Abstract
The Lygaeoidea, representing the second largest superfamily in the infraorder Pentatomomorpha, are one of the most diverse groups of Heteroptera with about 700 genera and more than 4,200 species in the world. In the Neotropics, about 184 genera and 836 species are included in 12 of the world’s 16 families. For each family, we provide a diagnosis; an overview of the classification; information on the general life history, ecology, and economic importance; and comprehensive keys to subfamilies, tribes, and genera for the Neotropical Region, including Mexico, Central and South America, and the West Indies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The Lygaeoidea represent the second largest superfamily within the Pentatomomorpha with more than 4,200 species worldwide (Henry 2009). Though the superfamily currently is recognized by most contemporary workers, its status has fluctuated. For example, Štys (1961, 1967) grouped the Lygaeoidea and Pyrrhocoroidea with the Coreoidea but kept the superfamily Piesmatoidea; Henry and Froeschner (1988) accepted the Lygaeoidea but retained Piesmatoidea as a separate superfamily; Schaefer (1993) also recognized Lygaeoidea but included the Piesmatidae and Idiostolidae; and Henry (1997a) recognized six superfamilies within the infraorder based on a cladistic analysis, merging the Piesmatoidea with the Lygaeoidea and giving nearly all subfamilies within the Lygaeidae (sensu lato) family status. More recently, Li et al. (2005), in using molecular sequence data, concluded that the Berytidae and Piesmatidae nested within the Pyrrhocoroidea, making the Lygaeoidea paraphyletic. Given the strong morphological support for a monophyletic Lygaeoidea Henry (1997a), including the Berytidae and Piesmatidae, the acceptance of such a novel hypothesis needs further investigation (Forero 2008).
We recognize 12 of the 16 families within the Lygaeoidea as occurring in the Neotropical Region, following Henry (1997a). For each family, we give a diagnosis, an overview of the classification, and keys to all of the subfamilies, tribes, and genera. In addition, we provide a brief overview of the general life history, habits, ecology, and economic importance of the major lygaeoid species.
The Artheneidae are all Old World, except for two Palearctic species, Chilacis typhae (Perrin) (Wheeler and Fetter 1987) and Holcocranum saturejae (Kolenati) (Hoffman and Slater 1995), recently established in the United States. Polychisme poecilus (Spinola), a South American species previously placed in Artheneidae (Slater and Brailovsky 1986), has been shown to belong in the lygaeid subfamily Ischnorhynchinae (Kerzhner 1997). The Cryptorhamphidae, containing only two genera and four species, are restricted to the Australian Region (Hamid 1971; Cassis and Gross 2002), and the Malcidae, with three genera and about 29 species, are restricted to the Oriental and Palearctic regions (Štys 1967; Kerzhner 2001). The Meschiidae, the most recently recognized family of Heteroptera, was described from Australia and India to accommodate the genus Meschia Distant, containing four species, the new genus Neomeschia Malipatil, and the new species N. queenslandicus Malipatil (Malipatil 2014).
Our classification closely aligns with Sweet’s (2000a) interpretation of the Lygaeoidea, with the exception of his proposal to elevate the lygaeid (sensu stricto) subfamilies Ischnorhynchinae and Orsillinae to family status, but without giving specific character information to support his hypothesis. As a consequence, we feel that the strong character support for a monophyletic Lygaeidae (Henry 1997a), which includes these two subfamilies and the Lygaeinae, precludes any argument to separate them until new evidence is offered.
Slater (1964a) and Slater and O’Donnell (1995) cataloged the Lygaeidae (sensu lato) of the world; Ashlock and Slater (1988) cataloged the Nearctic species, which includes numerous taxa also occurring in the Neotropics; Schuh and Slater (1995) provided a good overview of the subfamilies and tribes; and Henry (1997a) provided a key to help distinguish 15 of the 16 recognized families of Lygaeoidea. There have been a number of outstanding treatments of the Lygaeoidea with keys, including Froeschner’s (1981) list and keys to the Ecuadorian Heteroptera and (Froeschner 1985) synopsis of the Galapagos fauna; Slater and Baranowski’s (1990) Lygaeidae of Florida; Slater and Brailovsky’s (2000) Lygaeidae of Mexico, with keys to tribes and a checklist of species; Peck’s (2001) list and keys to the Heteroptera of the Galapagos Islands; Baranowski and Slater’s (2005) Lygaeidae of the West Indies; and Dellapé’s (2014) Lygaeoidea of Argentina, with keys to genera and a checklist of species. More regional checklists include Paula and Ferreira’s (1998, 2000) list for Minas Gerais, Brazil; Cervantes and Brailovsky’s (2011) list for Veracruz, Mexico; and the lists for Argentina by Melo et al. (2004) (Corrientes), Melo et al. (2011) (Chaco National Park), and Dellapé and Carpintero (2012) (Buenos Aires Hills). Also, although aimed primarily at the North American fauna, this author went by Torre-Bueno’s (1946) synopsis and keys and Slater and Baranowski’s (1978) How to Know the True Bugs are useful for many taxa ranging into Mexico, Central America, and the West Indies.
Sweet (1964a, b) detailed the ecology and feeding habits of many widespread New World Lygaeoidea. Sweet (2000a, b) provided an especially thorough overview of the economic importance of many of the most important taxa in the superfamily.
2 Life History, Ecology, and Economic Importance
The Lygaeoidea are a diverse, highly successful group of true bugs found in all zoogeographic regions. Important reviews of the habits, life history, and economic importance include Sweet (1960, 1964a, b, 2000a) and Schuh and Slater (1995) and the many papers cited in these works. Many lygaeoids have attracted considerable attention as model species for the study of insect physiology (Slater and O’Donnell 1995) and agriculture (Sweet 2000a). More recently, Burdfield-Steel and Shuker (2014) provided an overview emphasizing their behavior, evolution, and ecology.
Lygaeoid nymphs typically are associated with the adults (Schuh and Slater 1995) and are often gregarious (Aller and Caldwell 1979). Although parental care is widespread in the Heteroptera, no evidence has been found for this behavior within the Lygaeoidea. A number of species in temperate areas, including Lygaeus equestris (L.) and L. simulans Deckert, show reproductive diapause and migratory capabilities affected by temperature and photoperiod (Solbreck 1979; Dingle et al. 1980). These adaptations allow them to avoid or survive low temperatures during the winter months, as well as to migrate by following seasonal patterns and host plant abundance (Dingle et al. 1980; Attisano et al. 2013).
Wing modifications within the Lygaeoidea fall into four categories (Slater 1977): (1) aptery, or the complete absence of wings; (2) sub-brachyptery, where the forewings extend only to the end of the fifth abdominal tergite; (3) brachyptery, where the forewings are reduced and do not cover the sixth and seventh abdominal terga and the hind wings are reduced but usually not flaplike; and (4) macroptery, where the clavus and corium are distinct, the membrane is well developed, and the hind wings are elongate. Flightless morphs are favored by habitat permanency (Slater 1977).
Slater (1977) and Slater and Baranowski (1990) recognized three major lygaeoid habitats: arboreal, geophilic, and laminophilic. Many arboreal species live on plants above ground level. These species are fully winged and usually readily fly when disturbed. Geophiles live on the ground in the litter layer, where they feed on fallen seeds. A number of the geophilous species may climb plants to feed on mature seeds, but most spend most of their lives on the ground. Laminaphiles live between the sheaths of leaves and stems of grasses, sedges, and rushes. Unlike arboreal species, they often are brachypterous (Slater and Baranowski 1990).
The feeding habits of seed bugs were extensively discussed by Sweet (1960, 1964a, b, 2000a). Most lygaeids feed on seeds or plant sap, whereas only a few are predatory. They have piercing-sucking mouthparts, which are made up of the mandibles and maxillae modified to form needlelike stylets lying within a grooved labium (Schuh and Slater 1995). Feeding methods can be divided into two “types”: “stylet-sheath” feeders and “lacerate-flush” feeders (Schuh and Slater 1995; Sweet 2000a). The majority of Lygaeoidea are lacerate-flush feeders, a method commonly used by Miridae and other heteropterans that feed on portions of the plant rich in nutrients, such as seeds (Schuh and Slater 1995; Wheeler 2001), whereas the families Blissidae, Malcidae, and Colobathristidae are predominantly sap feeders.
3 General Characteristics and Diagnoses of the Lygaeoidea
Henry (1997a) documented the paraphyly of Lygaeidae as treated by previous authors (e.g., Slater 1964a; Slater and O’Donnell 1995; Schuh and Slater 1995). That the Berytidae, Colobathristidae, Malcidae, and Piesmatidae nested within the Lygaeidae in his analysis provided sufficient evidence to either reduce these well-defined families to subfamily status within the Lygaeidae (sensu lato) or to elevate many of the subfamilies within the paraphyletic Lygaeidae to family level. Henry (1997a) chose the latter as the more informative strategy to reflect his hypothesized phylogeny, based on three synapomorphies, the reduced venation nearly always lacking closed cells on the hemelytral membrane, and the incrassate fore femora found in all basal taxa but lost in a number of distal groups. Thus, 16 families are now recognized in the superfamily Lygaeoidea.
The following key modified from Henry (1997a) and Dellapé (2014) will distinguish the Neotropical lygaeoid families.
Key to the Neotropical families of Lygaeoidea
1. Abdominal spiracles on segment II ventral..........................................................2 |
– Abdominal spiracles on segment II dorsal...........................................................4 |
2. Trichobothria present on head; ovipositor, at most, dividing abdominal sternite VII; suture between abdominal sterna IV and V usually curving anteriorly and usually ending before attaining lateral abdominal margin, except in Plinthisinae.................................................................................................Rhyparochromidae |
– Trichobothria never present on head; ovipositor dividing at least sternites VI and VII; suture between abdominal sterna IV and V straight, always attaining lateral abdominal margin................................................................................................3 |
3. Profemora weakly incrassate, little thicker than metafemora; base of hemelytral membrane with a distinct closed cell............................................Heterogastridae |
– Profemora strongly incrassate, much thicker than metafemora; base of hemelytral membrane without a closed cell..................................................Pachygronthidae |
4. Tarsi two segmented; ocelli present or absent; trichobothria lacking on abdominal segments IV and V, often lacking on other segments as well.............Piesmatidae |
– Tarsi three segmented; ocelli present; trichobothria present on abdominal segments IV and V...............................................................................................5 |
5. Each ocellus nearly encircled by a distinct groove..............................................6 |
– Each ocellus not encircled by a groove..............................................................11 |
6. Abdominal spiracles on segments V and VI ventral....................Colobathristidae |
– Abdominal spiracles on segments V and VI dorsal..............................................7 |
7. Connexiva on abdominal segments V to VI produced into conspicuous dentate lobes............................................................................Malcidae (Old World only) |
– Connexiva on abdominal segments V to VII simple, never produced into conspicuous lobes................................................................................................8 |
8. First antennal segment long, slender, often apically clavate, subequal in thickness to and nearly always longer than segments II and III.............................Berytidae |
– First antennal segment short, stout, barrel shaped, much shorter and thicker than segments II and III...............................................................................................9 |
9. Hemelytra impunctate or with only a few indistinct punctures, corium hyaline to translucent beyond constricted base; apex of scutellum bifid; head broad, eyes substylate, vertex wider than anterior width of pronotum.........................Ninidae |
– Hemelytra distinctly punctate on clavus and corium, corium opaque throughout, never constricted; apex of scutellum rounded or acute; head not broadened, eyes never substylate, vertex always narrower than anterior width of pronotum......10 |
10. Buccula short, not extending posteriorly beyond bases of antennae; abdominal trichobothria present on sternites II to VII.............................................Cymidae |
– Buccula long, extending posteriorly to base of head; abdominal trichobothria present only on sternites V and VI.............Cryptorhamphidae (Old World only) |
11. Abdominal spiracles on segments III and IV ventral.......................................12 |
– Abdominal spiracles on segments III and IV dorsal........................................13 |
12. Lateral pronotal margin explanate or with a wide flattened carina; female abdomen rounded caudally; male abdominal sternite VII without clusters or combs of setae ventrally.......................................................................................................Artheneidae (Old World, except for two introduced Nearctic species) |
– Lateral pronotal margin rounded or, at most, weakly carinate; female abdomen often rounded caudally; male abdominal segment VII with transverse combs or clusters of setae ventrally...............................................................Oxycarenidae |
13. Abdominal spiracles on segments V to VI ventral; sutures between tergites 4/5 and 5/6 curving forward through middle...........................................Geocoridae |
– Abdominal spiracles on segments V to VI dorsal; all abdominal tergites transverse, sutures never curving forward.......................................................14 |
14. Abdominal spiracles on segment VII dorsal; each pronotal callus with an impressed, transverse, usually shiny groove; scutellum usually with a cross-shaped carina........................................................................................Lygaeidae |
– Abdominal spiracles on segment VII ventral; pronotal calli without impressed grooves; scutellum without a cross-shaped carina.................................Blissidae |
4 Family Berytidae
4.1 General Characteristics and Diagnosis
Berytidae, commonly referred to as stilt bugs, comprise a small group of morphologically diverse lygaeoids. Typical stilt bugs, such as the metacanthine genera Jalysus Stål and Metacanthus Costa, are elongate, slender insects, with long slender legs and antennae, often as long as or longer than the body. Other taxa, however, deviate from this general appearance. Members of the genus Hoplinus Stål have spindle-shaped bodies and shorter and stouter legs and antennae and often are armed with spines on the head, pronotum, and hemelytra, species of Parajalysus Distant are more robust and armed with three long, erect spines on the pronotum, and the recently described Cuscohoplininus pagoreni Dellapé and Carpintero has only a single spine on the middle of the anterior pronotal lobe (Dellapé and Carpintero 2007). Other taxa, such as Pronotacantha Uhler, have spines on the pronotum and scutellum and distinctly banded appendages, those of Phaconotus Harris are adorned with pearl-like tubercles on the anterior lobe of the pronotum, and the genus Diabolonotus Henry has two anteriorly directed “devil-like,” pronotal horns.
Synapomorphies defining the family are the elongate bilobed head; basally tapered buccula; long, slender, apically clavate first antennal segment; basally narrowed scutellum; subparallel to basally constricted hemelytra; grooved metasternum; dentate claws; grooved, quadrate abdominal segment II; undivided abdominal segment VII in females; hidden dorsal spiracles; and midlateral position of the trichobothria on abdominal segment III (Henry 1997b, c).
4.2 Classification and Diversity
Thirty-seven genera and about 174 species of Berytidae are known in the world (Henry and Froeschner 1998; Henry 2002, 2007; Dellapé and Carpintero 2007; Cai et al. 2011, 2013). The family is separated into three subfamilies and six tribes: the Berytinae (and Berytini and Berytinini), Gampsocorinae (and Gampsocorini and Hoplinini), and Metacanthinae (and Metacanthini and Metatropini) (Henry 1997b). All three subfamilies but only four of six tribes are known from the Neotropical Region. The Neotropical Berytinae are represented only by the widespread North American Neoneides muticus (Say), which also occurs in Baja California, Mexico. The Neotropical Gampsocorinae are represented only by the genus Gampsocoris Fuss and four species in the nominate tribe Gampsocorini and nine genera and 36 species in the Hoplinini (Henry and Froeschner 1998; Henry 2002; Dellapé and Carpintero 2007). Two genera and 12 species of Neotropical Metacanthinae are known, all of which are placed in the nominate tribe Metacanthini (Henry and Froeschner 1998; Henry 2007).
Most early work on Neotropical berytids was limited to descriptions of a few new genera and species. Stål (1874) provided the first synopsis of the family with the first keys to genera and the species of Jalysus. Most subsequent papers were mostly descriptive (e.g., Distant 1880–1893; Horváth 1905), until McAtee’s (1919) review of the Nearctic fauna, which included several Neotropical genera. Harris (1943) added additional South American genera and species but little was provided to aid identification until berytid specialist J. M Štusák (e.g., 1967, 1968, 1971, 1973, 1977) provided a series of well-illustrated descriptions of new genera and species and clarification of previously confused taxa. Other important works include Štusák and Cobben’s (1975) keys to the Antillean species and Froeschner’s (1981) checklist and keys to the Ecuadorian genera and species. More recently, Henry (1997c) monographed the family for the Western Hemisphere, treating 13 genera and 52 species, including 12 genera and 49 species from the Neotropics. Subsequently, Henry (2002) reviewed the genus Hoplinus, clarified the identity of the type species, H. spinosissimus Signoret and described the new species H. paulai from Brazil; Henry (2007) described the new species of Jalysus ossesae from Brazil; and Dellapé and Carpintero (2007) described the new hoplinine genus and species Cuscohoplininus pagoreni from Peru.
The following keys to the Neotropical subfamilies, tribes, and genera of Berytidae are modified from Henry (1997c).
Key to the Neotropical subfamilies of Berytidae
1. Head, pronotum, and undersurface of thorax bordering rostral sulcus always clothed with appressed sericeous or woolly pubescence; ventral surface of abdomen deeply punctate (except Old World genus Yemmatropis).................................................................................................................Berytinae |
– Head, pronotum, and undersurface of thorax along the rostral sulcus without appressed sericeous or woolly pubescence; ventral surface of abdomen never punctate................................................................................................................2 |
2. Metathoracic scent channel smooth, extended onto an elongate spout or digitiform spine (except Old World genus Metatropis)....................................Metacanthinae |
– Metathoracic scent channel lined with overlapping scalelike plates, extended onto a pouchlike structure, or scent channel and spout completely absent, at most, with a blunt to elongate tubercle comprised of honeycombed chambers.......................................................................................................Gampsocorinae |
4.2.1 Subfamily Berytinae Puton
Only one genus and species of this subfamily, belonging to the tribe Berytini Puton, occurs in the Neotropical Region. The widespread Neoneides muticus (Say) occurs throughout much of the United States and Canada and into northern Mexico (Henry 1997c).
4.2.2 Subfamily Metacanthinae
Only the nominate tribe Metacanthini occurs in the Neotropical Region.
Key to the genera of Neotropical Metacanthini
1. Ostiolar spout ending in an acutely produced apical spine (Fig. 1)....Jalysus Stål |
– Ostiolar spout without an apical spine, instead apically rounded and weakly recurved near the level of the hemelytra.................................Metacanthus Costa |
4.2.3 Subfamily Gampsocorinae Southwood and Leston
Key to the Neotropical Tribes of Gampsocorinae
1. Ostiolar scent channel distinct, set within a pouchlike spout lined with overlapping scalelike plates; each side of pronotal collar with an erect spine or tubercle..........................................................................................................Gampsocorini |
– Ostiolar scent channel absent, never with a pouchlike spout; pronotum often variously armed with spines or tubercles, but never with a distinct collar having a spine or tubercle on either side...............................................................Hoplinini |
4.2.3.1 Tribe Gampsocorini
Gampsocoris Fuss (Fig. 1) is the only Neotropical genus belonging to this tribe. Henry (1997c) provided a key to the four known species.
