Abstract
Apicomplexans are eukaryotic parasites of major medical and veterinary importance. They have complex life cycles through frequently more than one host, interact with many cell types in their hosts, and can breach host cell membranes during parasite traversal of, or egress from, host cells. Some of these parasites make a strikingly heavy use of the pore-forming MACPF domain, and encode up to 10 different MACPF domain-containing proteins. In this chapter, we focus on the two most studied and medically important apicomplexans, Plasmodium and Toxoplasma, and describe the known functions of their MACPF polypeptide arsenal. Apicomplexan MACPF proteins appear to be involved in a variety of membrane-damaging events, making them an attractive model to dissect the structure-function relationships of the MACPF domain.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The membrane attack complex/perforin (MACPF) domain is conserved in many kingdoms of life. In vertebrates, it is present in the five components of the complement membrane attack complex (C6, C7, C8α, C8β, and C9) and perforin. The MACPF domain is found in proteins that confer plant immunity, such as the constitutively activated cell death 1 (CAD1) protein of Arabidopsis thaliana, and in cytolytic toxins from venomous sea anemones [25, 33, 42]. Bacteria also express MACPF domain-containing proteins, including non-pathogenic species such as most Bacteroides spp. of the microbiota of the human intestinal tract [43], and pathogenic species such as Chlamydia [29] or the insect pathogen P. luminescens [32]. Protozoa also produce MACPF proteins, including free-living predatory organisms, such as Paramecium and Tetrahymena, and parasites of the Apicomplexa phylum [20].
Apicomplexan parasites are an interesting case with respect to the MACPF domain. These organisms, some of which are of major medical and veterinary importance, convert between multiple forms during their life cycle, interact with many cell types in their hosts, and can breach host cell membranes from both sides. MACPF proteins are widespread in apicomplexan parasites, with many genera expressing multiple (up to seven) different versions [20]. Apicomplexan MACPF proteins thus constitute a valuable system to investigate the function of the MACPF domain. In addition, since all MACPF proteins studied so far are critical to parasite infectivity, they are attractive targets for anti-parasite control strategies.
Here, we overview the conservation of the MACPF domain in apicomplexans and the structural singularities of MACPF proteins in these parasites, and focus on the known contributions of the MACPF proteins in the life cycles of Plasmodium and Toxoplasma.
MACPF Proteins in Apicomplexa Parasites
Apicomplexans
The Apicomplexa phylum of protozoa contains almost exclusively parasitic organisms, which frequently exhibit complex life cycles involving more than one host. Some infect blood cells and rely on blood feeding invertebrates for transmission, such as Plasmodium, the agent of malaria, or Theileria and Babesia, which cause severe diseases in cattle [6]. Others invade the intestinal tract of animals and are transmitted via the fecal-oral route, such as Toxoplasma and Cryptosporidium, which cause severe pathologies and death in immuno-compromised patients [15, 39].
Most apicomplexan parasites are obligatorily intracellular, i.e., multiply only inside host cells, and continuously switch between an intracellular and an extracellular position in their hosts. Their extracellular stages (zoites) are typically polarized and motile cells. They use a sub-membrane actin-myosin motor to power a unique type of substrate-dependent motility, termed gliding motility, which propels them at high speeds (up to 5 μm/sec) without overt change in parasite shape [23]. In most cases apicomplexan zoites invade host cells inside a parasitophorous vacuole (PV), formed upon invagination of the host cell plasma membrane around the entering zoite [4, 8]. Inside the PV, the parasite multiplies into daughter zoites, which then egress the host cell and go on to invade new host cells [5, 12]. Some apicomplexan stages, including in Plasmodium, can traverse host cells, i.e., breach their plasma membrane and glide through their cytosol before egressing, without PV formation. Motility, cell traversal and cell invasion require the discharge of apical secretory organelles called micronemes, which are a defining feature of the phylum [37].
Apicomplexan MACPF Proteins
All genomes of apicomplexan parasites that have been sequenced so far encode MACPF domains, except that of Cryptosporidium, an early-branching clade in the phylum. Remarkably, among studied apicomplexans those transmitted by insects, e.g. Plasmodium, Theileria and Babesia, appear to produce more MACPF proteins (Fig. 12.1). Plasmodium, transmitted by Anopheles mosquitoes, expresses five MACPF proteins, while Theileria and Babesia, which are transmitted by ticks, produce at least six and seven MACPF proteins, respectively. Unlike Plasmodium (Haemosporididae), Theileria and Babesia (Piroplasmidae) escape from the enclosing PV shortly after invasion and replicate free within the host cell cytoplasm.
