Summary
Photosynthesis in lichens is intimately linked to the photosynthetic capacities of the photobiont, i.e. autotrophic algae and cyanobacteria, that form the lichen association together with a fungal partner. Lichen photosynthesis in nature is also affected by a complex mixture of internal and external factors.
Intrathalline locatization of photobiont cells, structure of photobiont layer, functional photobiont-mycobiont interlink, and physico-chemical properties of the fungal part of thallus are considered important internal characteristics affecting photosynthesis and utilization of photosynthetic products in lichens. In this chapter, a brief introduction into the anatomy and morphology is provided from a view point of function. Special attention is given to cellular structure of photobionts, and especially to the chloroplast of unicellular alga Trebouxia, the most abundant symbiotic alga in lichen association. Since lichens are typical poikilohydric organism with no active control of their hydration status, the photosynthetic responses of lichens to full, partial and severely limited water supply are described. In addition the protective mechanisms activated during thallus desiccation are discussed. Several aspects of lichen photosynthesis including light-response curves, photoinhibition, activation of photoprotective mechanisms and reactive oxygen species-induced changes in the amount and activity of antioxidative substances are reviewed. Lichens can photosynthesize over a wide temperature range, including subzero temperature. The photobiont also exhibits response depending on nitrogen availability and exposure to heavy metals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chlorophyll Fluorescence
- Lichen Species
- Chlorophyll Fluorescence Parameter
- Usnic Acid
- High Light Treatment
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
I. Introduction
Photosynthesis is a key metabolic function of lichens. Therefore a understanding of mechanisms and limitations of photosynthesis in this symbiotic organisms is desirable. Since many lichens are considered extremophilic organisms, that exhibit great tolerance against a variety of stress factors, lichen photosynthesis in response to several of these stress factors has been investigated in detail. Defining and characterizing the range of conditions that lichen can tolerate continuous to be an active area of research. The photosynthetic machinery of lichens responds to these challenges with a number of biophysical and biochemical protective mechanisms. Although some of these protective mechanisms, may have well-characterized homologous counterparts in plants, many mechanisms in lichens are not yet fully understood. This lack of knowledge has given generations of lichenologist, plant physiologist and ecophysiologist the motivation to study lichen photosynthesis under natural and controlled conditions. In recent years, molecular, proteomic and metabolomic approaches are increasingly employed to study lichen photosynthesis. In addition miniaturized and automated measuring devices are now available that enable detailed long-term studies of photosynthesis in the field. This chapter gives an overview of our current knowledge of lichen photosynthesis, with an emphasis on mechanisms that have been revealed under stress conditions.
II. Lichen Anatomy and Morphology
A. Symbionts and Thallus Forms
Liches are symbiotic organisms consisting of at least two partners: A photobiont and mycobiont that form the structures of a lichenized association, i.e. the lichen thallus. The mycobiont, i.e. fungal partner (in most cases Ascomycetes) forms the majority of the lichen thallus biomas. The mycobiont is with only a few exceptions, responsible for thallus morphology and growth form. The photobiont, i.e. photosynthetizing partner, is an alga or cyanobacterium. The relation between these two partners is considered a mutualistic symbiosis.
While bipartite lichens consist of one mycobiont and one photobiont species, tripartite lichens have two photobionts in a single mycobiont. Tripartite lichens have typically an alga (see Chap. 17) and cyanobacteria (see Chap. 14) in association with the mycobiont. Areas where these two photobionts are located have anatomical differences in the majority of cases – see e.g. Placopsis contortuplicata (Fig. 20.1).
Regarding thallus morphology, lichens are divided into three main and several additional morphological groups. In this chapter, only the main ones are treated: crustose liches, foliose lichens, and fruticose lichens (for more details see e.g. Büdel and Scheidegger 1996).
Crustose lichens are tightly attached to the substrate (upper surface of stones and rocks) with their lower surface (see Fig. 20.2). A typical feature that distinguishes crustose lichens from the foliose and fruticose lichens is that crustose lichens cannot be removed from the surface without a lot of force and destruction of the thallus structure.
Foliose lichens possess generally a flat leaf-like thallus, which is attached to the surface only partially by special structures: rhizines, cilia, tomentum or umbilicus (see Fig. 20.3). Foliose lichens exhibit considerable difference in coloration and surface structure between the upper and lower thallus surface. The thallus of foliose lichens is often divided into lobes that show various degree of branching. The size of the lobes can vary from several milimeters to more than 10 cm. Among foliose lichens, two subgroups can be distinguished: laciniate and umbilicate. While laciniate thalli typically exhibit numerous lobes arranged radially or forming overlapping structures, umbilicate lichens create circular thalli consisting of one unbranched lobe.
Foliose thalli of Umbilicaria antarctica, a macrolichen, are abundant in maritime Antarctica. In the dry state, the upper side of the thallus exhibits a pale brown color (left), while the lower side of the thallus is black (right). There is only one point that connects the thallus to a surface (typically rock); this umbilicus is located in the center of the thallus (right) (Photo M. Barták).
Lichens with fruticose thallus morphology typically form three-dimensional branching structures. These structures may be arranged upward (shrub-like structures) or form hanging branched structures oriented downward from a basal holdfast attached to the substrate (typically tree branches or stems). Fruticose lichens form a cluster of free-standing, branching tubes, which are usually round in cross section (see Fig. 20.4). Some fruticose lichens, however, may have flattened branches such as e.g. Ramalina sp.
Cluster of fruticose thalli (left) and cross section of the middle part of a single thallus of Usnea aurantiaco-atra (right) showing typical anatomical structures. From the thallus periphery to the thallus center, the outer cortex (brown), medulla with symbiotic algae (individual dark points) forming round-shaped layer, and a cell-free internal space (light red) can be distinguished (Photos M. Barták (left), J. Rotkovská (right)).
B. Intrathalline Location of Photobionts and Their Photosynthetic Properties
More than 40 algal and cyanobacterial genera have been described that participate as photobionts in lichen association (Ahmadjian 1993). The most frequent algal photobionts include species of the Trebouxia, Trentepohlia, Coccomyxa, and Dictyochloropsis clades. In cyanobacterial lichens, Nostoc, Scytonema, Stigonema, Gloeocapsa, and Calothrix are the most common genera. Recently, thanks to advanced molecular biology-based taxonomic approaches, a majority of species previously assigned to the genus Trebouxia have been reclassified as Asterochloris (Peksa and Škaloud 2010). In this chapter, however, the term Trebouxia is used even for those newly reclassified algal species. For majority of lichens, symbiotic algae and/or cyanobacteria are located beneath the upper cortex layer that is formed by densely packed fungal hyphae (see Figs. 20.4 and 20.5).
Cross sections of the foliose lichen Lasallia pustulata that show the marginal (a), intemediate (b), and central thallus part, with hemispherical outgrowth called pustulus (c). The upper cortex contains a algal layer with different thickness in panels a, b & c. The medulla, and lower cortex with rhizines can be seen from top to the bottom at individual panels (a, b & c) (Photos J. Dubová).
Since more than 50 % of all known lichens possess the unicellular alga Trebouxia in their thalli, anatomical and physiological properties are briefly overviewed here. More than 40 species of Trebouxia isolated from lichen thalli are currently described. Trebouxia species are defined using shape of chloroplast (see Fig. 20.6), as well as pyrenoid (see Chap. 7) and pyrenoglobuli configuration as critical features (Peksa and Škaloud 2011). The pyrenoid is a large central part of the chloroplast, rich in proteins and lipids. It contains the photosynthetic enzyme RuBisCo and is the place of starch synthesis. Within a pyrenoid, there are several pyrenoglobuli that are lipid-rich storage inclusions (Ahmadjian 2001). The pyrenoids contained in Trebouxia cells also possess a carbon-concentrating mechanism that can enhance photosynthetic performance (Smith and Griffiths 1998).
Carbon concentrating mechanism (CCM) is a term that refers to the ability of pyrenoid-containing algal chloroplasts, as well as carboxysome-containing cyanobacteria to locally increase the amount of inorganic carbon that can so be used to efficiently perform carbon fixation. Lichens use CCM during favourable conditions, when the thallus is hydrated and light is sufficiently available. Studies made on isolated photobionts (e.g. Palmqvist and Badger 1996) indicate a key role of carbonic anhydrase (CA) in such lichens. It has been shown for Trebouxia sp. and Coccomyxa sp. that CA is located both intra- and extracellularly. Species-specific differences in total CA content have been determined by Palmqvist and coworkers (1997).