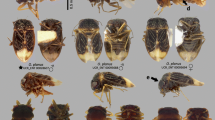
Figs. 1–12. 1, Gampsocoris tuberculatus Štusák (Berytidae: Gampsocorinae). 2, Jalysus sobrinus Stål (Berytidae: Metacanthinae). 3, Blissus parasigaster Drake (Blissidae). 4, Patritius grossus Haglund (Blissidae). 5, Colobasiastes similis Horváth (Colobathristidae). 6, Cymodema breviceps Stål (Cymidae: Cyminae). 7, Geocoris callosulus Berg (Geocoridae: Geocorinae). 8 Isthmocoris imperialis Distant (Geocoridae: Geocorinae). 9, Epipolops frondosus Herrich-Schaeffer (Geocoridae: Pamphantinae). 10, Cephalocattarus waorani Slater and Henry (Geocoridae: Pamphantinae). 11, Kleidocerys virescens (F.) (Lygaeidae: Ischnorhynchinae). 12, Polychisme ferruginosus (Stål) (Lygaeidae: Ischnorhynchinae) (Photos by G Ouellette)
4.2.3.2 Tribe Hoplinini
The nine genera included in this tribe are restricted to the Western Hemisphere. Henry (1997c) revised the group and included keys to species.
Key to the Neotropical genera of Hoplinini
1. Posterior lobe of pronotum armed with erect spines...........................................2 |
–Posterior lobe of pronotum without spines..........................................................4 |
2. Head with five or more median spines.............................................Hoplinus Stål |
– Head without median spines, at most, with a small rounded tubercle on vertex or a slender spine on middle of posterior lobe.........................................................3 |
3. Pronotum with three rows of long, slender spines, one row along each lateral margin and one along meson; posterior lobe of head with six white tubercles, the middle one often spinelike...................................................Pronotacantha Uhler |
– Pronotum with four stout spines, one on anterior lobe and three along basal margin of posterior lobe; posterior lobe of head without tubercles.................................................................................................Parajalysus Distant |
4. Base of pronotal disc with three high, acute ridges; head and pronotum with a well-developed median carina; legs and antennae with pilose setae three or more times the diameter of the segments.................................................................................................Metajalysus Štusák |
– Base of pronotal disc often convex, but never with three high ridges; appendages with or without pilose setae three or more times diameter of segment..................5 |
5. Scutellum hidden by a strongly explanate basal edge of pronotum; body and appendages with erect, bristlelike or long, simple setae; metafemora distinctly bowed...................................................................................................................6 |
– Scutellum clearly visible and armed with a distinct spine or tubercle; body and appendages without bristlelike or pilose setae; metafemora not bowed..............7 |
6. Anterior lobe of pronotum with two long, stout, anteriorly directed, blunt spines or tubercles; side of pronotum without a swollen protuberance visible from dorsal aspect; appendages with long, pilose setae, setae on tibiae three or four times longer than the diameter of the respective segments............................................................................................................................................Diabolonotus Henry |
– Anterior lobe of pronotum unarmed, without stout spines; side of pronotum with a swollen protuberance visible from dorsal aspect; appendages sometimes with erect, bristlelike setae, but those on tibiae at most subequal to diameter of respective segments.....................................................................Xenoloma Harris |
7. Anterior lobe of pronotum with an erect spine at middle between calli...............................................................Cuscohoplininus Dellapé and Carpintero |
– Anterior lobe of pronotum lacking an erect spine................................................8 |
8. Anterior lobe of pronotum with 6–11 white, rounded tubercles; posterior edge of pronotum unarmed; posterior lobe of head with a short white tubercle between ocelli and often with a white tubercle on either side of each ocellus......................................................................................................................Phaconotus Harris |
– Anterior lobe of pronotum with only five white, rounded tubercles restricted to narrow collar region (two rounded ones on either side and one in between two erect, white spines); posterior edge of pronotum with four upturned tubercles; posterior lobe of head without tubercles............................. Oedalocanthus Henry |
5 Family Blissidae
5.1 General Characteristics and Diagnosis
The Blissidae, or chinch bugs, are elongate to broadly oval, often flattened to access leaf sheaths of their hosts, and range in size from less than 3 mm to more than 15 mm. They are recognized by the often entirely or partially pruinose surfaces of head and pronotum, a tubercle on the genital capsule, the peculiarly winged sperm reservoirs in many taxa, and the dorsal position of the abdominal spiracles and by being the only lygaeoid group to feed entirely on plant sap, rather than seeds. Blissids range from fully winged to frequently short winged or brachypterous, having only remnant wing pads as in the genera Blissus Burmeister and Ischnodemus Fieber or even the complete loss of wing pads as in the genera Aulacoblissus Slater and Howdenoblissus Štys.
5.2 Classification and Diversity
The Blissidae comprise about 51 genera and 436 species worldwide (Cassis and Gross 2002; Henry 2009). Only 16 genera and about 109 species are known from the Neotropical Region (Slater 1979; Slater and O’Donnell 1995; Dellapé and Montemayor 2009). Of these, about 72 % are in the genera Blissus (12 spp.), Ischnodemus (34 spp.), Patricius Distant (12 spp.), and Toonglasa Distant (21 spp.; see discussion below). Slater (1979) monographed the world fauna and provided keys to all genera and most species except for the genus Blissus. Although Leonard (1968a) studied the species of Blissus for eastern North America and described a few new species (Leonard 1968b, 1970), members of the genus remain difficult to identify and are in great need of revision. Slater and Brailovsky (1983, 1990), in revising the primarily Neotropical genus Toonglasa Distant, synonymized Extarademus Slater and Wilcox (1966) and provided keys to the 21 known species. Slater (1986b) established the genus Aulacoblissus to accommodate a new micropterous species from Venezuela, Štys (1991) described Howdenoblissus slateri, a similar-appearing apterous species from Colombia, and Brailovsky and Barrera (2012) added Napoblissus foreroi, also with greatly abbreviated hemelytra from Ecuador. Dellapé and Montemayor (2009) described the most recently recognized species of Ischnodemus and provided descriptions of the male and immature stages of I. subflavus Slater and Wilcox. Henry (1997a) elevated Blissinae to family status.
The following key to genera was created in part from Slater (1979) and in part based on our original research. In our opinion, the genus Toonglasa is not monophyletic as now interpreted. As a consequence, until additional revisionary studies can be conducted, we are reestablishing Toonglasa as a monotypic genus, containing only the type species T. forficuloides Distant and resurrecting Extarademus to accommodate the remaining 20 species, including its type species, Macropes collaris Signoret, as designated by Slater and Wilcox (1966).
Key to the Neotropical genera of Blissidae
1. Fore coxal cavities open behind...........................................................................2 |
– Fore coxal cavities closed behind........................................................................9 |
2. Pronotum at least partially pruinose....................................................................3 |
– Pronotum shiny, without pruinose areas..............................................................5 |
3. Pronotum entirely pruinose (Fig. 3).........................................Blissus Burmeister |
– Anterior half of pronotum shiny; posterior half pruinose....................................4 |
4. Membrane entirely white; posterior margin of pronotum tan, contrasting with a darker anterior area............................................ Caveloblissus Slater and Wilcox |
– Membrane dark brown, with a subbasal pale band; posterior pronotal lobe entirely dark..........................................................................................Praeblissus Barber |
5. Macropterous to micropterous (with at least short wing pads)............................6 |
– Apterous (without wing pads)..............................................................................7 |
6. Abdominal venter with a prominent stridulitrum on segments 3 to 6; metathoracic scent-gland auricle simple and rounded; only fore femur with one large and several small spines..............................................................Heteroblissus Barber |
– Abdominal venter lacking a stridulitrum; metathoracic scent-gland auricle strongly produced anteriorly; all femora with spines...........Praetorblissus Slater |
7. Fore femur lacking spines....................................................Howdenoblissus Štys |
– Fore femur with one or two spines.......................................................................8 |
8. Fore femur with only one small spine; scutellum lacking a median elevation................................................................................................Aulacoblissus Slater |
– Fore femur with two spines, one large and one small; scutellum with a median elevation9 |
9. Labium short, not reaching fore coxae; pronotum pruinose................................................................................................................Patritiodemus Slater and Ahmad |
– Labium longer, always reaching fore coxae or beyond; pronotum shiny or in part pruinose..............................................................................................................10 |
10. Fore femur lacking spines....................................................Ischnodemus Fieber |
– Fore femur with one or more spines................................................................11 |
11. Fore femur with only one spine.......................................................................12 |
– Fore femur with two or more spines................................................................14 |
12. Membrane composed of numerous small reticulate cells..........................................................................................................Reticulatodemus Slater and Wilcox |
– Membrane without small reticulate cells.........................................................13 |
13. Broad flattened species; fore femur strongly incrassate; pro- and mesosternum grooved to receive labium; each side of seventh abdominal segment in males with a prominent posteriorly directed projection; scent-gland auricle relatively broad, slightly curving forward distally, and distinctly raised above the evaporative surface...........................................Toonglasa Distant, revised status |
– Slender, elongate species; fore femur not strongly incrassate; pro- and mesosternum not grooved to receive labium; seventh abdominal segment in males without projections; scent-gland auricle slender, curving forward, and flat against evaporative surface................................Extarademus Slater and Wilcox |
14. Fore femur with two spines; head and pronotum with scalelike setae...............................................................................................Xenoblissus Barber |
– Fore femur with three or more spines..............................................................15 |
15. Relatively slender species; only fore femur multispinose, middle and hind femora without spines.....................................Procellademus Slater and Wilcox |
– All femora with multiple spines.......................................................................16 |
16. Body broad and strongly flattened; head shiny; pruinose areas of pronotum confined to the area around collar and sometimes a narrow line across the transverse impression............................................................Riggiella Kormilev |
– Body elongate and not flattened; head pruinose; pronotum usually at least in part pruinose (Fig. 4).........................................................................Patritius Distant |
6 Family Colobathristidae
6.1 General Characteristics and Diagnosis
The Colobathristidae (Fig. 5) comprise a small group of tropical bugs with about half the known genera found in the Neotropical Region. They range from about 6.0 mm to over 20 mm. They are characterized by their slender elongate bodies, long slender legs and antennae, bulging eyes, quadrate bilobed thorax, slender often spined scutellum, hyaline hemelytra with few hardly visible membranal veins, and slender elongate abdomen constricted at the base. Colobathristids also possess characters appearing in part coreoid and lygaeoid (Henry 1997a). Štys (1966) indicated they have a platelike ovipositor similar to those in the Coreoidea or Pyrrhocoroidea but internal genital structures similar to those in the malcid line. Cobben (1968) indicated that the eggs were most similar to the Coreoidea. Kumar (1968), however, considered the three-lobed salivary glands and the fingerlike gastric ceca on the midgut “decidedly lygaeid features” (sensu lato), as well as the aedeagus, the arrangement of the trichobothria, and the position of the spiracles. In Henry’s (1997a) phylogenetic analysis, the colobathristids nest within the Lygaeoidea, forming a sister-group relationship with the Berytidae.
6.2 Classification and Diversity
Twenty-five genera and more than 80 species of Colobathristidae (Kerzhner 2001; Štys and Exnerová 2012) are placed in two subfamilies, the nominate Colobathristinae and the monogeneric Dayakiellinae, containing only two Indonesian species of Dayakiella Horváth (Štys 1966). Štys and Exnerová (2012) provided a key to the 13 Old World genera. Thirteen genera and about 40 species are recorded from the Neotropics. Phaenacantha saileri Kormilev, described from Guatemala, is the only representative of an otherwise exclusively Old World genus. Horváth (1904) monographed the family and Kormilev (1949a, 1949b, 1951) described additional new Neotropical taxa. Carvalho and Henry (1986) described the new genus Parathristes to accommodate P. carajaensis from Pará, Brazil, the largest species of the family yet discovered. Štys and Henry (2015) added the new genus Neolabradoria and new species N. inexpectata from Peru, provided the replacement name Bradaloria for the preoccupied Labradoria Kormilev, and gave a revised key to the Neotropical genera.
Kormilev (1951) provided a key to the Neotropical genera, modified from Horváth (1904), and Carvalho and Costa (1989) provided a well-illustrated update, with the addition of Parathristes. The following key is modified from Štys and Henry (2015). The genus Curupira Distant (1888), tentatively placed as a synonym of Colobathristes Burmeister by Carvalho and Costa (1989), is not included until type material is studied and its identity clarified.
Key to the genera of Neotropical Colobathristidae
1. Side of head with a distinct nearly straight to lunate stridulitrum.......................2 |
– Side of head without a stridulitrum......................................................................7 |
2. Scutellum with a long, erect, spine, usually longer than the scutellum...............3 |
– Scutellum without an erect spine, apex spiniform, horizontal or subhorizontal, usually shorter than the scutellum........................................................................6 |
3. Distance between ocelli greater than space from an ocellus to an eye; sternite IV as long as III..........................................................................Diascopoea Horváth |
– Distance between ocelli much less than distance between an ocellus and an eye...................................................................................................................4 |
4. Antennal segment IV with a distinct white ring at base..........................................................................................................................Neocolobrathristes Kormilev |
– Antennal segment IV without a white ring at base..............................................5 |
5. Scutellar spine nearly glabrous; length of antennal segments III and IV subequal; vertex with one groove...........................................................Calliseidus Horváth |
– Scutellar spine with numerous long setae; antennal segment IV distinctly longer than III; vertex with two grooves.......................................Trichocentrus Horváth |
6. Anterior lobe of pronotum bulbous or horn shaped, higher than head; scutellar spine horizontal or subhorizontal; undersurface of fore tibia with distinct denticles........................................................................................................Peruda Distant |
– Anterior lobe of pronotum not bulbous, lower than the head and posterior lobe; scutellum without horizontal spine, upper surface with a small dent or impression; fore tibia without distinct denticles.........................................Perudella Kormilev |
7. Scutellum with a long, erect, spine, usually longer than scutellum.....................8 |
– Scutellum without an erect spine, apex spiniform, horizontal or subhorizontal, usually shorter than scutellum...........................................................................11 |
8. Head and anterior lobe of pronotum almost glabrous, with only thin, simple setae..............................................................................................................................9 |
– Head and anterior lobe of pronotum densely coated with adpressed, woolly, silvery pubescence.............................................................................................10 |
9. Rostrum extending only to bases of fore coxae; anterior lobe of pronotum much longer than posterior lobe.................................Parathristes Carvalho and Henry |
– Rostrum extending nearly to middle coxae; anterior lobe of pronotum shorter than posterior lobe.............................................................Phaenacantha Horváth |
10. Distance from an ocellus to an eye about twice the distance between ocelli; rostral segment IV longer than III; ventral edge of genital capsule with a sharp, protruding process..............................................................Carvalhoia Kormilev |
– Distance from an ocellus to an eye subequal or slightly greater than the distance between ocelli; rostral segments III and IV equal in length; ventral edge of genital capsule lacking a sharp process.....................Colobathristes Burmeister |
11. Distance from an ocellus to an eye less than distance between ocelli; vertex with a median groove in front of ocelli....................................................................12 |
– Distance from an ocellus to an eye three times greater than distance between ocelli; vertex in front of ocelli with two shallow grooves................................13 |
12. Distance between ocelli slightly greater than distance from an ocellus to an eye; scutellum subtriangular, with a slender, pointed, horizontal spine (meso- and metanotum covered); hemelytra narrower than abdomen.......................................................................................................................Bradaloria Štys and Henry |
– Distance between ocelli subequal to distance from an ocellus to an eye; scutellum transverse, oval, with only a low dentiform tubercle (medial parts of meso- and metanotum exposed); hemelytra as wide as abdomen......................................... .............................................................................Neolabradoria Štys and Henry |
13. Scutellum with subhorizontal spine visible in lateral aspect; fore femora lacking or with only a few tiny denticles on distal third; antennal segment III short, only about one-third of body length...........................................Piptocentrus Horvath |
– Scutellum with horizontal spine, not visible in lateral aspect; fore femur with numerous small denticles over entire length; antennal segment III long, longer than half the body length..................................................Colobasiastes Breddin |
7 Family Cymidae
7.1 General Characteristics and Diagnosis
New World cymids are small, punctate, usually yellowish-brown bugs, measuring from 3.0 to nearly 5.0 mm. Members of this family have the ocelli nearly encircled by a groove, a short barrel-shaped antennal segment I, a short buccula not extending posteriorly beyond the level with the bases of the antennae, spiracles II to VI dorsal, and the dorsal abdominal scent-gland scars appearing singly between terga 4/5 (Cymodema and a few Cymus), in twos between terga 3/4 and 4/5 (Cymus), and threes between terga 3/4, 4/5, and 5/6 (Ontiscinae).
7.2 Classification and Diversity
Nine genera and about 54 species known worldwide (Hamid 1975; Henry 2009) are placed in two subfamilies, the Cyminae and Ontiscinae, of which only the former occurs in the New World. In the Neotropical Region, only two genera and eight species are known. Cymodema Spinola contains only two species, with C. breviceps (Stål) (Fig. 6) the most widespread, occurring from the United States to Argentina and Brazil, and C. barberi Hamid, known only from Mexico. Cymus Hahn contains six species, with one known only from Brazil, four recorded from Mexico, and one from Guatemala and Mexico. Hamid (1975) monographed the group as a subfamily and provided keys to the genera and species of the world. Henry (1997a) gave Cyminae family status (minus the Ninini) and hypothesized it as the sister group to the remainder of the “malcid line,” including in sequence the Ninidae, Malcidae, Colobathristidae, and Berytidae.
The following key will separate the only two Neotropical cymid genera.
Key to the Neotropical genera of Cymidae
1. First antennal segment not exceeding apex of clypeus; with two dorsal abdominal scent-gland scars between terga 3/4 and 4/5; seventh abdominal spiracle ventral............................................................................................................Cymus Hahn |
– First antennal segment exceeding the apex of clypeus; with only one dorsal abdominal scent-gland scar between terga 4/5; seventh abdominal spiracle dorsal (Fig. 6)....................................................................................Cymodema Signoret |
8 Family Geocoridae
8.1 General Characteristics and Diagnosis
Members of this subfamily are readily recognized by their kidney-shaped to stylate eyes, extending laterally beyond the anterior margins of the pronotum; the broad heads; the relatively stout ovoid bodies; and the posteriorly curved abdominal sutures between terga 4/5 and 5/6. In addition, the abdominal spiracles on segments II, III, and IV are dorsal and those on V, VI, and VII usually are ventral (Henry 2009), except for members of the Australian tribe Australocorinae, in which all abdominal spiracles (II–VII) are dorsal (Malipatil 2012).
8.2 Classification and Diversity
The Geocoridae, or big-eyed bugs, are a worldwide group comprising 27 genera and about 280 species (Henry 2009, 2013; Malipatil 2012; Rengifo-Correa et al. 2013). The family is separated into five subfamilies: the Australocorinae Malipatil, Bledionotinae Reuter, Geocorinae Stål, Henestarinae Douglas and Scott, and Pamphantinae Barber and Bruner (Slater 1999; Henry 1997a, 2009, 2013; Malipatil 2012). The Australocorinae, including one genus and four species restricted to Australia (Malipatil 2012); the Bledionotinae, containing one genus and species restricted to the Palearctic (Slater 1964a; Péricart 2001); and the Henestarinae, with three genera, are restricted to the Old World, with the exception of the problematic Coriantipus inopinatus Bergroth described from Argentina. The Geocorinae occur in all zoogeographic regions and the Pamphantinae are found only in the New World tropics (Henry 2013; Rengifo-Correa et al. 2013), with the exception of one genus and species described from Queensland, Australia (Slater 1981).
