Abstract
It is consensus that plant growth-promoting bacteria (PGPB) be studied extensively in the last two decades, but several of them are not fully investigated/explored especially in arid and semiarid regions worldwide. They have been deployed as potent source of bioactive compounds useful in prospecting of sustainable agricultural. In the present scenario to meet food security, a number of different approaches have been employed to cultivate crops in salt- and drought-prone area. Hence, nowadays, the use of microbial inoculation to alleviate abiotic stress and amelioration of crops could be considered a more cost-effective eco-friendly approach. By keeping current approaches available for plant-microbe interaction, it is needed to pursue prospective research in this area. In the present chapter, authors will emphasize the role of benign PGPB in crop cultivation under stress through produced elicitors/determinants. It is very urgent need to explore this approach for sustainable agriculture grown under stress and also to understand the mutual interactive activities belowground. Therefore, an exploitation of PGPB-plant interactions may be opted in the amelioration of plant health in arid and semiarid area.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Abiotic stress
- 1-Amino cyclopropane carboxylic acid
- Plant growth-promoting microorganisms
- Plant stress-responsive molecules
1 Introduction
Among the total agricultural production around world, legumes encompass 25 % which include mainly pulses and oil seeds, namely, soybean and peanut. Based on the report generated by FAOSTAT (2012), India ranks first in worldwide pulse production and soybean ranks fourth. Abiotic and biotic stresses are major constraints for agriculture production worldwide. Therefore, an immediate and imperative integrated approach is required to avoid stresses and dissemination of the low-cost technologies in legume production (Reddy et al. 2013). In food web life does not exist without producers and, in natural resources wherein plants represent huge diversity in agroecosystems (AES), provides benign to detrimental metabolites. Among the benign are foods rich in proteins, feeds, and organic manures, and fix dinitrogen (N2) improves soil structural characteristics and encourages beneficial microorganisms and the reclamation and revegetation of barren/degraded lands (Chaer et al. 2011). Based on these attributes, legumes are one of the most promising components of the Climate Smart Agriculture concept (FAO 2013). It finds major application as livestock forage and silage, grain, blooms, pharmaceutical/industrial, fallow/green manure, and human consumption as these are the good source of protein and rich in iron and vitamin B complex.
In India top legume producers constitute of Madhya Pradesh, Uttar Pradesh, Maharashtra, Rajasthan, Andhra Pradesh, Karnataka, Chhattisgarh, Gujarat, Jharkhand, and Bihar. Rajasthan ranks good enough in this list (Fig. 7.1a, b). The Thar Desert (Great Indian Desert) is a part of Rajasthan (constitutes 60 % of its area in Thar Desert). Geographically, Rajasthan lies between 23° 3′ to 30° 12′ longitude and 69° 30′ to 78° 17′ latitude. It occupies 342,239 km2 land area which solely implies 10.41 % of the total land area of India. The Thar Desert lies between 24° to 28° N latitude and 68° to 71° E longitude, occupying an area of about 200,000 km2. Its vegetation describes 911 wild species belonging to 780 genera and 154 families. Rajasthan consist of three climatic zones, namely, arid zone, semiarid temperate zone, and semiarid tropical zone (Fig. 7.2). Enduring flora of the Thar Desert (arid zone) involves tree and shrubs including cultivated leguminous plants, e.g., Vigna (V. aconitifolia, V. mungo, V. radiata, V. unguiculata, etc.), Pisum sativum, Cicer arietinum, Trigonella foenumgraecum, Cajanus cajan, Cyamopsis tetragonoloba, Lens culinaris, Vicia faba, Phaseolus lunatus, Lablab purpureus, Canavalia ensiformis, Arachis hypogea, etc. (http://dst.rajasthan.gov.in/), whereas semiarid zone is rich in Glycine max, Arachis hypogaea, Cajanus cajan, Cicer arietinum, V. unguiculata, etc. Legumes grown in these regions posses problem of abiotic stresses like salinity, alkalinity, high temperature, and drought, which lead to dehydration and osmotic stress in soil and thereby reduction in crop yields worldwide. Around 70 % of yield losses in major crops occur due to abiotic stress (da Silva et al. 2014).
