell line by using electron microscopy [12]. However, the viral genome of EBV B95.8 strain was fully sequenced until 20 years later [13].
In contrast to EBV, Helicobacter pylori was the first bacterium bug found to associate with gastritis and peptic ulceration by Robin Warren and Barry Marshall in 1984 [14, 15], which led to the award of Nobel Prize for Physiology or Medicine in 2006. Initially, Warren failed to culture the organism by standard 48-h culture protocol and succeed by a chance for 5 days of culture due to leftovers from Easter holiday [16]. Although stomach ulcers were previously ascribed to diet (too many alcohol and spicy food), increasing evidence demonstrated that chronic inflammation is linked with cancer, and H. pylori was considered as a carcinogen that directly contributes to malignant transformation of stomach ulcers.
During the development history of infectious cause of cancers, we have to mention two key events. One is the discoveries of retroviruses and reverse transcriptase by Baltimore, Dulbecco, and Temin in 1975; another is the understanding of the oncogene and the tumor suppressor gene, which were initially introduced as of virus origin by Huebner and Todaro in 1969 [17] and were later termed cellular oncogenes or proto-oncogenes (their normal functions are to promote cell growth and division, while malignant cell occurs due to they are expressed aberrantly) by Bishop and Vermus in 1976 [18], which subsequently led them to receive a Nobel Prize in 1989.
Based on the discovery of cellular oncogenes, Harald zur Hausen began series of studies to demonstrate the relationship between HPV and cervical cancer in the 1970s [19], which eventually led to a Nobel Prize in 2008. In addition, it is worthy to mention that two important tumor viruses, namely, Kaposi’s sarcoma-associated virus (KHSV) and Merkel cell polyomavirus (MCV), were discovered by Drs. Yuan Chang and Patrick Moore (a couple who from the University of Pittsburgh) in 1994 and 2008, which are the etiology causes of Kaposi’s sarcoma and Merkel carcinoma, respectively [20,21,22]. This indicates that more and more new tumor viruses will be discovered as our researches are ongoing in the future.
1.2 Basic Molecular Mechanisms of Cancer Caused by Infection
It is well known now that carcinogenesis caused by infection may be direct or indirect. For example, insertion of viral genes into the host cell’s genome will trigger cell malignant transformation, while induction of chronic inflammation (i.e., cirrhosis, chronic gastritis) will create a local microenvironment with a greatly increased risk of cell transformation, or when the infective organism suppresses the host immune response, it will trigger tumorigenesis. In general, direct cause increases the risk of individual cells to malignant transformation, while indirect cause usually acts at tissue microenvironment level to increase the risk of emergence of a malignant clone.
1.2.1 Direct Cause by Infection
To date, it is well known that chromosome instability is a virtual feature of cancer cells. To understand the molecular biology of infectious causes of cancer, we need to know normal cell biology and then to interpret how the malignant cell subverts the normal processes. Some key concepts have been demonstrated in cell malignant transformation, which include apoptosis (one type of cell death, which is distinct from necrosis that induces inflammatory response) and the cell cycle (cells divide in an ordered sequence of G1, S, G2, and M phase under the control of genes including cyclins and cyclin-dependent kinases, while some cells are not dividing or preparing to divide, called G0 or rest phase). During the process of cell malignant transformation, a normal cell usually occurs as a complex of genetic changes, which regulate cell growth, division, and death, as well as escape from localization controls of basement membrane integrity (metastases). The major types of these essential genes usually included oncogenes (which drive pathological cell division), tumor suppressor genes (which normally inhibit growth and division), and DNA repair genes (which lose ability to maintain genomic integrity).