Polyols (i.e. sugar alcohols) are photosynthetic products of symbiotic algae. In lichens, polyols (mainly ribitol, arabitol, mannitol) have several physiological roles (see below). Natural levels of polyols in lichens vary within 1.4–8.8 mg/g dry weight (ribitol) and 0.4–29.0 mg/g (mannitol) between species and physiological state – for review see Hájek et al. (2009). The polyols can make up between 2 and 10 % of the thallus dry weight (Palmqvist 2000). The stored polyols are utilized for supplying the mycobiont with carbon, as energy-storage compound within algae, and as a cryoprotectant. In agreement with these functions is that the highest content of polyols is typically found in late summer (Sundberg et al. 1999).
In the majority of lichens, ribitol is produced in algal cells and then transported to fungal part of a lichen, where it is transformed to mannitol and can then be utilized for metabolic processes. When Trebouxia is in lichen association it produces ribitol, but when cultured axenically at laboratory conditions, sucrose is the main photosynthate. This observation may indicate that sucrose is the preferred carbon storage component in autotrophic conditions, while ribitol is produced to exchange carbon with the fungal partner. Interestingly Trebouxia, when isolated from a lichen thallus and cultivated on agar, can grow both autotrophically and heterotrophically (Archibald 1977) suggesting that some carbon may also be imported form the fungal partner by the alga. In contrast to lichens in association with alga, cyanolichens utilize glucose as the main transport compound to the mycobiont.
III. Dependence of Photosynthesis on Physical Factors
A. Hydration-Dependent Photosynthesis
Due to their poikilohydric nature, lichens can respond to the changes in the thallus water content rapidly. While dehydrated lichens are virtually metabolically inactive, increases of the thallus water content leads to gradual re-activation of physiological processes (including photosynthesis and respiration) – Palmqvist and Sundberg 2000. The majority of lichens exhibit dry thalli that are more contracted and possess a different spatial organization compared to their wet state. Extensive packing of thalli layers decreases the volume occupied by the lichen in the dry state. On a cell level, symbiotic algae and cyanobacteria as well as the fungal hyphae are a lot reduced in volume. The cells of the algal photobionts are far from the round shape observed in wet lichens, but are typically collapsed into star-shape structures and densly packed in-between mycobiont cells (de los Rios et al. 1999).
Dessication also has consequences for the ultrastructure in severely desiccated cells of photobionts. For example the pyrenoglobuli may be re-distributed in a pyrenoid and pyrenoglobuli-free pyrenoid matrix areas may be observed in pyrenoid cross section (Ascaso et al. 1988). Moreover, pyrenoglobuli density may be altered according to severity and rapidity of drying. Due to cytorhisis, i.e. loss of intracellular water resulting in cell collapse (protoplast, however remains attached to cell wall), the cell wall of photobionts is much less resistant to desiccation-induced changes than the thick cell wall of mycobionts (Scheidegger et al. 1995).
During rehydration, both photo- and mycobiont cells undergo volumetric changes accompanied with gradual re-activation of physiological processes including photosynthesis (see e.g. Sancho and Kappen 1989). When rewetted from the dry state, lichen thalli show an almost linear increase in photosynthesis and respiration with time of hydration (Green et al. 2011). Duration of rehydration and the rate of restoration of photosynthetic processes are, however, species-specific and also dependent on the ecophysiology of the lichen in the preceding period (i.e. number of hydration/dehydration cycles, prevailing temperature, the length of period spent dehydrated etc.). At optimum hydration, i.e. when the full cellular turgor of photosyntesizing symbiont is reached, the lichens exhibit maximum photosynthetic rate. The optimum water content in a lichen thallus varies amongst species. When the content is above the optimum, surface or extracellular water represents a physical barrier for CO2 transfer from the air to a photobiont. Over hydration therefore leads to the limitation of CO2 supply and inhibits the rate of photosynthesis. This phenomenon is called hypersuprasaturation effect on photosynthesis (Lange and Green 1996) and was observed in several lichen species such as e.g. Ramalina maciformis Lange (1980), Xanthoria calcicola and Lecanora muralis Lange (2002), and Teloschistes capensis (Lange et al. 2006).
It is well established that, lichens can perform close-to-maximum or even maximum photosynthesis at hydration levels below full thallus hydration. Several studies that applied gasometric measurements of lichen photosynthesis reported such high photosynthesis rates in partially dehydrated thalli (see below). However, such photosynthetic studies expressed water status of thalli as relative water content (RWC, %, Eq. 20.1).
where FWa is fresh weight of actually hydrated lichen thallus, FWw is fresh weight of fully hydrated lichen thallus, and DW is dry weight of fully dehydrated thallus. In majority of lichens, maximum photosynthetic rates are reached within the RWC range of 60–90 %. However, species-specific differences in ability to hold water in their thalli (per unit of dry weight) exist. Therefore gasometric measurements of photosynthesis are difficult to compare between lichen species, especially when these measurements were obtained in the field.
In contrast to gasometric measurements, the hydration-response curves of photosynthesis measured by chlorophyll fluorescence can be related directly to water potential (WP). In this approach, WP of lichen thallus is measured directly in a chamber of a chilled-mirror dewpoint meter. Immediately after the WP is evaluated, the lichen sample is exposed to light and effective quantum yield of photosystem II (ϕPSII) is measured using a saturation pulse method. In this way, simultaneous measurements of water status and photosynthetic activity can be performed on gradually desiccating lichens (see e.g. Jupa et al. 2012). Using the approach, photosynthesis under water stress can be compared between poikilohydrous lichens (Fig. 20.7) and homoihydrous higher plants.
Effective quantum yield of PSII (ϕPSII)) in response to dehydration. With gradual dehydration of the lichen thallus, no substantial decrease of ϕPSII is between a water potential (WP) of 0 (full hydration) to −12 MPa. Further thalli dehydration (WP below −12 MPa) leads to a rapid decrease of ϕPSII. The critical WP, at which full inhibition of ϕPSII occurs is typically about −25 MPa (see Stereocaulon glabrum – right). However, several species may exhibit higher tolerance of ϕPSII against thallus dehydration (see Umbilicaria antarctica – left).
While higher plants can hardly perform positive photosynthetis below −2.5 MPa, the majority of lichens do not show any significant decrease within the range of WP −10.0 to 0 MPa (M. Barták, unpublished data). With further dehydration of the lichen thalli, photosynthesis, assessed by effective quantum yield of PSII (ϕPSII), declines in a curvilinear manner towards critical WP at which ϕPSII = 0. For the majority of lichens, critical WP is about −30.0 MPa (e.g. Xanthoria elegans – Barták et al. 2005), however, some species of the genus Umbilicaria have critical WP of as low as −40.0 MPa (Fig. 20.7). The gradual inhibition of photosynthetic processes with ongoing thallus dehydration leads to the activation of protective mechanisms that involves mainly interconversion of xanthophyll-cycle pigments (Calatayud et al. 1997). Such changes are manifested in an dehydration-dependent increase of non-photochemical quenching (NPQ). The rate of NPQ increase is higher when the WP decreases from 0 to −15 MPa than at more pronounced thalli dehydration within the WP range of −30 to −15 MPa (Lasallia pustulata, Moudrá 2009).
Dehydration-induced inhibition of photosynthetic processes may be altered by the rapidity of desiccation and light conditions during desiccation. Generally, fast dehydration leads to more pronounced stress to PSII. Therefore, after fast dehydration, a slower and incomplete recovery is observed. This behavior is also observed in the isolated photobiont Trebouxia erici (Gasulla et al. 2009).
Stuctural and functional properties of the lichen thallus may affect dehydration-induced inhibition of photosynthesis as well. Kosugi et al. (2009) showed that an association of the photobionts with the mycobionts in lichen thalli increases tolerance to photoinhibition under drying conditions, compared to isolated photobiont.