Different climatic zones of Rajasthan state (Source: http://www.nicra-icar.in/nicrarevised/index.php/component/content/article?layout=edit&id=195)
The major limiting factors affecting the agricultural productivity worldwide are environmental stresses. Ecosystem of Rajasthan’s Thar Desert is mainly affected with high temperature, salinity/alkalinity, low pH and several other abiotic factors. Apart from decreasing yield these introduces devastating impact on plant growth (Suzuki et al. 2014). High salinity and severe drought are the major constraints affecting the agricultural practices in Rajasthan. Out of this, soil degradation through salinization accounts the most wherein the main cause of salinization is irrigation. However, annual precipitation of rainfall (APRF) is poorly disseminated to make certain harvestable crops in arid and semiarid regions, resulting in gradual degradation (Singh et al. 2012). It has been reported that APRF affects approx. 50 % of irrigated areas worldwide and causes very stern threat to AES and leads to decline of natural resources (Gabrijel et al. 2009). In India, 8.4 Mha land is affected by soil salinity and alkalinity per se, of which about 5.5 Mha are waterlogged (Singh et al. 2012). And hence, over recent decades, soil salinization threatening environment health and sustainable development induced by human activities had developed sound land-use policies and planning actions for integrated land management to come in scenario (Zhang et al. 2011).
Soil salinization is considered as the occurrence of suspended inorganic ions that include Cl−, SO2 4, Mg++, Ca++, K+, Na+, HCO3 −, and CO3 2− in the aqueous phase of soil milieu. The change in soil salinity affects the survival of salt-sensitive plants so-called glycophytes, e.g., soybean. Soil with ECe greater than 40 mM NaCl (4 dS/m) is considered saline (USDA Salinity Laboratory). Hence, increase in these limits leads to two major stresses for the plant osmotic and ionic stress. The occurrence of osmotic stress outside the plant root is the result of a rise in salt over threshold level which reflects hassles in H2O uptake, cell growth, and expansion of lateral bud (Munns and Tester 2008). The ionic stress rose upon increase in toxic level of Na+ that accumulates in leaf tissues over threshold level and causes leaf mortality with chlorosis/necrosis, whereby hindering cellular metabolic and enzyme activities (Chaves et al. 2009; Nawaz et al. 2013). To reduce salt-led phytotoxicity, the halophytes develop strategies to limit Na+ uptake; further accumulation in shoot tissues is significant for survival (Zhang et al. 2008a, b).
According to crisis management plan (national) 2014, arid region of Rajasthan has shown drought efficiency of 2 in 5 years and semiarid region has 1 in 3 years. Drought has been considered as subtle peril of natural ecosystem and so-called creeping phenomenon and varied from one place to another. Land becomes dry when it gets light rain and sleet and leads to deep drought that cause noteworthy harm to the confined economy. Drought may also affect cropping system and threatens lasting erosion of AES enterprises (Kasotia and Choudhary 2014a, b). Water deficit caused by drought results in reduced turgor pressure of plant cells which thereby affects worth and measure of crop yield worldwide. It affects phenetic and genetic parameters of the plant and reflects reduction in cell division, enlargement, and differentiation including overall plant growth (Huang et al. 2012).
There is a cross talk between drought and salt stress as they eventually result in osmotic imbalance and lead to dehydration of the cell (Nakashima et al. 2014). This comprises three parameters: (1) restoration of ionic and osmotic equilibrium of the cell to develop homeostasis, (2) production of detoxification mechanisms to restore stress damage, and (3) induction of cell signaling to control cell division and metabolic pathways. Soil drying and salinization alter optimal supply of water, mineral nutrients, small organic molecules, proteins, and hormones in xylem (Pérez-Alfocea et al. 2011). Under stress condition plant cell implies signal transduction pathway that leads to production of secondary messengers, e.g., Ca+2, ROS, and IMP. When plant possesses abiotic and biotic stresses, cytosolic level of calcium increases in the plant cell. Thereafter several simultaneous pathways are activated by calcium-interacting proteins (Kim et al. 2009). Mainly two stress responses are revealed by salinity stress, i.e., osmotic stress and ionic stress, whereas drought stress shows only osmotic stress (Huang et al. 2012). Osmotic stress produced by drought stress and salinity stress leads to ABA-dependant and ABA-independent signaling (Saibo et al. 2009), while ionic stress is alleviated by salt overly sensitive pathway (SOS pathway). Upon occurrence of salt stress, ion homeostasis of plant gets distressed that results in the rise of Na+ and lack of K+ in the cytoplasm. To mitigate such imbalance, ion transporters (plasma membrane Na+/H+ antiporter SOS1 and the high-affinity K+ transporter 1 (HKT1)), located in the cell membrane, reflect exclusion of Na+ entry into and exit out of cells and regulate Na+/K+ ration (Huang et al. 2012; Brini and Masmoudi 2012).