Since oncogene was discovered in about four decades ago, it has been well demonstrated that oncogenes are frequently active and typically act in a dominant fashion to drive forward the cell cycle and cell division. The normal functions of these oncogenes are usually acting as growth factors (messages communicate between cells in blood), cell surface receptors (receive and pass chemical messages from one to other cells), transcription factors (regulate genes on or off), or signal transmission proteins (carry the signal from the cell surface receptors to the nucleus). In contrast to oncogene functions as an accelerator of cell division, tumor suppressor gene is a braker [23]. In general, oncogene and tumor suppressor gene operate cooperatively during the cell cycle. The main function of tumor suppressor genes is to activate DNA repair process once any abnormal DNA occurs. If repair is unsuccessful, the cell will initiate apoptosis and sacrifice itself. Therefore, it is not surprising that the arrest of the cell division process will fail and lead to generation of faulty daughter cells with high-potential malignant property, once tumor suppressor genes are absent or defective. Many cellular tumor suppressors have been discovered so far. One of the most important tumor suppressors is p53. It is well known that p53 is the key gene (also called the “guardian of the genome”) to initiate cellular repair pathways in response to DNA damage and to ensure apoptosis of any cell with irreparably defective genome. Other prominent examples include BRCA1, BRCA2, and retinoblastoma (Rb). Given that the DNA repair genes are of great importance to the cell and highly conserved across species in eukaryote evolution, the dysfunction of DNA repair process by infection of organisms through various different pathways will lead to chromosome abnormality. To directly increase the cell dividing and risk of a cell acquiring mutation, one of common strategies used by pathogen infection is to deregulate the promoters of oncogene and tumor suppressor genes and another is to encode oncoproteins which may directly deregulate cell cycle, alter apoptotic or other cell signal pathways. These are basic molecular mechanisms why infection of organism could trigger cell transformation.
1.2.2 Indirect Cause by Tissue Microenvironment
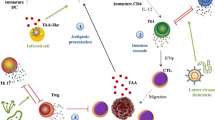
Another important trigger of cancer malignancy caused by infection is induction of chronic inflammation within tumor tissue microenvironment. Inflammation is a protective response of body tissues to different harmful stimuli by activating immune cell, blood vessels, and molecular mediators. It can be classified as two types: acute or chronic. Acute inflammatory response will increase movement of plasma and leukocyte (especially granulocytes) from the blood into the injured tissues to eliminate the initial cause of cell injury and repair, while prolonged inflammation, also known as chronic inflammation, will result into a progressive shift of cell types such as mononuclear cells at the injury tissues and stimulate destruction and healing of the tissue during inflammatory process. The role of chronic inflammation in cell malignancy is to create a background in which oncogenesis is more likely to occur, while inflammation alone is not sufficient to induce malignant diseases. It is known that persistent organism infection may cause chronic inflammation by producing cytotoxic molecules into tissue microenvironment, which will alter cellular immune response and eventually lead to inflammation. For instances, the persistent infection of hepatitis viruses can cause cirrhosis and potentially hepatocellular carcinoma. Another example is that the chronic inflammation induced by H. pylori infection and chronic gastritis.
During the inflammatory process of tumor development, one of key events is angiogenesis (also named vasculogenesis). It is a normal physiological process in which new blood vessels form from preexisting vessels in tissue growth and development as well as wound healing. However, as tumors grow, to overcome the requirement for oxygen and nutrient supply, the cancer cells were also found by Judah Folkman in 1971 to release different cytokines to induce angiogenesis around tumor. It has been demonstrated that one of the most famous cytokines associated with angiogenesis is called VEGF. It has been well demonstrated that many organism infection could greatly enhance expression of VEGF through the HIF1α pathway and highly associate with tumor angiogenesis [24]. Given the role of angiogenesis in driving cancer development, it has long been proposed as a potential target for anticancer therapy. In 1994, although Judah Folkman and his team in the first time reported promising results of endostatin as an antiangiogenic compound in inhibition of new vessel development both in vitro and in murine models, there is no effective anti-angiogenesis agent applied in clinical cancer therapy.