The Neotropical Geocorinae are represented by only four genera and about 25 species (Slater 1964a; Slater and O’Donnell 1995), which represents only about 10 % of the world fauna (Readio and Sweet 1982). The eastern US species of Geocoris Fallén and Isthmocoris McAtee were revised by Readio and Sweet (1982). Brailovsky (2013) provided a key to the fourteen Neotropical species of Ninyas Distant. The monobasic geocorine genus Stenogeocoris and the species S. horvathi were described by Montandon (1913) based on one specimen from Córdoba Province, Argentina; the type specimen is lost and there have been no additional records since Montandon’s description.
The Pamphantinae, comprising ten genera and 48 species (Henry 2013; Rengifo-Correa et al. 2013), are separated into three tribes, the Cattarini Slater, the Epipolopini Slater, and the nominate Pamphantini (Slater 1999). Brailovsky (1989b) described one new genus and two new species of Pamphantini and provided a key to the genera. Slater and Henry (1999) reviewed the cattarine genus Cattarus, with four new species, and described the remarkable ant-mimetic Cephalocattarus waorani from Ecuador. Baranowski and Slater (2005) gave a key to the three genera and 13 species known from the West Indies. Henry (2006) revised the stalk-eyed genus Epipolops, described five new species, and provided a key to distinguish the 14 known species, and Henry (2013) described the new genus and species Cymapamphantus valentineorum from the British Virgin Islands and provided a checklist and keys to the tribes and genera of the Pamphantinae. Rengifo-Correa et al. (2013) described two additional species of Epipolops and provided a revised key to species and a phylogenetic analysis of the genus.
The following key to the genera of Geocorinae is modified from Readio and Sweet (1982) and Baranowski and Slater (2005). The keys to tribes and genera of the Pamphantinae are from Henry (2013).
Key to the Neotropical subfamilies of Geocoridae
1. Sutures on abdominal sterna II, III, and IV fused and without lateral trichobothria; body elongate, antlike......................................................................Pamphantinae |
– Sutures on abdominal sterna II, III, and IV entire and with distinct, lateral trichobothria; body more stout, not antlike............................................Gecorinae |
Key to the Neotropical genera of Geocorinae
1. Claval commissure well developed; clypeus with a slight sulcus; basal three abdominal sterna often fused..........................................................Ninyas Distant |
– Claval commissure not present; clypeus with a complete median sulcus; basal three abdominal sterna not fused.........................................................................2 |
2. Rostral segment II longer than III; eyes stylate, remote from anterior angles of pronotum; ocular suture incomplete, extending laterally from the ocellus around base of eye stalk to apical margin of head, ending at anterior margin of eye (Fig. 8)...................................................................................Isthmocoris McAtee |
– Rostral segment II shorter than III; eyes semistylate, sometimes near or in contact with anterior angles of pronotum; ocular suture absent or suture complete, extending laterally from the ocellus, around base of eye stalk to apical margin of head and posteriorly back to ocellus (Fig. 7).......................................................3 |
3. Body elongate, about three times longer than wide; pronotum subquadrangular, wider at the level of and slightly constricted behind the calli......................................................................................................................Stenogeocoris Montandon |
– Body short and stout, never more than twice as long as wide. Pronotum subquadrangular............................................................................Geocoris Fallén |
Key to the Neotropical tribes of Pamphantinae
1. Males and females with a distinct lunate stridulitrum on side of head below eyes and a plectron on inner face of fore femur; male abdomen with a distinct tubercle on each side of segment III; anterior and posterior pronotal lobes separated by a deep transverse impression......................................................................Cattarini |
– Males and females without a stridulitrum on head or a plectron on fore femur; male abdominal segment III without a tubercle, though sometimes slightly swollen; pronotal lobes usually not separated by a deep transverse impression.2 |
2. Eyes strongly stylate, extending laterally well beyond outer margin of head; pronotum usually with lobes and/or spines along lateral margins; includes only Epipolops (Fig. 9)...............................................................................Epipolopini |
– Eyes not stylate, never extending beyond outer margin of head; pronotum entire, without lateral lobes or spines............................................................Pamphantini |
Key to the Neotropical genera of Cattarini
1. Lateral margins of pronotal lobes unarmed......................................Cattarus Stål |
– Lateral margin of anterior pronotal lobe with a broad winglike process and each humeral angle of posterior lobe with a spinelike projection (Fig. 10).........................................................................Cephalocattarus Slater and Henry |
Key to the Neotropical genera of Pamphantini
– Anterior femur without a distinct subapical spine...............................................2 |
– Anterior femur with one or two distinct subapical spines....................................3 |
2. Anterior and posterior lobes of pronotum little separated, without a deep transverse impression; posterior lobe of pronotum almost flat, without swellings..........................................................................................................Parapamphantus Barber |
– Anterior and posterior lobe of pronotum separated by a deep transverse impression; posterior lobe with a distinct swelling or blunt tubercle on each side......................................................................Tropicoparapamphantus Brailovsky |
3. Head lacking ocelli..............................................................................................4 |
– Head with distinct ocelli between eyes................................................................5 |
4. Anterior pronotal lobe greatly swollen or globose; narrow posterior lobe with a long, slender, curving spine arising at each humeral angle................................................................................................................................Abpamphantus Barber |
– Anterior pronotal lobe not greatly swollen, two and half times as long and only slightly wider than posterior lobe; humeral angles unarmed.........................................................................................................................Cymapamphantus Henry |
5. Eyes large and substylate, inner margin of eye extending past anterior angle of pronotum; distance between ocelli less than to subequal to the distance from an ocellus to an eye; posterior half of hemelytra convex or rounded; profemur with two spines (apical spine broken on three specimens examined).......................................................................Neopamphantus Barber and Bruner |
– Eyes prominent, but not substylate, inner margin of eye not extending past anterior angle of pronotum; each ocellus closer to eye than to each other; hemelytra subparallel throughout; profemur with only one spine...............Pamphantus Stål |
9 Family Heterogastridae
9.1 General Characteristics and Diagnosis
This family is recognized by the ventral abdominal spiracles, the lack of trichobothria on the head, the weakly incrassate fore femora, the distinct closed cell at the base of the hemelytral membrane, and the deeply inserted ovipositor often extending to abdominal segment V (Scudder 1962a; Henry 1997a, 2009).
9.2 Classification and Diversity
The Heterogastridae are primarily an Old World group comprising about 24 genera and 100 species (Cassis and Gross 2002; Henry 2009), with only two native (Ashlock and Slater 1988) and one introduced (Wheeler and Hoebeke 2013) species of Heterogaster Schilling occurring in North America. Only H. behrensii (Uhler) gets into northern Mexico as the sole Neotropical member of the family. Scudder (1962a) provided a key to the genera of the world and Henry (1997a) elevated Heterogastrinae to family status.
10 Family Lygaeidae
10.1 General Characteristics and Diagnosis
The Lygaeidae are recognized by the impressed, often shiny, transverse line across the calli; the Y-shaped pattern on the scutellum; and the dorsal position of abdominal spiracles II through VII. Members of the subfamily Ischnorhynchinae are dull brown to reddish brown, elongate-oval, nearly glabrous bugs having the clavus punctate and the base of the pronotum entire and not depressed on either side of the scutellum. The Lygaeinae are usually moderate to large, often aposematically colored, orange and red bugs having an impunctate clavus, the base of the pronotum depressed on either side of the scutellum, and a distinct subcosta on the hemelytra. The Orsillinae are relatively small, dull, yellowish brown, often pubescent bugs having a depressed area at the base of the pronotum and lacking punctures on the clavus and a subcosta.
10.2 Classification and Diversity
The Lygaeidae (sensu stricto) comprise about 102 genera and 970 species worldwide (Slater and O’Donnell 1995; Henry 2009). The family is separated into three subfamilies, the Ischnorhynchinae, Lygaeinae, and Orsillinae (Henry 1997a). Sweet (2000a) suggested that the Ischnorhynchinae and Orsillinae should be given family status. He did not, however, provide information to support his hypothesis; thus, we maintain the family as documented by Henry (1997a). Brailovsky (1982a) and A. (“A” used to distinguish Alex Slater from James A. Slater – i.e., Slater without initials throughout text) Slater (1992) provided keys to the Neotropical genera; Baranowski and Slater (2005) monographed the Lygaeoidea of the West Indies and gave keys to all of the genera and species. Henry (1997a) accorded the Lygaeidae (sensu stricto), including the Ischnorhynchinae, Lygaeinae, and Orsillinae, family status.
The Ischnorhynchinae comprise about 15 genera and 77 species worldwide, with only four genera and nine species known from the Neotropics (Slater 1964a; Slater and O’Donnell 1995; Baranowski and Slater 2005). The primarily Holarctic genus Kleidocerys Stephens contains four Neotropical species. Neokleidocerys Scudder, with three Neotropical species and previously treated as a subgenus of Kleidocerys, was elevated to generic rank by Slater and Brailovsky (1989). Polychisme Kirkaldy, with only one species, previously was placed in the family Artheneidae (Slater and Brailovsky 1986) but was returned to Ischnorhynchinae by Kerzhner (1997). Syzygitis Bergroth, previously considered a synonym of Polychisme (Slater 1967), was reinstated by Slater and Brailovsky (1986). Scudder (1962a) revised the subfamily and provided a key to the world genera.
Lygaeinae is largest of the three subfamilies, with about 57 genera and 500 species worldwide. Twenty-two genera and about 175 species are known in the Neotropics (A. Slater 1992; A. Slater and Baranowski 2001). Brailovsky (1982a) and Baranowski and Slater (2005) provided keys to all or a substantial number of the Neotropical genera, and A. Slater (1992) revised the subfamily and provided keys to all New World genera and a checklist of species. Slater and O’Donnell (1995) summarized the many new combinations resulting from A. Slater’s (1992) work. A. Slater and Baranowski (2001) described the most recent Neotropical genus, Melanopleuroides, from the Dominican Republic. Other important works include Brailovsky’s revisions of Lygaeus F. (1978), Craspeduchus Stål (1979), Acroleucus Stål (1980), Ochrimnus Stål (1982b), and Torvochrimnus Stål (1983), among other important papers. Many lygaeines, such as the genera Lygaeus F. and Oncopeltus Stål, are aposematically colored red and orange. The vast literature base involving mostly laboratory studies of Oncopeltus fasciatus (Dallas), commonly called the large milkweed bug, was compiled by Slater and O’Donnell (1995).
The Orsillinae comprise about 30 genera and 250 species, separated into four tribes (Ashlock 1967a; Henry 2009). In the Neotropics, three tribes, nine genera, and about 46 species are known. Ashlock and Lattin (1963) established Xyonysius for ten species previously placed mostly in Nysius Dallas, Ashlock (1967a) revised and provided a key to the orsilline genera of the world, and Hamilton (1983) established Neortholomus for the New World species of Ortholomus Stål. Barber (1947) revised the North America species of Nysius, but this work is badly outdated and the Neotropical species remain confused and difficult to identify.
We provide keys to the subfamilies and keys to the genera of Ischnorhynchinae modified from Scudder (1962b), Lygaeinae modified from A. Slater (1992) and Baranowski and Slater (2005), and the Orsillinae modified from Ashlock (1967a).
Key to the Neotropical subfamilies of Lygaeidae
1. Clavus punctate; base of pronotum entire, not depressed..........Ischnorhynchinae |
– Clavus impunctate; base of pronotum bordering either side of scutellum depressed or flattened...........................................................................................................2 |
2. Hemelytra with a distinct subcosta and membrane lacking intervannal veins; coloration often in large part red or orange; most species nearly glabrous................................................................................................................Lygaeinae |
– Hemelytra lacking a subcosta and membrane with intervannal veins; coloration dull gray to yellowish brown; species often pubescent..........................Orsillinae |
Key to the Neotropical genera of Ischnorhynchinae
1. Lateral margin of pronotum lacking a distinct carina; corium with a short row of punctures along claval suture near inner angle and a complete row extending the entire length; middle of hemelytra largely hyaline..............................................2 |
– Lateral margin of pronotum distinctly carinate; corium with only a single row or two or more entire rows of punctures; middle of corium more opaque...............3 |
2. Anterior half of pronotum narrowed and elongate; corium with apical margin and apex with a rectangular or L-shaped macula...........................................................................................................Neokleidocerys Slater and Brailovsky |
– Anterior half of pronotum not narrowed and elongate; corium at most with two small maculae apically (Fig. 11)..........................................Kleidocerys Stephens |
3. All abdominal spiracles dorsal; basal two-thirds of lateral margin of hemelytra broadly explanate, broader at middle than diameter of antennal segment I..................................................................................................Syzygitis Bergroth (Chile) |
– Spiracles on abdominal segments III to VII ventral; basal two-thirds of lateral margin of hemelytra only narrowly explanate, subequal at middle to diameter of antennal segment II (Fig. 12)................................................Polychisme Kirkaldy |
Key to the Neotropical genera of Lygaeinae
1. Eyes on stalks................................................................................Nicuesa Distant |
– Eyes not on stalks.................................................................................................2 |
2. Callus depressed on either side of median carina; hemelytral membrane dark with apical margin or rounded macula clear or hyaline........................Acroleucus Stål |
– Callus not depressed or interrupted medially; hemelytral membrane not dark with a hyaline apical area.............................................................................................3 |
3. Pronotum with four transverse depressions behind the calli.........Ochrimnus Stål |
– Pronotum without four transverse depressions behind the calli..........................4 |
4. Scutellum swollen, raised above hemelytra; basal margin of pronotum on either side of scutellum flattened and produced posteriorly (Fig. 14)....Oncopeltus Stål |
– Scutellum not swollen; basal margin of pronotum not produced posteriorly......5 |
5. Posterior pronotal lobe, at most, as high mesally as lateral margins; disc flat or nearly flat; median carina on basal third distinct.................................................6 |
– Posterior pronotal lobe higher mesally than at lateral margins; disc distinctly convex; median carina absent..............................................................................9 |
6. Clavus pale, with a dark vein on inner and outer margin....................................................................................................................................Neacoryphus Scudder |
– Clavus unicolorous...............................................................................................7 |
7. Dorsal pubescence short, usually semierect, and dense; membrane uniformly dark............................................................................Dalmochrimnus Brailovsky |
– Dorsal pubescence short, recumbent, and sparse; membrane usually marked with white.....................................................................................................................8 |
8. Larger species, length 6 mm or more; membrane dark with narrow lateral margin white or entirely white, with dark veins; brachypters uncommon...................................................................................................................Melacoryphus A. Slater |
– Smaller species, length usually less than 6 mm; membrane usually with a large, central white spot, if largely white with dark veins, then basal third dark; brachypters common.............................................................Lygaeospilus Barber |
9. Pronotum coarsely punctate, punctures extending onto disc...............................................................................Oxygranulobaphus Brailovsky |
– Pronotum finely punctate, punctures restricted to depressed areas just before and after calli............................................................................................................10 |
10. Pronotum dark, at most with anterior angles paler..........................................11 |
– Pronotum extensively pale, at least with more than anterior angles pale........12 |
11. Head with pale spots; posterior metapleural margin nearly straight (Fig. 13)...............................................................................................Melanopleurus Stål |
– Head without pale spots; posterior metapleural margin distinctly concave (Dominican Republic)..........................Melanopleuroides Slater and Brailovsky |
12. Claval veins either paler or darker than surrounding area...............................13 |
– Claval veins concolorous with surrounding area.............................................14 |
13. Lateral margin of the pronotum dark brown; veins on corium and membrane darker than surrounding areas (Fig. 15).....................Torvochrimnus Brailovsky |
– Lateral margin of pronotum pale; veins on corium paler than surrounding area; veins on membrane concolorous with surrounding area...............................................................................................................................Hadrosomus A. Slater |
14. Claval margin bordering scutellum pale, contrasting with dark margin bordering corium or a semicircular, submedian spot.......................................................15 |
– Clavus unicolorous or pale basally and dark apically or with only the commissural margin pale.......................................................................................................16 |
15. Apical margin of corium red or orange with a narrow outer black border......................................................................................Anochrostomus A. Slater |
– Apical margin of the corium yellow................................................................17 |
16. Postcallar impression interrupted on either side of median line forming a short accessory branch....................................................................Craspeduchus Stål |
– Postcallar impression entire, not interrupted on either side of median line..................................................................................................Ochrostomus Stål |
17. Pronotum longer medially than wide at anterior margin; hemelytral membrane brown with a pale median line........................................ Ektyphonotus A. Slater |
– Pronotum distinctly shorter medially than wide anteriorly; hemelytral membrane not brown with a pale median line...................................................................18 |
18. Costal margin of corium pale apically and basally, dark brown to black between; dark markings on corium either restricted to the costal margin and a small discal spot or a triangular spot with the longest side at costal margin; thoracic pleura almost entirely gray or black...................................................Lygaeus Fabricius |
– Costal margin of corium usually entirely pale, if interrupted, then dark markings of corium forming a subrectangular spot with anterior and posterior edges straight; thoracic pleura with wide pale margins.............................................19 |
19. Length more than 10 mm; pronotum pale orange with a small, transverse, brown spot on either side of basal margin and a dark quadrate spot on each callus....................................................................................Biblochrimnus Brailovsky |
– Length 7 mm or less; pronotum with pale orange to red, but with at least a large dark quadrate spot on the posterior lobe..........................................................20 |
20. Pronotum uniformly dark brown, with only median line pale.......................................................................................... Achlyosomus A. Slater |
– Anterior pronotal lobe orange to red; posterior lobe dark, with median line pale...........................................................................................................................21 |
21. Head slightly swollen; ocelli lower than vertex when viewed laterally............................................................................ Pseudacroleucoides Brailovsky |
– Head slightly less swollen; ocelli about level with vertex when viewed laterally......................................................................................Latochrimnus Brailovsky |
Key to the Neotropical genera of Orsillinae
1. Costal margin of hemelytron straight to at least level with apex of clavus; fore femur sometimes spined; connexivum often exposed (Orsillini)........................2 |
– Costal margin of hemelytron straight only to level with apex of scutellum; fore femur never spined; connexivum not exposed.....................................................4 |
2. Mesopleuron and propleuron evenly meeting, not overlapping (widespread) (Fig. 16)............................................................................Neortholomus Hamilton |
– Mesopleuron appearing to overlap the propleuron..............................................3 |
3. Vertex with a carina extending from each ocellus to antenniferous tubercle; head short, anteocular length less than two times length of an eye; labium extending to hind coxae (Brazil).....................................................................Aborsillus Barber |
– Vertex lacking carinae; head elongate, anteocular length more than two times length of an eye; labium long, extending to apex of abdomen (North America and Mexico)...................................................................................Belonochilus Uhler |
4. Hemelytron without a complete row of punctures on either side of claval suture; buccula impunctate (Nysiini) (widespread) (Fig. 17)......................Nysius Dallas |
– Hemelytron with a distinct row of punctures on either side of claval suture; buccula usually punctate (Metrargini).................................................................5 |
5. Buccula not extending beyond level with middle of eye (widespread)................................................................................Xyonysius Ashlock and Lattin |
– Buccula nearly reaching base of head..................................................................6 |
6. Antenniferous tubercle acute or quadrate; explanate costal margin of hemelytra with alternating light and dark spots....................................................................7 |
– Antenniferous tubercle not produced; costal margin of hemelytra without alternating light and dark spots............................................................................8 |
7. Scutellum longer than prothorax; veins of corium raised; hemelytral membrane reduced (Juan Fernandez Islands)................................. Robinsonocoris Kormilev |
– Scutellum shorter than prothorax; veins of corium not raised; hemelytral membrane fully developed (Galapagos Islands).................. Darwinysius Ashlock |
8. Apex of scutellum rounded; macropterous (brachypters unknown) (Argentina)................................................................................................Balionysius Ashlock |
– Apex of scutellum acute; hemelytra coleopteriform; hemelytral membrane greatly abbreviated (Colombia).........................................................Coleonysius Ashlock |
11 Family Ninidae
11.1 General Characteristics and Diagnosis
Most Ninidae range from about 3.0 to 4.0 mm long and are recognized by the broad head, the stylate eyes, the broad vertex, the transverse sulcus before the ocelli, the mostly hyaline and often basally constricted hemelytra, the bifid apex of the scutellum, and the dorsal position of abdominal spiracles II–IV (Scudder 1957; Henry 1997a). The head and pronotum of several taxa are coated with a powdery white residue.
