Abstract
This chapter summarizes more than 200 compounds from fungi that have been shown to possess nematicidal activities. These compounds belong to diverse chemical groups including alkaloid, quinone, isoepoxydon, pyran, furan, peptide, macrolide, terpenoid, fatty acid, diketopiperazine, aphthalene and simple aromatics. They have mainly been isolated from a variety of ascomycetous and basidiomycetous fungal taxa. Their nematicidal activities are described and their potential roles in the biocontrol of nematodes are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Nematodes can attack, infect and consume a wide variety of organisms, including animals, microorganisms and plants. In recent years, the use of synthetic chemical compounds has been the most common strategy for controlling parasitic nematodes (Haydock et al. 2006). While these have been effective in certain circumstances, the widespread use of man-made chemical nematicides has caused significant problems to both the environment and human health. Consequently, their use for pest control in agriculture and forestry has been reduced significantly. The reduced use of synthetic chemical nematicides has generated significant demands for environmentally friendly alternative strategies (Meira et al. 2006). Biological control is a potentially effective alternative for the management of nematode pests. Biological agents include both live organisms as well as their metabolic products (Willis and Thomas 1998). Fungi are known to possess a huge diversity of metabolic pathways and they have provided several large classes of commercial compounds, including many antibiotics used in medicine (Harvey 2000). Therefore, secondary metabolites in fungi could have much potential in their novel structures and nematicidal activities.
Many nematicidal compounds had been discovered from nematode-toxic fungi, a group of nematophagous fungi. The aim of this chapter is to review the nematode-toxic fungi, the different types, their structure and the nematicidal activity of compounds isolated from nematode-toxic fungi.
Nematode-Toxic Fungi and Their Nematicidal Metabolites
About 280 fungal species in 150 genera of Ascomycota and Basidiomycota have been reported to possess nematicidal activity as they produce toxic compounds which are active against nematodes. More than 200 of these compounds with nematicidal activities are summarized in this chapter.
Nematode-Toxic Ascomycetes and Their Nematicidal Metabolites
About 80 genera comprising more than 120 species have been reported to produce nematicidal active components. Lachnum papyraceum (Hyaloscyphaceae) is one of the most prolific producers of nematicidal secondary metabolites and a number of nematicidal metabolites have been isolated from this taxa, culturing under different conditions. The structures of 30 compounds isolated from L. papyraceum have been elucidated to be isoepoxydon, isocoumarin, mycorrhizin and furan, and 24 compounds including 15 new isolates having nematicidal activities (Stadler and Anke 1993a, b; Stadler et al. 1995a, b, c, d, e; Shan et al. 1996).
The production of nematicidal compounds by three species of Nematoctonus have been demonstrated by Giuma and Cooke (1971) and Giuma et al. (1973), and these compounds were termed nematoctoxins. The result indicated that Nematoctonus species quickly retard and kill their nematode hosts by the production of toxin, but the toxin had not been identified. Nematoctonus robustus was shown to have nematode-immobilizing activity in culture filtrate (Kennedy and Tampion 1978). The nematicidal effect of filtrates from 15 asexual ascomyctes were tested against Meloidogyne incognita, and Acremonium strictum, Alternaria alternata, Curvularia pallescens, Nigrospora sphaerica, Paecilomyces lilacinus, Penicillium spinulosu, Trichoderma harzianum, were most effective hatch inhibitors of root-knot nematodes and the nematicidal action of culture filtrates against nematode might be attributed to the production of certain toxic metabolites (Khan and Kgan 1992).
Besides producing special structures that trap nematodes, some trapping fungi can produce nematicidal compounds at the same time as the process of trapping nematodes. The nematicidal compound linoleic acid was isolated from the nematophagous Arthrobotrys brochopaga, A. conoides, A. dactyloides, A. oligospora, and oligosporon, with 4’,5’-dihydro-oligosporon and arthrobotrisin A being obtained from A. oligospora (Stadler et al. 1993; Anke et al. 1995; Anderson et al. 1995; Wei et al. 2011). These compounds have nematicidal activity and thus the process by which these fungi overcome and capture the nematode is complex.
Beauveria bassiana is an important insect pathogenic fungus which produces the bioactive substance beauvericin which has nematicidal activity against Meloidogyne incognita (Hamill et al. 1969; Mayer 1995), Caenorhabditis elegans and Bursaphelenchus xylophilus (Shimada et al. 2010). The compound is also produced by Fusarium sp. (Mayer 1995) and Paecilomyces fumoso-roseus (Bernardini et al. 1975).
Isolates of 130 freshwater fungal taxa have been assayed for nematicidal activity against B. xylophilus and 22 filtrates and 13 water-soluble extracts of broken fungal mycelia were found to be active against the nematode. The mobility of over 90 % of nematodes were inhibited by filtrates from Annulatascus sp., Caryospora callicarpa, Massarina thalassioidea, Ophioceras commune, Pseudohalonectria adversaria, Pseudohalonectria lignicola, and mycelia extracts from Helicomyces roseus, Phomatospora berkeleyi and P. lignicola (Dong et al. 2003). Several new nematicidal compounds were obtained from these freshwater fungi (Dong et al. 2007, 2008, 2010). A novel class of potent nematicidal thermolides was isolated from a thermophilic fungus Talaromyces thermophilus. Thermolides A and B showed the strongest activities against nematodes with similar activity of avermectins (Guo et al. 2012). These and other nematode-toxic ascomycetes (and anamorphs), and their nematicidal compounds are lised in Table 7.1.