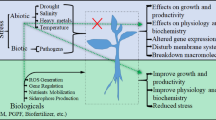
To alleviate such stressful conditions in plant, plant growth-promoting bacteria (PGPB) have been reported to implicate in the metabolism and growth of plants (Kang et al. 2014; Kasotia and Choudhary 2014b). In addition, PGPB that resides in the spermosphere (sphere that surround the seed) and rhizosphere (area around roots with 1–10 mm) enhance plant growth, after attaching to root surface. To alleviate abiotic stress, PGPB strains have been reported and include Bacillus, Burkholderia, Acinetobacter, Enterobacter, Azospirillum, Beijerinckia, Rhizobium, Serratia, Erwinia, Flavobacterium, Alcaligenes, etc. (Bharti et al. 2013). These microbes secrete bacterial AAC-deaminase, volatiles, antioxidants, cytokinin, IAA, and unknown metabolites in response to plant’s ethylene, HKT1, ROS, and ABA under salt and drought stress (Yang et al. 2009). These microbial determinants result in “induced systemic tolerance (IST)” in plants, and further IST has been utilized to overcome the harmful effects of abiotic stress (Yang et al. 2009).
In higher plants, ethylene is produced under various abiotic stresses. It is a simple gaseous hydrocarbon that regulates many physiological processes, including root and shoot growth, seed germination, flower development, ripening of fruits, and senescence of plant organs. Under various abiotic stress conditions (salinity, chilling, drought, wounding, temperature, and heat), the level of C2H4 increases in plants (Li et al. 2013). Synthesis of ethylene mediated through l-methionine via the intermediates, S-adenosyl-l-methionine (SAM) and 1-aminocyclopropane-1-carboxylic acid (ACC) following Yang cycle (Yang and Hoffman 1984). The instant originator of C2H4 is ACC (Chen et al. 2013). It has been described that ACC-deaminase secreted by PGPB reduces the deleterious levels of ethylene. Bacteria use ACC as nitrogen source and degrade it to ammonia and α-ketobutyrate that are readily assimilated.5 Salinity results in elevated levels of Na+:K+ which can be reduced by HKT1. HKT1 plays main physiological role in Na+ homeostasis and thereby protects both mono- and dicotyledonous plants upon toxic level of Na+ (Almeida et al. 2013). Among transporters, tt is one of Na+ transporters that allows to transport Na+ back to the soil by coupling to H+ (Shi et al. 2002), transporters that avoid toxic level of Na+ in the leaf tissues (Byrt et al. 2007), and antiporters that seize Na+ in the vacuoles along with H+-ATPase/H+-PPase (Apse et al. 1999). Bacterial volatiles help the plant to regulate expression of HKT1 gene in maintaining low Na+:K+ ratio in plant (Zhang et al. 2008a, b).
Upon induction of salinity and drought, there is a rise in variety of ROS species which include radical (O2 −, OH, HO2, and RO) and non-radical forms (H2O2, and 1O2) synthesized in plant cells (Gill and Tuteja 2010). To alleviate toxic level of ROS species, plant tissues per se contain several enzymatic (superoxide dismutase, catalase, glutathione reductase, peroxidase, etc.) and nonenzymatic (phenolic compounds, ascorbate, glutathione, carotenoid, and α-tocopherol) scavenging mechanisms (Jaleel et al. 2009; Gill and Tuteja 2010). The balance between the generation of ROSs and further the sequestration of antioxidants for ROSs gets disturbed under environmental stress conditions and leads to oxidative damage (Miller et al. 2010).