11.2 Classification and Diversity
Ninids are a small family comprising five genera and 16 species worldwide (Slater 1964a; Slater and O’Donnell 1995). Scudder (1957) revised the Ninini as a tribe of the Cyminae and provided a key to genera and species. Only three genera and six species of Ninidae occur in the Neotropics. Cymoninus notabilis (Distant) is the most widespread New World species, ranging from the southern United States, throughout the West Indies, and into much of South America (Scudder 1957), and C. wilcoxae Brailovsky is known only from Mexico (Brailovsky 1975). Neoninus illustris Distant occurs from Mexico to Brazil, N. argentinus Kormilev is known only from Argentina, and N. montanellus Brailovsky occurs in Brazil and Venezuela (Brailovsky 1989a). The monotypic genus Paraninus Scudder is represented by P. gracilis Scudder, described from Brazil and British Guiana. This small family previously was included as a tribe of the Cyminae prior to its elevation to family status (Henry 1997a). The following key is modified from Scudder (1957).
Key to the Neotropical genera of Ninidae
1. Apical half of first labial segment slender................................Paraninus Scudder |
– Apical half of first labial segment swollen...........................................................2 |
2. Antennal segments II and III more slender than segment IV; distal half of clavus clear or transparent (Fig. 18)..................................................Cymoninus Breddin |
– Antennal segments II and III as thick as segment IV; distal half of clavus pruinose and opaque..................................................................................Neoninus Distant |
12 Family Oxycarenidae
12.1 General Characteristics and Diagnosis
Oxycarenids are characterized by the punctate porrect head; hyaline often explanate hemelytra; abdominal spiracles III to VII ventral and II dorsal; lack of lateral trichobothria on sterna III, IV, and V; a median trichobothrium on sternum V; abdominal sutures complete to lateral margins (segments III, IV, and V often fused); a truncate female abdomen; and a transverse comb of glandular setae on the male abdomen (Henry 1997a, 2009; Henry and Dellapé 2009). Previously treated as a subfamily of the Lygaeidae (sensu lato), the group was accorded the group family status by Henry (1997a).
12.2 Classification and Diversity
The Oxycarenidae include about 24 genera and 150 species, most of which occur in the Old World (Péricart 2001; Henry 2009; Henry and Dellapé 2009; Brailovsky and Cervantes 2011). Five genera and 22 species are found in the Neotropics (Slater 1964a; Dellapé and Cheli 2007; Henry and Dellapé 2009; Brailovsky and Cervantes 2011). The largest genus Crophius Stål, comprising 19 species, including eight from the Neotropics (Slater 1964a; Dellapé and Cheli 2007), was synonymized by Hoberlandt (1987) under the Palearctic Anomaloptera Amyot and Serville, an action followed by a number of subsequent authors (e.g., Slater and O’Donnell 1995; Péricart 1998, 2001; Dellapé and Cheli 2007). Henry et al. (2015), however, have shown that Crophius is distinct from Anomaloptera, as is Mayana Distant (containing the two species M. costatus Distant and M. diruptus Distant), also previously placed in synonymy with Crophius (Van Duzee 1910). The genus Dycoderus Uhler (1901) is represented by the species D. picturatus (Uhler), known only from the western United States; Neaplax Slater (1974) contains two species, N. mexicanus Slater and N. baja Brailovsky and Cervantes (2011), both known only from Mexico; and Notocoderus Henry and Dellapé (2009) was described to accommodate N. argentinus Henry and Dellapé from Argentina. The large Palearctic genus Oxycarenus Costa is represented in the New World by the introduced O. hyalinipennis (Costa) (Fig. 20), a pest of cotton first detected in Brazil as early as 1917, now known to occur throughout much of South America and the West Indies (Slater and Baranowski 1994). More recently, it was detected in the Florida Keys on naturalized wild cotton, Gossypium sp. (Nagoshi et al. 2012), but apparently it has been eradicated (NAPPO 2014).
The following key is modified from Henry et al. (2015).
Key to the Neotropical Genera of Oxycarenidae
1. Ocelli absent......................................................................................................... 2 |
– Ocelli present ....................................................................................................... 3 |
2. Head strongly globose in front of eyes; pronotum trapeziform, not separated into two lobes, but with a wide collar-like area; costal margin of hemelytra explanate .........................................................................................................Neaplax Slater |
– Head not strongly globose; pronotum lacking a collar and separated into two distinct lobes, with anterior lobe two or more times longer than posterior lobe; costal margin of hemelytra not explanate.............Notocoderus Henry and Dellapé |
3. Fore femora with only one spine; labium usually extending only to middle coxae, if longer, never onto abdomen.......................................................... Crophius Stål |
– Fore femora with two or more spines; labium extending to or beyond middle coxae..........................................................4 |
4. Fore femora with two spines; labium extending only to middle coxae or slightly beyond; hemelytral membrane with numerous closed cells .................................................................................................................... Mayana Distant |
– Fore femora with four spines; labium extending beyond hind coxae, well onto abdomen; hemelytral membrane without closed cells (Fig. 19)...................................................................................................... Oxycarenus Costa |
13 Family Pachygronthidae
13.1 General Characteristics and Diagnosis
Members of this family are separated into two subfamilies, the Pachygronthinae and the Teracriinae (Slater 1955). Pachygronthines are distinguished by the elongate bodies, strongly incrassate spined fore femora, and the frequently unusually long first antennal segment. Teracriines are more stout bodied, have shorter antennae, and are held together as a subfamily by the ventral position of the abdominal spiracles (Henry 1997a).
13.2 Classification and Diversity
The Pachygronthidae comprise about 13 genera and 78 species worldwide (Cassis and Gross 2002; Henry 2009). Only the two pachygronthine genera Oedancala Amyot and Serville, with 14 species, and Pachygrontha Germar, with six species, are known from the Neotropics (Slater 1955, 1964a; Slater and O’Donnell 1995). The teracriine genus Phlegyas Stål contains only three species, two of which occur in the Neotropical Region. Phlegyas annulicrus Stål is known from the western United States and Mexico and P. patruelis Berg has been reported from Argentina, Paraguay, and Uruguay. All members of the family feed on monocots of the families Cyperaceae (sedges), Juncaceae (rushes), and Poaceae (grasses) (Slater 1955). Slater (1955) revised the family for the world and gave keys to genera and species, and Henry (1997a) gave the group family status. The following key is modified from Slater (1955) and Dellapé (2014).
Key to the Neotropical subfamilies and genera of Pachygronthinae
1. Antennal segment I not or barely attaining apex of clypeus, always much shorter than antennal segments II–IV (Teracriinae) (Fig. 22)...................... Phlegyas Stål |
– Antennal segment I greatly exceeding apex of clypeus, usually as long or longer than antennal segments II–IV (Pachygronthinae) ...................... 2 |
2. Eye as wide as or wider than long; distance between base of the antenna and anterior margin of eye as long as or longer than length of eye; antennal segment I clavate; antennal segment IV shorter than either segment II or III (Fig. 21)............................................................................................. Pachygrontha Germar |
– Eye longer than wide; distance between base of antenna and anterior margin of eye less than length of eye; antennal segment I gradually swollen to apex; antennal segment IV longer than either segment II or III............................................................................................. Oedancala Amyot and Serville |
14 Family Piesmatidae
14.1 General Characteristics and Diagnosis
The Piesmatidae, often called ash-gray plant bugs, are recognized by the small size (5 mm or less); the dull yellowish-gray coloration; the broad head; the produced juga often surpassing the apex of the clypeus; the widely placed ocelli (often reduced or absent in brachypters); the uni-, bi-, or tricarinate pronotum; the reticulate or areolate hemelytra; the dorsal spiracle on abdominal segment II; the absence of trichobothria on abdominal segments IV and V; and the two-segmented tarsi.
14.2 Classification
The Piesmatidae superficially resemble certain Tingidae because of the reticulate hemelytra and the two-segmented tarsi. Drake and Davis (1958), however, showed that they belonged in the infraorder Pentatomomorpha based on the presence of abdominal trichobothria, a true spermatheca, and a differentiated endophallus. Their superfamily placement has fluctuated from being included in their own superfamily, the Piesmatoidea, to their current position in the Lygaeoidea (Henry 1997a). Worldwide nine genera and about 45 species are separated into two subfamilies, the widespread Piesmatinae and the Afrotropical Psamminae (Henry 1997a, 2009; Dellapé 2014).
Drake and Davis (1958) treated the world genera and Heiss and Péricart (1983) revised the Palearctic fauna. Péricart (1974) established two new subgenera, Afropiesma and Parapiesma, for the widely distributed genus Piesma Le Peletier and Serville, and Heiss and Péricart (1997) later gave these subgenera generic status. As a consequence, most of the New World species previously placed in Piesma now belong in Parapiesma Péricart, including the widespread P. cinereum (Say). Two genera and two species occur in the Neotropics. Miespa reedi (Drake) is known only from Chile, and Parapiesma cinereum (Say) occurs throughout much of the New World.
Key to the genera and species of Neotropical Piesmatidae
1. Spiracles VI and VII subventral laterally; pronotum tricarinate (Fig. 23)................................................................................................Parapiesma Péricart |
– Spiracle VI dorsal, only VII subventral laterally; pronotum with only a single median carina...................................................................................Miespa Drake |
15 Family Rhyparochromidae
15.1 General Characteristics and Diagnosis
The Rhyparochromidae are commonly called seed bugs because most feed on mature seeds (Sweet 1964a, b). They are recognized by the presence of head trichobothria; a fused suture between sterna IV and V, usually curving forward anterolaterally from the midline of the sternum and not reaching the dorsal margin of the abdomen (except in Plinthisinae); and the fore femora usually incrassate and armed below with spines (Schuh and Slater 1995; Henry 1997a; Dellapé 2014). Most are dull brown to black, often with contrasting pale or white marks or mottled patterns on the hemelytra. They range from very small (e.g., Antillocorini, Lilliputocorini) to large (e.g., some Lethaeini and Myodochini).
Rhyparochromids have a broad range of feeding habits. Many species live on the ground, feeding on fallen seeds. Other species climb vegetation when mature seeds are available, some live on weedy vegetation (Slater 1977; Slater and Baranowski 1990), and others are arboreal and live in forests canopies (Slater et al. 2009; Dellapé and Henry 2010). The Cleradini, which feed on vertebrate blood, are the exception to the seed-feeding habit within the family.
Numerous species are myrmecomorphic, and although many are not extremely similar to ants in their morphology, adults and especially nymphs of some genera (such as the myodochines Neopamera Harrington, Pseudopachybrachius Malipatil, and Heraeus Stål) mimic ants in their movements (Slater and Baranowski 1990).
15.2 Classification and Diversity
The Rhyparochromidae are the most diverse group of the Lygaeoidea, comprising more than 2,000 species in 14 tribes or about a half the world’s fauna. According to Henry’s (1997a) phylogenetic classification, the family, defined by the presence of head trichobothria, contains the subfamilies Plinthisinae and Rhyparochrominae. The Plinthisinae, with only the nominate tribe, have complete abdominal sutures reaching the dorsal margins of the abdomen, whereas most other rhyparochromines have abdominal sutures IV–V abbreviated and not reaching the dorsal margin of abdomen. The apparent presence of a complete abdominal sternal suture on segments IV–V in at least 11 tribes of Rhyparochrominae suggests multiple independent origins of this character as discussed by Slater et al. (2009). We note (personal observation), however, that in most of these exceptions segments IV–V are modified in some way, indicating that the previous oversimplified presence or absence interpretation of the abbreviated abdominal sutures needs to be redefined and expanded.
Key to the subfamilies of Rhyparochromidae
1. Males with a stridulatory mechanism on abdominal segment I and hind wing; females with a conjunctival membrane between abdominal sterna 4/5; pronotum wider across anterior one-third than across humeral angles.............................................................................................................Plinthisinae |
– Males without abdominal and hind-wing stridulatory mechanisms; both sexes usually with abdominal sterna 4/5 fused; pronotum variable, usually wider across humeral angles than across anterior lobe.................................Rhyparochrominae |
15.3 Subfamily Plinthisinae
The Plinthisinae possess head trichobothria, similar feeding habits, and an overall general appearance but lack the fused suture between the abdominal terga 4/5 found in most rhyparochromids.
Plinthisines usually have the pronotum expanded across the anterior lobe; the fore femora are heavily incrassate and spined; the wings are often greatly reduced or staphylinoid; males have a stridulatory mechanism on abdominal segment I and the hind wing; and females have a conjunctival membrane between sterna 4/5 (Slater and Woodward 1982; Schuh and Slater 1995; Slater and Brailovsky 2000). Putshkov (1958) argued that plinthisines merit subfamily status. According to Slater and Woodward (1982), the Plinthisini represent the basal group of the family, and Henry (1997a) corroborated the monophyly of the group based on the head trichobothria and maintained its subfamily status.
The subfamily includes only two genera, the monotypic Bosbequius Distant from Myanmar and the large genus Plinthisus Stephens (Fig. 24), with most of the species distributed in the Palearctic, a few species in North America, and only three Neotropical species described by Slater (1971).
Figs. 13–24. 13, Melanopleurus bistriangularis (Say) (Lygaeidae: Lygaeinae). 14, Oncopeltus sexmaculatus Stål (Lygaeidae: Lygaeinae). 15, Torvochrimnus poeyi Guérin-Méneville (Lygaeidae: Lygaeinae). 16, Neortholomus jamaicensis (Dallas) (Lygaeidae: Orsillinae). 17, Nysius irroratus (Spinola) (Lygaeidae: Orsillinae). 18, Cymoninus notabilis (Distant) (Ninidae). 19, Crophius convexus Barber (Oxycarenidae). 20, Oxycarenus hyalinipennis (Costa) (Oxycarenidae). 21, Pachygrontha compacta Distant (Pachygronthidae: Pachygronthinae). 22, Phlegyas annulicrus Stål (Pachygronthidae: Teracriinae). 23, Parapiesma cinereum (Say) (Piesmatidae). 24, Plinthisus parvioculatus Slater (Rhyparochromidae: Plinthisinae) (Photos by G Ouellette)
15.4 Subfamily Rhyparochrominae
Key to the tribes of Rhyparochrominae
1. Abdominal spiracles II, III, and IV dorsal...........................................................2 |
– All abdominal spiracles ventral............................................................................3 |
2. Inner laterotergites absent; lateral pronotal margins almost always rounded.....................................................................................................................Myodochini |
– Inner laterotergites present; lateral pronotal margins variable from rounded to carinate.................................................................................................Udeocorini |
3. Posterior pair of trichobothria on abdominal sternum V positioned one above the other.....................................................................................................................4 |
– Posterior pair of trichobothria on abdominal sternum V positioned one in front of the other in a linear series on segments IV and V................................................7 |
4. Ocelli lateral and behind eyes; suture between abdominal sterna IV and V attaining a lateral connexival margin; abdominal tergum III usually not sclerotized; labial segment II usually not attaining the base of the head...................Cleradini |
– Ocelli between and slightly posterior to eyes; suture between abdominal sterna IV and V usually not attaining lateral connexival margin and usually markedly curving anteriorly from venter dorsally; labium variable, but usually with segment II reaching or exceeding base of head..................................................................5 |
5. Medium sized, length usually more than 5 mm; apical corial margin straight..............................................................................................................Ozophorini |
– Very small to minute, length usually less than 3.5 mm; apical corial margin usually concave....................................................................................................6 |
6. Inner laterotergites absent; metathoracic scent-gland auricle strongly curved anteriorly; tarsi two segmented.......................................................Lilliputocorini |
– Inner laterotergites present; metathoracic scent-gland auricle straight or curved posteriorly; tarsi three segmented........................................................................7 |
7. Apical corial margin deeply concave; inner laterotergites present; head lacking iridescent areas; abdominal scent-gland scars between terga III/IV, IV/V, and V/VI.......................................................................................................Antillocorini |
– Apical corial margin straight; inner laterotergites absent; head with iridescent areas basally; abdominal scent-gland scars between terga V/VI minute or absent.................................................................................................................Lethaeini |
15.4.1 Tribe Antillocorini
The Antillocorini are a cosmopolitan group, with about 100 species occurring in tropical and subtropical regions of the world. Seventeen of the 34 currently recognized genera and 34 species occur in the Neotropics (Slater 1964a; Slater and O’Donnell 1995; Brambila 2000).
The Antillocorini are characterized by the bucculae joined by a carina well behind the labium; a deeply concave apical corial margin; the ventral abdominal spiracles; the linear trichobothria on abdominal sternum V; the presence of inner laterotergites; and immatures with a double or “troughed” suture between terga 3/4 and 4/5 and with a field of spines or tubercles laterally, with three pairs of scent glands between abdominal terga 3/4, 4/5, and 5/6 (Ashlock 1964; Sweet 1977; Slater and Woodward 1982; Schuh and Slater 1995; Slater and Brailovsky 2000).
Slater (1980) described several new taxa, discussed the phylogenetic relationships, and provided a key to the genera of the Western Hemisphere. Some Neotropical species lack a deeply concave apical corial margin and the linear arrangement of abdominal trichobothria. Schuh and Slater (1995) stated that the group may not be monophyletic.
The following key is modified from Slater’s (1980) key.