A detailed analysis of non-photochemical quenching components (qE, qT, qI) reveals that they differ between lichen thalli passing fast and slow dehydration. Apart from well-known protective mechanisms activated during dehydration of lichen thalli, i.e. light-dependent xanthophyll cycle pigments conversion, and state 1–2 transition, there is another effective quencher in the photosynthetic apparatus of PSII of desiccating lichens. It is not related to zeaxanthin formation and independent on light. According to Heber et al. (2006), it may involve conformational changes in pigment-protein complexes in PSII. In this way, highly-effective dissipation centers are formed in which excitation energy is trapped (Heber et al. 2010). Recently, the presence of a strong quencher located in the PSII core and/or PSII antennas has been reported from Parmelia sulcata desiccating on light (Veerman et al. 2007), however its chemical structure is not known.
B. Light-Dependent Photosynthesis
1. Light Response Curves
Optimally-hydrated, lichens respond to light in a similar manner to higher plants and light response curves of photosynthesis can be determined in both systems and differences evaluated. At low light intensities of photosynthetically active radiation (PAR), lichens exhibit linear increase in net photosynthetic rate (Pn) with increasing light followed by a curvilinear Pn-PAR relationship until constant photosynthesis (Pnmax) in saturating light is reached.
The great majority of lichens is adapted to low light and thus reach typical light-saturated photosynthesis in the PAR range of 100–400 μmol m−2 s−1 (Demmig-Adams et al. 1990a). However, species from sun-exposed habitats exist that exhibit light-saturated photosynthesis at 1,100 μmol photons m−2 s−1 (Green et al. 1997). In general, lichens have much lower maximum (light-saturated) photosynthetic rates expressed per area or dry weight unit of thallus than higher plant leaves. This is because, photobionts (alga, cyanobacterium or both) constitute only a small proportion to the lichen thalli, while the bulk is made up of the fungal partner. Typically, maximum rate of net photosynthesis (Pnmax) does not exceed 6 μmol (CO2) m−2 s−1 as shown e.g. for cyanobacterial lichen Collema tenax (Lange et al. 1998), algal species Xanthoria elegans (Barták et al. 2005), and Lecanora muralis (Lange and Green 2008). For Lecanora muralis a Pnmax as high as 7.5 was reported for optimally hydrated thalli when measured under controlled laboratory conditions. Many lichens, however, have much lower Pnmax ranging 1.0–2.0 μmol (CO2) m−2 s−1 as shown for Lobaria pulmonaria and Platismatia glauca (Sundberg et al. 1997).
2. Photoinhibition
Photoinhibition of photosynthesis frequently occurs in hydrated lichens exposed to high light intensities. Such high-exposure to lichen can occur in wet open habitats such as rocks and light-exposed clear cuts or forest edges. It is well established that the sensitivity to photoinhibition is higher in the lichens possessing cyanobacterial photobionts than those having algal partners (Demmig-Adams et al. 1990b). When photoinhibited, cyanobacterial lichens do not show fast return of photosynthetic parameters to pre-photoinhibition parameters during dark recovery. This in contrast to lichens with algal photobiont, which exhibit recovery to initial values typically within tens of minutes.
When the photosynthetic apparatus of the lichen photobionts is exposed to high light doses, reactive oxygen species (ROS) are formed that are destructive to the photosynthetic machinery (see Chap. 11), especially photosystem II. ROS may cause damage or destruction of pigment-protein complexes and inhibition of photochemical processes. To avoid or reduce the negative consequences of ROS formation, lichen photobionts activate several photoprotective mechanisms that are similar to higher plants.
a. Zeaxanthin Formation
Several laboratory-based studies with high light-treated lichens demonstrated zeaxanthin formation in algal lichens (e.g. Vráblíková et al. 2005). Zeaxantin is involved in effective thermal energy dissipation from overenergized photosystem II of algal photobionts (Demmig-Adams et al. 1990a), i.e. high light-induced conversion of violaxanthin to zeaxanthin (DEPS value). The recovery to pre-photoinhition violaxanthin to zeaxanthin ratios is relatively fast ranging from 3 to 10 h (Barták et al. 2008). While the zeaxanthin formation is well established in algal photobionts, cyanobacterial photobionts do not possess such mechanism.
b. Involvement of Glutathione
Glutathione is low-weight thiol that has the function of a antioxidant and plays a role in the photoprotection of lichens. Phytochelatines, another antioxidant is synthesized from glutathione. The amount of intrathaline glutathione ranges typically in lichens between 1.2 and 3.3 μmol/g dry weight. Glutathione levels respond not only to accual light conditions and functioning of photosynthetic apparatus of algal and fungal symbionts during light-induced oxidative stress, but also possess seasonal variation as well as spatial variation within the thallus (Kranner and Grill 1996).
The amount of glutathion Increases during lichen dehydration (Kranner and Birtić 2005; Tretiach et al. 2012), while both increases and decreases can be induced by heavy metals and toxic compound (Mrak et al. 2010).
If exposed to strong, brief high light treatment, reduced glutathione (GSH) is converted to oxidized glutathione (GSSG) and the amount of total glutathione (GSHt) decreases in lichens as algal cells are increasingly photoinhibited. Severe photoinhibtion leads to degradation of GSH (down to 12–30 % of GSHt before light treatment Štěpigová et al. 2007) to glutamyl-cystein. Glutamyl-cystein in turn can be used to synthesis reduced glutathione during recovery from high light treatment. An increase of glutathione content by long-term high light treatment is reported for higher plants (Burritt and MacKenzie 2003), but has never been demonstrated for lichens.
The extent of photoinhibition and recovery can be sensitively monitored in a lichen thallus by several chlorophyll fluorescence techniques and the derived chlorophyll fluorescence parameters. In chloroplasts, photoinhibition can lead to a gradual inactivation and photodestruction of chlorophyll molecules. This leads to a decrease in chlorophyll fluorescence (variable fluorescence – Fv) and efficiency of absorbed energy transfer through PSII and PSI. Figures 20.8 and 20.9 show photoinhibition-induced changes in the shape of fast and slow chlorophyll fluorescence induction kinetics in wet lichen thalli exposed to high doses of photosynthetically active radiation.
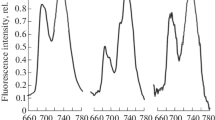
Photoinhibition and recovery in Usnea antarctica assessed by fast chlorophyll fluorescence induction curves (OJIP). The fluorescence kinetics are caused by a short light pulse applied to a dark-adapted lichen thallus. Photoinhibition is characterized by a decrease in chlorophyll fluorescence yield. Partial inhibition (B) and full inhibition (C) can be compared to the uninhibited control (A). After photoinhibition treatment, a 30 or 60 min dark recovery period leads to partial (D) and almost complete (E) restoration of PSII fluorescence characteristics. This fast recovery indicates high resistance of PSII in U. antarctica to short-term high light treatment.
Slow chlorophyll fluorescence kinetics and maximum fluorescence induced by saturation light pulses applied to liches in dark-adapted and light-adapted states. Untreated Usnea antarctica (solid line) is exposed to a 30/60 min high light treatment (solid bold line) and consequent dark recovery (dashed line). The recovery in the dark is faster when thalli are exposed for longer periods (60 min) indicating the involvement of photoprotective and regulatory process in chloroplasts (Reprinted from Barták et al. 2012 with the permission of the Masaryk University Press).
c. Antioxidative Enzymes
In symbiotic lichen algae, antioxidative enzymes are involved in cell protection when ROS are formed (del Hoyo et al. 2011). Several studies reported altered levels of catalase (CAT), superoxide dismutase (SOD) and ascorbate peroxidase (APX) during dehydration stress (e.g. Mayaba and Beckett 2001; Gasulla et al. 2009), heavy metal-induced stress (Weissman et al. 2006), and exposure to SO2 (Kong et al. 1999). It appears very likely that CAT, SOD and APX would be also involved in protecting lichens against ROS formed during high-light stress in hydrated lichens, but experimental efficidence is lacking.
3. Effects of UV-B Radiation
Lichens are generally resistant against high doses of UV radiation, especially when dehydrated and thus physiologically inactive. Some algal lichens, however, show limited resistance (Unal and Uyanikgil 2011). Experiments conducted so far led to the conclusion that UV enhancement typically lead to an increase of UV-absorbing pigments and compounds such as phenolics (Buffoni Hall et al. 2002), usnic acid (Bjerke et al. 2002), rhizocarpic acid (Rubio et al. 2002), parietin (Solhaug and Gauslaa 2004). However, these protective substances cannot fully prevent DNA damage and cell death (Unal and Uyanikgil 2011). UV screening compounds are typically located in the upper cortex of lichen thallus and thus reduce the amount of harmful radiation reaching the algal layer.
