Abstract
Nutraceuticals are food products that have health and medical benefits, hence promoting their use in prevention and treatment of diseases. Recently, nutraceuticals have come to limelight because of current lifestyle trends which lead to improper nutrition thereby creating a need for food supplements. Due to improper systemic delivery and poor oral bioavailability, potential use of nutraceuticals is limited. Most of the nutraceuticals show difficulty in adsorption through intestinal epithelium due to the presence of bilayer lipid membrane. Other factors such as low solubility or stability of nutraceuticals under harsh gastric conditions limit their beneficial use in health industry. Nanoencapsulation of nutraceuticals can improve their bioavailability thereby increasing health benefits. Recently there has been extensive research on encapsulation of nutraceuticals into biodegradable nanocarriers so as to increase their absorption and hence the therapeutic potential. Various materials have been used for formation of protective shell of encapsulates. The material should be food-grade and biodegradable and must be able to protect the internal phase from its surroundings. Of all the materials of choice, most frequently used are polysaccharides. Proteins and lipids are also promising candidates for appropriate encapsulation. The potential of nanotechnology to overcome the various limitations with the nutraceuticals based on the recent developments in this area are being reported.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
Drug delivery field is currently being revolutionized by nanoencapsulation of bioactives. Main sources for bioactives are the natural products which are believed to be the most important discovery of modern medicine (Molinski 1993; Grabley and Thiericke 1999). Identification of novel pharmacophores and active compounds has always been in demand because still synthetic medicinal chemistry has yet to find substitutes to many of the natural compounds (Leach et al. 2010). But several phytochemicals have low solubility leading to their poor bioavailability. Encapsulation of such bioactives in appropriate carrier matrix can enhance their bioavailability because of altered pharmacokinetics and biodistribution (Huang et al. 2013). Therapeutic index of drugs has been shown to increase using controlled drug delivery systems due to increase in their localization to specific tissues (Riehemann et al. 2009; Ferrari 2005). Recent trends in medical field have shown that nanoparticle-based therapeutic products have the potential of being commercialized and there is an upsurge in number of commercially available nanoparticle-based products. These days not only researchers but ordinary people are also attracted towards natural dietary agents due to their proven benefits in healthcare and fitness (Amin et al. 2009). Recently taurine, a major constituent of cactus pear, has become an active constituent of nutraceuticals after the reported health benefit spectrum of cactus plant such as anticancer, antiviral and antidiabetic properties. Due to the increasing interest of scientists and researchers in traditional medicines, the World Health Organization has initiated a global strategy to deal with the concerns related to traditional medicine. The European Commission has also resolved to put the disease risk reduction at priority in their coming plans. Researches on nanoencapsulation of nutraceuticals have been in recent trends to eliminate the limitations associated with them and maximize their health benefits. The present review focuses on different nanocarriers for nutraceuticals and major challenges in the way of commercialization of nano-nutraceuticals.
2 Nutraceuticals
The term nutraceuticals arises from the combination of nutrition and pharmaceuticals. These are a part of food or foods which offers nutritional and pharmaceutical benefits, that is, give nutrients to the body, provide resistance against several diseases and also help in curing of some diseases (Trottier et al. 2010). In ancient time, the knowledgeable people working in the field of medicine thought of developing such food which could be used as medicine to prevent and cure diseases. Those brilliant ideas gave birth to the field of nutraceuticals. Nutraceuticals can be divided into three main categories—dietary supplements, functional foods and functional beverages. Further the dietary supplements can be subdivided into vitamin and mineral supplements, herbal supplements, plant extracts and protein supplements. Functional foods include omega fatty acid foods and probiotics, whereas functional beverages can be subsegmented into energy drinks, sports drinks and fortified juices. Some common words related to nutraceuticals or used as synonym for nutraceuticals are functional food, multifunctional food, dietary supplements, etc. Functional foods are just the same as basic foods providing nutritions, incorporated with special and specific ingredients which provide health benefits to the body (Kalra 2003). The recent advancements in the field of food technology have opened gates for the development of functional foods exclusively produced to promote good health for human being. Some basic steps taken into consideration while scrutinizing are identification, isolation, purification and characterization of the properties of incorporated food components, that is, the nutritional value, medicinal value, etc. Primary food elements comprise of carbohydrates, proteins and lipids which are the basic necessity for proper functioning of the body and its normal energy requirements. Vitamins are secondary food elements which are commonly not synthesized within the human body, so these must be taken in food diet for proper functioning of the body. Nutraceuticals are also minor food elements which improve the body functioning by fighting against some persistent diseases (McClements 2012b).