Key to the Neotropical genera of Antillocorini
1. Apical corial margin straight or, at most, very slightly sinuate near inner angle..............................................................................................................................2 |
– Apical corial margin with inner third deeply and conspicuously concave..............................................................................................................................5 |
2. Suture between abdominal sterna 4/5 straight, reaching dorsal margin....................................................................................................Caeneusia Strand |
– Suture between abdominal sterna 4/5 curving anteriorly, not reaching dorsal margin..................................................................................................................3 |
3. Lateral pronotal margins rounded; metathoracic scent-gland auricle weakly curved posteriorly........................................................................Paradema Slater |
– Lateral pronotal margins bluntly calloused or subcarinate; metathoracic scent-gland auricle curved posteriorly...........................................................................4 |
4. Body elongate and slender, more than 3 mm long, macropterous ................................................................................................. Schuhocoris Slater |
– Body small to minute, less than 2 mm long, robust, coleopteroid ......................................................................................Branstettocoris Brailovsky |
5. Lateral pronotal margins rounded or faintly calloused........................................6 |
– Lateral pronotal margins calloused or carinate....................................................7 |
6. Body above and below polished and shining...............................Acolhua Distant |
– Head and abdominal sternum shining, constrasting with dull pruinose or subpruinose surface on remainder of body ............................... Bathydema Uhler |
7. Trichobothria on abdominal sternum V linear, the posterior trichobothrium directly below or well behind the spiracle...........................................................8 |
– Trichobothria on abdominal sternum V not linear; if linear then the posterior trichobothrium anterior to the spiracle.................................................................9 |
8. Fore femur mutic; posterior trichobothrium on abdominal sternum V well behind spiracle...........................................................Arimacoris Baranowsky and Slater |
– Fore femur with two rows of spines, larger in males; posterior trichobothrium on abdominal sternum V directly below or slightly caudad of spiracle (Fig. 26)........................................................................................................Valeris Brambila |
9. Trichobothria on abdominal sternum V linear, anterior to spiracle...................10 |
– Trichobothria on abdominal sternum V not linear, somewhat dorsoventral relative to one another, usually below or behind spiracle...............................................11 |
10. Prosternum with a deep median groove.....................................Cligenes Distant |
– Prosternum lacking a deep median groove..............................Botocudo Kirkaldy |
11. Dorsal surface almost or entirely shiny and polished......................................12 |
– Dorsal surface either completely pruinose or with head shiny, contrasting with the pruinose pronotum.............................................................................................14 |
12. Dorsal surface completely shiny, not pruinose................................................13 |
– Dorsal surface almost entirely shiny and polished, pruinose only narrowly across the base of head between ocelli.................... Germacoris Baranowsky and Slater |
13. Fore femora with 8–9 sharp spines; head and anterior pronotal lobe smooth and impunctate............................................................................... Terenocoris Slater |
– Fore femora with only 2–3 spines; head and pronotum with numerous, irregular, coarse punctures................................................................... Trachinocoris Slater |
14. Lateral pronotal margins sharply and acutely carinate............ Paurocoris Slater |
– Lateral pronotal margins with a blunt calloused ridge, but lacking a sharp, acute edge..................................................................................................................15 |
15. Dorsal surface with strongly variegated coloration; metathoracic scent-gland auricle broad, lobate, not strongly curved caudad; evaporative area with grooves................................................................................................ Antillodema Slater |
– Dorsal surface dull yellow, brown or chestnut, without bright variegated contrasting coloration; metathoracic scent-gland auricle slender and strongly curved posteriorly; evaporative area lacking conspicuous grooves................. 16 |
16. Outer margin of metathoracic scent-gland evaporative area elevated into a raised subcarinate ridge, only slightly removed from outer lobe of scent-gland auricle; head pruinose, similar in texture to that of pronotum; spiracle of abdominal segment IV below lateral shelf; paramere with an elongate, acutely pointed inner projection........................................................................ Scythinus Distant |
– Outer margin of metathoracic scent-gland evaporative area not strongly elevated into a ridge, well removed from outer lobe of scent-gland auricle; head shiny, strongly contrasting with texture of pronotum; spiracle on abdominal segment IV on lateral shelf; paramere lacking a well-developed inner projection........................................................................................................ Antillocoris Kirkaldy |
15.4.2 Tribe Cleradini
The Cleradini, with 20 genera and 54 species, are confined to the Old World tropics, except for Clerada apicicornis Signoret (Fig. 27) introduced into the Western Hemisphere (Schuh and Slater 1995). This species has been recorded from Cuba, the Dominican Republic, Grenada, Haiti, Jamaica, Puerto Rico, St. Vincent, St. Thomas, and the Virgin Islands in the West Indies and Brazil, Colombia, and Venezuela from South America (Slater 1964a; Morales et al. 1969; Torres et al. 2000; Baranowski and Slater 2005).
Figs. 25–36. 25, Bathydema jamaicensis Slater and Baranowski (Rhyparochromidae: Rhyparochriminae: Antillocorini). 26, Valeris subcavicola (Scudder) (Rhyparochromidae: Rhyparochriminae: Antillocorini). 27, Clerada apicicornis Signoret (Rhyparochromidae: Rhyparochriminae: Cleradini). 28, Cistalia signoretii (Guérin-Méneville) (Rhyparochromidae: Rhyparochriminae: Lethaeini). 29, Paragonatas divergens (Distant) (Rhyparochromidae: Rhyparochriminae: Lethaeini). 30, Distingphyses insignis Distant (Rhyparochromidae: Rhyparochriminae: Myodochini). 31, Myodocha froeschneri A. Slater (Rhyparochromidae: Rhyparochriminae: Myodochini). 32, Prytanes confusus (Barber) (Rhyparochromidae: Rhyparochriminae: Myodochini). 33, Ptochiomera nodosa Say (Rhyparochromidae: Rhyparochriminae: Myodochini). 34, Xenydrium formiciforme Bergroth (Rhyparochromidae: Rhyparochriminae: Myodochini). 35, Ozophora costaricensis Slater and O’Donnell (Rhyparochromidae: Rhyparochriminae: Ozophorini). 36, Tempyra biguttula Stål (Rhyparochromidae: Rhyparochriminae: Udeocorini) (Photos by G Ouellette)
Members of the Cleradini have laterally placed ocelli; a short antennal segment III and a short labium not exceeding the base of the head; an unarmed, slender fore femur; the abdomen with all spiracles ventral and without inner laterotergites; an expanded connexival membrane; a secondary longitudinal abdominal suture; complete abdominal sutures between sternal suture IV–V; bifurcate parameres; and eggs with a pseudoperculum (Slater and Woodward 1982; Schuh and Slater 1995; Slater and Brailovsky 2000; Slater et al. 2009).
15.4.3 Tribe Lethaeini
The tribe Lethaeini, with 38 genera and more than 160 species worldwide (Li et al. 2011), has its greatest diversity in the tropical and subtropical parts of the Afrotropical, Oriental, and Australian regions (Slater 1986a). It is one of the most diverse tribes in the Neotropics, with 13 genera and 36 species known.
Lethaeines are recognized by the linear placement of the trichobothria on abdominal sternum V; a rounded buccular groove joined immediately behind the labium; a carinate juga; a trichobothrium at each anterior corner of the pronotum; the abdomen with all spiracles ventral and with or without inner laterotergites; the absence of a Y chromosome; the extreme modification of the sperm reservoir; the immatures with a double or “troughed” suture between terga 3/4, 4/5, and 5/6 and with a field of spines or tubercles laterally; the reduced scent gland between abdominal terga 5/6; and the presence of an iridescent area or areas on the head (Ashlock 1964; Slater and Woodward 1982; O’Donnell 1991).
The following key is modified from Baranowski and Slater’s key (2005) to the West Indian lethaeines.
Key to the Neotropical genera of Lethaeini
1. Pronotal trichobothria absent; eyes large, occupying more than half the length of the head; juga reduced; densely setose species.......................Lipostemmata Berg |
– Pronotum with a pair of trichobothria on anterolateral region; eyes small; juga well developed; less setose species......................................................................2 |
2. Dorsal surface of body strongly shiny.................................................................3 |
– Dorsal surface of body only partly shiny.............................................................5 |
3. Pronotum trapeziform, narrowing anteriorly; head with two iridescent patches; fore femur slightly incrassate...............................................................................4 |
– Pronotum subquadrangular; head with one iridescent patch; fore femur strongly incrassate and compressed.................................................................Rhaptus Stål |
4. Body with long setae dorsally; eyes with two long, forward-curving setae.............................................................................................Xestocoris Van Duzee |
– Body almost glabrous or with only a few long, scattered setae dorsally; eyes without two long, forward-curving setae...................................Bubaces Distant |
5. Head with two iridescent patches......................................................................6 |
– Head with one iridescent patch..........................................................................8 |
6. Inner laterotergites absent; body with short setae dorsally........................................................................................................................Stictolethaeus O’Donnell |
– Inner laterotergites present; body with long setae dorsally................................7 |
7. Dorsal surface weakly punctate; evaporative area extensive; macropterous or brachypterous species...............................................................Valtissius Barber |
– Dorsal surface strongly punctate; evaporative area reduced, restricted to areas surrounding auricle; coleopteroid species...........................................Esuris Stål |
8. Transverse pronotal impression distinct, dividing pronotum into anterior and posterior lobes; lateral pronotal margins broadly explanate and sinuate; collar triangular, well separated from the pronotum by a row a punctures........................................................................................Neopetissius O’Donnell |
– Transverse pronotal impression indistinct; lateral pronotal margins carinate and narrowly explanate; collar not differentiated.....................................................9 |
9. Evaporatory area extended anteriorly over the mesopleura, reaching or nearly reaching dorsal margin.....................................................................................10 |
– Evaporatory area not extended anteriorly, distant from dorsal margin of the mesopleura...........................Cryphula Stål |
10. Antennal segment I with a pale annulus (Fig. 28)...........................Cistalia Stål |
– Antennal segment I without a pale annulus.....................................................11 |
11. Larger, length over 6 mm...........................................................Petissius Distant |
– Smaller, length less than 5 mm........................................................................12 |
12. Lateral margin of pronotum explanate....................................Gonatoides Slater |
– Lateral margin of pronotum rounded (Fig. 29)...................Paragonatas Distant |
15.4.4 Tribe Lilliputocorini
The Lilliputocorini are circumtropical and comprise the nominotypical genus Lilliputocoris Slater and Woodward and ten species (Slater and O’Donnell 1995). The only record from the Neotropics is L. neotropicalis Slater and Woodward, described from a female from Pará, Brazil.
The Lilliputocorini are minute bugs, less than 2 mm long, recognized by the clavate antennal segments II and III; the reduced and modified ovipositor; the two-segmented tarsi; the uniquely formed metathoracic scent-gland auricle and adjacent evaporative area; the abdomen with all spiracles ventral and without inner laterotergites; the immatures with a double or “troughed” suture between terga 3/4, 4/5, and a field of spines or tubercles laterally; and the reduction or loss of the scent gland between abdominal terga 5/6 (Slater and Woodward 1982; Schuh and Slater 1995; Slater and Brailovsky 2000).
15.4.5 Tribe Myodochini
The tribe Myodochini is among the most diverse groups of Rhyparochromidae (Dellapé and Henry 2010), comprising 75 genera and 307 species worldwide, with 36 genera and 117 species occurring in the Neotropics.
The Myodochini usually possess a deeply incised transverse impression across the pronotum and a rounded anterior pronotal lobe; the abdomen has the spiracles on segments II, III, and IV dorsal and lacks inner laterotergites; and immatures have a Y-suture between abdominal terga 3/4 and three pairs of scent glands between abdominal terga 3/4, 4/5, and 5/6 (Slater and Woodward 1982; Schuh and Slater 1995; Slater and Brailovsky 2000).
Three Neotropical species now assigned to the genera Pseudocnemodus Barber, Ptochiomera Say, and Sisamnes Distant are misplaced. Pseudocnemodus martinezi Brailovsky from Paraguay probably should be included in Bergicoris Dellapé and probably is a synonym of B. multifarious Berg; the identity and position of the Chilean species in Ptochiomera are doubtful; and Sisamnes annulicollis (Berg) from Argentina probably belongs in Neopamera Harrington. Clarification of these taxa will be presented in a forthcoming paper by PMD.
Some myodochines, such as species in the genera Neopamera, Pseudopachybrachius, and Prytanes, are abundant and commonly collected in disturbed habitats and generally climb vegetation, whereas other taxa are strict geophiles, presumably in specialized habitats, and are poorly represented in collections. Most genera have fewer than ten species, and in the case of genera with more species (e.g., Paromius), only a few are Neotropical. The actual diversity of this group is much higher than present numbers indicate, and many new taxa remain to be described.
Harrington (1980) revised Myodochini and provided a generic key and a cladistic and biogeographic analysis. Nine Neotropical genera have been described since her work. Ashlockobius Slater and A. Slater (1999) was synonymized with Villalobosothignus Brailovsky 1984 by Dellapé and Montemayor (2011). The following key is modified from the Harrington’s (1980) key to the myodochine genera of the world.
Key to the Neotropical genera of Myodochini
1. Interocular distance less than postocular distance...............................................2 |
– Interocular distance equal to or exceeding postocular distance...........................4 |
2. Posterior margins of ocelli posterior to posterior margins of eyes; eyes round (Fig. 31)...................................................................................Myodocha Latreille |
– Posterior margins of ocelli anterior to posterior margins of eyes; eyes elongate-oval.......................................................................................................................3 |
3. Fore femur incrassate, with strong spines; ocelli sessile; antennal segment I not extending beyond clypeus (tylus).............................................Pephysena Distant |
– Fore femur weakly incrassate, slender, with only a few medium-sized and minute spines; ocelli protuberant; antennal segment I extending beyond clypeus.......................................................................................Dushinckanus Brailovsky |
4. Ocelli absent ...................................... Andercnemodus Brailovsky and Cervantes |
– Ocelli present ..................................................................................................... 5 |
5. Crescent-shaped, striated stridulitrum present ventrolaterally on anterior portion of abdomen...........................................................................................................6 |
– Striated stridulitrum lacking on anterior portion of abdomen...........................10 |
6. Head prolonged into a distinct neck; stridulitrum confined to abdominal sterna II and III; plectrum on hind femur consisting of two chisel-like projections..................................................................................................... Erlacda Signoret |
– Head not prolonged into a neck; stridulitrum extending onto sternum IV; plectrum on hind femur consisting of a line or scattered field of minute tubercles............7 |
7. Pronotum shiny; length of antennal segment I greater than interocular distance; pronotum shiny, not pruinose, and never deeply punctate; male fore tibia typically armed with a spine or spines..................................................Pseudomera Distant |
– Pronotum pruinose; length of antennal segment I less than interocular distance; pronotum pruinose, in some cases very deeply punctate; male fore tibia unarmed..............................................................................................................................8 |
8. Stridulitrum diffuse, not readily apparent; pronotum and head deeply punctate; abdomen with a band of long silvery setae covering much of sternum IV in lateral view.................................................................................Stridulocoris Harrington |
– Stridulitrum readily apparent; pronotum and head not deeply punctate; abdomen of uniform vestiture, without a band of long silvery setae...................................9 |
9. Lateral margin of posterior pronotal lobe angled posterolaterad at approximately a 45 degree angle; pronotum markedly bilobed, with a deeply incised, transverse impression; always macropterous; antennal segment IV with a pale proximal band; fore femoral spines clearly in two rows (present on both the inner and outer edge of ventral surface)...................................................Froeschneria Harrington |
– Lateral margin of the posterior pronotal lobe angled posterolaterad at less than a 45 degree angle; transverse impression not deeply incised; often submacropterous; antennal segment IV usually uniformly dark; if with a pale band, then fore femoral spines in a single row (present only along inner edge of ventral surface)......................................................................................................Ligyrocoris Stål |
10. Evaporative area reduced, occupying much less than half of metapleural area; claval punctation usually in three rows; pronotum shiny, never pruinose............11 |
– Evaporative area not reduced, occupying much more than half of metapleural area; claval punctation in more than three rows; pronotum dull, shagreened, or pruinose............................................................................................................14 |
11. Antennae with enlarged segments; segment III strongly swollen, distally of much greater diameter than segment I (Fig. 33).......................Ptochiomera Say |
– Antennae filiform; segment III generally filiform, if somewhat clavate, diameter no greater distally and often more slender than segment I..............................12 |
12. Collar not apparent on the anterior pronotal lobe; ventral surface of head not grooved; buccular juncture not near labial insertion, occurring at level of eyes; pronotum dorsoventrally compressed, lateral margins subcarinate.................13 |
– Collar apparent on anterior pronotal lobe but not demarked posteriorly; ventral surface of head grooved; buccular juncture U shaped near labial insertion; lateral margins of both pronotal lobes rounded (Fig. 32)....................Prytanes Distant |
13. Buccular juncture broadly U shaped; fore femur with spines only along the inner edge of the ventral surface; anterior margin of abdominal sternum II scalloped.................................................................................................Kolenetrus Barber |
– Buccular juncture elongate, terminating in a V-shaped end; fore femur with spines not confined to a single row; anterior margin of abdominal sternum II not scalloped......................................................Scintillororis Slater and Brailovsky |
14. Pronotum dorsoventrally compressed with a distinct lateral carina on both lobes.......................................................................................Megacholula Harrington |
– Pronotum not compressed; lateral pronotal margins ecarinate.........................15 |
15. Both lobes of pronotum and head uniformly and coarsely punctate; head broad, posterior margin of eyes touching anterior pronotal angles; sternal scalloping prolonged and clearly visible on sternum II; a very narrow anterior pronotal collar vaguely indicated but never demarked posteriorly by a groove.............16 |
– Head and anterior pronotal lobe impunctate or only vaguely punctate; head generally with eyes removed from anterior pronotal angles; sterna scalloping usually lacking, if present, largely hidden under metapleuron and not prolonged; in most cases, with a distinct anterior pronotal collar demarked posteriorly by a distinct groove..................................................................................................18 |
16. Clavus with three regular rows of punctures; apex of scutellum rounded, elevated, and distinctly pale; buccula with a posterior projection...............................................................................................................Paracholula Harrington |
– Clavus often with more than three rows of punctures; scutellum unicolorous; buccula without a posterior projection.............................................................17 |
17. Abdomen with a large glabrous area or diffuse stridulitrum on lateral portions of sterna II and III; hind femur with a plectrum of scattered spines on basal half; abdomen often with a band of long silvery setae covering much of sternum IV in lateral view; lateral pronotal margins ecarinate...................................................................................................................Stridulocoris Harrington |
– Abdomen without a stridulitrum or band of long setae as above; hind femur devoid of spines; lateral margin of posterior pronotal lobe with a blunt carina, sometimes forked over humeral angles.......................................Cholula Distant |
18. Mesacetabulum with mesepimeron emergent (or barely) from between the meso- and metepisternum................................................................................19 |
– Mesepimeron enclosed by metepisternum; touching mesepisternum.............28 |
19. Head elongate, often with a distinct neck; postocular distance equal to or greater than distance between ocelli............................................................................20 |
– Head less elongate, never with a distinct neck; postocular distance less than distance between ocelli....................................................................................24 |
20. Eyes elongate-oval; posterior margin of ocelli anterior to posterior margin of the eyes..................................................................................................................21 |
– Eyes rounded; posterior margin of ocelli posterior to posterior margin of eyes..........................................................................................................................22 |
21. Head with a clearly defined cylindrical neck; vertex of head convex; anterior pronotal lobe pruinose.............................................................Pephysena Distant |
– Head with a much less well-defined neck; vertex of head flat, depressed between the eyes; anterior pronotal lobe shiny..............Tenuicoris Slater and Harrington |
22. Head essentially flat when viewed laterally, not showing a gradual rounded constriction from eyes to insertion of head; fore coxa without a spine....................................................................................................................Catenes Distant |
– Head when viewed laterally showing gradual rounded constriction from eyes to insertion of head; fore coxa armed with a spine..............................................23 |
23. Ventral portion of collar produced anteriorly..................................Heraeus Stål |
– Ventral portion of collar not produced anteriorly.......................Paisana Dellapé |
24. Juga rounded; pygophore with anterior margin of dorsal aperture with small denticles; aedeagus with large, stout spines on conjunctiva and vesica.........................................................................................Myodacanthus Dellapé |
– Juga forming a ridge; pygophore with anterior margin of dorsal aperture smooth; aedeagus spined or unspined............................................................................25 |
25. Eyes not protruding or surpassing dorsal margin of head in lateral view..........................................................................................................................26 |
– Eyes protruding, inserted high, and surpassing dorsal margin of head in lateral view..................................................................................................................27 |
26. Head and anterior pronotal lobe lower than posterior pronotal lobe in lateral aspect; fore coxal spine poorly developed or absent; color predominantly blackish brown, posterior pronotal lobe with characteristic orange areas along lateral margins and paired orange maculae medially adjacent to transverse impression....................................................................................Orthaea Dallas |
– Dorsal surface of head and both lobes of pronotum essentially in the same plane; fore coxal spine(s) well developed; posterior pronotal lobe lacking orange markings along lateral margins.......................................Neopamera Harrington |
27. Profemur elongate and slender, with a few spines restricted to distal end; mesepimeron clearly emergent; aedeagus spined..............................................................................................Neomyocoris Dellapé and Montemayor |
– Profemur incrassate with two rows of spines ventrally; mesepimeron barely emergent; aedeagus without spines...............Thoraea Dellapé and Montemayor |
28. Head broad, interocular distance equal to or greater than width of pronotal collar..........................................................................................................................29 |
– Head not broad, interocular distance less than width of pronotal collar..........................................................................................................................31 |
29. Strikingly ant mimetic, with lateral margin of head between eye and insertion of antenna expanded into a platelike curving ridge; lateral margin of pronotal collar with a rounded but distinct spine (Fig. 34).........................................................................Xenydrium Poppius and Bergroth |
– Not ant mimetic, lateral margin of head between eye and base of antenna not expanded into a ridge; lateral margin of pronotal collar without a spine..........................................................................................................................30 |
30. Head elongate behind eyes, forming a short cylindrical neck; V-shaped buccular juncture with a strong midventral carina; antennal segment I short, remote from apex of head; scutellum pruinose on basal half, shiny on black tumid central area and apex....................................................Acrolophyses Dellapé and Henry |
– Head with a shorter neck; midventral carina of buccular junction less developed; antennal segment I longer, almost attaining apex of head; scutellum completely pruinose (Fig. 30).............................................................Distingphyses Scudder |
31. Head and anterior pronotal lobe, including collar, impunctate, or collar with only a few faint punctures; pronotal collar narrow and ringlike..................................................................................................Perigenes Distant |
– Pronotal collar usually broad and distinctly punctate; rest of anterior pronotal lobe and head also often punctate, though sometimes with indistinct, minute punctures..........................................................................................................32 |
32. Pronotum tapering cephalad with anterior lobe flattened and weakly convex; anterior lobe distinctly lower than posterior lobe in lateral view; collar with a characteristic median depression to posterior margin; abdomen equal to or longer than combined length of head and pronotum.................Paromius Fieber |
– Anterior pronotal lobe usually strongly convex, not lower than posterior lobe in lateral view; collar without median depression; abdomen shorter than combined length of head and pronotum...........................................................................33 |
33. Small, length 4.0 mm or less; ant mimetic, head strongly swollen in lateral view; eyes small, rounded, protruding, and beadlike............Bacacephalus Harrington |
– Larger than above; if small, then not ant mimetic with a swollen head and beadlike eyes....................................................................................................34 |
34. Large, usually greater than 6 mm long; head broad and jugal ridge above antennal segment I distinct...............................................................................35 |
– Small, generally less than 5 mm long; jugal ridge above antennal segment I usually narrow and poorly developed...............Pseudopachybrachius Malipatil |
35. Hemelytra with a broad, dark, transverse band at level of corial apex; male fore tibia unarmed; aedeagus with serrated projections on vesica and two strong spines on conjunctiva..............................................Pseudoparomius Harrington |
– Hemelytra without a broad, dark, transverse band at level of corial apex; male fore tibia with or without spines; aedeagus spined or unspined but without serrate projections............................................................................................36 |
36. Antennal segment IV with apex paler; anterior pronotal lobe elongate, at least 1.5 times as long as posterior pronotal lobe, but never globose; male fore tibia not strongly curved, with only a few short, sharp spines; aedeagus unspined..............................................................................Villalobosothignus Brailovsky |
– Antennal segment IV with or without a broad yellowish band subbasally, but never with apex paler; anterior pronotal lobe elongate and globose; male fore tibia curved on anterior half and straight distally, with a large spine medially; aedeagus spined.....................................................................Bergicoris Dellapé |
15.4.6 Tribe Ozophorini
The Ozophorini have a worldwide distribution, with most taxa occurring in the Neotropical and Oriental regions (Slater 1986a; Schuh and Slater 1995; Cassis and Gross 2002). Twenty-eight genera and 194 species are currently included in the tribe, with 13 genera and about 87 species known from Neotropics, mostly in the large genus Ozophora Uhler.