Photosynthesis in most lichens appears not to be negatively effected by UV radiation. For example, Lud et al. (2001) reported no change of fluorometric parameters in Turgidosculum complicatulum exposed to extra UV-B radiation in Antarctica. Lichens from shade habitats, however, may exhibit some limited sensitivity of supplemental UV and resulting species-specific effects on photosynthetic rate and biomass production (Larsson et al. 2009). For a majority of lichens, exposure to increased quantities of UV-B radiation leads to increased synthesis of photoprotective, UV-absorbing compounds, such as phenolics (Buffoni Hall et al. 2002) parietin, melanic compounds (Solhaug and Gauslaa 2004; Solhaug et al. 2003) and usnic acid (McEvoy et al. 2006). In lichen thalli – in the upper cortex layer, in particular – these compounds protect photosynthetic pigments located in a photobiont cells in underlying layer.
C. Temperature-Dependence of Photosynthesis
Similarly to higher plants, the rate of photosynthesis and production in lichens is also temperature-dependent including aquatic lichens (Davis et al. 2002). Therefore, temperature response curves can be measured and interspecific differences in temperature tolerance can be evaluated. Due to poikilohydric character of the lichen thallus, however, an increase in thallus temperature is accompanied with gradual loss of water. In natural conditions, temperature-induced change in photosynthetic rate can therefore not be distinguished from dehydration-dependent changes. Several studies evaluated photosynthetic temperature-response curves in lichens that were maintained optimally hydrated in laboratory studies. For many lichens, the temperature optimum for photosynthesis varies between 10 and 22 °C. Individual temperature optima depend on lichen adaptation/acclimation to particular growing habitats (tropical, mountainous, polar etc.), as well as other physical environmental factors including light intensity. If a lichen is exposed to a lower than optimum light level, a decrease in optimum temperature for photosynthesis is observed (Reiter et al. 2008). Many lichens are capable of maintaining high photosynthetic rates (of about 80 % of maximum photosynthesis) within a temperature range of 5–20 °C (Hájek et al. 2001). The critical maximum temperature for lichen photosynthesis is about 40 °C for both tropical and high-mountain lichen species (Lange et al. 2004; Hájek et al. 2001). However this parameter (maximum temperature) has mostly theoretical value, as such high temperature will usually also cause rapid dessication of the lichen in most environments. At 40 °C, wet and fully photosynthetically active thalli can hardly be found in the field.
The only exception are tropical lichens. At temperature above optimum, absolute rate of lichen respiration increases as well as proportion of respiration to photosynthesis. This is attributed mainly to the respiration of mycobiont which forms substantial part of overall thallus CO2 release. Short-term high temperature stress in hydrated lichens leads to the loss of effectivity of photosynthetic processes in photosystem II and, if the capacity of protective mechanisms is low, to the injury of PSII structure in photosynthetizing symbiont (e.g. destruction of the Mn-cluster – Oukarroum et al. 2012), as well as partial disintegration of thylakoid membrane structure.
Contrastingly to higher plants, majority of lichens can perform photosynthesis at temperature far below zero even under snow cover (Kappen 1993, Pannewitz et al. 2006). This capability is facilitated by the presence of molecules in lichen thallus, such as e.g. polyols that prevent freezing. The critical temperature for lichen photosynthesis can drop to values about −20 °C as has been demonstrated by gasometric (Kappen et al. 1996) and fluorometric studies (Barták et al. 2007) in lichens from polar and alpine regions. In many field and laboratory studies, photosynthetic parameters, such as e.g. Fv/Fm and ϕPSII are used as indicators of cryoresistance of lichens and their ability to survive long-term periods at freezing temperature (in dry state) without significant damage to photosynthetic apparatus. However, freezing experiments with lichen species from cold habitats showed that wet thalli may perform substantial photosynthesis at below zero temperature (e.g. Usnea antarctica – see Fig. 20.10). During rapid cooling, both Fv/Fm and ϕPSII of lichen thallus decline, in a S-curve type manner. While Fv/Fm is more or less constant within the range of 20 to −10 °C, ϕPSII tends to decline more rapidly indicating gradual temperature-dependent inhibition of Photosystem II.
Temperature-dependent decrease of potential quantum yield of PSII (Fv/Fm – potential yield of PSII photosynthesis) recorded during linear cooling of Usnea antarctica thallus from +20 to −30 °C (cooling rate 0.5 °C/ min). Photochemical processes in PSII show slight decrease in the temperature range (−12 to +20 °C), dramatic decrease within the range of −12 to −20 °C. The critical point for photosynthesis (assessed by chlorophyll fluorescence) is about −22 °C.
The algal photobionts are remarkable resilient to freezing. Even when shock-frozen in liquid nitrogen, lichen symbiotic algae of the genus Trebouxia survive to a certain extent (typically 30 % of algal population, Hájek et al. 2012) and are able to restore their photosynthetic performance during subsequent cultivation.
IV. Important Chemical Factors Affecting Photosynthesis
A. Availability of Nutrients
The typical lichen habitat only provides poor access to nutrients. This limitation also effects photosynthetic performance. Consequently, lichens can increase photosynthetic rate when nutrients – nitrogen in particular – are available (see e.g. Davis et al. 2000). A study by Palmqvist et al. (1998) demonstrated that lichens with higher nitrogen content exhibit higher gross photosynthetic rate, but lower photosynthetic nitrogen use efficiency. It is well established that when nitrogen is available to a lichen, larger amount of nitrogen is invested in both, the algal and fungal partner. The nitrogen partiniting between the algal and fungal partner is likely species specific (Palmqvist et al. 2002). Nitrogen fertilization experiments show that additional nitrogen is used to synthesize proteins, chlorophyll and ribitol, the photosynthetic product of algal photobiont that can be supplied to the fungal partner (Dahlman et al. 2003).
B. Heavy Metals
With few exceptions of heavy metal-tolerant species (e.g. Xanthoria parietina), lichens are considered good indicators of toxic environmental pollution (Beckett and Brown 1983). It is well established that lichen abundance and distribution decreases with heavy presence of heavy metals. This susceptibility is a result of lichens’ disposition to accumulate heavy metals. Heavy metal accumulation is thought to lead to ROS formation, and consequently to lipid peroxidation and damages to proteins and nucleic acids.
On the level of the photobiont, a decrease in the integrity of chlorophyll molecules that can in extreme cases also lead to a severe decrease in of chlorophyll content, that limits and inhibits photosynthetic processes (Bačkor and Fahsett 2008; Bačkor and Loppi 2009). Interestingly evidence from laboratory studies suggest that heavy metal-sensitive and – tolerant Trebouxia strains exist.
While there is amply evidence from laboratory experiments, field studies that investigate the effect of heavy metals on photosynthesis are less frequent. Garty et al. (2002) reported a decrease of capacity of photosynthetic processes in PSII (Fv/Fm) in epilithic fruticose lichen Ramalina maciformis exposed to a mixture of heavy metal ions from industrial pollution. The decrease in photosynthetic performance is attributed to the degradation of chlorophylls, leading to a decrease in variable chlorophyll fluorescence. Therefore, heavy metal exposure in lichens may be assessed as an decrease in minimum (Fo) fluorescence, variable chlorophyll fluorescence (Fv) and chlorophyll fluorescence parameters derived from those signals (Kupper et al. 1998).
The extent of heavy metal-induced inhibition of photosynthetic processes differs among lichen species. Several species, especially those grown near copper mines, exhibit increased tolerance to heavy metals. Such organisms have increased content of phytochelatins, glutathione (Bačkor et al. 2009) compared to similar organisms living away from pollution sources.
V. Lichen Photosynthesis in the Field
A. Overview of Typical Environments
Over last several decades, numerous short- and long-term studies have been conducted that involved measurements of lichen photosynthesis in the field. In addition to the pioneering studies of O. Lange and his co-workers, who started photosynthesis measurements of lichens in semi-desert ecosystems (Lange et al. 1970), a wide range ecosystems and biotopes has been investigated (see below). Initially these studies have utilized infra-red gas (CO2) analysers, that were later complemented by a variety of chlorophyll fluorescence measurements and environmental sensors.