Efficacy of any nutraceutical product depends on its bioavailability. In terms of nutritional concept, bioavailability means that some nutrients in food are partially available, whereas in terms of pharmacology it refers to rate and extent to which a drug reaches to its site of action. With increasing popularity of nutraceuticals as preventive medicine, their bioavailability has become a major issue to the regulators and manufacturers of health-related products (Rapaka and Coates 2006). When administered orally, various parameters such as insufficient gastric residence time, low permeability and/or solubility within the gut and instability under conditions encountered in food processing or in the gastrointestinal (GI) tract limit the activity and hence health benefits of various nutraceuticals (Bell 2001). Apart from their low bioavailability and poor water solubility, other challenges such as chemical instability and crystallization need to be overcome before the incorporation of these bioactive molecules into commercial food products (Augustin and Sanguansri 2012; McClements et al. 2012a, c). Patenting of new delivery systems like nanotechnology has been in trend for improving the efficacy of nutraceuticals.
3 Nanocarriers as Delivery Platforms
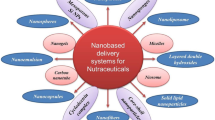
There has been a boom of nanotechnology in most of the sectors with major impact in health and food industry. Encapsulation of active food ingredients in nano-based drug delivery system is an important application of this technology, in the field of food and nutrition (Fig. 7.1).
Advantages of nanocarriers over the conventional methods of encapsulation (McClements 2012a)
Nanoformulations of nutraceuticals also follow the fundamental principles of nanotechnology. The nanotechnology platforms are mainly used to prepare delivery systems for nutraceuticals with poor water solubility. The technology possesses a great potential for commercialization of the bioactives by overcoming the limitations associated with them and therefore a multifold increase for this technology is eminent in the coming years (Augustin and Hemar 2009). However before its use in commercial food products, it is a must to ensure that these newly developed nanoscale delivery systems are safe to be incorporated in commercialized food products. There are several desirable characteristics of nanoscale delivery systems which need to be taken into consideration. First of all, as reduced to nanoscale, they will behave differently within gastrointestinal tract in comparison to conventional particulate matter (Tiede et al. 2008; Bouwmeester et al. 1998). If the digestion product of nanoscale delivery system is the same as that of conventional particulate matter, then they can’t be expected to be more toxic; otherwise toxicity concern may arise. So it is very important to assess potential toxicity of these food-grade nanoscale delivery systems to ensure their safety. Use of food-grade ingredients for fabrication of these delivery systems is preferable. These nanoscale systems should be economically viable, strong enough to confront storage conditions as well as robust enough for practical applications. Further, their incorporation into final food product should not adversely affect its quality aspects.
-
Delivery system should be chemically and physically stable to environmental stresses while preserving its functional characteristics (McClements 2012a).
-
Delivery system should be able to improve gastric stability of labile bioactive nutrients.
-
Should be able to maintain constant dosage level within systemic circulation.
-
In case of highly lipophilic compound, it should be capable of facilitating lymphatic transport.
-
Should be able to extend gastric retention time (Ting et al. 2014).
Over the past few years, several researchers have described the use of nanotechnology for nutraceutical compounds. Oral absorption and bioavailability of phenolic compounds have been reported to increase thereby promoting their nutraceutic effect (Munin and Edwards-Levy 2011; Rein et al. 2013). The nanoparticles have mainly been prepared using lipids, polysaccharides or proteins, loaded with different bioactive compounds. Some of the nanodelivery systems used for nutraceuticals are discussed below.
3.1 Lipid-Based Nanostructures
Lipid-based structures such as solid lipid nanoparticles, liposomes, nanoemulsions and self-emulsifying systems hold a great potential for encapsulation and delivery of sensitive bioactives and may serve as bioreactors for release of aroma compound and flavours. They are advantageous on the part that most of the natural bioactives can be encapsulated in them. Moreover they can incorporate high loads of different molecules and target them on specific sites via active or passive mechanisms (Mozafari et al. 2006; Taylor et al. 2005; Mozafari and Mortazavi 2005; Mozafari et al. 2009).