Apicomplexan PLPs. Schematic representation of the genes encoding MACPF domains in Plasmodium falciparum (Pf), Babesia bovis (Bb), Theileria annulata (Ta), Toxoplasma gondii (Tg), Neospora caninum (Nc) and Eimeria tennela (Et). The MACPF domain is represented in red. Other classical domains such as C2 (blue), Thrombospondin type I repeat (TSP-1, green) and Low Density Lipoprotein receptor domain A (LDL-A, purple) are found, respectively, in perforin and complement factor 9. In apicomplexan PLPs only a kinase domain (yellow) is found in Eimeria PLP1
Structural homology modeling of the MACPF domain in PLP1 of T. gondii [19] confirmed that the characteristic structural features of MACPF domains, which comprise a twisted, four-stranded β-sheet placed on top of two helical clusters [13, 32], are conserved in the MACPF domains of apicomplexans (MACPFapi). The helical clusters of MACPFapi domains exhibit the pattern of alternating hydrophobic and hydrophilic amino acids found in other MACPF domains [19], which convert into amphipathic β-hairpins and insert into the target membrane during pore-formation. The MACPFapi domains also contain a conserved insertion of a pair of anti-parallel α-helices, which is absent from canonical MACPF domains. This insertion is modeled to reside on the external face of the MACPFapi domain in a region that contains important residues for oligomerization of membrane-bound perforin monomers [19, 20]. MACPF domains exhibit limited sequence similarity but contain a signature [YW]-G-[TS]-H-[FY]-x(6)-G-G motif [32, 36], while MACPFapi domains display a signature pattern of W-x(2)-[FL]-[FI]-x(2)-[FY]-G-T-H-x(7)-G-G.
The MACPF domain is commonly found associated with N- and C-terminal domains, such as TSR, LDLRA, Sushi/CCP/SCR, FIMAC or C2, which are thought to control and/or target MACPF function. The MACPFapi domains characterized to date all share similar domain architectures with an N-terminal region, followed by a centrally located MACPFapi domain, and a C-terminal β-pleated sheet-rich domain unique to the apicomplexan phylum. However, the N-terminal region of MACPFapi proteins is not conserved across the phylum, varying considerably in length and sequence, suggesting that they might have unique functions in each MACPFapi protein. The C-terminal region of Toxoplasma PLP1, which consists of a β-sheet-rich domain, is reminiscent of C-terminal domains in other MACPF proteins, which play critical roles in binding to lipids or protein receptors in target membranes [35]. These C-terminal domains typically consist of β-pleated sheets, e.g., an EGF-like domain followed by a TSR in C8α or the β-sandwich immunoglobulin domain in cholesterol-dependent cytolysins (CDCs) [28]. The C-terminal domains of MACPFapi proteins contain multiple copies of a domain predicted to possess five β-strands [20]. This domain, named Apicomplexan Perforin-like protein C-terminal β-sheet (APC-β) domain, is ~55-residue long, is generally present in three tandem copies, is found associated with MACPFapi domains across the apicomplexan phylum, and appears to be unique to that phylum [20].
MACPF Proteins in Plasmodium
Plasmodium Life Cycle: In and Out and Through Host Cells
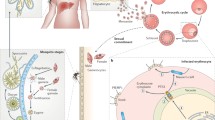
Malaria, caused by Plasmodium, remains the most deadly parasitic disease in humans (WHO 2011 report, [24]), causing up to one million deaths per year. The Plasmodium parasite is transmitted during the bite of an Anopheles mosquito. During its life, the parasite adopts distinct forms adapted to the various tissues encountered and parasitized in both hosts, as schematized in Fig. 12.2. Central to the parasite life and causing the symptoms in the mammalian host are the cycles of parasite multiplication inside RBC. The parasite invades a RBC inside a PV and either produces 10–30 new parasites during asexual multiplication or differentiates into sexual forms. In either case, the parasite(s) ultimately leave the RBC. Sexual reproduction takes the parasite inside a mosquito, successively in the midgut lumen, across the midgut epithelium, inside the mosquito circulation and through the salivary gland system. Once injected in the skin of the host during the mosquito bite, the parasite rapidly invades capillaries in the dermis and egresses sinusoids in the liver and eventually invades hepatocytes inside a PV, where it re-transforms into the RBC-infecting form.