Detailed field studies can assess the diurnal courses of lichen photosynthesis simultaneously with microclimatological and environmental parameters. Such approaches enables the identification of the key factors that limit photosynthesis in particular location (see e.g. Lange 2002), as well as primary productivity of lichens (e.g. Green and Lange 1991; Uchida et al. 2006).
In tropical rain forests, net photosynthetic rate of algal and cyanobacterial lichens is generally high, but also show season-dependent variation (Lange et al. 2004). During moist period (i.e. overcast days), light is limiting, while during the periods with high photosynthetically active radiation, thalli dehydration is limiting lichen photosynthesis.
Water limitation is the key factor that determins lichen photosynthesis in deserts and semideserts, where lichens are components of soil crusts. It has been shown for a variety of lichen species from the Negev desert (Lange et al. 1977; Palmer and Friedmann 1990) and Namib desert (Lange et al. 1994, 2006) that aerial moisture is a crucial factor for photosynthesis and the survival of crust-forming lichens. Nocturnal fog and/or early morning dew hydrates lichen so that they are able to perform positive net photosynthesis for a short period of time after sunrise.
Several long-term studies on lichen photosynthesis in mountainous ecosystems have been conducted (Reiter and Turk 2000; Reiter et al. 2008). These studies addressed interaction of water availability and temperature and their effect on photosynthetic performance of Xanthoria elegans, Umbilicaria cylindrica, and Brodoa atrofusca, respectively. Continuous field measurements of net photosynthesis and respiration revealed that in the particular alpine ecosystem, the species were photosynthetically active only 16–25 % of the observation time. The most limiting factor for photosynthesis was dehydration of thalli. Interspecific comparison showed that X. elegans is the most photosynthetically active among the three species.
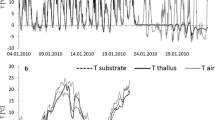
Similar long-term studies were carried out in polar regions, particularly in Antarctica. Using automation the effective quantum yield (ϕPSII) of lichen photosynthesis was continously evaluated. Measurements can be recorded over weeks (see Fig. 20.11) and even several seasons (Schroeter et al. 2011). These measurements clearly show the limitation of lichen photosynthesis by thallus dehydration, freezing of thalli and unavailability of light during the polar night.
While continuous long-term field measurements of lichen photosynthesis provide unparalleled insights in the ecophysiology of lichens, field studies that acquire data in week or month intervals can also be very informative. Such studies can reveal general trends in acclimation of lichen photosyntheis and provide additional data that require direct interactions with the sample (e.g. quantification of photosynthetic pigments) (Piccotto and Tretiach 2010).
B. Special Environments
In addition to ecosystems on Earth, lichen photosynthesis has been also investigated in extraterrestrial space. Here lichen photosynthesis may serve as a marker for surviving conditions experienced in space (Gomez et al. 2012). Within the last decade, several preflight tests have performed to test the resistance of lichens to freezing temperature, vacuum and UV radiation using chlorophyll fluorescence characteristics in Rhizocarpon geographicum and Xanthoria elegans.
Short-term exposure of lichens to extraterrestrial (orbital) conditions was evaluated in the year 2005 within the FOTON_M2 spacecraft mission using the BIOPAN facility. Based on these studies, a long-term (18 months) lichen exposure experiment was performed within the EXPOSE-E experiment onboard the International Space Station (ISS) in 2008/2009. These experiments showed that the experimental lichen species survive open space conditions in a dry state and restore their photosynthetic activity with only minor signs of damage when rewetted under laboratory conditions (de la Torre et al. 2010).
VI. Methods for Assessing Lichen Photosynthesis
A wide array of methods is available to study photosynthesis in lichens and their isolated photobionts. Assessment of photosynthetic CO2 fixation by measuring gas exchange is frequently employed in the field and laboratory. Due to the small dimensions of lichen thalli, necessity of maintaining a constant hydration state, impossibility of deattaching crustose lichen thalli from stone surfaces, these gasometric measurements typically require specifically-modified measuring chambers (e.g. clap-cuvette – Lange et al. 1986, 2007). In spite of these difficulties, many studies demonstrate the acquisition of gas exchange measurements to characterize lichen photosynthesis.
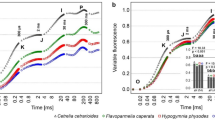
Measurements of chlorophyll fluorescence parameters are also widely used in lichen photosynthesis studies. Fluorometers are relatively robust and cost-effective instruments. Apart of generally well-established indicators of potential (Fv/Fm) and actual (ϕPSII ) effectivity of PSII processes, there are several parameters used in the evaluation of stress in lichen photosynthesis, such as e.g. non-photochemical quenching (NPQ, qN) and its components related to the involvement of energy-dependent (qE), state one-state two transition (qT) and photoinhibitory (qI) processes in PSII. Such approach enables to determine negative effects of particular stress factors in chloroplast of a lichen photobiont. Almost all chlorophyll fluorescence measuring techniques are used in lichens to address specific aspects of lichen photosynthesis. Fast chlorophyll fluorescence transients (OJIPs) were exploited to characterize absorbed light energy transformation in PSII and photochemical photosynthetic machinery. At OJIP curves measured on symbiotic lichen alga Trebouxia, Ilík et al. (2006) reported unusual dip that differs from evidence from higher plant. It was attributed to fast reoxidation of electron carriers in chloroplastic thylakoid membranes caused by activation of ferredoxinNADP + oxidoreductase or Mehlerperoxidase. Oukarroum et al. (2012) used OJIPs to evaluate heat stress effect in PSII of optimally hydrated thallus of Parmelia tiliacea. The study focused on high temperature-induced occurrence of K and M peaks at the OJIP transient. K and M values are indicative for heat stress effects in PSII. Recently, visualization techniques are increasingly used in many fluorometric studies (e.g. Barták et al. 2000) enabling to distinguish intrathalline differences in lichen photosynthetic performance (see Fig. 20.12). Tens of chlorophyll fluorescence parameters are available to evaluate the heterogenetity of photosynthesis caused over lichen thalli by patterned distribution of photosynthetizing photobionts (Jensen et Siebke 1997) photoinhibitory (Barták et al. 2006), temperature and osmotic stress (Hájek et al. 2006). Recently, development of automated and even robotic fluorometers that are used in many applications in higher plants is very promising and future application chlorophyll fluorescence imaging in ecophysiological studies of lichen photosynthesis might be expected.
Chlorophyll fluorescence imaging visualizes several physiological and photosynthetic processes in lichens. (a) Image of the chlorophyll fluorescence signal (intensity indicated by color) reveals higher amount of chlorophyll (red) and less developed dark pigmentation (yellow) of the central (young and growing) thallus part of Lasallia pustulata: Older and more dark-pigmented marginal parts show low chlorophyll fluorescence (green and blue). (b) Intrathalline heterogeneity of effective quantum yield of photosynthetic processes (ϕPSII) in Umbilicaria antarctica. The blue line across the thallus area indicates inhibited photosynthesis due to mechanical stress caused by repeated dehydration-dependent movements. In response to desiccation, the thallus bends along by the blue line. (c, d) Distribution of photoinhibition (assessed by Fv/Fm) in a fully-hydrated Lasallia pustulata thallus induced by high light treatment. The reduction of Fv/Fm from pre-photoinhibory state (Fv/Fm = 0.648) to high light state (Fv/Fm = 0.530) is indicated by color shift form orange (see c) to yellow and green (see d). (e, f) Photoinhibition caused by high light (650 μmol m−2 s−1 for 2 d) in the dry thallus of foliose lichen Lobaria pulmonaria. Maximum photosynthetic activity (ϕPSII) is observed close to the tips of thallus lobes in the untreated control (red and yellow in panel e). In the desiccated state, a part of thallus bends and so provides a shield against incident radiation. As a consequence, one part of thallus exhibits very limited photoinhibition (indicated in yellow in panel f) while the rest of thallus (unshielded by overlaped part) is severely photoninhibited (blue).
Oxymetric methods in lichen photosynthesis research have only been employed recently and only to a limited extent. In principle, the method is based on a Clark-type oxygen electrode housed in a chamber that interfaces with the lichen. Typically these instruments are used to assess photosynthetic O2 evolution in response to light, temperature and hydration status of lichens (Charles 2011; Aubert et al. 2007). Due to the small chamber volumes of these instruments a constant hydration state of lichen thallus can be maintained for longer periods than during CO2 exchange measurements.