3.1.1 Solid Lipid Nanoparticles (SLN)
Solid lipid nanoparticles are colloidal dispersions of lipid in water or aqueous surfactant solution (Weber et al. 2014). They have many advantages such as protection of incorporated molecule from external environment, better stability of the encapsulated compound, ability to carry both lipophilic as well as hydrophilic drugs, controlled release and easy to scale up. These lipidic systems may also help to improve organoleptic and functional properties. Moreover these systems include the compounds with GRAS (generally recognized as safe) status (Severino et al. 2011). Disadvantages including relatively high water content and increased particle size, flocculation, aggregation and compound release may occur during storage (Das and Chaudhury 2010).
3.1.2 Nanoliposomes
Liposomes are lipid bilayer membrane structures with hydrophilic heads and hydrophobic fatty acid tails (Lasic 1998). They can carry both hydrophobic and hydrophilic bioactives, hydrophilic in aqueous core whereas hydrophobic component in lipid bilayers (Langer 1990). Nanoliposomes specifically refer to liposomes with nanometric size (Mozafari and Mortazavi 2005). Route of administration for nanoliposomes could be parenteral, oral, topical or nasal (Shoji and Nakashima 2004; Li et al. 2013). Their disadvantage is that they are considered as foreign particles by circulatory system and hence rapidly get cleared by reticuloendothelial system (Tang et al. 2013). Furthermore they can get disintegrated by various forces such as electrostatic, hydrophobic and van der Waals forces that can disintegrate nanoliposomes (Lasic et al. 1991). Hence some stability mechanism is required such as steric stabilization using inert polymers (Momekova et al. 2007).
3.1.3 Nanoemulsions
Emulsions are basically biphasic systems which are composed of an inner phase, i.e. dispersed phase, and an outer phase, i.e. continuous phase. There is an interphase made up of surfactant molecule. Nanoemulsions are emulsions which are extremely small in size and appear transparent or translucent. Their size range is usually 50–200 nm which is much smaller than the conventional emulsions (Solans et al. 2005). In general, the size of a surfactant molecule is 2 nm long, and therefore a micelle is usually 5 nm or more in diameter. But incorporation of oil phase into micellar core can cause increases in its size sometimes to a large extent (Huang et al. 2013). Nanoemulsion is a better option to incorporate poorly soluble nutraceuticals into food matrix, and it is well known that most of the bioactive phytochemicals are either poorly soluble or lipophilic in nature. Systemic bioavailability of these active components is considerably influenced by their poor solubility in water or oil because their properties, such as solubility, partition coefficient, lipophilicity, etc., decide their route of administration, transport and target sites. Entrapment of such bioactives into nanoemulsions can prove advantageous as the small particle size of nanoemulsions will increase their surface area thereby resulting in enhanced digestion rates, rapid diffusion across mucus membrane and increased epithelium cell permeability (Sivakumar et al. 2014; Ting et al. 2014; Yu and Huang 2013). Moreover, nanoemulsions may protect the chemically labile bioactives from oxidation, thereby resulting in increased shelf life and reduced degradation in the gastrointestinal tract (GIT) (Augustin et al. 2011; Frede et al. 2014). There are a large number of reports on entrapment of bioactives into nanoemulsions, and recent trends have shown the use of food-grade nanoemulsions (Sun et al. 2015). Carrier oil is an important component in preparation of food-grade nanoemulsions as it determines the bioavailability of encapsulated components (Qian et al. 2012; Zheng et al. 2014). The carrier oil should be able to form mixed micelles with a high solubilization capacity for active component and should be fully digestible (Li et al. 2012).