Ozophorines usually have a porrect, grooved head; the hind wing lacks a hamus and secondary veins; the abdomen lacks inner laterotergites and all spiracles are ventral; and the immatures possess a Y-suture between abdominal terga 3/4 and three pairs of scent glands between abdominal terga 3/4, 4/5, and 5/6 (Slater and Woodward 1982; Schuh and Slater 1995; Slater and Brailovsky 2000). The genus Ozophora is one of the most speciose taxa of Rhyparochromidae in the Western Hemisphere. Slater (1995) provided a key to the 51 mainland Neotropical species.
The following key is based on Ashlock and Slater’s (1982) key to the New World Ozophorini and Ashlock’s (1985) key to the Bergidea group.
Key to the Neotropical genera of Ozophorini
1. Fore femur unarmed beneath, at most with a patch of 20 or more minute spicules..............................................................................................................................2 |
– Fore femur beneath with one or more stout, compound spines set on the tubercles in both sexes.........................................................................................................5 |
2. Ocelli present.......................................................................................................3 |
– Ocelli absent.........................................................................................................4 |
3. Fore femur with a patch of 20 or more minute spicules, more evident in males; hemelytra with clavus and corium differentiated, macropterous......................................................................................................Pamozophora Ashlock and Slater |
– Fore femur unarmed; hemelytra with clavus and corium indistinguishably fused and nearly uniformly, coarsely punctate........Allotrophora Slater and Brailovsky |
4. Body short, stout, elliptical; vertex of head strongly convex; eyes large; pronotum extremely short and broad, width more than two times median length, disc strongly convex, lateral margin broadly explanate........Icaracoris Slater |
– Body elongate-oval; vertex of head flattened, eyes smaller, dorsoventrally elongate; pronotum nearly quadrate, width two times median length, disc less convex, lateral margins not explanate.......................................................Longinischus Brailovsky |
5. Basal segments of abdomen laterally with a cross-striated, crescent-shaped stridulitrum; plectrum consisting of scattered small tubercles on base of hind femur..............................................................................Lygofuscanellus Scudder |
– Basal segments of abdomen laterally without stridulatory structures..................6 |
6. Lateral edge of corium (viewed laterally) with a finely striated stridulitrum; hind femora with plectrum (with a regular and dense field of tubercles on apical half); lateral margins of pronotum sharply and broadly explanate, gently narrowing to humeral angles, without a posterior notch.....................................Balboa Distant |
– Lateral edge of corium without a stridulitrum, at most with minute crenulations on narrow lateral edge; hind femora without a plectrum; lateral margins of pronotum bluntly carinate or, if explanate (some species of Ozophora), then ending abruptly to form a notch at humeral angles..............................................7 |
7. Pronotal collar and pronotal lobes usually distinct..............................................8 |
– Pronotal collar and pronotal lobes always indistinct.........................................10 |
8. Coleopteroid; ocelli absent..................................................................................9 |
– Usually macropterous, if hemelytra modified, then not extremely so, or if coleopteroid (Ozophora ovalis, Colombia), ocelli present (Fig. 35)......................................................................................................Ozophora Uhler |
9. Eyes protruding laterally on short stalks; costal margin of corium clearly emarginate; claval commissure shorter than length of head and pronotum combined..........................................Pseudomenotelus Brailovsky and Cervantes |
– Eyes large, sessile, not protruding laterally; costal margin of corium weakly emarginate; claval commissure longer than length of head and pronotum combined...........................................................Pseudomenus Ashlock and Slater |
10. Coleopteroid, claval commissure longer than pronotum; ocelli absent; metapleural evaporative area covering less than one-third of metapleuron....................................................................................................11 |
– Often submacropterous or brachypterous, but never coleopteroid, claval commissure shorter than pronotum; ocelli present; metapleural evaporative area occupying at least one-half of metapleuron (Chile and Argentinean Patagonia).......................................................................................Bergidia Breddin |
11. Body short, elliptical, strongly convex; hemelytra without any trace of membrane, claval commissure longer than head and pronotum taken together Brailovskyocoris Slater |
– Body elongate oval; hemelytra with membrane reduced, claval commissure shorter than head and pronotum taken together.................................................12 |
12. Antennal segment III longer than segment I and more than half as long as segment II; pronotum and hemelytra with surface, including punctures, dull, not sculptured or rugose; texture of anterior lobe of pronotum and base of scutellum not distinct from remainder of dorsum; evaporative area covering mesepimeron (Juan Fernandez Islands)...................................................Micrymenus Bergroth |
– Antennal segment III shorter than segment I and less than half as long as segment II; pronotum and hemelytra with surface, especially punctures, shiny, obviously rugose and sculptured; anterior lobe of pronotum and basal area of scutellum with a finely pebbled texture, strongly contrasting with surrounding areas; evaporative area covering only posterior part of mesepimeron (Juan Fernandez Islands)...............................................................................................Rugomenus Ashlock |
15.4.7 Tribe Udeocorini
The Udeocorini, comprising 17 genera and 38 species, are most abundant in the Australian Region (Slater 1964a; Slater and O’Donnell 1995). Only three genera and five species have been recorded from the Neotropics. The presence of the Nearctic Tempyra biguttula Stål in Argentina probably represents an introduction. Slater (1986a) argued for a Gondwanan origin of the group.
Members of the tribe have rounded, carinate, or explanate pronotal margins; the abdomen has dorsal spiracles on segments II, III, and IV and inner laterotergites; and the immatures possess a Y-suture between abdominal terga 3/4 and have three pairs of scent glands between abdominal terga 3/4, 4/5, and 5/6 (Sweet 1967; Slater and Woodward 1982; Schuh and Slater 1995; Slater and Brailovsky 2000).
Key to the Neotropical genera of Udeocorini
1. Body surface shiny and uniformly dark............................Astemmoplitus Spinola |
– Body surface dull and not uniformly dark...........................................................2 |
2. Hemelytra covering abdomen in dorsal view; corium with a pale, round macula distally (Fig. 36)................................................................................Tempyra Stål |
– Hemelytra narrower than abdomen, connexiva visible in dorsal view; corium with a pale, elongate macula distally.................................................Bathycles Distant |
16 Main Species
The Lygaeoidea include many species of economic importance. Sweet (2000a) gave a detailed account of the most important ones, which are concentrated in the families Blissidae, Geocoridae, Lygaeidae, and Oxycarenidae, though the diverse family Rhyparochromidae has a large number of economically important seed-feeding species (e.g., Henry and Froeschner 1993) and numerous nuisance pests that often cluster in and around homes and commercial buildings (Henry and Adamski 1998; Henry 2004).
The Blissidae, also known as chinch bugs, are probably the most economically important group of Lygaeoidea (Sweet 2000a; Samuels et al. 2002). The chinch bugs are sap suckers specialized for feeding on monocotyledonous plants (Slater 1976). Many blissids are among the most important pests of grasses (Poaceae), including barley, corn (maize), millets, oats, rice, rye, sorghum, and wheat (Sweet 2000a). Blissus leucopterus (Say) is considered the most important New World species (Slater 1976; Sweet 2000a). Pereira and da Silva (1988) reported B. leucopterus (Say) attacking Brachiaria radicans Napper in Minas Gerais, Brazil. Valério et al. (1999) documented Blissus antillus Leonard damaging more than 800 acres of Tangola grass pastures in southern Mato Grosso.
Members of the family Geocoridae are in large part predaceous, although they also feed on seeds and foliage of plants, especially as early instars (Sweet 1960; Tamaki and Weeks 1972). As noted by Sweet (2000b), geocorids have complex nutritional requirements and evidently require plant food for optimal development. Species such as Geocoris uliginosus (Say) may be considered omnivorous (Carstens et al. 2008). Nevertheless, their importance as biological control agents is well documented (Naranjo and Gibson 1996; Coll and Ruberson 1998; Hagen et al. 1999). A list of the prey for species of Geocoris in the United States was compiled by Readio and Sweet (1982).
The family Lygaeidae is separated into three subfamilies, the Ischnorhynchinae, Lygaeinae, and Orsillinae. The life cycles and habitats of most of the species of Ischnorhynchinae are not known. Scudder (1962b) gave available host records, Wheeler (1976) studied the seasonal history and summarized hosts of the Holarctic birch catkin bug, Kleidocerys resedae (Panzer) and Cervantes and Baez (2010) presented the life histories of K. punctatus Distant and K. virescens Fabricius. Only a few species of Lygaeinae are recorded as pests on agricultural crops (Slater 1964b). Aposematically colored members of the genus Oncopeltus are restricted to milkweeds and other plants of the family Asclepiadaceae (Wheeler 1983), from which they sequester toxic cardenolides, making them unpalatable to potential predators (Scudder and Duffy 1972).
Other species, such as Lygaeus kalmii Stål (Wheeler 1983) and Neacoryphus bicrucis (Say) (Solbreck and Pehrson 1979), have been found feeding on numerous plant families, even though asclepiads remain their preferred hosts (Wheeler 1983). The Orsillinae include species that feed on seeds, as well as on vascular tissues. Much of the recorded damage occurs when large populations migrate from wild hosts to crop plants, especially during times of water stress (Ashlock 1967b). Economically important population of Nysius develop when the seed supply of the preferred host becomes insufficient for a large population, and the bugs move to a new host, often one of agricultural importance. Nysius simulans Stål has been reported from São Paulo state, attacking corn, cotton, and rice, and Neopamera bilobata (Say), from Minas Gerais, attacking tobacco (Costa Lima 1940).
The Oxycarenidae feed on both seeds and sap, chiefly of the plant family Malvaceae. The introduced Oxycarenus hyalinipennis (Costa) often is of major economic importance on cotton and other Malvaceae, sometimes developing large numbers and feeding between the fibers of the cottonseeds, causing staining of the cotton bolls (Annecke and Moran 1982). Almost nothing is known of the feeding habits of the largest New World genus, Crophius, though (Blatchley 1926) reported C. disconotus (Say) in numbers on goldenrod, Solidago sp. (Asteraceae).
Only a scattering of records are available for the economic species of Rhyparochromidae (Sweet 2000a). Species such as Neopamera bilobata (Say), Pseudopachybrachius vincta (Say), and their relatives sporadically damage crops, especially strawberries (Slater and Baranowski 1990). Sweet (1964b) found Cryphula trimaculata (Distant) inhabiting old fields, feeding on seeds of perennial bunch grasses, such as Schizachyrium scoparium J. Presl, Festuca rubra L., and Panicum sp. Elasmolomus sordidus (F.) and Dieuches armatipes (Walker) are potential pest of peanuts (Slater 1972; Henry and Froeschner 1993). Though documented as feeding on the blood of small rodents (Harrington 1983, 1990), Clerada apicicornis Signoret apparently prefers feeding on other insects, including the blood-feeding reduviid Rhodnius prolixus Stål (Torres et al. 2000).
Although the Berytidae were long thought to be primarily phytophagous (Wheeler and Schaefer 1982; Péricart 1984), many have strong predatory tendencies (Henry 1997a, 2000). Parajalysus spinosus Distant is said to cause serious damage on cacao, though a later study considered it an important predator of Heliothis virescens (F.) eggs (Wille 1951). Other species, such as Parajalysus andinus (Horváth), may play an important role in pollinating cacao (Henry 1997b). Henry and Froeschner (1998) documented the known hosts, and Henry (2000) provided an overview of the feeding habits in the family.
The Colobathristidae and Piesmatidae are also sap feeders (Sweet and Schaefer 1985; Heiss and Péricart 1983; Sweet 2000a). Colobathristids, found in the Neotropical and the Oriental-Australian regions, all feed on grasses, including bamboos. No economically important colobathristids have been reported in the Neotropics, although Phaenacantha (Anorygma) saccharicida (Karsch) is a pest of sugar cane in Australia (Cassis and Gross 2002). Many piesmatids specialize on plants of the family Chenopodiaceae, including the widespread Parapiesma cinereum (Say) (Schaefer 1981; Heiss and Péricart 1983).
17 Concluding Remarks
The Lygaeoidea, though predominantly seed feeders, include some of the most economically important species of Heteroptera, such as the sap-feeding chinch bugs. A lesser number of others, such as the geocorids, are in large part predatory. The group represents the second largest superfamily of the infraorder Pentatomomorpha, with 16 families, more than 700 genera, and 4,200 species worldwide (Henry 2009). The Neotropical fauna treated in this chapter comprises about 184 genera and 836 species, representing 26 % of the world’s genera and only 20 % of the species, suggesting that the number of new taxa awaiting description is immense. That nearly 30 new species of Heraeus Stål are being described in a forthcoming revision (Dellapé et al. in review) further illustrates the amount of work that remains to be done in the Neotropics. We hope this review of the superfamily, which includes information on hosts, habits, economic importance, classification, and keys to all Neotropical genera in 12 families, will stimulate interest in this large, diverse group of taxonomically and ecologically important insects.