Plasmodium life cycle through the mosquito vector and the mammalian host, and parasite-induced disruption of host cell membranes. During a blood meal, an infected anopheline mosquito injects sporozoites into the skin (red asterisk). The motile sporozoites traverse cells in the skin using PPLP1/SPECT2 to reach blood vessels, finding their way to the liver. In the liver sinusoids, sporozoites use PPLP1 to resist clearance by Kupffer cells and cross the sinusoids, getting access to hepatocytes. After traversing several hepatocytes, a sporozoite invades a final hepatocyte inside a PV, where it transforms into tens of thousands of merozoites. Merozoites egress hepatocytes inside merosomes, which are vesicles bound by the hepatocyte membrane shed into the sinusoid circulation that protects them from local macrophages (Kupffer cells, KC) in the liver sinusoids. Merozoites invade RBC inside a PV and asexually multiply, generating 10–30 new merozoites at each cycle and causing the symptoms of the disease. Intra-RBC parasites can also differentiate into gametocytes and, when ingested by the mosquito, into gametes. One female macrogamete and eight male microgametes egress from RBC; egress of the male microgametes from the RBC membrane requires PPLP2. Fertilization in the mosquito gut lumen/blood meal generates a zygote, which transforms into a motile ookinete. The ookinete traverses epithelial cells, using at least PPLP3 and PPLP5, and lodges underneath the basal lamina that separates the gut epithelium from the mosquito body cavity/circulation. Inside an oocyst, thousands of sporozoites are formed, which are released in the cavity, attach to the salivary glands, traverse cells in a MACPF-independent way and finally reach the salivary cavities and ducts, where they await transmission to a new host
MACPF Proteins in Plasmodium
Five genes encoding a MACPF domain are present in all Plasmodium genomes sequenced so far: P. falciparum, P. vivax and P. knowlesi, which cause malaria in humans, and P. yoelli, P. berghei and P. chabaudi, which infect rodents. Their products were initially called Plasmodium perforin-like proteins (PPLPs) [21]. The role that these proteins play in the parasite life cycle was addressed in P. berghei, a species that is particularly amenable to genetic manipulation [26]. Parasites in which the PPLP1-, PPLP2-, PPLP3- and PPLP5-encoding genes were inactivated were selected and cloned (only erythrocytic stages of Plasmodium can be genetically manipulated). This indicates that these genes are not important for parasite multiplication in, and egress from, erythrocytes, in agreement with gene expression analysis in P. yoelii and P. falciparum showing that the family is not expressed in blood-stage parasites [21]. Nonetheless, inactivation of the PPLP4-encoding gene, which is mostly expressed in the sexual and/or ookinete stages [14, 30] generated mutants that could not be cloned, raising the possibility that PPLP4 might act during parasite multiplication in the blood [11]. All characterized MACPF proteins locate to parasite micronemes [10, 17, 18, 21].
PPLP2: Male Gamete Egress from Erythrocytes
To egress erythrocytes, gametocytes must rupture both the parasitophorous vacuole membrane (PVM) and the erythrocyte plasma membrane (EPM) to fertilize as gametes in the mosquito midgut lumen (Fig. 12.2). In P. berghei, male, but not female gametocytes produce PPLP2. P. berghei PPLP2(-) parasites generate normally fertile female gametes, but infertile male gametes [9]. In the wild-type (WT), eight male flagellated gametes (one axoneme and one nucleus surrounded by a flagellar membrane) normally break out from the cell to rapidly attach to erythrocytes, forming exflagellation centers. In contrast, PPLP2(-) male gametocytes, while rupturing the PVM, fail to rupture the EPM, forming flagella that remain bundled and trapped in the intact EPM. However, the fertilization block is not complete. Ookinetes are occasionally formed, which in turn do not display any detectable defect in crossing the mosquito midgut epithelium, showing that the contribution of PPLP2 is restricted to egress of the male gametocyte.