The induced activities resulted by PGPB detoxify plant cell by elevating antioxidant enzyme levels in plant cells (Kohler et al. 2008). The rise in ABA in plants showed a developmental process and allows an adaption to environmental stimuli in plants (Figueiredo et al. 2008; Fujita et al. 2011). Characteristically, it gets increased in roots, xylem sap, and shoots under osmotic stress (Albacete et al. 2008). For this cytokinin-producing bacteria are known to confer resistance (Nishiyama et al. 2011; Liu et al. 2013). Above all, PGPB secretes some more hormones such as IAA and GA that helps in the promotion of amplified root growth which leads to nutrient uptake in plants under stress (Kochar et al. 2011; Duca et al. 2014; Kang et al. 2014). They also act as signaling molecule in bacteria (Bashan and de-Bashan 2010). Nitrogen fixation via rhizobia-legume symbiosis is a well-known mechanism employed by PGPB to fix atmospheric nitrogen. PGPB convert atmospheric nitrogen to ammonia, a form that can be used up by plants (Franche et al. 2009). These bacteria contain enzyme complex nitrogenase that fixes atmospheric nitrogen to ammonia (Santi et al. 2013). Moreover PGPB influence soil fertility by solubilizing organic and precipitated phosphates in soil (Khan et al. 2009). PGPB excretes organic acids, namely, gluconic/citric acid, that dissolve calcium phosphates in the form of Pi and PO4 3− (orthophosphate) and solubilize inorganic phosphate available largely in soil to bioavailable phosphorous. Besides, many phosphatase and cellulolytic enzymes are released for enzyme-labile soil organic phosphorous in favor of plant availability (Richardson and Simpson 2011).
To chelate iron in soil, PGPB also produce siderophore (Fe-III chelating agent) which can solubilize and sequester iron, whereby alleviating stress and allowing plant growth. Kintu et al. (2001) reported that microbially produced siderophores are of size <10,000 Da and showed the ability to chelate ferric ion as scavenging agent to fight against low iron stress (Kintu et al. 2001). Proteases secreted by PGPB break down complex proteins available in soil into plant-usable amino acids. They catalyze total hydrolysis of proteins to peptides and thereby function as degradative enzymes (Zhang et al. 2008a). In response to osmotic stress in soil, PGPB secretes compatible solutes which help them to adapt in external osmolarity (Paul and Nair 2008). Compatible solutes are low molecular weight hydrophilic molecular osmolytes including carbohydrates, amino acids, and their modified forms (Wood 2011). PGPB colonizes plant roots and alleviates the debilitating effects of salt stress (Paul 2013). Production of microbial EPS in soil under stress helps in removal of drought stress and whereby develops water retention capacity of soil (Sandhya et al. 2009). It is reported that EPS also binds to positively charged ions including Na+ and therefore reduces the toxic level of Na+ in soil and ameliorates plant growth (Nunkaew et al. 2014).
PGPB have been proven to be best eco-friendly remedy to accelerate the growth of plant in nutrient-deficient soil with respect to chemical fertilizers which are least available to plant. PGPB solubilizes nutrient and makes them available for uptake by plants (Choudhary 2011). There are some legumes like mung bean and soybean which are incapable of growing in drought and salt stresses as they may be devoid of mechanisms to survive in stressed conditions or due to unavailability of nutrient or increased secretion of ethylene hormone or decreased secretion of plant growth-promoting hormones.
References
Albacete A, Ghanem ME, Martínez-Andújar C, Acosta M, Sánchez-Bravo J, Martínez V, Lutts S, Dodd IC, Pérez-Alfocea F (2008) Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J Exp Bot 59:4119–4131
Almeida P, Katschnig D, de Boer AH (2013) HKT transporters—state of the art. Int J Mol Sci 14:20359–20385
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285:1256–1258
Bashan Y, de-Bashan LE (2010) How the plant growth-promoting bacterium Azospirillum promotes plant growth-A critical assessment. In: Sparks DL (ed) Advances in agronomy, 108, Elsevier, Academic Press. Adv Agro 108:77–136