3.2 Polysaccharide-Based Nanoparticles
Most of the naturally occurring polysaccharides are considered to be cheap and adequate raw materials for nanoencapsulation of different bioactives. Nanoparticles of different shape and sizes have been prepared using various methods. Selection of method and polysaccharides depends on factors such as safety, economic and environmental considerations, etc. Different processes can be used for nanoencapsulation of bioactives depending upon physical and chemical properties of bioactives and polysaccharides. Polysaccharides are able to encapsulate both hydrophilic and hydrophobic compounds (Renard et al. 2002). Their structural versatility and site-specific digestion properties project them as suitable carriers for the targeted and controlled delivery of nutraceuticals along the human gastrointestinal tract (GIT) (Sinha and Kumria 2001). Their non-toxic, biocompatible, stable structure, low-cost, hydrophilic nature along with availability of reactive sites for chemical modifications makes them the material of choice (Sinha and Kumria 2001). Commonly used polysaccharides include starch, pectin and guar gum, chitosan, chondroitin sulphate, alginate, etc. (Kosaraju 2005; Augustin and Hemar 2009). They are advantageous on the part that they can be used for delivery of synergistic combinations, but when considered for food applications, high molecular weight of polysaccharides sometimes limits its application, e.g. for delivery in clear drinks, because it is difficult to prepare transparent system based on biopolymeric nanoparticles (Livney 2008).
Vast research has been conducted to encapsulate wide variety of bioactive components in nutraceuticals and functional foods (McClements et al. 2009) as shown in Table 7.1, but clear in vitro or in vivo evidences of their biological efficacies are still limited.
4 Nanotechnology-Enabled Food Products
According to the Food Standards Agency, fumed silica, nanosilver, nanoclay and titanium nitride are the nanomaterials that are permitted to be used in food provided they follow the relevant legislation. Centre for Food Safety has released a database which enlists near about 300 food products/food contact products that use nanotechnology. Those with food supplement and additives category are summarized in Table 7.2.
Apart from these, Chinese Nano tea, Nano silver and Nano gold have been used as mineral supplements; carotenoid nanoparticles have been used in fruit drinks; patented “Nano drop” delivery systems have been used for encapsulation of materials such as vitamins; and Nano cages or Nano clusters have been used in nanoceutical products such as chocolate drink thereby imparting sweetness without addition of any sugar or sweeteners (Paul and Dewangan 2016).
5 Regulatory Issues
Nutraceuticals products claiming medicinal benefits would be required to abide by regulatory requirements for medicinal products regarding safety, efficacy, quality testing and marketing authorization procedures (Pandey et al. 2010). Use of nanodelivery systems for designing not only fresh food but also healthier food has been in trend, but however many of them can cause serious threats to safety of people (Pradhan et al. 2015). A number of regulatory bodies such as the European Food and Safety Authority (EFSA), Environmental Protection Agency (EPA), Food and Drug Administration (FDA), National Institute for Occupational Safety and Health (NIOSH), Occupational Safety and Health Administration (OSHA), US Department of Agriculture (USDA), Consumer Product Safety Commission (CPSC) and US Patent and Trademark Office (USPTO) govern application of nanosystems in food (Qi et al. 2004). In 2012, FDA released two draft guidance documents regarding nanotechnology. The documents cover the category of food and cosmetics, but none of them points out to dietary supplements specifically because as per FDA dietary supplements are considered to be a category of food. As per FDA guidance document, bioavailability of a food substance is changed if its physical or chemical properties are changed. Further, such changes can also have an effect on its toxic levels. In its new dietary ingredient (NDI) draft guidance, the FDA considered nanotechnology as an example of a process that creates a new dietary ingredient and hence requires a notification to the FDA. But nanoceuticals are not regulated and therefore can be launched to the market with little or no verification of safety. The FDA anticipates that these nanotechnology products should come under the jurisdiction of the Office of Combination Products (Javeri 2016). As per EC Food Law Regulation, several points need to be considered, while designing nanomaterials for food applications such as the nanomaterials use should be free of toxic and heavy metals and mycotoxins (Scampicchio et al. 2008). Directive 89/107/EEC states that nanomaterials intended for use in food packaging should be initially evaluated as a direct food additive (Sondi and Salopek-Sondi 2004).