PPLP3 and PPLP5: Ookinete Traversal of Insect Cells
Fertilization in the mosquito midgut generates a motile zygote, called ookinete, which needs to cross the midgut epithelium to reach the basal lamina, where it transforms into an oocyst. The ookinete wounds and traverses midgut epithelial cells [34], usually killing the traversed cell [45]. P. berghei parasites deficient in PPLP3/MAOP (membrane-attack ookinete protein) [18] or in PPLP5 [10] display the same phenotype: ookinetes are normally formed but fail to cross midgut epithelial cells and do not generate oocysts. In both cases, mutant ookinetes remain attached to the microvilli of the apical side of the midgut epithelium, sometimes causing the epithelial cell membrane to invaginate, without breaking [18]. Interestingly, injection of PPLP5-deficient ookinetes in the mosquito hemocoel (body cavity), i.e., on the other side of the midgut epithelium, restores parasite infectivity in subsequent stages, showing that PPLP5 activity is restricted to ookinete passage through the midgut epithelium barrier.
PPLP1: Sporozoite Traversal of Mammalian Cells
The oocyst, which derives from the ookinete, generates thousands of sporozoites, the only parasite stage that infects cells in both the mosquito and mammalian hosts. The oocyst releases sporozoites in the mosquito hemocoel, which invade the salivary gland cells to reach the salivary cavities and ducts. Sporozoites then reside extracellularly in the mosquito salivary system, where they await transmission during a mosquito bite [27].
The motile sporozoite can breach the plasma membrane of, and traverse host cells, including macrophages [41], hepatocytes and epithelial cell types [22]. PPLP1/SPECT2 (sporozoite protein essential for cell traversal 2) was the first MACPF-containing product characterized in Plasmodium [17, 21]. SPECT2 is expressed only in sporozoites and specifically in those present in the mammalian host, and is the sole PPLP produced by sporozoites. P. berghei SPECT2(-) parasites are normally infective until sporozoites have invaded the mosquito salivary glands [17]. They fail to wound hepatocytes and other host cell types in vitro and are poorly infective after injection into mice [17]. Intravital imaging showed that SPECT2 was important to (i) traverse host cells and freely locomote in the skin [1], (ii) traverse and resist killing by Kupffer cells, the resident macrophages in the liver sinusoids [40], and (iii) cross the sinusoidal barrier by breaching through endothelial cells and/or Kupffer cells [40]; Fig. 12.3). The SPECT2-mediated host cell traversal activity thus serves multiple purposes during the sporozoite journey, including progression through cellular barriers in the skin and across endothelia and preventing phagocytosis by host immune cells.
Host cell traversal (CT) by Plasmodium sporozoites in the liver. Sporozoites are capable of cell traversal, i.e., breaching host cell plasma membranes, gliding through the cytosol and egressing. Sporozoite CT requires PPLP1/SPECT2 and can be detected by the incorporation of membrane-impermeant dye on the extracellular medium or alternatively by the loss of intracellular contents. a Time-lapse microscopy of a sporozoite (SPZ-red) traversing a primary liver sinusoidal GFP+ endothelial cell (EC-green) isolated from a flk1-gfp mouse in the presence of propidium iodide (PI) and triggering the specific decrease of the GFP fluorescence intensity of EC and the simultaneous incorporation of PI in the EC (red). b Intravital imaging in the liver of mice showing the fading of the GFP and the incorporation of PI by the EC when a sporozoite (SPZ-red) crosses the sinusoid (Flk1-GFP-EC, green) and enters the black parenchyma. c During their journey in the sinusoids, motile sporozoites (SPZ-red) frequently interact and wound Kupffer cells (KC, resident macrophages) as detected by the incoporation of PI (red) in F4/80+ cells (blue). d In contrast, PPLP1/SPECT2-deficient sporozoites (SPECT2− SPZ) associate with and are cleared by F4/80+-KC (blue)
TgPLP1 of Toxoplasma gondii: Tachyzoite Egress from Host Cells
Toxoplasma causes congenital birth defects and severe encephalitis in immuno-compromised individuals. Its life cycle consists of a sexual phase in cats and an asexual phase that can occur in virtually any warm-blooded animal. In the latter, Toxoplasma sporozoites invade intestinal epithelial cells, where they transform into tachyzoites, a rapidly multiplying parasite stage that lacks cell traversal ability. Tachyzoites spread via the blood stream to all organs and tissues of the body, where they invade host cells and convert into bradyzoites, the dormant stage that forms tissue cysts and can reactivate infection upon host immune depression.