1.3 Epidemiological Distribution of Infection-Associated Cancers
The chief contributors to the burden of infection-associated cancer discovered so far are viruses including EBV (Epstein-Barr virus, review in Chaps. 5 and 6) [25], hepatitis virus (HBV and HCV, review in Chaps. 2 and 8, respectively) [26], human papillomaviruses (HPV 16 and 18, review in Chap. 3) [27], HLTV-1 (human T lymphotropic virus-1, review in Chap. 9), KSHV (Kaposi’s sarcoma-associated herpesvirus, review in Chap. 7), and MCV (Merkel cell polyomavirus, review in Chap. 4). These are estimated to account for over 90 % of infection-associated cancers. In addition to viruses, nonviral infections including bacterium such as Helicobacter pylori (gastric cancer and lymphoma, review in Chap. 11) [28] and parasite including Schistosoma species and liver flukes (review in Chap. 12) as well as prion (review in Chap. 13) are also known to associate with cancer. Although some pathogens like parasites are rare in the developed world, knowledge of their associated cancer is necessary as it may be encountered in any clinical environment. In many cases, parasite-associated cancers can be found due to very brief exposure, tourists who are vulnerable, or population movement after the long latency between infection and cancer diagnosis. Thus, those who have experience in the developing world may be diagnosed with a parasite-link cancer after they spent many years living in the developed world.
In addition to those infectious organisms definitely identified as causes of specific cancers, there are many others which are suspected of carcinogenic potential and various pathways by which infection may lead to cancer. For example, the patient who is infected with human immunodeficiency virus (HIV) not only cause acquired immunodeficiency syndrome (AIDS) but also increase the risk of EBV- or KSHV-induced lymphoma due to coinfection. Chapters 10 and 15 will review the basic concepts of HIV-associated cancer biology and microbiology, to explore the current understanding of HIV infections which may induce or drive malignant transformation. Given the critical role of tumor microenvironment and animal model in studying infectious causes of cancer, we not only address the recent progress on interplay between microenvironmental abnormalities and infectious agents in tumorigenesis in Chap. 16 but also include Chap. 14 to address the recent understanding of murine gammaherpesvirus 68 (MHV68) as an animal model in studying EBV- and KSHV-associated disease in vivo.
Virtually, most cases of infection-associated cancers occur after an extended latency – sometimes decades long from initial infection to eventual diagnosis of cancer. Commonly, only a small percentage of infected individuals will develop cancer. For instance, in the case of Helicobacter pylori, India has one of the highest infection rates in the world, but the incidence of gastric cancer is very low [29]. Therefore, the risk of gastric cancer in Helicobacter pylori carriers appears to be determined by a combination of several factors including host genetics, bacterial genetics, and habits of diet, smoking, etc. [30] In conclusion, there is geographical variation in incidence rates of each infection-associated cancer due to several cofactors. The reason why only small population and certain population develop cancer after infection could be due to the consequence of different interactions among several factors as follows: (1) different incidence of relevant infections, (2) timing of infection, (3) biological variability of the infectious agents, (4) genetic variation in host susceptibility to infection, and (5) incidence of external cofactors, e.g., diet and smoking.
1.4 Future Perspective
Given the incredible amount of infectious organisms in the world, it is almost impossible to estimate the amount of infectious organism within an average person’s body in his/her whole life. Despite the rate of host cell malignant transformation which is low, the incidence of many of the infections is very high. Due to infection-associated cancers which usually have a very long latency between infection and development of malignancy, reduction of the burden of infection-associated cancer will require a combination of primary prevention (blocking transmission route of infection, boosting host immune resistance against infection by vaccination), and secondary prevention (preventing progression from chronic infection to malignant transformation), based on different infection-associated cancers. For example, in hepatitis B, a compelling evidence has been found that infection during early infancy carries a high risk of eventual liver cancer, while infection in adult confers a much lower risk. Therefore, the priority strategy for prevention of HBV-associated liver cancer is to block transmission from mother to child. Another case is high-risk HPV-associated cervical cancer and, in almost all cases of infection, is acquired early after a woman becomes sexually active. The best strategy for prevention is to ensure vaccination before young women first experience penetrative sex. In contrast, the tropical infections of schistosomiasis and fluke appear to be potentially carcinogenic at any age of population, and the effective interruption of the associated cancer requires a program and may take decades. Thus, to effectively prevent the infection-associated cancers, it requires all medical scientists and healthcare professionals continue to work together and explore the nature of each infection-associated cancers.