6 Conclusion
Production of NPs using the environment-friendly processes is quite a promising area of research to develop the various food products. Although many important goals have been reached in achieving controlled release of food products, cost is the overriding factor that has hindered the introduction of sophisticated controlled release technologies in food products. We all are aware about the potential health benefits of nutraceuticals and probiotics. So the added value of nutraceutical ingredients justifies the additional cost of nanoencapsulation technology used to maintain the stability of these ingredients. The published literature indicates that in the near future, nanoparticle-based delivery systems will have more commercial status in the market than in the past. It seems these new technologies are viable and promising strategy for the food product industries and provoke various manufacturers to introduce nano-based ingredients into their food products and as a part of their marketing strategy. Nanoparticles can minimize some of these food products unique problems by safeguarding stability and preserving their safety, low cost, appeal (taste, odour, colour and texture), stability and nutritional value. The published literature indicates that in the near future, nanoparticles based delivery systems will have more commercial status in the market than in the past.
References
Altındal DC, Gümüşderelioglu M (2015) Melatonin releasing PLGA micro/nanoparticles and their effect on osteosarcoma cells. J Microencapsul 33(1):1–12
Amin AR, Kucuk O, Khuri FR, Shin DM (2009) Perspectives for cancer prevention with natural compounds. J Clin Oncol 27:2712–2725
Antônio E, Khalil NM, Mainardes RM (2016) Bovine serum albumin nanoparticles containing quercetin: characterization and antioxidant activity. J Nanosci Nanotechnol 16:1346–1353
Augustin MA, Hemar Y (2009) Nano- and micro-structured assemblies for encapsulation of food ingredients. Chem Soc Rev 38(4):902–912
Augustin MA, Sanguansri L (2012) Challenges in developing delivery systems for food additives, nutraceuticals, and dietary supplements. In: Garti N, McClements DJ (eds) Encapsulation technologies and delivery systems for food ingredients and nutraceuticals. Woodhead Publishing, Cambridge, UK, pp 19–48
Augustin MA, Abeywardena MY, Patten G, Head R et al (2011) Effects of microencapsulation on the gastrointestinal transit and tissue distribution of a bioactive mixture of fish oil, tributyrin and resveratrol. J Funct Foods 3:25–37
Bell LN (2001) Stability testing of nutraceuticals and functional foods. In: Wildman REC (ed) Handbook of nutraceuticals and functional foods. CRC Press, New York, pp 501–516
Bernela M, Ahuja M, Thakur R (2016a) Enhancement of anti-inflammatory activity of bromelain by its encapsulation in katira gum nanoparticles. Carbohydr Polym 143:18–24
Bernela M, Ahuja M, Thakur R (2016b) Enhancement of anti-inflammatory activity of glycyrrhizic acid by encapsulation in chitosan-katira gum nanoparticles. Eur J Pharm Biopharm 105:141–147
Blazevic F, Milekic T, Romic MD, Juretic M et al (2016) Nanoparticle-mediated interplay of chitosan and melatonin for improved wound epithelialisation. Carbohydr Polym 146:445–454
Bourbon AI, Cerqueira MA, Vicente AA (2016) Encapsulation and controlled release of bioactive compounds in lactoferrin-glycomacropeptide nanohydrogels: curcumin and caffeine as model compounds. J Food Eng 180:110–119
Bouwmeester H, Dekkers S, Noordam MY, Hagens WI et al (1998) Nutraceuticals: poised for a healthy slice of the healthcare market? Nat Biotechnol 16:728–731
Chaiyasan W, Srinivas SP, Tiyaboonchai W (2016) Development and characterization of topical ophthalmic formulations containing lutein-loaded mucoadhesive nanoparticles. Int J Pharm Pharm Sci 8(3):261–266
Das S, Chaudhury A (2010) Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS Pharm Sci Tech 12:62–76