Another application of oxymetric methods is the measurement of oxygen exchange in algal/cyanobacterial cultures of photobionts isolated from lichen thalli and cultivated in liquid media. However the isolated photobionts cultivated in cultures differ in their photosynthetic performance from their natural behaviour of their lichenized forms. Studies that contrast the performance of mycobiont and photobiont collective with the isolated photobiont can be used to characterize features that arise from interactions within lichens.
Carbon isotopes are also used in the evaluation of lichen photosynthesis. Stable isotope (13C) discrimination is typically used to distinguish between photosynthesis and respiration and also between the presence or absence of carbon concentration mechanisms (Máguas et al. 1993). Radioactive carbon isotope (14C) is used for identification of photosynthetic products of lichen photobionts and secondary metabolites such as sugars, polyols and lichen acids (Eisenreich et al. 2011). Recently, the approach has been used as an effective tool to assess lichen metabolomics in Cladonia portentosa (Freitag et al. 2012).
Abbreviations
- APX:
-
– Ascorbate peroxidase;
- CA:
-
– Carbonic anhydrase;
- CAT:
-
– Catalase;
- CCM:
-
– Carbon concentrating mechanism;
- DEPS:
-
– Deepoxidation state of xantophyll-cycle pigments;
- DW:
-
– Dry weight;
- Fo:
-
– Background chlorophyll fluorescence;
- Fm :
-
– Maximal fluorescence yield;
- Fv :
-
– Variable chlorophyll fluorescence;
- Fv/Fm :
-
– Potential quantum yield of photochemical processes in photosystem II;
- FW:
-
– Fresh weight;
- GSH:
-
– Reduced glutathione;
- GSSG:
-
– Oxidised glutathione;
- NPQ:
-
– Non-photochemical quenching;
- PAR:
-
– Photosynthetically active radiation;
- Pn:
-
– Net photosynthesis;
- Pnmax :
-
– Maximum rate of net photosynthesis;
- PSII:
-
– Photosystem II;
- ϕPSII :
-
– Effective quantum yield of photochemical processes in photosystem II;
- qE:
-
– Energy dependendt quenching;
- qI:
-
– Photoinhibitory quenching;
- qT:
-
– State 1-state 2 transition quenching;
- ROS:
-
– Reactive oxygen species;
- RuBisCo:
-
– Ribulose bis phosphate carboxylase oxygenase;
- RWC:
-
– Relative water contents;
- SOD:
-
– Superoxid dismutase;
- UV-A:
-
– Ultraviolet radion (B);
- UV-B:
-
– Ultraviolet radion (B);
- WP:
-
– Water potential
References
Ahmadjian V (1993) The lichen symbiosis. Wiley, Chichester, 250p
Ahmadjian V (2001) Trebouxia: reflections on a perplexing and controversial lichen photobiont. In: Seckbach J (ed) Symbiosis. Mechanisms and model systems. Kluwer Academic Publishers, Dordrecht, pp 373–383
Archibald PA (1977) Physiological characteristics of Trebouxia (Chlorophyceae, Chlorococcales) and Pseudotrebouxia (Chlorophyceae, Chlorosarcinales). Phycologia 16:295–300
Ascaso C, Brown HD, Rapsch S (1988) The effect of desiccation on pyrenoid structure in the oceanic lichen Parmelia laevigata. Lichenologist 20:31–39
Aubert S, Juge C, Boisson AM, Gout E, Bligny R (2007) Metabolic processes sustaining the reviviscence of lichen Xanthoria elegans (Link) in high mountain environments. Planta 226:1287–1297
Bačkor M, Fahselt D (2008) Lichen photobionts and metal toxicity. Symbiosis 46:1–10
Bačkor M, Loppi S (2009) Interactions of lichens with heavy metals. Biol Plant 53:214–222
Bačkor M, Kováčik J, Dzubaj A, Bačkorová M (2009) Physiological comparison of copper toxicity in the lichens Peltigera rufescens (Weis) Humb. and Cladina arbuscula subsp. mitis (Sandst.) Ruoss. Plant Growth Regul 58:279–286
Barták M, Hájek J, Gloser J (2000) Heterogeneity of chlorophyll fluorescence over thalli of several foliose macrolichens exposed to adverse environmental factors: interspecific differences as related to thallus hydration and high irradiance. Photosynthetica 38:531–537
Barták M, Gloser J, Hájek J (2005) Visualized photosynthetic characteristics of the lichen Xanthoria elegans related to daily courses of light, temperature and hydration: a field study from Galindez Island, maritime Antarctica. Lichenologist 37:433–443
Barták M, Solhaug KA, Vráblíková H, Gauslaa Y (2006) Curling during desiccation protects the foliose lichen Lobaria pulmonaria against photoinhibition. Oecologia 149:553–560
Barták M a, Váczi P, Hájek J, Smykla J (2007) Low-temperature limitation of primary photosynthetic processes in Antarctic lichens Umbilicaria antarctica and Xanthoria elegans. Polar Biol 31:47–51
Barták M, Vráblíková-Cempírková H, Štepigová J, Hájek J, Váczi P, Večeřová K (2008) Duration of irradiation rather than quantity and frequency of high irradiance inhibits photosynthetic processes in the lichen Lasallia pustulata. Photosynthetica 46:161–169
Barták M, Hájek J, Očenášová P (2012) Photoinhibition of photosynthesis in Antarctic lichen Usnea antarctica. I. Light intensity- and light duration-dependent changes in functioning of photosystem II. Czech Polar Rep 2:42–51
Beckett RP, Brown DH (1983) Natural and experimentally-induced zinc and copper resistance in the lichen genus Peltigera. Ann Bot 52:43–50
Bjerke JW, Lerfall K, Elvebakk A (2002) Effects of ultraviolet radiation and PAR on the content of usnic and divaricatic acids in two arctic-alpine lichens. Photochem Photobiol Sci 1:678–685
Büdel B, Scheidegger C (1996) Thallus morphology and anatomy. In: Nash TH (ed) Lichen biology. Cambridge University Press, Cambridge, pp 37–64
Buffoni Hall RS, Bornman JF, Bjorn LO (2002) UV-induced changes in pigment content and light penetration in the fruticose lichen Cladonia arbuscula ssp. Mitis. J Photochem Photobiol B 66:13–20
Burritt DJ, Mackenzie S (2003) Antioxidant metabolism during acclimation of Begonia × erythrophylla to high light levels. Ann Bot 91:783–794
Calatayud A, Deltoro VI, Barreno E, del Valle-Tascon S (1997) Changes in in vivo chlorophyll fluorescence quenching in lichen thalli as a function of water content and suggestion of zeaxanthin-associated photoprotection. Physiol Plant 101:93–102
Charles HC (2011) The physiological response of sub-Arctic lichens to their abiotic environment. Masters thesis, Durham
Dahlman L, Persson J, Näsholm T, Palmqvist K (2003) Carbon and nitrogen distribution in the green algal lichens Hypogymnia physodes and Platismatia glauca in relation to nutrient supply. Planta 217:41–48
Davis WC, Gries C, Nash TH III (2000) The ecophysiological response of the aquatic lichen Hydrothyria venosa to nitrate in terms of weight and photosynthesis over long periods of time. Biblio Lichenol 75:201–208
Davis WC, Gries C, Nash TH III (2002) The influence of temperature on the weight and net photosynthesis of the aquatic lichen Peltigera hydrothyria over long periods of time. Biblio Lichenol 83:233–242
de la Torre R, Sancho LG, Horneck G, de los Ríos A, Wierzchos J, Olsson-Francis K, Cockell CS, Rettberg P, Berger T, de Vera J-PP, Ott S, Frías JM, Melendi PG, Lucas MM, Reina M, Pintado A, Demets R (2010) Survival of lichens and bacteria exposed to outer space conditions – results of the Lithopanspermia experiments. Icarus 208:735–748
de los Ríos A, Ascaso C, Wierzchos J (1999) Study of lichens with different state of hydration by the combination of low temperature scanning electron and confocal laser scanning microscopies. Internat Microbiol 2:251–257
del Hoyo A, Álvarez R, Del Campo EM, Gasulla F, Barreno E, Casano LM (2011) Oxidative stress induces distinct physiological responses in the two Trebouxia phycobionts of the lichen Ramalina farinacea. Ann Bot Lond 107:109–118