References
de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D et al (2012) Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 13:607–615
Javier RT, Butel JS (2008) The history of tumor virology. Cancer Res 68:7693–7706
Rous P (1911) A sarcoma of the fowl transmissible by an agent separable from the tumor cells. J Exp Med 13:397–411
Rous P (1910) A transmissible avian neoplasm. (Sarcoma of the common fowl.) J Exp Med 12:696–705
Shope RE, Hurst EW (1933) Infectious papillomatosis of rabbits : with a note on the histopathology. J Exp Med 58:607–624
Hitchcock CR, Bell ET (1952) Studies on the nematode parasite, Gongylonema neoplasticum (spiroptera neoplasticum), and avitaminosis A in the forestomach of rats: comparison with Fibiger’s results. J Natl Cancer Inst 12:1345–1387
Raju TN (1998) The nobel chronicles. 1926: Johannes Andreas Grib Fibiger (1867–1928). Lancet 352:1635
Vogt PK (1996) Peyton Rous: homage and appraisal. FASEB J Off Publ Fed Am Soc Exp Biol 10:1559–1562
Jackson RB, Little CC (1933) The existence of non-chromosomal influence in the incidence of mammary tumors in mice. Science 78:465–466
Burkitt D (1958) A sarcoma involving the jaws in African children. Br J Surg 46:218–223
Coakley D (2006) Denis Burkitt and his contribution to haematology/oncology. Br J Haematol 135:17–25
Epstein MA, Achong BG, Barr YM (1964) Virus particles in cultured lymphoblasts from Burkitt’s lymphoma. Lancet 1:702–703
Baer R, Bankier AT, Biggin MD, Deininger PL, Farrell PJ, Gibson TJ et al (1984) DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature 310:207–211
Marshall BJ, Warren JR (1984) Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1:1311–1315
Warren JR, Marshall B (1983) Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1:1273–1275
Warren JR (2006) Helicobacter: the ease and difficulty of a new discovery (Nobel lecture). ChemMedChem 1:672–685
Huebner RJ, Todaro GJ (1969) Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A 64:1087–1094
Stehelin D, Varmus HE, Bishop JM, Vogt PK (1976) DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature 260:170–173
Zur Hausen H, Meinhof W, Scheiber W, Bornkamm GW (1974) Attempts to detect virus-secific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int J Cancer 13:650–656
Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM et al (1994) Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 266:1865–1869
Shuda M, Feng H, Kwun HJ, Rosen ST, Gjoerup O, Moore PS et al (2008) T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci U S A 105:16272–16277
Feng H, Shuda M, Chang Y, Moore PS (2008) Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 319:1096–1100
Knudson AG (2001) Two genetic hits (more or less) to cancer. Nat Rev Cancer 1:157–162
Zhu C, Zhu Q, Wang C, Zhang L, Wei F, Cai Q (2016) Hostile takeover: manipulation of HIF-1 signaling in pathogen-associated cancers (review). Int J Oncol 49:1269–1276
Andersson J (2000) An overview of Epstein-Barr virus: from discovery to future directions for treatment and prevention. Herpes: J IHMF 7:76–82
McGlynn KA, London WT (2005) Epidemiology and natural history of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol 19:3–23
Zur Hausen H (2002) Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2:342–350
Ahmed N, Sechi LA (2005) Helicobacter pylori and gastroduodenal pathology: new threats of the old friend. Ann Clin Microbiol Antimicrob 4:1
Prabhu SR, Amrapurkar AD, Amrapurkar DN (1995) Role of Helicobacter pylori in gastric carcinoma. Natl Med J India 8:58–60
Megraud F, Lehours P (2004) Helicobacter pylori and gastric cancer prevention is possible. Cancer Detect Prev 28:392–398
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Cai, Q., Yuan, Z. (2017). Overview of Infectious Causes of Human Cancers. In: Cai, Q., Yuan, Z., Lan, K. (eds) Infectious Agents Associated Cancers: Epidemiology and Molecular Biology. Advances in Experimental Medicine and Biology, vol 1018. Springer, Singapore. https://doi.org/10.1007/978-981-10-5765-6_1
Download citation
DOI: https://doi.org/10.1007/978-981-10-5765-6_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5764-9
Online ISBN: 978-981-10-5765-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)