Fakhoury IH, Saad WS, Gali-Muhtasib HU, Schneider-Stock R (2014) Thymoquinone nanoparticle formulation and in vitro efficacy in: materials for drug & gene delivery & cancer nanotech. Nanotech (2014) 2:367–370
Fakoor Yazdan Abad M, Rajabzadeh G, Taghvaei Ganjali S, Tavakoli R (2016) Preparing allicin nanocapsules and determining the factors controlling their particle size through artificial intelligence. Int J Food Eng 12(3):257–264
Fathi M, Varshosaz J, Mohebbi M, Shahidi F (2013) Hesperetin-loaded solid lipid nanoparticles and nanostructure lipid carriers for food fortification: preparation, characterization, and modeling. Food Bioprocess Technol 6:1464–1475
Ferrari M (2005) Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer 5:161–171
Frede K, Henze A, Khalil M, Baldermann S, Schweigert FJ, Rawel H (2014) Stability and cellular uptake of lutein-loaded emulsions. J Funct Foods 8:118–127
Grabley S, Thiericke R (1999) Bioactive agents from natural sources: trends in discovery and application. Adv Biochem Eng Biotechnol 64:101–154
Guan X, Gao M, Xu H, Zhang C et al (2016) Quercetin-loaded poly (lactic-co-glycolic acid)-d-α-tocopheryl polyethylene glycol 1000 succinate nanoparticles for the targeted treatment of liver cancer. Drug Deliv 23(9):3307–3318
Hong DY, Lee J-S, Lee HG (2015) Chitosan/poly-rmgamma-glutamic acid nanoparticles improve the solubility of lutein. Int J Biol Macromol 85:9–15
Huang Q, Yu H, Ru Q (2013) Bioavailability and delivery of nutraceuticals using nanotechnology. J Food Sci 75:50–57
Javeri I (2016) Application of “nano” nutraceuticals in medicine. In: Nutraceuticals: efficacy, safety and toxicity. Elsevier, Amsterdam, pp 189–192
Joung HJ, Choi M-J, Kim JT, Park SH, Park HJ, Shin GH (2016) Development of food-grade curcumin nanoemulsion and its potential application to food beverage system: antioxidant property and in vitro digestion. J Food Sci 81:745–753
Jourghanian P, Ghaffari S, Ardjmand M, Haghighat S, Mohammadnejad M (2016) Sustained release curcumin loaded solid lipid nanoparticles. Advan Pharma Bulletin 6:17–21
Kalra EK (2003) Nutraceutical – definition and introduction. AAPS Pharm Sci 5:27–28
Kim JH, Park EY, Ha HK, Jo CM et al (2016) Resveratrol-loaded nanoparticles induce antioxidant activity against oxidative stress. Asian-Aust J Anim Sci 29(2):288–298
Kosaraju SL (2005) Colon targeted delivery systems: review of polysaccharides for encapsulation and delivery. Crit Rev Food Sci Nutr 45(4):251–258
Krausz AE, Adler BL, Cabral V, Navati M et al (2015) Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomed: Nanotechnol Biol Med 11(1):195–206
Langer R (1990) New methods of drug delivery. Science 249:1527–1533
Lasic DD (1998) Novel applications of liposomes. Trends Biotechnol 16:307–321
Lasic DD, Martin FJ, Gabizon A, Huang SK, Papahadjopoulos D (1991) Sterically stabilized liposomes: a hypothesis on the molecular origin of the extended circulation times. Biochim Biophys Acta 1070:187–192
Leach AR, Gillet VJ, Lewis RA, Taylor R (2010) Three-dimensional pharmacophore methods in drug discovery. J Med Chem 53:539–558
Li Y, Xiao H, McClements DJ (2012) Encapsulation and delivery of crystalline hydrophobic nutraceuticals using nanoemulsions: factors affecting polymethoxyflavone solubility. Food Biophys 7(4):341–353
Li C, Zhang X, Huang X, Wang X, Liao G, Chen Z (2013) Preparation and characterization of flexible nanoliposomes loaded with daptomycin, a novel antibiotic for topical skin therapy. Int J Nanomedicine 8:1285–1292
Livney YD (2008) Complexes and conjugates of biopolymers for delivery of bioactive ingredients via food. In: Delivery and controlled release of bioactives in foods and nutraceuticals, Woodhead Publishing series in Food Science, Technology and Nutrition. Woodhead, Cambridge, pp 234–242