References
Aller T, Caldwell RL (1979) Investigation of the possible presence of an aggregation pheromone in the milkweed bugs, Oncopeltus fasciatus and Lygaeus kalmii. Physiol Entomol 4:287–290
Annecke DP, Moran VG (1982) Insects and mites of cultivated plants in South Africa. Butterworths, Durban
Ashlock PD (1964) Two new tribes of Rhyparochrominae: a re-evaluation of the Lethaeini (Hemiptera-Heteroptera: Lygaeidae). Ann Entomol Soc Am 57:414–422
Ashlock PD (1967a) A generic classification of the Orsillinae of the world (Hemiptera-Heteroptera: Lygaeidae). University of California Press, Berkeley
Ashlock PD (1967b) New records and name changes of North American Lygaeidae (Hemiptera: Heteroptera: Lygaeidae). Proc Entomol Soc Wash 79:575–582
Ashlock PD (1985) A revision of the Bergidea group: a problem in classification and biogeography (Hemiptera-Heteroptera: Lygaeidae). J Kansas Entomol Soc 57:675–688
Ashlock PD, Lattin JD (1963) Stridulatory mechanisms in the Lygaeidae, with a new American genus of Orsillinae (Hemiptera: Heteroptera). Ann Entomol Soc Am 56:693–703
Ashlock PD, Slater JA (1982) A review of the genera of Western Hemisphere Ozophorini with two new genera from Central America (Hemiptera-Heteroptera: Lygaeidae). J Kansas Entomol Soc 55:737–750
Ashlock PD, Slater JA (1988) Family Lygaeidae Schilling, 1829. Infericornes Amyot and Serville, 1843; Myodochidae Kirkaldy, 1899; Geocoridae Kirkaldy, 1902. In: Henry TJ, Froeschner RC (eds) Catalog of the Heteroptera, or true bugs, of Continental United States. E. J. Brill, Leiden/New York, pp 167–245
Attisano A, Tregenza T, Moore AJ, Moore PJ (2013) Oosorption and migratory strategy of the milkweed bug, Oncopeltus fasciatus. Anim Behav 86:651–657
Baranowski RM, Slater JA (2005) The Lygaeidae of the West Indies. Florida Agric Exp Station Bull 402:1–266
Barber HG (1947) Revision of the genus Nysius in the United States and Canada (Hemiptera: Heteroptera: Lygaeidae). J Acad Sci Washington 37:354–366
Blatchley WS (1926) Heteroptera or true bugs of Eastern North America, with especial reference to the faunas of Indiana and Florida. Nature Publishing Company, Indianapolis
Brailovsky H (1975) Contribución al estudio de los Hemiptera-Heteroptera para México IV. Una nueva especie de Cymonimus Breddin (Lygaeidae-Cyminae-Ninini). Rev Soc Mexicana Hist Nat 36:177–181
Brailovsky H (1978) Estudio de género Lygaeus Fabricius 1794, del Nuevo Mundo, con descripción de cinco nuevas especies (Hemiptera-Heteroptera-Lygaeidae-Lygaeinae). An Inst Biol Univ Nac Aut México Ser Zool 49:123–166
Brailovsky H (1979) Revisión de género Craspeduchus Stal con descripción de dos nuevas especies (Hemiptera-Heteroptera-Lygaeidae-Lygaeinae). An Inst Biol Univ Nac Aut México Ser Zool 50:205–226
Brailovsky H (1980) Revisión del género Acroleucus Stal (Hemiptera-Heteroptera-Lygaeidae-Lygaeinae). Folia Entomol Mexicana 44:39–120
Brailovsky H (1982a) Un nuevo arreglo nomenclatorial y descripción de tres nuevos géneros y dos nuevas especies americanas de la subfamilia Lygaeinae (Hemiptera-Heteroptera-Lygaeidae). An Inst Biol Univ Nac Aut México Ser Zool 52:259–276
Brailovsky H (1982b) Revisión del complejo Ochrimnus, con descripción de nuevas especies y nuevo géneros (Hemiptera-Heteroptera-Lygaeidae-Lygaeinae). Folia Entomol Mexicana 51:1–163
Brailovsky H (1983) Revisión del género Torvochrimnus Brailovsky (Hemiptera-Heteroptera-Lygaeidae-Lygaeinae). An Inst Biol Univ Nac Aut México Ser Zool 53:285–320
Brailovsky H (1984) Un nuevo género y una nueva especie sudamericanos de la tribu Myodochini (Hemiptera: Heteroptera: Lygaeidae). An Inst Biol Univ Nac Aut México Ser Zool 54:79–85
Brailovsky H (1989a) Una especie nueva del género Neoninus Distant (Hemíptera-Heteroptera-Lygaeidae-Cyminae-Ninini) de Sudamérica. An Inst Biol Univ Nac Aut México Ser Zool 59:153–158
Brailovsky H (1989b) Un género y dos especies nuevas de hemipteros (Lygaeidae, Bledionotinae, Pamphantinae) del Brasil. An Inst Biol Univ Nac Aut México Ser Zool 59:193–202
Brailovsky H (2013) Description of four new species of Ninyas from Venezuela, a key to the known species and some new records (Hemiptera: Heteroptera: Geocoridae). Acta Mus Moraviae Sci Biol (Brno) 98:395–406
Brailovsky H, Barrera E (2012) A remarkable new micropterous Blissidae (Hemiptera, Heteroptera, Lygaeoidea) from South America. Dtsch Entomol Z 59:43–45
Brailovsky H, Cervantes Peredo L (2011) A second species of the genus Neaplax Slater 1974 from Mexico (Heteroptera: Lygaeidae: Oxycarenidae). Proc Entomol Soc Wash 110:1–6
Brambila J (2000) A review of Cligenes with the description of a new genus, Valeris (Hemiptera: Rhyparochromidae: Antillocorini). Florida Entomol 83:303–315
Burdfield-Steel E, Shuker DM (2014) The evolutionary ecology of the Lygaeidae. Ecol Evol 4:2278–2301
Cai B, Dang K, Bu W (2011) Paraberytus Štusák, a new record genus from China, with description of a new species (Hemiptera, Heteroptera, Berytidae, Berytini). Acta Zootax Sinica 36:241–245
Cai B, Ye Z, Bu W (2013) A review of Yemmalysus Štusak, 1972 from China, with description of one new species (Hemiptera: Heteroptera: Berytidae). Zootaxa 3736:338–344
Carstens JD, Baxendale FP, Heng-Moss TM, Wright RJ (2008) Predation of the Chinch Bug, Blissus occiduus Barber (Hemiptera: Blissidae) by Geocoris uliginosus (Say) (Hemiptera: Lygaeidae). J Kansas Entomol Soc 81:328–338
Carvalho JCM, Costa LAA (1989) Chave para identificaçno dos gLneros neotrópicos da família Colobathristidae (Hemiptera). Rev Brasil Biol 49:271–277
Carvalho JCM, Henry TJ (1986) Sobre um gênero novo peculiar da família Colobathristidae (Hemiptera) da regino de Carajás (Pará, Brasil). Bol Museu Paraense Emilio Goeldi Zool 2:85–91
Cassis G, Gross GF (2002) Hemiptera: Heteroptera (Pentatomomorpha). In: Houston WK, Maynard GV (eds) Zoological catalogue of Australia, vol 27.3B. CSIRO, Melbourne, pp 1–737
Cervantes Peredo L, Baez MB (2010) Life histories of the seed bugs, Kleidocerys punctatus and Kleidocerys virescens. J Insect Sci 10:91; insectscience.org/10.91
Cervantes Peredo LM, Brailovsky H (2011) Chinches: Lygaeoidea (Insecta: Heteroptera). In: Cruz-Angón A, Lorea-Hernández F, Hernández-Ortiz V, Morales-Mavil JE (eds) La biodiversidad en Veracruz. Estudio de estado. Diversidad de especies: conocimiento actual, vol 2. CONABIO, Gobierno del Estado de Veracruz, Universidad Veracruzana, Instituto de Ecología, A. C. Distrito Federal, pp 327–337
Cobben RH (1968) Evolutionary trends in Heteroptera. Part I. Eggs, architecture of the shell, gross embryology, and eclosion. Centre for Agricultural Publishing and Documentation, Wageningen
Coll M, Ruberson JR (1998) Predatory Heteroptera: their ecology and use in biological control. Thomas Say Publications in Entomology, Lanham
Costa Lima A (1940) Insetos do Brasil – Hemípteros – 2° Tomo. Escola Nacional de Agronomia, Rio de Janeiro
Dellapé PM (2014) Lygaeoidea. In: Roig-Juñent L, Claps E, Morrone JJ (eds) Biodiversidad de artrópodos Argentinos, vol 3. Editorial INSUE, Argentina, pp 89–106
Dellapé PM, Carpintero D (2007) Cuscohoplininus pagoreni: a new genus and species of Hoplinini stilt bug from Peru (Heteroptera: Berytidae). Rev Biol Trop 55:673–676
Dellapé PM, Carpintero D (2012) Relevamiento de los Heteroptera (Insecta: Hemiptera) de las sierras de Tandil, provincia de Buenos Aires, Argentina. Rev Museo Arg Cien Nat 14:125–134
Dellapé PM, Cheli GH (2007) A new species of Anomaloptera Amyot & Serville from Patagonia (Hemiptera: Lygaeoidea: Oxycarenidae). Zootaxa 1528:65–68
Dellapé PM, Henry TJ (2010) Acrolophyses, a new seed bug genus and two new species (Hemiptera: Heteroptera: Rhyparochromidae: Myodochini) from forest-canopy fogging in Ecuador and Peru. Insect Syst Evol 41:75–89
Dellapé PM, Melo MC, Henry TJ. A phylogenetic review of the true bug genus Heraeus Stål 1862 (Hemiptera: Rhyparochromidae: Myodochini), with the description of two new genera and 30 new species. Zool J Linnean Soc (in review)
Dellapé PM, Montemayor SI (2009) Description of a new species of Ischnodemus from Peru, and the male and immature stages of I. subflavus (Hemiptera: Heteroptera: Lygaeoidea: Blissidae). Rev Mexicana Biodiv 80:687–691
Dellapé PM, Montemayor SI (2011) On the identity of Ashlockobius Slater & Slater and Villalobosothignus Brailovsky (Hemiptera: Heteroptera: Rhyparochromidae: Myodochini), with the description of a new arboreal species from Ecuador. Zootaxa 2748:47–52
Dingle H, Alden BM, Blakley NR, Kopec D, Miller ER (1980) Variation in photoperiodic response within and among species of milkweed bugs (Oncopeltus). Evolution 34:356–370
Distant WL (1880–1893) Insecta. Rhychota. Hemiptera-Heteroptera. In Goodman FD, Salvin O (eds) Biologia Centrali-Americana, vol I. London. x + 462 p, 39 pls (1880: 1–88; 1881: 89–168; 1882: 169–224; 1883: 225–264; 1884: 265–304; 1889: 305–328; 1893: i–xx + 329–462)
Distant WL (1888) Enumeration of the Van Volxem Collection of Rhynchota contained in the Brussel’s Museum. Part II. In: Comptes-Rendus des Séances. Serie III, No. 95. Bull Soc Entomol Belgique 33:VII–XIII
Drake CJ, Davis NT (1958) The morphology and systematics of the Piesmatidae (Hemiptera), with keys to world genera and American species. Ann Entomol Soc Am 51:567–581
Forero D (2008) The systematics of the Hemiptera. Rev Colomb Entomol 34:1–21
Froeschner RC (1981) Heteroptera or true bugs of Ecuador: A partial catalog. Smithsonian Contr Zool 322:1–147
Froeschner RC (1985) Synopsis of the Heteroptera or true bugs of the Galápagos Islands. Smithsonian Contr Zool 407:1–84
Hagen KS, Mills NJ, Gordh G, McMurtry JA (1999) Terrestrial arthropods predators of insect and mite pests. In: Bellows TS, Fisher TW (eds) Handbook of biological control: principles and applications of biological control. Academic, San Diego, pp 383–503
Hamid A (1971) A revision of the Cryptorhamphinae (Heteroptera: Lygaeidae) including the description of two new species from Australia. J Australian Entomol Soc 10:163–174
Hamid A (1975) A systematic revision of the Cyminae (Heteroptera: Lygaeidae) of the world with a discussion of the morphology, biology, phylogeny and zoogeography. Occas Pap Entomol Soc Nigeria 14:1–179
Hamilton SW (1983) Neortholomus, a new genus of Orsillini (Hemiptera–Heteroptera: Lygaeidae: Orsillinae). Univ Kansas Sci Bull 52:197–234
Harrington BJ (1980) A generic level revision and cladistic analysis of the Myodochini of the world (Hemiptera, Lygaeidae, Rhyparochrominae). Bull Am Mus Nat Hist 167:49–116
Harrington BJ (1983) A new species of Cleradini (Hemiptera, Lygaeidae, Rhyparochrominae) from the Central–African – Republic and Ghana. J New York Entomol Soc 91:63–67
Harrington BJ (1990) Detecting evidence of hematophagy in dry museum specimens of Clerada apicicornis (Hemiptera: Lygaeidae: Rhyparochrominae). Ann Entomol Soc Am 83:545–548
Harris HM (1943) Art. XVI. New Neididae (Hemiptera) from South America, with notes on some little-known species. Ann Carnegie Mus 29:443–450
Heiss E, Péricart J (1983) Revision of Palaearctic Piesmatidae (Heteroptera). Mitteilungen Münchner Entomol Gesellschaft 73:61–171
Heiss E, Péricart J (1997) Revised taxonomic status of some Old World Piesmatidae (Heteroptera). Zeitschrift Arbeitsgeminschaft Österreichischer Entomol 49:119–120
Henry TJ (1997a) Phylogenetic analysis of family groups with the infraorder Pentatomomorpha (Hemiptera: Heteroptera), with emphasis on the Lygaeoidea. Ann Entomol Soc Am 90:275–301
Henry TJ (1997b) Cladistic analysis and revision of the stilt bug genera of the world (Heteroptera: Berytidae). Contr Am Entomol Inst 30:1–100
Henry TJ (1997c) Monograph of the stilt bugs, or Berytidae (Heteroptera), of the Western Hemisphere. Mem Entomol Soc Washington 19:1–149
Henry TJ (2000) Stilt bugs (Berytidae). In: Schaefer CW, Panizzi AR (eds) Heteroptera of economic importance. CRC Press, Boca Raton, pp 725–735
Henry TJ (2002) Review of the stilt bug genus Hoplinus with the description of a new species and notes on other Hoplinini (Heteroptera: Berytidae: Gampsocorinae). J New York Entomol Soc 110:182–191
Henry TJ (2004) Raglius alboacuminatus (Goeze) and Rhyparochromus vulgaris (Schilling) (Lygaeoidea: Rhyparochromidae): Two Palearctic bugs newly discovered in North America. Proc Entomol Soc Wash 106:513–522
Henry TJ (2006) Revision of the New World lygaeoid genus Epipolops (Heteroptera: Geocoridae: Pamphantinae: Epipolopini), with descriptions of five new species. Can Entomol 138:504–530
Henry TJ (2007) A newly discovered Brazilian species of the stilt bug genus Jalysus (Hemiptera: Heteroptera: Berytidae) associated with myrmecophytic plants. Proc Entomol Soc Wash 109:324–330
Henry TJ (2009) Biodiversity of Heteroptera. In: Foottit R, Adler P (eds) Insect biodiversity: science and society, 1st edn. Blackwell Publishing, Oxford, UK, pp 223–263
Henry TJ (2013) Cymapamphantus valentineorum, a new genus and species of Pamphantinae (Heteroptera: Lygaeoidea: Geocoridae) from the British Virgin Islands, with a checklist of the species and keys to the tribes and genera of the subfamily. Proc Entomol Soc Wash 115:392–401
Henry TJ, Adamski D (1998) Rhyparochromus saturnius (Rossi) (Heteroptera: Lygaeoidea: Rhyparochromidae), a Palearctic seed bug newly discovered in North America. J New York Entomol Soc 106:132–140
Henry TJ, Dellapé PM (2009) A new genus and species of Oxycarenidae (Hemiptera, Heteroptera, Lygaeoidea) from Argentina. ZooKeys 25:49–59
Henry TJ, Froeschner RC (eds) (1988) Catalog of the Heteroptera, or true bugs, of Canada and the Continental United States. E. J. Brill, Leiden
Henry TJ, Froeschner RC (1993) Dieuches armatipes (Walker) (Heteroptera: Lygaeidae) newly discovered in the Western Hemisphere. Proc Entomol Soc Wash 95:449–452
Henry TJ, Froeschner RC (1998) Catalog of the stilt bugs, or Berytidae, of the world (Insecta: Hemiptera: Heteroptera). Contr Am Entomol Inst 30:1–72
Henry TJ, Dellapé PM, Scudder GGE (2015) Resurrection of the genera Crophius Stål and Mayana Distant from synonymy with Anomaloptera Amyot and Serville, and a key to the New World genera (Hemiptera: Heteroptera: Oxycarenidae). Proc Entomol Soc Wash 117: 367–380
Hoberlandt L (1987) Results of the Czechoslovak-Iranian Entomological Expeditions to Iran 1970,1973 and 1977. Heteroptera, Lygaeidae, Oxycareninae. Acta Entomol Musei Nat Pragae 42:12–29
Hoffman RL, Slater JA (1995) Holcocranum saturejae, a Palearctic cattail bug established in eastern United States and tropical Africa (Heteroptera: Lygaeidae: Artheneinae). Banisteria 26:12–15
Horváth G (1904) Monographia Colobathristinarum. Ann Musei Nat Hungarici 2:117–172
Horváth G (1905) Berytidae novae a Dre G. Horváth descriptae. Ann Musei Nat Hungarici 3:56–60
Kerzhner IM (1997) East Palaearctic species of the genus Artheneis (Heteroptera: Lygaeidae). Zoosyst Rossica 6:213–232
Kerzhner IM (2001) Family Malcidae. In: Aukema B, Rieger C (eds) Catalogue of Heteroptera of the Palaearctic Region. Netherlands Entomological Society, Amsterdam, pp 227–229
Kormilev NA (1949a) Notas sobre los Colobathristidae de Bolivia con la descripción de un género y una especies nuevos (Hemiptera). Univ Nac La Plata Not Museo La Plata 14(Zool 124):167–176
Kormilev NA (1949b) La Familia Colobathristidae Stal en la Argentina. Acta Zool Lilloana 7:359–383
Kormilev NA (1951) Notas sobre Colobathristidae neotropicales (Hemiptera), con la descripción de tres géneros y siete especies nuevas. Rev Brasil Biol 11:63–84
Kumar R (1968) Aspects of the morphology and relationships of the superfamilies Lygaeoidea, Piesmatoidea and Pyrrhocoroidea (Hemiptera: Heteroptera). Entomol Mon Mag 103:251–261
Leonard DE (1968a) A revision of the genus Blissus (Heteroptera: Lygaeidae) in eastern North America. Ann Entomol Soc Am 61:239–250
Leonard DE (1968b) Three new species of Blissus from the Antilles (Heteroptera: Lygaeidae). Proc Entomol Soc Wash 70:150–153
Leonard DE (1970) A new North American species of Blissus (Heteroptera: Lygaeidae). Can Entomol 102:1531–1533
Li H-M, Den R-Q, Wang J-W, Chen Z-Y, Jia F-L, Wang X-Z (2005) A preliminary phylogeny of the Pentatomomorpha (Hemiptera: Heteroptera) based on nuclear 18S rDNA and mitochondrial DNA sequences. Mol Phylogen Evol 37:313–326
Li J, Gao C, Bu W (2011) Review of the tribe Lethaeini Stål (Hemiptera: Heteroptera: Lygaeoidea: Rhyparochromidae) from China, with a key to Chinese genera and species. Zootaxa 3126:28–38
Malipatil MB (2012) Australocorinae, a new subfamily of Geocoridae (Hemiptera: Heteroptera, Lygaeoidea) from Australia, with descriptions of a new genus and two new species. Zootaxa 3554:75–88
Malipatil MB (2014) Meschiidae, a new family of Lygaeoidea (Hemiptera: Heteroptera) from India and Australia, with descriptions of a new genus and two new species. Zootaxa 3815:233–248
McAtee WL (1919) Key to the Nearctic genera and species of Berytidae (Heteroptera). J New York Entomol Soc 27:79–92