The Toxoplasma gondii genome encodes two MACPF proteins, TgPLP1 and TgPLP2. Only TgPLP1 was functionally characterized. TgPLP1(-) tachyzoites are impaired in egressing from host cells and rupturing the PVM [19]. Interestingly, in cells containing both TgPLP1(-) and WT tachyzoites in distinct PVs, TgPLP1(-) tachyzoites readily egress their PV via the activity of TgPLP1 produced by WT tachyzoites, which shows that TgPLP1 can function from either side of the PVM.
Like all MACPF proteins, TgPLP1 contains N- and C-terminal domains flanking the MACPF domain. Biochemical analysis of TgPLP1 showed that it forms large, multimeric membrane-embedded complexes, and that recombinant TgPLP1 was sufficient for membrane disruption [31]. Structure-function analysis also showed that each of the central MACPF domain and the ß-sheet-rich C-terminal domain, but not the N-terminal domain, were required for activity, and that each of the N- and C-terminal domains were sufficient for membrane association.
Conclusions and Perspectives
It appears from the initial characterization of the roles of MACPFapi proteins that they mediate many, but not all host membrane-disruption events imposed by the life cycles of apicomplexan parasites. In Plasmodium, MACPF proteins are mainly used for cell traversal by the ookinete and the sporozoite, although the sporozoite appears to traverse salivary gland cells in a MACPF-independent way. A MACPF protein used by the P. berghei male, but not female gamete permits egress from erythrocytes, by rupturing the EPM. Merozoites, however, do not use a MACPF protein for egressing host cells. In contrast, T. gondii tachyzoites use a MACPF protein for egressing, but by rupturing the PVM.
Since the merozoite appears to be the only Plasmodium stage that does not use a MACPF to egress the host cell, it is interesting to note that merozoites egress hepatocytes by a complex mechanism that separates the rupturing of the PVM and of the host cell/hepatocyte membrane (HPM) [38]. After the rupture of the PVM, and the release of thousands of merozoites in the hepatocyte cytosol, the HPM initially remains intact. Merozoite-filled vesicles bound by the HPM (merosomes) bud off from infected hepatocytes into the sinusoid circulation. The merosome/hepatocyte membrane shields merozoites from local macrophages patrolling in the liver sinusoids, and only ruptures when merosomes have reached the lungs, where released merozoites safely invade erythrocytes [3].
Why does the ookinete use two, and possibly three, MACPF proteins to cross a single epithelial cell in the insect when the sporozoite use only one to traverse multiple cell types in different tissues in the mammal? Do the two or three MACPF proteins secreted by the ookinete act as a complex, analogous to the interaction of C6-C9 in the membrane attack complex? If so, what specific function is conferred by multiple MACPF domains in the pore-forming complex of the ookinete? The PPLP1/SPECT2 of the sporozoite might also act as a complex with non-MACPF proteins, including SPECT that has a similar contribution to SPECT2 in sporozoite infectivity [1, 16, 17]. Reconstitution of the entire pore-forming machineries at each stage will be essential for understanding their mode of action.
How do apicomplexan MACPF proteins act? Formation of large pore might be sufficient for a motile parasite (egressing or traversing) to squeeze in and move through. Pore formation may also permit the translocation of other effectors, e.g., membrane-damaging proteases or lipases (in the case of egress) or molecules facilitating the degradation of the cortical actin network (in the case of traversal). The pore may also cause ion fluxes that influence parasite egress. For example, ATP or K+ efflux or Ca2+ influx might induce host cell contraction, initiate parasite motility, or activate parasite (SUB1, SERAs; [2, 44]) or host (calpains; [7]) proteases.
The receptor(s) recognized by apicomplexan MACPF proteins on the various membranes parasites encounter in insect and mammalian hosts are unknown. Toxoplasma PLP1 acts on either side of the PVM, and Plasmodium sporozoites need to rupture the membrane of the traversed cell from the outside and the inside. Both the N-terminal and C-terminal domains of TgPLP1 bind to membranes, although only the latter is required for MACPF activity while the former may regulate MACPF activity via a processing event [31]. Understanding how MACPF activity is regulated might help explain how Plasmodium sporozoites, which invade host cells by secreting micronemes and thus MACPF proteins, prevent the rupture of the nascent PVM.