Manea A-M, Vasile BS, Meghea A (2014) Antioxidant and antimicrobial activities of green tea extract loaded into nanostructured lipid carriers. C R Chim 17:331–341
McClements DJ, Decker EA, Park Y, Weiss J (2009) Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit Rev Food Sci Nutr 49(6):577–606
McClements DJ (2012a) Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr Opin Colloid Interface Sci 17(5):235–245
McClements DJ (2012b) Requirements for food ingredient and nutraceutical delivery systems. In: Encapsulation technologies and delivery systems for food ingredients and nutraceuticals. Woodhead Publishing, Cambridge, pp 3–18
McClements DJ (2012c) Nanoemulsions versus microemulsions: clarification of differences, similarities and terminology. Soft Matter 8(6):1719–1729
Molinski TF (1993) Developments in marine natural products, receptor-specific bioactive compounds. J Nat Prod 56:1–8
Momekova D, Rangelov S, Yanev S, Nikolova E et al (2007) Long-circulating, pH-sensitive liposomes sterically stabilized by copolymers bearing short blocks of lipid-mimetic units. Eur J Pharm Sci 32:308–317
Mozafari MR, Mortazavi SM (2005) Nanoliposomes: from fundamentals to recent developments. Trafford, Pub Ltd, Oxford. UK
Mozafari MR, Flanagan J, Matia-Merino L et al (2006) Recent trends in the lipid-based nanoencapsulation of antioxidants and their role in foods. J Sci Food Agric 86(13):2038–2045
Mozafari MR, Pardakhty A, Azarmi S, Jazayeri JA et al (2009) Role of nanocarrier systems in cancer nanotherapy. J Liposome Res 19:310–321
Munin A, Edwards-Levy F (2011) Encapsulation of natural polyphenolic compounds: a review. Pharmaceutics 3:793–829
Neves AR, Martins S, Segundo MA, Reis S (2016) Nanoscale delivery of resveratrol towards enhancement of supplements and nutraceuticals. Forum Nutr 8:131
Pandey M, Verma RK, Saraf SA (2010) Nutraceuticals: new era of medicine and health. Asian J Pharm Clin Res 3:11–15
Paul SD, Dewangan D (2016) Nanotechnology and nutraceuticals. Int J Nanomater Nanotechnol Nanomed 2(1):9–12
Pradhan N, Singh S, Ojha N, Shrivastava A et al (2015) Facets of nanotechnology as seen in food processing, packaging, and preservation industry. Bio Med Res Int 2015:365672
Puglia C, Offerta A, Tirendi GG, Tarico MS et al (2016) Design of solid lipid nanoparticles for caffeine topical administration. Drug Deliv 23(1):36–40
Qi LF, Xu ZR, Jiang X, Hu C, Zou X (2004) Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res 339(16):2693–2700
Qian C, Decker EA, Xiao H, McClements DJ (2012) Nanoemulsion delivery systems: influence of carrier oil on beta-carotene bioaccessibility. Food Chem 135:1440–1447
Rani R, Dilbaghi N, Dhingra D, Kumar S (2015) Optimization and evaluation of bioactive drug-loaded polymeric nanoparticles for drug delivery. Int J Biol Macromol 78:173–179
Rapaka RS, Coates PM (2006) Dietary supplements and related products: a brief summary. Life Sci 78:2026–2032
Ravanfar R, Tamaddon AM, Niakousari M, Moein MR (2016) Preservation of anthocyanins in solid lipid nanoparticles: optimization of a microemulsion dilution method using the Plackett–Burman and Box–Behnken designs. Food Chem 199(15):573–580
Rein MJ, Renouf M, Cruz-Hernandez C, Actis-Goretta L, Thakkar SK, da Silva MP (2013) Bioavailability of bioactive food compounds: a challenging journey to bioefficacy. Br J Clin Pharmacol 75:588–602
Renard D, Robert P, Lavenant L, Melcion D et al (2002) Biopolymeric colloidal carriers for encapsulation or controlled release applications. Int J Pharm 242(1–2):163–166
Riehemann K, Schneider SW, Luger TA, Godin B (2009) Nanomedicine-challenge and perspectives. Angew Chem Int Ed Eng 48:872–897
Scampicchio M, Ballabio D, Arecchi A, Cosio SM, Mannino S (2008) Amperometric electronic tongue for food analysis. Microchim Acta 163:11–21
Severino P, Andreani T, Macedo AS, Fangueiro JF et al (2011) Current state-of-art and new trends on lipid nanoparticles (SLN and NLC) for oral drug delivery. J Drug Deliv 12:1–10