Bharti N, Yadav D, Barnawal D, Maji D, Kalra A (2013) Exiguobacterium oxidotolerans, a halotolerant plant growth promoting rhizobacteria, improves yield and content of secondary metabolites in Bacopa monnieri (L.) Pennell under primary and secondary salt stress. World J Microbiol Biotechnol 29:379–387
Brini F, Masmoudi K (2012) Ion transporters and abiotic stress tolerance in plants. ISRN Mol Biol. doi:10.5402/2012/927436
Byrt CS, Platten JD, Spielmeyer W, James RA, Lagudah ES, Dennis ES, Tester M, Munns R (2007) HKT1; 5-like cation transporters linked to Na+ exclusion loci in wheat, Nax2 and Kna1. Plant Physiol 143:1918–1928
Chaer GM, Resende AS, de Balieiro FC, Boddey RM (2011) Nitrogen-fixing legume tree species for the reclamation of severely degraded lands in Brazil. Tree Physiol 31:139–149
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Chen L, Dodd IC, Theobald JC, Belimov AA, Davies WJ (2013) The rhizobacterium Variovorax paradoxus 5C-2, containing ACC deaminase, promotes growth and development of Arabidopsis thaliana via an ethylene-dependent pathway. J Exp Bot. doi:10.1093/jxb/ert031
Choudhary DK (2011) Plant growth-promotion (PGP) activities and molecular characterization of rhizobacterial strains isolated from soybean (Glycine max L. Merril) plants against charcoal rot pathogen, Macrophomina phaseolina. Biotechnol Lett 33:2287–2295
da Silva GJ, Costa de Oliveira A (2014) Genes acting on transcriptional control during abiotic stress responses. Adv Agric. doi:10.1155/2014/587070
Duca D, Lorv J, Patten CL, Rose D, Glick BR (2014) Indole-3-acetic acid in plant-microbe interactions. Anton Leeuw 106:85–125
FAO (2013) Climate-smart agriculture sourcebook. FAO, Rome
Figueiredo MV, Burity HA, Martínez CR, Chanway CP (2008) Alleviation of drought stress in the common bean (Phaseolus vulgaris L.) by co-inoculation with Paenibacillus polymyxa and Rhizobium tropici. Appl Soil Ecol 40:182–188
Franche C, Lindström K, Elmerich C (2009) Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 321:35–59
Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K (2011) ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res 124:509–525
Gabrijel O, Davor R, Zed R, Marija R, Monika Z (2009) Cadmium accumulation by muskmelon under salt stress in contaminated organic soil. Sci Total Environ 407:2175–2182
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica. doi:10.6064/2012/963401
Huang GT, Ma SL, Bai LP, Zhang L, Ma H, Jia P, Liu J, Zhong M, Guo ZF (2012) Signal transduction during cold, salt, and drought stresses in plants. Mol Biol Rep 39:969–987
Jaleel CA, Riadh K, Gopi R, Manivannan P, Inès J, Al-Juburi HJ, Chang-Xing Z, Hong-Bo S, Panneerselvam R (2009) Antioxidant defense responses: physiological plasticity in higher plants under abiotic constraints. Acta Physiol Plant 31:427–436
Kang SM, Khan AL, Waqas M, You YH, Kim JH, Kim JG, Hamayun M, Lee IJ (2014) Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact 9:673–682
Kasotia A, Choudhary DK (2014a) Induced inorganic phosphate solubilization through N-Methyl-N′-Nitro-N-Nitrosoguanidine treated mutants of Pseudomonas koreensis strain AK-1 (MTCC Number 12058) under polyethylene glycol. Proc Natl Acad Sci, India, Sect B Biol Sci 86:115–123
Kasotia A, Choudhary DK (2014b) Pseudomonas-mediated mitigation of salt stress and growth promotion in Glycine max L. Merrill Agric Res. doi:10.1007/s40003-014-0139-1
Khan AA, Jilani G, Akhtar MS, Naqvi SS, Rasheed M (2009) Phosphorus solubilizing bacteria: occurrence, mechanisms and their role in crop production. J Agric Biol Sci 1:48–58
Kim MC, Chung WS, Yun D-J, Cho MJ (2009) Calcium and calmodulin-mediated regulation of gene expression in plants. Mol Plant 2:13–21
Kintu K, Dave BP, Dube HC (2001) Detection and chemical characterization of siderophores produced by certain fungi. Indian J Microbiol 41:87–91
Kochar M, Upadhyay A, Srivastava S (2011) Indole-3-acetic acid biosynthesis in the biocontrol strain Pseudomonas fluorescens Psd and plant growth regulation by hormone overexpression. Res Microbiol 162:426–435
Kohler J, Hernandez JA, Caravaca F, Roldàn A (2008) Plant-growth-promoting rhizobacteria and arbuscular mycorrhizal fungi modify alleviation biochemical mechanisms in water-stressed plants. Funct Plant Biol 35:141–151
Li B, Sang T, He L, Sun J, Li J, Guo S (2013) Exogenous spermidine inhibits ethylene production in leaves of cucumber seedlings under NaCl stress. J Am Soc Hortic Sci 138:108–113