Further studies in apicomplexans promise to yield interesting insights into the MACPF domain. The genetic tractability of these parasites, along with the diversity of membrane disruption events they cause, should help dissect the structure-function relationships and adaptive potential of the fascinating MACPF fold.
Abbreviations
- APC-β:
-
Apicomplexan perforin-like protein C-terminal β-sheet
- CAD1:
-
Constitutively activated cell death 1
- CCP:
-
Complement control protein
- CDCs:
-
Cholesterol-dependent cytolysins
- EPM:
-
Erythrocyte plasma membrane
- FIMAC :
-
Factor I membrane attack complex
- HPM:
-
Hepatocyte plasma membrane
- LDL-A:
-
Low density lipoprotein receptor domain A
- MACPF:
-
Membrane-attack complex/perforin
- MACPFapi :
-
MACPF domains of apicomplexans
- MAOP:
-
Membrane-attack ookinete protein
- PLP:
-
Perforin-like protein
- PPLP:
-
Plasmodium perforin-like protein
- PV:
-
Parasitophorous vacuole
- PVM:
-
Parasitophorous vacuole membrane
- RBC:
-
Red blood cell
- SCR:
-
Short consensus repeats
- SPECT:
-
Sporozoite microneme protein essential for cell traversal
- TSR:
-
Thrombospondin type I repat
- WT:
-
Wild type
References
Amino R, Giovannini D, Thiberge S, Gueirard P, Boisson B, Dubremetz JF, Prévost MC, Ishino T, Yuda M, Ménard R (2008) Host cell traversal is important for progression of the malaria parasite through the dermis to the liver. Cell Host Microbe 3:88–96
Arastu-Kapur S, Ponder EL, Fonović UP, Yeoh S, Yuan F, Fonović M, Grainger M, Phillips CI, Powers JC, Bogyo M (2008) Identification of proteases that regulate erythrocyte rupture by the malaria parasite Plasmodium falciparum. Nat Chem Biol 4:203–213
Baer K, Klotz C, Kappe SH, Schnieder T, Frevert U (2007) Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoS Pathog 3:e171
Bargieri D, Lagal V, Tardieux I, Ménard R (2012) Host cell invasion by apicomplexans: what do we know? Trends Parasitol 28:131–135
Blackman MJ (2008) Malarial proteases and host cell egress: an ‘emerging’ cascade. Cell Microbiol 10:1925–1934
Chalmers RM, Giles M (2010) Zoonotic cryptosporidiosis in the UK—challenges for control. J Appl Microbiol 109:1487–1497
Chandramohanadas R, Davis PH, Beiting DP, Harbut MB, Darling C, Velmourougane G, Lee MY, Greer PA, Roos DS, Greenbaum DC (2009) Apicomplexan parasites co-opt host calpains to facilitate their escape from infected cells. Science 324:794–797
Cowman AF, Berry D, Baum J (2012) The cellular and molecular basis for malaria parasite invasion of the human red blood cell. J Cell Biol 198:961–971
Deligianni E, Morgan RN, Bertuccini L, Wirth CC, Silmon de Monerri NC, Spanos L, Blackman MJ, Louis C, Pradel G, Siden-Kiamos I (2013) A perforin-like protein mediates disruption of the erythrocyte membrane during egress of Plasmodium berghei male gametocytes. Cell Microbiol 15:1438–1455
Ecker A, Pinto SB, Baker KW, Kafatos FC, Sinden RE (2007) Plasmodium berghei: Plasmodium perforin-like protein 5 is required for mosquito midgut invasion in Anopheles stephensi. Exp Parasitol 116:504–508
Ecker A, Bushell ES, Tewari R, Sinden RE (2008) Reverse genetics screen identifies six proteins important for malaria development in the mosquito. Mol Microbiol 70:209–220
Graewe S, Rankin KE, Lehmann C, Deschermeier C, Hecht L, Froehlke U, Stanway RR, Heussler V (2011) Hostile takeover by Plasmodium: reorganization of parasite and host cell membranes during liver stage egress. PLoS Pathog 7:e1002224
Hadders MA, Beringer DX, Gros P (2007) Structure of C8alpha-MACPF reveals mechanism of membrane attack in complement immune defense. Science 317:1552–1554