Nematicidal Metabolites from Alternaria, Ascochyta, Aspergillus, Hemicarpenteles, Paecilomyces and Penicillium Species
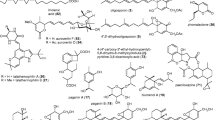
Brefeldin A (1) is identical to two known chemicals ascotoxin and decumbin. It was first obtained from Penicillium decumbens (Singleton et al. 1958), and subsequently isolated from several other fungal species including P. brefeldianis (Kima and Kochevar 1995), P. camemberti (Abraham and Arfmann 1992), Hemicarpenteles paradoxus (Anke et al. 1995), Alternaria carthami, A. zinniae (Vurro et al. 1998), Paecilomyces sp., Aspergillus clavatus (Wang et al. 2002) and Ascochyta imperfecta (Suzuki et al. 1970). Screening against the nematode A. aceti with brefeldin A (1) resulted in significant nematicidal activity (BačÍkovÁ et al. 1965). A symmetric 16-membered macrodiolide helmidiol (2) was produced by Alternaria alternata (Kind et al. 1996). The compound had activity against Haemunchus cortortus (Kind et al. 1996) and Meloidogyne incognita (Khan and Kgan 1992).

Nematicidal Metabolites from Apiocrea Chrysosperma
Four linear lipophilic peptides chrysospermins A (3), B (4), C (5) and D (6) were isolated from the mycelium of Apiocrea chrysosperma Ap101 (Dornberger et al. 1995). These compounds have been patented as nematicidal and anthelminthic agents (Metzger et al. 1994). Each of these four peptides contains 19 amino acids and possesses a C-terminal Trpol and one labile Aib-Pro bond (Table 7.2, Bodo et al. 1985).
Nematicidal Metabolites from Nematode-Trapping Fungi Arthrobotrys, Chlorosplenium, Dactylella and Monacrosporium
An aliphatic compound linoleic acid (7) was detected in the mycelial extracts of the nematode-trapping fungi Arthrobotrys conoides, A. brochopaga, A. dactyloides, A. oligospora, Dactylella candida and Monacrosporium doedycoides. The LD50 of the compound towards Caenorhabditis elegans was 10 µg mL−1 and the LD30 to Meloidogyne incognita was 100 µg mL−1 (Stadler et al. 1994c; Anke et al. 1995). Besides linoleic acid (7), two isoepoxydon compounds oligosporon (8) and its dihydro-derivative 4’,5’-dihydro-oligosporon (9) were isolated from Arthrobotrys oligospora. These two compounds were active against Haemunchus cortortus with LD50 values of 25 and 50–100 µg mL−1. However, they were inactive against the nematode Caenorhabditis elegans at concentrations up to 100 µg mL−1 (Anderson et al. 1995; Stadler et al. 1993). Recently, three novel oligosporons, named arthrobotrisins A-C (10–12) were isolated from A. oligospora, but only arthrobotrisin A (10) had nematicidal activity against Panagrellus redivivus (Wei et al. 2011). Linoleic acid (7) was also found in Chlorosplenium sp. (Anke et al. 1995), and the basidiomycete Hericium coralloides (Xiang and Feng 2001) and Pleurotus pulmonarius (Stadler et al. 1994c).