Demmig-Adams B, Adams WW III, Czygan F-C, Schreiber U, Lange OL (1990a) Differences in the capacity for radiationless energy dissipation in the photochemical apparatus of green and blue-green algal lichens associated with differences in carotenoid composition. Planta 180:582–589
Demmig-Adams B, Máguas C, Adams WW, Meyer A, Kilian E, Lange OL (1990b) Effect of high light on the efficiency of photochemical energy conversion in a variety of lichen species with green and blue-green phycobionts. Planta 180:400–409
Eisenreich W, Knispel N, Beck A (2011) Advanced methods for the study of the chemistry and the metabolism of lichens. Pytochem Rev 10:445–456
Freitag S, Feldmann J, Raab A, Crittenden PD, Hogan EJ, Squier HA, Boyd KG, Thain S (2012) Metabolite profile shifts in the heathland lichen Cladonia portentosa in response to N deposition reveal novel biomarkers. Physiol Plant 146:160–172
Garty J, Tamir O, Cohen Y, Lehr H, Goren AI (2002) Changes in the potential quantum yield of photosystem II and the integrity of cell membranes relative to the elemental content of the epilithic desert lichen Ramalina maciformis. Environ Toxicol Chem 21:848–858
Gasulla F, de Nova PG, Esteban-Carrasco A, Zapata JM, Barreno E, Guéra A (2009) Dehydration rate and time of desiccation affect recovery of the lichen alga Trebouxia erici: alternative and classical protective mechanisms. Planta 231:195–208
Gomez F, Barták M, Bell EM (2012) Extreme environments on earth as analogues for life on other planets: astrobiology. In: Bell EM (ed) Life at extremes. Environments, organisms and strategies for survival. CABI, Walingford, pp 522–536
Green TGA, Lange OL (1991) Ecophysiological adaptations of the lichen genera Pseudocyphellaria and Sticta to South temperate rainforests. Lichenologist 23:267–282
Green TGA, Büdel B, Meyer A, Zellner H, Lange OL (1997) Temperate rainforest lichens in New Zealand: light response of photosynthesis. NZ J Bot 35:493–504
Green TGA, Sancho LG, Pintado A (2011) Ecophysiology of desiccation/rehydration cycles in mosses and lichens. In: Luttge U et al (eds) Plant desiccation tolerance, ecological studies 215, Part 2. Springer, Berlin, pp 89–120
Hájek J, Barták M, Gloser J (2001) Effects of thallus temperature and hydration on photosynthetic parameters of Cetraria islandica from contrasting habitats. Photosynthetica 39:427–435
Hájek J, Barták M, Dubová J (2006) Inhibition of photosynthetic processes in foliose lichens induced by temperature and osmotic stress. Biol Plant 50:624–634
Hájek J, Vaczi P, Barták M, Smejkal L, Lipavská H (2009) Cryoproective role of ribitol in Xanthoparmelia somloensis. Biol Plant 53:677–684
Hájek J, Váczi P, Barták M, Jahnová L (2012) Interspecific differences in cryoresistance of lichen symbiotic algae of genus Trebouxia assessed by cell viability and chlorophyll fluorescence. Cryobiology 64:215–222
Heber U, Bilger W, Shuvalov VA (2006) Thermal energy dissipation in reaction centres and in the antenna of photosystem II protects desiccated poikilohydric mosses against photo-oxidation. J Exp Bot 57:2993–3006
Heber U, Bilger W, Türk R, Lange OL (2010) Photoprotection of reaction centres in photosynthetic organisms: mechanisms of thermal energy dissipation in desiccated thali of the lichen Lobaria pulmonaria. New Phytol 185:459–470
Ilík P, Schansker B, Kotabová E, Váczi P, Strasser RJ, Barták M (2006) A dip in the chlorophyll fluorescence induction at 0.2–2 s in Trebouxia-possessing lichens reflects a fast reoxidation of photosystem I. A comparison with higher plants. Biochim Biophys Acta 1757:12–20
Jensen M, Siebke K (1997) Fluorescence imaging of lichens in the macro scale. Symbiosis 23:183–195
Jupa R, Hájek J, Hazdrová J, Barták M (2012) Interspecific differences in photosynthetic efficiency and spectral reflectance in two Umbilicaria species from Svalbard during controlled desiccation. Czech Polar Rep 2:31–41
Kappen L (1993) Plant activity under snow and ice, with particular reference to lichens. Arctic 46:297–302
Kappen L, Schroeter B, Scheidegger C, Sommerkorn M, Hestmark G (1996) Cold resistance and metabolic activity of lichens bellow 0° C. Adv Space Res 18:119–128
Kong FX, Hu W, Chao SY, Sang WL, Wang LS (1999) Physiological responses of the lichen Xanthoparmelia mexicana to oxidative stress of SO2. Environ Exper Bot 42:201–209
Kosugi M, Arita M, Shizuma R, Moriyama Y, Kashino Y, Koike H, Satoh K (2009) Responses to desiccation stress in lichens are different from those in their photobionts. Plant Cell Physiol 50:879–888
Kranner I, Birtić S (2005) A modulating role for antioxidants in desiccation tolerance. Integrat Comparat Biol 45:734–740
Kranner I, Grill D (1996) Significance of thiol-disulphide exchange in resting stages of plant development. Bot Acta 109:8–14
Küpper H, Küpper F, Spiller M (1998) In situ detection of heavy metal substituted chlorophylls in water plants. Photosynth Res 58:123–133
Lange OL (1980) Moisture content and CO2 exchange of lichens. Oecologia 45:82–87
Lange OL (2002) Photosynthetic productivity of the epilithic lichen Lecanora muralis: long-term field monitoring of CO2 exchange and its physiological interpretation - I. Dependence of photosynthesis on water content, light, temperature and CO2 concentration from laboratory measurements. Flora 197:233–249
Lange OL, Green TGA (1996) High thallus water content severely limits photosynthetic carbon gain of central European epilithic lichens under natural condition. Oecologia 19:111–118
Lange OL, Green TGA (2008) Diel and seasonal courses of ambient carbon dioxide concentration and their effect on productivity of the epilithic lichen Lecanora muralis in a temperate, suburban habitat. Lichenologist 40:449–462
Lange OL, Schulze ED, Koch W (1970) Experimentell-ökologische Untersuchungen an Flechten der Negev-Wüste.II.CO2-Gaswechsel und Wasserhaushalt von Krusten- und Blattflechten am natürlichen Standort während der sommerlichen Trocken-periode. Flora 159:525–538
Lange OL, Geiger IL, Schulze E-D (1977) Ecophysiological investigations on lichens of the Negev desert. Oecologia 28:247–259
Lange OL, Kilian E, Ziegler H (1986) Water vapor uptake and photosynthesis of lichens: performance differences in species with green and blue-green algae as phycobionts. Oecologia 71:104–110
Lange OL, Meyer A, Zellner H, Heber U (1994) Photosynthesis and water relations of lichen soil crusts: field measurements in the coastal fog zone of the Namib desert. Funct Ecol 8:253–264
Lange OL, Belnap J, Reichenberger H (1998) Photosynthesis of the cyanobacterial soil-crust lichen Collema tenax from arid lands in Southern Utah, USA: role of water content on light and temperature responses of CO2 exchange. Funct Ecol 12:195–202
Lange OL, Budel B, Meyer A, Zellner H, Zotz G (2004) Lichen carbon gain under tropical conditions: water relations and CO2 exchange of Lobariaceae species of a lower montane rainforest in Panama. Lichenologist 36:329–342
Lange OL, Green TGA, Melzer B, Meyer A, Zellner H (2006) Water relations and CO2 exchange of the terrestrial lichen Teloschistes capensis in the Namib fog desert: measurements during two seasons in the field and under controlled conditions. Flora 201:268–280
Lange OL, Reichenberger H, Walz H (2007) Continuous monitoring of CO2 exchange of lichens in the field: short-term enclosure with an automatically operating cuvette. Lichenologist 29:259–274
Larsson P, Večeřová K, Cempírková H, Solhaug KA, Gauslaa Y (2009) Does UV-B influence biomass growth in lichens deficient in sun-screening pigments? Environ Exp Bot 67:215–221
Lud C, Huiskes A, Ott S (2001) Morphological evidence for the symbiotic character of Turgidosculum complicatulum Kohlm. & Kohlm. (= Mastodia tesselata Hook.f. & Harvey). Symbiosis 31:141–151