Hall N, Karras M, Raine JD, Carlton JM, Kooij TW, Berriman M, Florens L, Janssen CS, Pain A, Christophides GK, James K, Rutherford K, Harris B, Harris D, Churcher C, Quail MA, Ormond D, Doggett J, Trueman HE, Mendoza J, Bidwell SL, Rajandream MA, Carucci DJ, Yates JR 3rd, Kafatos FC, Janse CJ, Barrell B, Turner CM, Waters AP, Sinden RE (2005) A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science 307:82–86
Innes EA (2010) A brief history and overview of Toxoplasma gondii. Zoonoses Public Health 57:1–7
Ishino T, Yano K, Chinzei Y, Yuda M (2004) Cell-passage activity is required for the malarial parasite to cross the liver sinusoidal cell layer. PLoS Biol 2:E4
Ishino T, Chinzei Y, Yuda M (2005) A Plasmodium sporozoite protein with a membrane attack complex domain is required for breaching the liver sinusoidal cell layer prior to hepatocyte infection. Cell Microbiol 7:199–208
Kadota K, Ishino T, Matsuyama T, Chinzei Y, Yuda M (2004) Essential role of membrane-attack protein in malarial transmission to mosquito host. Proc Natl Acad Sci USA 101:16310–16315
Kafsack BF, Pena JD, Coppens I, Ravindran S, Boothroyd JC, Carruthers VB (2009) Rapid membrane disruption by a perforin-like protein facilitates parasite exit from host cells. Science 323:530–533
Kafsack BF, Carruthers VB (2010) Apicomplexan perforin-like proteins. Commun Integr Biol 3:18–23
Kaiser K, Camargo N, Coppens I, Morrisey JM, Vaidya AB, Kappe SH (2004) A member of a conserved Plasmodium protein family with membrane-attack complex/perforin (MACPF)-like domains localizes to the micronemes of sporozoites. Mol Biochem Parasitol 133:15–26
Mota MM, Pradel G, Vanderberg JP, Hafalla JC, Frevert U, Nussenzweig RS, Nussenzweig V, Rodríguez A (2001) Migration of Plasmodium sporozoites through cells before infection. Science 291:141–144
Münter S, Sabass B, Selhuber-Unkel C, Kudryashev M, Hegge S, Engel U, Spatz JP, Matuschewski K, Schwarz US, Frischknecht F (2009) Plasmodium sporozoite motility is modulated by the turnover of discrete adhesion sites. Cell Host Microbe 6:551–562
Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD (2012) Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379:413–431
Oshiro N, Kobayashi C, Iwanaga S, Nozaki M, Namikoshi M, Spring J, Nagai H (2004) A new membrane-attack complex/perforin (MACPF) domain lethal toxin from the nematocyst venom of the Okinawan sea anemone Actineria villosa. Toxicon 43:225–228
Philip N, Orr R, Waters AP (2013) Transfection of rodent malaria parasites. Methods Mol Biol 923:99–125
Pimenta PF, Touray M, Miller L (1994) The journey of malaria sporozoites in the mosquito salivary gland. J Eukaryot Microbiol 41:608–624
Polekhina G, Giddings KS, Tweten RK, Parker MW (2005) Insights into the action of the superfamily of cholesterol-dependent cytolysins from studies of intermedilysin. Proc Natl Acad Sci USA 102:600–605
Ponting CP (1999) Chlamydial homologues of the MACPF (MAC/perforin) domain. Curr Biol 9:R911–913
Raibaud A, Brahimi K, Roth CW, Brey PT, Faust DM (2006) Differential gene expression in the ookinete stage of the malaria parasite Plasmodium berghei. Mol Biochem Parasitol 150:107–113
Roiko MS, Carruthers VB (2013) Functional dissection of toxoplasma gondii perforin-like protein 1 reveals a dual domain mode of membrane binding for cytolysis and parasite egress. J Biol Chem 288:8712–8725
Rosado CJ, Buckle AM, Law RH, Butcher RE, Kan WT, Bird CH, Ung K, Browne KA, Baran K, Bashtannyk-Puhalovich TA, Faux NG, Wong W, Porter CJ, Pike RN, Ellisdon AM, Pearce MC, Bottomley SP, Emsley J, Smith AI, Rossjohn J, Hartland EL, Voskoboinik I, Trapani JA, Bird PI, Dunstone MA, Whisstock JC (2007) A common fold mediates vertebrate defense and bacterial attack. Science 317:1548–1551