Nematicidal Metabolites from Aspergillus Fumigatus, Penicillium Nigricans, Gliocladium Fimbriatum and Penicillium Sp.
A terpenoid, fumagillin (13), isolated from both Aspergillus fumigatus and Penicillium nigricans, was reported to be moderately active against nematode Anguillula aceti (Tarbell et al. 1960; BačÍkovÁ et al. 1965; Beecham et al. 1966). Gliotoxin (14), a known antibiotic, was also weakly active against A. aceti (BačÍkovÁ et al. 1965). Gliotoxin (14) had been isolated from Gliocladium fimbriatum, Aspergillus fumigatus, Penicillium sp. and other fungi (Tarbell et al. 1960; Beecham et al. 1966). Besides the two compounds, five compounds including two new active fumiquinones A (15), B (16) and three known spinulosin (17), LL-S490 (18) and pseurotin A (19) were also isolated from A. fumigatus. Fumiquinone A (15) showed effective nematicidal activities against Pratylenchus penetrans and Bursaphelenchus xylophilus, but fumiquinone B (16) and the three known compounds only showed activity against B. xylophilus. All of these five compounds had no nematicidal activities against Caenorhabditis elegans (Hayashi et al. 2007).

Nematicidal Metabolites from Aspergillus Glaucus, A. Melleus and A. Niger
A widely distributed anthraquinone in plants, emodin (20) was also obtained from Aspergillus glaucus (Anke et al. 1980a, b). Emodin (20) has shown activity against Meloidogyne incognita (Mayer 1995). Aspyrone (21) was isolated from Aspergillus melleus and showed a nematicidal activity against Pratylenchus penetrans with killing rates of 39 % and 80.8 % at concentrations of 100 mg L−1 and 300 mg L−1, respectively (Kimura et al. 1996). Nafuredin (22) was isolated as an inhibitor of an anaerobic electron transporter from the culture broth of Aspergillus niger FT-0554 (Ui et al. 2001; Ōmura et al. 2001). In vivo trials with sheep indicated that nafuredin (22)had significant nematicidal activity against Haemunchus cortortus. Nafuredin (22) could be easily converted to nafuredin-γ (23) by weak alkaline treatment. The latter also showed an inhibitory activity similar to nafuredin (22) (Shiomi et al. 2005). The IC50 values of nafuredin (22) and nafuredin-γ (23) were 9.7 nM and 6.4 nM respectively in their inhibition against NADH-fumarate reductase (NFRD) of Ascaris suum (Shiomi et al. 2005). Two new nematicidal ophiobolins, ophiobolin K (24) and 6-epiophiobolin K (25) were obtained from Aspergillus ustus (Singh et al. 1991). The two compounds were also isolated from Cochliobolus heterostrophus (Rosegay et al. 1996).