Máguas C, Griffiths H, Ehleringer J, Serôdio J (1993) Characterization of photobiont associations in lichens using carbon isotope discrimination techniques. In: Ehleringer J, Hall T, Farquhar G (eds) Physiological ecology series: perspectives on carbon and water relations from stable isotopes, vol 11. Academic, London, pp 201–212
Mayaba N, Beckett RP (2001) The effect of desiccation on the activities of antioxidant enzymes in lichens from habitats of contrasting water status. Symbiosis 31:113–121
McEvoy M, Nybakken L, Solhaug KA, Gauslaa Y (2006) UV triggers the synthesis of the widely distributed secondary compound usnic acid. Mycol Prog 5:221–229
Moudrá A (2009) Activation of non-photochemical quenching mechanisms of absorbed light energy in lichen thalli exposed to desiccation stress. Master thesis, Masaryk University
Mrak T, Jeran Z, Batič F et al (2010) Arsenic accumulation and thiol status in lichens exposed to As(V) in controlled conditions. Biometals 23:207–219
Oukarroum A, Strasser RJ, Schansker G (2012) Heat stress and the photosynthetic electron transport chain of the lichen Parmelina tiliacea (Hoffm.) Ach. in the dry and the wet state: differences and similarities with the heat stress response of higher plants. Photosynth Res 111:303–314
Palmer RJ, Friedmann EI (1990) Water relations and photosynthesis in the cryptoendolithic microbial habitat of hot and cold deserts. Microb Ecol 19:111–118
Palmqvist K (2000) Carbon economy of lichens. New Phytol 148:11–36
Palmqvist K, Badger MR (1996) Carbonic anhydrase(s) associated with lichens: in vivo activities, possible locations and putative roles. New Phytol 132:627–639
Palmqvist K, Sundberg B (2000) Light use efficiency of dry matter gain in five macro-lichens: relative impact of microclimate conditions and species-specific traits. Plant Cell Environ 23:1–14
Palmqvist K, de los Rios A, Ascaso C, Samuelsson G (1997) Photosynthetic carbon acquisition in the lichen photobionts Coccomyxa and Trebouxia (Chlorophyta). Oecologia 101:67–76
Palmqvist K, Campbell D, Ekblad A, Johansson H (1998) Photosynthetic capacity in relation to nitrogen content and its partitioning in lichens with different photobionts. Plant Cell Environ 21:361–372
Palmqvist K, Dahlman L, Valladares F, Tehler A, Sancho LG, Mattsson J-E (2002) CO2 exchange and thallus nitrogen across 75 contrasting lichen associations from different climate zones. Oecologia 133:295–306
Pannewitz S, Green TGA, Schlensog M, Seppelt R, Sancho LG, Schroeter B (2006) Photosynthetic performance of Xanthoria mawsonii C. W. Dodge in coastal habitats, Ross Sea region, continental Antarctica. Lichenologist 38:67–81
Peksa O, Škaloud M (2010) Evolutionary inferences based on ITS rDNA and actin sequences reveal extensive diversity of the common lichen alga Asterochloris (Trebouxiophyceae, Chlorophyta). Mol Phyl Evol 54:36–46
Peksa O, Škaloud M (2011) Do photobionts influence the ecology of lichens? A case study of environmental preferences in symbiotic green alga Asterochloris (Trebouxiophyceae). Mol Ecol 20:3936–3948
Piccotto M, Tretiach M (2010) Photosynthesis in chlorolichens: the influence of the habitat light regime. Ital J Plant Res 123:763–775
Reiter R, Türk R (2000) Investigations on the CO2 exchange of lichens in the alpine belt. II. Comparative patterns of net the CO2 exchange in Cetraria islandica and Flavocetraria nivalis. Phyton Ann Rei Botanicae 40:161–177
Reiter R, Höftberger M, Green TGA, Türk R (2008) Photosynthesis of lichens from lichen-dominated communities in the alpine/ nival belt of the Alps – II: laboratory and field measurements of CO2 exchange and water relations. Flora 203:34–46
Rubio C, Fernández E, Hidalgo ME, Quilhot W (2002) Effects of solar UV-B radiation in the accumulation of rhizocarpic acid in a lichen species from alpine zones of Chile. Bol de la Soc Chil de Quím 47:1–10
Sancho LG, Kappen L (1989) Photosynthesis and water relations and the role of anatomy in Umbilicariaceae (lichenes) from Central Spain. Oecologia 81:473–480
Scheidegger C, Schroeter B, Frey B (1995) Structural and functional processes during water vapour uptake and desiccation in selected lichens with green algal photobionts. Planta 197:399–409
Schroeter B, Green TGA, Pannewitz S, Schlensog M, Sancho LG (2011) Summer variability, winter dormancy: lichen activity over 3 years at Botany Bay, 77°S latitude, continental Antarctica. Polar Biol 34:13–22
Smith EC, Griffiths H (1998) Intraspecific variation in photosynthetic responses of trebouxioid lichens with reference to the activity of a carbon-concentrating mechanism. Oecologia 113:360–369
Solhaug K-A, Gauslaa Y (2004) Photosynthates stimulate the UV-B induced fungal anthraquinone synthesis in the foliose lichen Xanthoria parietina. Plant Cell Environ 27:167–176
Solhaug KA, Gauslaa Y, Nybakken L, Bilger W (2003) UV-induction of sunscreening pigments in lichens. New Phytol 158:91–100
Štěpigová J, Vráblíková H, Lang J, Večeřová K, Barták M (2007) Glutathione and zeaxanthin formation during high light stress in foliose lichens. Plant Soil Environ 53:340–344
Sundberg B, Campbell D, Palmqvist K (1997) Predicting CO2 gain and photosynthetic light acclimation from fluorescence yield and quenching in cyano-lichens. Planta 201:138–145
Sundberg B, Ekblad A, Näsholm T, Palmqvist K (1999) Lichen respiration in relation to active time, temperature, nitrogen and ergosterol concentrations. Funct Ecol 13:119–125
Tretiach M, Pavanetto S, Pittao E, Sanità di Toppi L, Piccotto M (2012) Water availability modifies tolerance to photo-oxidative pollutants in transplants of the lichen Flavoparmelia caperata. Oecologia 168:589–599
Uchida M, Nakatsubo T, Kanda H, Koizumi H (2006) Estimation of the annual primary production of the lichen Cetrariella delisei in a glacier foreland in the high arctic, Ny-Ålesund, Svalbard. Polar Res 25:39–49
Unal D, Uyanikgil (2011) UV-B induces cell death in the lichen Physcia semipinnata (J.F.Gmel). Turk J Biol 35:137–145
Veerman J, Vasil’ev S, Paton GD, Ramanauskas J, Bruce D (2007) Photoprotection in the Lichen Parmelia sulcata: the origins of desiccation-induced fluorescence quenching. Plant Physiol 145:997–1005
Vráblíková H, Barták M, Wonisch A (2005) Changes in glutathione and xanthophyll cycle pigments in high light-stressed lichens Umbilicaria antarctica and Lasallia pustulata. J Photochem Photobiol B 79:35–41
Weissman L, Fraiberg M, Shine L, Garty J, Hochman A (2006) Responses of antioxidants in the lichen Ramalina lacera may serve as an early-warning bioindicator system for the detection of air pollution stress. FEMS Microbiol Ecol 58:41–53
Acknowledgments
I am very thankful to my colleagues from the Laboratory of Photosynthetic Processes (Masaryk University, Brno) who have collaborated with me in many field- and laboratory-based studies focussed on ecophysiology of lichen photosynthesis within last decade. Their particular help during the preparation of manuscript of this chapter is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Barták, M. (2014). Lichen Photosynthesis. Scaling from the Cellular to the Organism Level. In: Hohmann-Marriott, M. (eds) The Structural Basis of Biological Energy Generation. Advances in Photosynthesis and Respiration, vol 39. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-8742-0_20
Download citation
DOI: https://doi.org/10.1007/978-94-017-8742-0_20
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-8741-3
Online ISBN: 978-94-017-8742-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)