Shoji Y, Nakashima H (2004) Nutraceutics and delivery systems. J Drug Target 12:385–391
Singhal NK, Agarwal S, Bhatnagar P, Tiwari MN et al (2015) Mechanism of nanotization-mediated improvement in the efficacy of caffeine against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism. J Biomed Nanotechnol 11(12):2211–2222
Sinha VR, Kumria R (2001) Polysaccharides in colon-specific drug delivery. Int J Pharm 224:19–38
Sivakumar M, Tang SY, Tan KW (2014) Cavitation technology – a greener processing technique for the generation of pharmaceutical nanoemulsions. Ultrason Sonochem 21:2069–2083
Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma MJ (2005) Nanoemulsions. Curr Opin Colloid Interface Sci 10(3–4):102–110
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gramnegative bacteria. J Colloid Interface Sci 275(1):177–182
Sun Y, Xia Z, Zheng J, Qiu P, Zhang L, McClements DJ, Xiao H (2015) Nanoemulsion-based delivery systems for nutraceuticals: influence of carrier oil type on bioavailability of pterostilbene. J Funct Foods 13:61–70
Tang S, Gao D, Zhao T, Zhou J, Zhao X (2013) An evaluation of the anti-tumor efficacy of oleanolic acid-loaded PEGylated liposomes. Nanotechnology 24:235102
Taylor TM, Davidson PM, Bruce BD, Weiss J (2005) Liposomal nanocapsules in food science and agriculture. Crit Rev Food Sci Nutr 45(7–8):587–605
Tiede K, Boxall ABA, Tear SP, Lewis J, David H, Hassellov M (2008) Detection and characterization of engineered nanoparticles in food and the environment. Food Addit Contam Part A-Chem Anal Control Expo Risk Assess 25(7):795–821
Ting YW, Jiang Y, Ho CT, Huang QR (2014) Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J Funct Foods 7:112–128
Trottier G, Boström PJ, Lawrentschuk N, Fleshner NE (2010) Nutraceuticals and prostate cancer prevention. A Curr Rev Nat Rev Urol 7:21–30
Vidal SL, Rojas C, Padin RB, Rivera MP, Haensgen A, Gonzalez M, Rodriguez-Llamazares S (2016) Synthesis and characterization of polyhydroxybutyrate-cohydroxyvalerate nanoparticles for encapsulation of quercetin. J Bioact Compat Polym 31(5):1–14
Vijayakumar MR, Kumari L, Patel KK, Vuddanda PR et al (2016) Intravenous administration of trans-resveratrol-loaded TPGS-coated solid lipid nanoparticles for prolonged systemic circulation, passive brain targeting and improved in vitro cytotoxicity against C6 glioma cell lines. RSC Adv 6:50336–50348
Wagner V, Dullaart A, Bock AK, Zweck A (2006) The emerging nanomedicine landscape. Nat Biotechnol 24:1211–1217
Weber S, Zimmer A, Pardeike J (2014) Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharma Biopharm 86:7–22
Yu H, Huang Q (2013) Bioavailability and delivery of nutraceuticals and functional foods using nanotechnology. In: Di B, Bagchi M, Moriyama H, Shahidi F (eds) Bio-nanotechnology: a revolution in food, biomedical and health sciences, 1st edn. Blackwell Publishing Ltd., Oxford. https://doi.org/10.1002/9781118451915. ch35
Zheng J, Li Y, Song M, Fang X et al (2014) Improving intracellular uptake of 5-demethyltangeretin by food grade nanoemulsions. Food Res Int 62:98–103
Zhu Y, Peng W, Zhang J, Wang M et al (2014) Enhanced oral bioavailability of capsaicin in mixed polymeric micelles: preparation, in vitro and in vivo evaluation. J Funct Foods 8:358–366
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bernela, M., Kaur, P., Ahuja, M., Thakur, R. (2018). Nano-based Delivery System for Nutraceuticals: The Potential Future. In: Gahlawat, S., Duhan, J., Salar, R., Siwach, P., Kumar, S., Kaur, P. (eds) Advances in Animal Biotechnology and its Applications. Springer, Singapore. https://doi.org/10.1007/978-981-10-4702-2_7
Download citation
DOI: https://doi.org/10.1007/978-981-10-4702-2_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4701-5
Online ISBN: 978-981-10-4702-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)