Liu F, Xing S, Ma H, Du Z, Ma B (2013) Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl Microbiol Biotechnol 97:9155–9164
López-Otín C, Overall CM (2002) Protease degradomics: a new challenge for proteomics. Nat Rev Mol Cell Biol 3:509–519
Miller GAD, Susuki N, Ciftci-Yilmaz S, Mittler RON (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K (2014) The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Front Plant Sci. doi:10.3389/fpls.2014.00170
Nawaz K, Hussain K, Majeed A, Khan F, Afghan S, Ali K (2013) Fatality of salt stress to plants: morphological, physiological and biochemical aspects. Afr J Biotechnol 9:5475–5480
Nishiyama R, Watanabe Y, Fujita Y, Le DT, Kojima M, Werner T, Vankovad R, Yamaguchi-Shinozakib K, Shinozakia K, Kakimoto T, Sakakibara H, Schmülling T, Tran LSP (2011) Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23:2169–2183
Nunkaew T, Kantachote D, Nitoda T, Kanzaki H, Ritchie RJ (2014) Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions. Carbohydr Polym 115:334–341
Paul D (2013) Osmotic stress adaptations in rhizobacteria. J Basic Microbiol 53:101–110
Paul D, Nair S (2008) Stress adaptations in a plant growth promoting rhizobacterium (PGPR) with increasing salinity in the coastal agricultural soils. J Basic Microbiol 48:378–384
Pérez-Alfocea F, Ghanem ME, Gómez-Cadenas A, Dodd IC (2011) Omics of root-to-shoot signaling under salt stress and water deficit. Omics J Integr Biol 15:893–901
Reddy AA, Bantilan MCS, Mohan G (2013) Pulses production scenario: policy and technological options. Policy Brief 26, International Crop Research Institute for the Semi-Arid Tropics
Richardson AE, Simpson RJ (2011) Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol 156:989–996
Saibo NJM, Lourenςo T, Oliveira MM (2009) Transcription factors and regulation of photosynthetic and related metabolism under environmental stresses. Ann Bot 103:609–623
Sandhya V, Ali SZ, Grover M, Reddy G, Venkateswarlu B (2009) Alleviation of drought stress effects in sunflower seedlings by exopolysaccharides producing Pseudomonas putida strain P45. Biol Fertil Soils 46:17–26
Santi C, Bogusz D, Franche C (2013) Biological nitrogen fixation in non-legume plants. Ann Bot 111:743–767
Shi H, Lee BH, Wu SJ, Zhu JK (2002) Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat Biotechnol 21:81–85
Singh A, Nath Panda S, Flugel WA, Krause P (2012) Waterlogging and farmland salinisation: causes and remedial measures in an irrigated semi‐arid region of India. Irrig Drain 61:357–365
Suzuki N, Rivero RM, Shulaev V, Blumwald E, Mittler R (2014) Abiotic and biotic stress combinations. New Phytol 203:32–43
Wood JM (2011) Bacterial osmoregulation: a paradigm for the study of cellular homeostasis. Annu Rev Microbiol 65:215–238
Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol Plant Mol Biol 35:155–189
Yang J, Kloepper JW, Ryu C-M (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4
Zhang H, Kim MS, Sun Y, Dowd SE, Shi H, Paré PW (2008a) Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter HKT1. Mol Plant-Microbe Interact 21:737–744
Zhang H, Xie X, Kim MS, Kornyeyev DA, Holaday S, Pare PW (2008b) Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J 65:264–273
Zhang T, Zeng S, Gao Y, Ouyang Z, Li B (2011) Assessing impact of land uses on land salinization in the Yellow River Delta, China using an integrated and spatial statistical model. Land Use Policy 28:857–866
Acknowledgments
In the present review, some of the research has been partially supported by DBT and SERB grant no. BT/PR1231/AGR/021/340/2011 and SR/FT/LS-129/2012, respectively, to DKC. Authors would also like to acknowledge UGC-RGNF fellowship.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kasotia, A., Varma, A., Tuteja, N., Choudhary, D.K. (2016). Microbial-Mediated Amelioration of Plants Under Abiotic Stress: An Emphasis on Arid and Semiarid Climate. In: Choudhary, D., Varma, A., Tuteja, N. (eds) Plant-Microbe Interaction: An Approach to Sustainable Agriculture. Springer, Singapore. https://doi.org/10.1007/978-981-10-2854-0_7
Download citation
DOI: https://doi.org/10.1007/978-981-10-2854-0_7
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-2853-3
Online ISBN: 978-981-10-2854-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)