Rosado CJ, Kondos S, Bull TE, Kuiper MJ, Law RH, Buckle AM, Voskoboinik I, Bird PI, Trapani JA, Whisstock JC, Dunstone MA (2008) The MACPF/CDC family of pore-forming toxins. Cell Microbiol 10:1765–1774
Shahabuddin M, Pimenta PF (1998) Plasmodium gallinaceum preferentially invades vesicular ATPase-expressing cells in Aedes aegypti midgut. Proc Natl Acad Sci USA 95:3385–3389
Shatursky O, Bayles R, Rogers M, Jost BH, Songer JG, Tweten RK (2000) Clostridium perfringens beta-toxin forms potential-dependent, cation-selective channels in lipid bilayers. Infect Immun 68:5546–5551
Slade DJ, Lovelace LL, Chruszcz M, Minor W, Lebioda L, Sodetz JM (2008) Crystal structure of the MACPF domain of human complement protein C8 alpha in complex with the C8 gamma subunit. J Mol Biol 379:331–342
Singh S, Chitnis CE (2012) Signalling mechanisms involved in apical organelle discharge during host cell invasion by apicomplexan parasites. Microbes Infect 14:820–824
Sturm A, Amino R, van de Sand C, Regen T, Retzlaff S, Rennenberg A, Krueger A, Pollok JM, Menard R, Heussler VT (2006) Manipulation of host hepatocytes by the malaria parasite for delivery into liver sinusoids. Science 313:1287–1290
Suarez CE, Noh S (2011) Emerging perspectives in the research of bovine babesiosis and anaplasmosis. Vet Parasitol 180:109–125
Tavares J, Formaglio P, Thiberge S, Mordelet E, Van Rooijen N, Medvinsky A, Ménard R, Amino R (2013) Role of host cell traversal by the malaria sporozoite during liver infection. J Exp Med 210:905–915
Vanderberg JP, Chew S, Stewart MJ (1990) Plasmodium sporozoite interactions with macrophages in vitro: a videomicroscopic analysis. J Protozool 37:528–536
Voskoboinik I, Smyth MJ, Trapani JA (2006) Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol 6:940–952
Xu Q, Abdubek P, Astakhova T, Axelrod HL, Bakolitsa C, Cai X, Carlton D, Chen C, Chiu HJ, Clayton T, Das D, Deller MC, Duan L, Ellrott K, Farr CL, Feuerhelm J, Grant JC, Grzechnik A, Han GW, Jaroszewski L, Jin KK, Klock HE, Knuth MW, Kozbial P, Krishna SS, Kumar A, Lam WW, Marciano D, Miller MD, Morse AT, Nigoghossian E, Nopakun A, Okach L, Puckett C, Reyes R, Tien HJ, Trame CB, van den Bedem H, Weekes D, Wooten T, Yeh A, Zhou J, Hodgson KO, Wooley J, Elsliger MA, Deacon AM, Godzik A, Lesley SA, Wilson IA (2010) Structure of a membrane-attack complex/perforin (MACPF) family protein from the human gut symbiont Bacteroides thetaiotaomicron. Acta Crystallogr, Sect F: Struct Biol Cryst Commun 66:1297–1305
Yeoh S, O’Donnell RA, Koussis K, Dluzewski AR, Ansell KH, Osborne SA, Hackett F, Withers-Martinez C, Mitchell GH, Bannister LH, Bryans JS, Kettleborough CA, Blackman MJ (2007) Subcellular discharge of a serine protease mediates release of invasive malaria parasites from host erythrocytes. Cell 131:1072–1083
Zieler H, Dvorak JA (2000) Invasion in vitro of mosquito midgut cells by the malaria parasite proceeds by a conserved mechanism and results in death of the invaded midgut cells. Proc Natl Acad Sci USA 97:11516–11521
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Tavares, J., Amino, R., Ménard, R. (2014). The Role of MACPF Proteins in the Biology of Malaria and Other Apicomplexan Parasites. In: Anderluh, G., Gilbert, R. (eds) MACPF/CDC Proteins - Agents of Defence, Attack and Invasion. Subcellular Biochemistry, vol 80. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-8881-6_12
Download citation
DOI: https://doi.org/10.1007/978-94-017-8881-6_12
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-8880-9
Online ISBN: 978-94-017-8881-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)