Melo MC, Dellapé PM, Carpintero DL, Coscarón MC (2004) Reduviidae, Miridae, y Lygaeoidea colectados en Colonia Carlos Pellegrini (Esteros del Iberá, Corrientes, Argentina). Rev Soc Entomol Argentina 63:59–67
Melo MC, Dellapé PM, Carpintero DL, Montemayor SI (2011) Heteroptera (Hemiptera) from the Chaco National Park (Argentina). Zootaxa 2999:1–19
Montandon AL (1913) Nouvelles formes de Geocorinae appartenant aux collections du Muséum national Hongrois. Ann Musei Nat Hungarici 11:211–219
Morales A, Corredor-Arjona A, Osorno-Mesa E, Parra-Marquez J (1969) Infección natural de Mus musculus con Trypanosoma cruzi, en una región de Colombia, S.A. Rev Acad Colomb Cienc Exactas Fisicas Nat 13:375–377
NAPPO (2014) Official pest report: cotton seed bug (Oxycarenus hyalinipennis) eradicated from Florida. North American Plant Protection Organization (NAPPO), Phytosanitary Alert System http://www.pestalert.org/oprDetail.cfm?oprID=577
Naranjo SE, Gibson RL (1996) Phytophagy in predaceous Heteroptera: effects on life history and population dynamics. In: Alomar O, Wiedenmann RN (eds) Zoophytophagous Heteroptera: implications for life history and integrated pest management. Thomas Say Publications in Entomology, Lanham, pp 57–93
Nagoshi RN, Paraiso O, Brambila J, Kairo MT (2012) Assessing the usefulness of DNA barcoding to identify Oxycarenus hyalinipennis (Hemiptera: Oxycarenidae) in Florida, a potentially invasive pest of cotton. Florida Entomol 95:1174–1181
O’Donnell JE (1991) A new coleopteroid Lethaeini from southern South America (Hemiptera: Lygaeidae: Rhyparochrominae). J New York Entomol Soc 99:87–96
Paula A, Ferreira PSF (1998) Fauna de Heteroptera de la “Mata do Córrego do Paraíso”, Viçosa, Minas Gerais, Brasil. I. Riqueza y diversidad específicas. An Inst Biol Univ Nac Aut Mex Ser Zool 69:39–51
Paula A, Ferreira PSF (2000) Fauna de Heteroptera de la “Mata do Corrego do Paraiso”, Viçosa, Minas Gerais, Brasil. II. Patrones temporales. Distribucion anual y estacionalidad. An Inst Biol Univ Nac Aut Mex Ser Zool 71:7–19
Peck SB (2001) Smaller orders of insects of the Galapagos Islands, Ecuador: Evolution, ecology, and diversity. NRC Research Press, Ottawa
Pereira RP, da Silva SC (1988) Ocorrência de Blissus leucopterus em pastagens do estado do Rio de Janeiro. Pesq Agropec Bras 23:551–553
Péricart J (1974) Subdivision du genre Piesma [Hem. Piesmatidae] et remarques diverses. Ann Soc Entomol France 10:51–58
Péricart J (1984) Hémiptères Berytidae Euro–Méditerranéens. Faune de France. France et régions limitrophes, 70th edn. Féderation Française des Sociétés de Sciences Naturelles, Paris
Péricart J (1998) Hémiptères Lygaeidae Euro-Méditerranéens. Vol. 2. Systématique: Seconde Partie. Oxycareninae, Bledionotinae, Rhyparochrominae (1). Faune France 84B:I–III, 1–453
Péricart J (2001) Family Lygaeidae Schilling, 1829 seed-bugs. In: Aukema B, Rieger C (eds) Catalogue of the Heteroptera of the Palaearctic Region. Pentatomomorpha I, vol 4. The Netherlands Entomological Society, Amsterdam
Putshkov VG (1958) Larvae of Hemiptera-Heteroptera. I. Lygaeidae. Revue d’Entomol l’URSS 37:392–413
Readio J, Sweet MH (1982) A review of the Geocorinae of the United States east of the 100th Meridian (Hemiptera: Lygaeidae). Misc Publ Entomol Soc Am 12:1–91
Rengifo-Correa L, Brailovsky H, Henry TJ, Morrone JJ (2013) Phylogenetics and evolutionary morphology of the Neotropical true bug genus Epipolops (Hemiptera: Heteroptera: Geocoridae). Syst Entomol 39:127–140 DOI:. doi:10.1111/syen.12039
Samuels RI, Coracini DLA, dos Santos CAM, Gava CAT (2002) Infection of Blissus antillus (Hemiptera: Lygaeidae) eggs by the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. Biol Control 23:269–273
Schaefer CW (1981) Improved cladistic analysis of the Piesmatidae and consideration of the known host plants. Ann Entomol Soc Am 74:536–539
Schaefer CW (1993) The Pentatomomorpha (Hemiptera: Heteroptera): an annotated outline of its systematic history. Europ J Entomol 90:105–122
Schuh RT, Slater JA (1995) True bugs of the world (Hemiptera: Heteroptera). Classification and natural history. Cornell University Press, Ithaca
Scudder GGE (1957) A revision of Ninini (Hemiptera-Heteroptera, Lygaeidae) including the description of a new species from Angola. Publ Cult Comp Diamantes Angola 34:91–108
Scudder GGE (1962a) New Heterogastrinae (Hemiptera) with a key to the genera of the world. Opuscula Entomol 27:117–127
Scudder GGE (1962b) The Ischnorhynchinae of the world (Hemiptera: Lygaeidae). Trans Royal Entomol Soc London 114:163–194
Scudder GGE, Duffy SS (1972) Cardiac glycosides in the Lygaeinae (Hemiptera: Lygaeidae). Can J Zool 50:35–42
Slater JA (1955) A revision of the subfamily Pachygronthinae of the world (Hemiptera: Lygaeidae). Philip J Sci 84:1–160
Slater JA (1964a) A catalogue of the Lygaeidae of the world. 2 vols. University of Connecticut, Storrs
Slater JA (1964b) Results of the Lund University expedition in 1950–1951. Chapter II. Hemiptera (Heteroptera): Lygaeidae. South Afric Anim Life 10:15–228
Slater JA (1967) Synonymy in the Lygaeidae (Hem.). Proc Entomol Soc Wash 69:244–245
Slater JA (1971) The first Neotropical records of the genus Plinthisus with the description of three new species. J Kansas Entomol Soc 44:377–384
Slater JA (1972) The occurrence of Elasmolomus sordidus (F), a potential pest of peanuts, in Brazil (Hemiptera: Lygaeidae). O Biologico 38:394–395
Slater JA (1974) Neaplax, a new genus of Oxycareninae from the Western Hemisphere (Hemiptera: Lygaeidae). J Kansas Entomol Soc 47:517–522
Slater JA (1976) Monocots and chinch bugs: a study of host plant relationships in the lygaeid subfamily Blissinae (Hemiptera: Lygaeidae). Biotropica 8:143–165
Slater JA (1977) The incidence and evolutionary significance of wing polymorphism in lygaeid bugs with particular reference to those of South Africa. Biotropica 9:217–229
Slater JA (1979) The systematics, phylogeny, and zoogeography of the Blissinae of the world (Hemiptera, Lygaeidae). Bull Am Mus Nat Hist 165:1–180
Slater JA (1980) Systematic relationships of the Antillocorini of the Western Hemisphere (Hemiptera: Lygaeidae). Syst Entomol 5:199–226
Slater JA (1981) Two new genera of Lygaeidae from northern Australia including the first member of the Pamphantini from the Eastern Hemisphere (Hemiptera: Heteroptera). Australian J Entomol 20:111–118
Slater JA (1986a) A synopsis of the zoogeography of the Rhyparochrominae (Heteroptera: Lygaeidae). J New York Entomol Soc 94:262–280
Slater JA (1986b) Aulacoblissus, a new genus of micropterous Blissinae from Venezuela (Hemiptera: Lygaeidae). Florida Entomol 69:661–665
Slater A (1992) A genus level revision of Western Hemisphere Lygaeinae (Heteroptera: Lygaeidae) with keys to species. Univ Kansas Sci Bull 55:1–56
Slater JA (1995) Fifteen new species of Ozophora from Central and South America with a key to mainland Neotropical species (Hemiptera: Lygaeidae). Am Mus Novit 3135:1–31
Slater JA (1999) The systematic position of the Pamphantinae with description of two new tribes and a new species of Cattarus (Hemiptera: Lygaeoidea: Geocoridae). Acta Soc Zool Bohemicae 63:199–208
Slater JA, Baranowski RM (1978) How to know the true bugs (Hemiptera–Heteroptera). Wm. C. Brown Co. Publ, Dubuque
Slater JA, Baranowski RM (1990) The Lygaeidae of Florida (Hemiptera: Heteroptera). Arthropods of Florida and neighboring lands areas. Florida Dep Agric Consumer Serv 14:1–211
Slater JA, Baranowski RM (1994) The occurrence of Oxycarenus hyalinipennis (Costa) (Hemiptera: Lygaeidae) in the West Indies and new Lygaeidae records for the Turks and Caicos Islands of Providenciales and North Caicos. Florida Entomol 77:495–497
Slater A, Baranowski RM (2001) Melanopleuroides dominicanus, a new lygaeine genus and species from the Dominican Republic (Heteroptera: Lygaeidae). Florida Entomol 84:131–132
Slater JA, Brailovsky H (1983) Review of the Neotropical genus Toonglasa (Hemiptera: Lygaeidae). Ann Entomol Soc Am 76:523–535
Slater JA, Brailovsky H (1986) The first occurrence of the subfamily Artheneinae in the Western Hemisphere with the description of a new tribe (Hemiptera: Lygaeidae). J New York Entomol Soc 94:409–415
Slater JA, Brailovsky H (1989) El género Neokleidocerys (Scudder) status nov. y descripción de una especie nueva (Hemiptera-Heteroptera-Lygaeidae-Ischnorhynchinae. An Inst Biol Univ Nac Aut México Ser Zool 59:181–191
Slater JA, Brailovsky H (1990) A further contribution to the systematics of the genus Toonglasa (Hemiptera: Lygaeidae: Blissinae). J New York Entomol Soc 98:406–423
Slater JA, Brailovsky H (2000) Lygaeidae (Hemiptera). In: Llorente-Bousquets JE, González-Soriano E, Papavero N (eds) Biodiversidad, taxonomía y biogeografía de artrópodos de México: Hacia una síntesis de su conocimiento, vol II. Univ Nac Aut México, México, pp 319–333
Slater JA, Henry TJ (1999) Notes and descriptions of new Pamphantinae, including four new species of Cattarus and a remarkable new myrmecomorphic genus and species (Heteroptera: Lygaeoidea: Geocoridae). J New York Entomol Soc 107:304–330
Slater JA, O’Donnell JE (1995) A catalogue of the Lygaeidae of the world (1960–1994). New York Entomological Society, New York
Slater JA, Slater A (1999) Ashlockobius, a new genus of Myodochini from Venezuela (Hemiptera: Lygaeoidea: Rhyparochromidae: Myodochini). Proc Entomol Soc Wash 101:138–142
Slater JA, Wilcox DB (1966) An analysis of three new genera of Neotropical Blissinae (Hemiptera: Lygaeidae). Ann Entomol Soc Am 59:61–76
Slater JA, Woodward TE (1982) Lilliputocorini, a new tribe with six new species of Lilliputocoris, and a cladistic analysis of the Rhyparochrominae (Hemiptera, Lygaeidae). Am Mus Novit 2754:1–23
Slater JA, Schuh RT, Cassis G, Johnson CA, Pedraza-PeZaloza P (2009) Revision of Laryngodus Herrich-Schaeffer, an Allocasuarina feeder, with comments on its biology and the classification of the family (Heteroptera: Lygaeoidea: Rhyparochromidae). Invertebr Syst 23:111–133
Solbreck C (1979) Induction of diapause in a migratory seed bug, Neacoryphus bicrucis (Say) (Heteroptera, Lygaeidae). Oecologia 43:41–49
Solbreck C, Pehrson I (1979) Relations between environment, migration and reproduction in a seed bug, Neacoryphus bicrucis (Say) (Heteroptera, Lygaeidae). Oecologia 43:51–62
Stål C (1870–1876) Enumeratio Hemipterorum: Bidrag till en Företeckning öfver alla hittills kända Hemiptera, jemte systematiska meddelanden. Parts 1–5. Kongliga Svenska Vetenskaps-Akademiens Handlingar, 1870, part 1, 9(1): 1–232; 1872, part 2, 10: 1–159; 1873, part 3, 11: 1–163; 1874, part 4, 12: 1–186; 1876, part 5, 14: 162
Štusák JM (1967) New stilt bugs from the tropics (Heteroptera, Berytidae). Acta Entomol Musei Nat Pragae 37:279–295
Štusák JM (1968) A new genus of Neotropical stilt-bugs (Hemiptera: Berytidae). J New York Entomol Soc 76:2–8
Štusák JM (1971) A new species of Parajalysus Distant from Brazil (Heteroptera, Berytinidae). Acta Entomol Bohemosl 68:149–152
Štusák JM (1973) A new species of Pronotacantha Uhler from Mexico (Heteroptera, Berytinidae). Acta Entomol Bohemosl 70:45–48
Štusák JM (1977) A new Neotropical stilt bug – Metajalysus horvathi gen. et sp. n. (Heteroptera: Berytidae). Acta Zool Acad Scient Hungaricae 23:421–426
Štusák JM, Cobben RH (1975) The Heteroptera of the Netherlands Antilles – X Berytinidae (stilt bugs). Stud Fauna Curação Caribbean Islands 159:63–78
Štys P (1961) Morphology of the abdomen and female ectodermal genitalia of the trichophorous Heteroptera and bearing on their classification. Proc XXth Int Congr Entomol 1:37–43
Štys P (1966) Revision of the genus Dayakiella Horv. and notes on its systematic position (Heteroptera; Colobathristidae). Acta Entomol Bohemosl 63:27–39
Štys P (1967) Monograph of Malcinae, with consideration of morphology and phylogeny of related groups (Heteroptera, Malcidae). Acta Entomol Musei Nat Pragae 37:351–516
Štys P (1991) First apterous genus and species of Lygaeidae: Blissinae (Heteroptera). Acta Entomol Bohemosl 88:265–271
Štys P, Henry TJ (2015) A new genus and species of Colobathristidae (Hemiptera: Heteroptera) from Peru, with a replacement name for the preoccupied Labradoria Kormilev, and a revised key to the Neotropical genera. Proc Entomol Soc Wash 117:27–35
Štys P, Exnerová A (2012) A new genus and species of Oriental Colobathristidae (Hemiptera: Heteroptera) with a key to the Eastern Hemisphere genera and morphological and functional considerations. Entomol Am 118:53–65
Sweet MH (1960) The seed bugs: a contribution to the feeding habits of the Lygaeidae (Hemiptera: Heteroptera). Ann Entomol Soc Am 53:317–321
Sweet MH II (1964a) The biology and ecology of the Rhyparochrominae of New England (Heteroptera: Lygaeidae). Part I. Entomol Am 43:1–124
Sweet MH II (1964b) The biology and ecology of the Rhyparochrominae of New England (Heteroptera: Lygaeidae). Part II. Entomol Am 44:1–201
Sweet MH II (1967) The tribal classification of the Rhyparochrominae (Heteroptera: Lygaeidae). Ann Entomol Soc Am 60:208–226
Sweet MD (1977) The systematic position of the seedbug genus Neosuris Barber, 1924 (Hemiptera: Lygaeidae) with a discussion of the zoogeographical significance of the genus and notes on the distribution and ecology of N. castanea (Barber, 1911) and N. fulgida (Barber, 1918). J Kansas Entomol Soc 50:569–574
Sweet MH II (2000a) Seed and chinch bugs (Lygaeoidea). In: Schaefer CW, Panizzi AR (eds) Heteroptera of economic importance. CRC Press, Boca Raton, pp 143–264
Sweet MH II (2000b) Economic importance of predation by big-eyed bugs (Geocoridae). In: Schaefer CW, Panizzi AR (eds) Heteroptera of economic importance. CRC Press, Boca Raton, pp 713–724
Sweet MH, Schaefer CW (1985) Systematic status and biology of Chauliops fallax Scott, with a discussion of the phylogenetic relationships of the Chauliopinae (Hemiptera: Malcidae). Ann Entomol Soc Am 76:526–536
Tamaki G, Weeks RE (1972) Biology and ecology of two predators, Geocoris pallens Stål and G. bullatus (Say). USDA Tech Bull 1446:1–46
Torre-Bueno JR de al (1946) A synopsis of the Hemiptera-Heteroptera of America north of Mexico. Part III. Family XI–Lygaeidae. Entomol Am 26:1–141
Torres M, Cárdenas E, Pérez S, Morales A (2000) Haematophagy and cleptohaematophagy of Clerada apicicornis (Hemiptera: Lygaeidae), a potential biological control agent of Rhodnius prolixus (Hemiptera: Reduviidae). Mem Inst O Cruz 95:131–133
Uhler PR (1901) Some new genera and species of North American Hemiptera. Proc Entomol Soc Wash 4:507–515
Valério JR, Vieira JM, Valle LCS (1999) Ocorrência de Blissus antillus Leonard (Hemiptera: Lygaeidae: Blissinae) em Pastagem no Estado de Mato Grosso do Sul. An Soc Entomol Brasil 28:527–529
Van Duzee EP (1910) Monograph of genus Crophius Stal. Bull Buffalo Soc Nat Sci 9:389–398
Wheeler AG Jr (1976) Life history of Kleidocerys resedae on European white birch and ericaceous shrubs. Ann Entomol Soc Am 69:459–463
Wheeler AG Jr (1983) The small milkweed bug, Lygaeus kalmii (Hemiptera, Lygaeidae): milkweed specialist or opportunist? J New York Entomol Soc 91:57–62
Wheeler AG Jr (2001) Biology of the plant bugs (Hemiptera: Miridae). Pests, predators, opportunists. Cornell University Press, Ithaca, 506 pp
Wheeler AG Jr, Fetter JE (1987) Chilacis typhae (Heteroptera: Lygaeidae) and the subfamily Artheneinae new to North America. Proc Entomol Soc Wash 89:244–249
Wheeler AG Jr, Hoebeke ER (2013) Establishment of the Palearctic Heterogaster urticae (F.) (Hemiptera: Lygaeoidea: Heterogastridae) in North America, with new British Columbia records of the native H. behrensii (Uhler). Proc Entomol Soc Wash 115:189–196
Wheeler AG Jr, Schaefer CW (1982) Review of stilt-bug (Hemiptera: Berytidae) host plants. Ann Entomol Soc Am 75:498–506
Wille JE (1951) Biological control of certain cotton insects and the application of new organic insecticides in Peru. J Econ Entomol 44:13–18
Acknowledgments
We are grateful to Gary Ouellette (Systematic Entomology Laboratory, Agricultural Research Service, US Department of Agriculture, c/o the National Museum of Natural History, Washington, DC) for preparing the color plates included in this chapter and to Pavel Štys (Charles University, Prague, Czech Republic) and Alfred G. Wheeler (Clemson University, Clemson, South Carolina, USA) for their careful reviews of the manuscript and suggestions for its improvement.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Henry, T.J., Dellapé, P.M., de Paula, A.S. (2015). The Big-Eyed Bugs, Chinch Bugs, and Seed Bugs (Lygaeoidea). In: Panizzi, A., Grazia, J. (eds) True Bugs (Heteroptera) of the Neotropics. Entomology in Focus, vol 2. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9861-7_16
Download citation
DOI: https://doi.org/10.1007/978-94-017-9861-7_16
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9860-0
Online ISBN: 978-94-017-9861-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)