Nematicidal Metabolites from Aspergillus Spp.
Four macrolides including a new compoundββ,γ-dehydrocurvularin (26) and three known ones αβ-dehydrocurvularin (27), 8-β-hydroxy-7-oxocurvularin (28) and 7-oxocurvularin (29) were obtained from Aspergillus sp. These four macrolides have shown nematicidal activities against the root-lesion nematode Pratylenchus penetrans (Kusano et al. 2003). However, none of the four compounds had any observable effects on Caenorhabditis elegans at the tested concentrations (1–1000 mg L−1). The three known compounds are produced by many species in the genera Alternaria, Cochliobolus, Curvularia and Penicillium (Munro et al. 1967; Hyeon et al. 1976; Robeson and Strobel 1981, 1985; Kobayashi et al. 1988; Arai et al. 1989; Lai et al. 1989, 1990; Ghisalberti and Rowland 1997). Two members of a new class of anthelmintics, aspergillimide (VM55598) (30) and 16-keto aspergillimide (SB202327) (31), were isolated from Aspergillus sp. IMI 337664 (Banks et al. 1997). In addition, three new paraherquamides SB203105 (32), SB200437 (33) and VM54159 (34) were also isolated from this strain. This study was the first to report paraherquamides from an organism outside the fungal genus Penicillium. These compounds had activity against Trichjostrongylus columbriformis. Tests showed that the 16-keto analogue of aspergillimide (31) was active against Haemunchus contortus L3 larvae in vitro but not in vivo (Banks et al. 1997). A new nematicide 5-hydroxymethyl-2-furoic acid (35) was obtained from the cultures of an Aspergillus sp. The compound showed effective nematicidal activities against Bursaphelenchus xylophilus and Caenorhabditis elegans (Kimura et al. 2007). A pyran compound patulin (36) was proven to be active against Meloidogyne incognita with the LD30 dose at 100 µg mL−1 and a oxygen heterocycle penicillic acid (37) was found to possess weak activities against Anguillula aceti (BačÍkovÁ et al. 1965; Mayer 1995). Patulin (3 6) was found in several fungi including Aspergillus spp. (Lopez-Diaz and Flannigan 1997), Penicillium spp. (Adams et al. 1976; Alfaro et al. 2003; Dombrink-Kurtzman and Blackburn 2005), and Byssochlamys spp. (Moulé and Hatey 1977). Penicillic acid (37) has been isolated from several fungal species and strains belonging to Aspergillus (He et al. 2004; Kang and Kim 2004), Penicillium (Wirth et al. 1956; Reimerdes et al. 1975), and Malbranchea aurantiaca (Martínez-Luis et al. 2005).

Nematicidal Metabolites from Beauveria Bassiana, Bulgaria Inquinans and Paecilomyces Fumoso-Roseus
The cyclic depsipeptide beauvericin (3 8) was weakly active against Meloidogyne incognita (Mayer 1995), and then was proved to be active against Caenorhabditis elegans and Bursaphelenchus xylophilus (Shimada et al. 2010). This peptide was isolated from Beauveria bassiana (Hamill et al. 1969), Paecilomyces fumoso-roseus (Bernardini et al. 1975), Fusarium spp. (Bernardini et al. 1975), Beauveria sp. FKI-1366 (Fukuda et al. 2004), Fusarium bulbicola (Shimada et al. 2010) and F. redolens (Xu et al. 2010), and the basidiomycete Polyporus sulphureus (Deol et al. 1978). Two new azaphilones, bulgarialactone A (39) and B (4 0) were isolated from both the mycelia and fruit bodies of the Bulgaria inquinans. The LD50 value of bulgarialactone A (39) and B (4 0) against the nematode Caenorhabditis elegans was 5 µg mL−1 and 10–25 µg mL−1 respectively (Stadler et al. 1995).

Nematicidal Metabolites from Caryospora Callicarpa
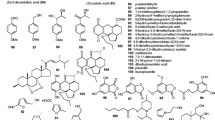
Three new macrolide caryospomycins A (4 1), B (4 2) and C (4 3), and four known compounds (4 4 –4 7) were isolated from the mycelium and fermentation broth of freshwater taxon C. callicarpa YMF1.01026 (Dong et al. 2007; Zhu et al. 2008). These compounds were active against Bursaphelenchus xylophilus (Dong et al. 2007; Zhu et al. 2008).

Nematicidal Metabolites from Chaetomium Globosum, Cladobotryum Rubrobrunnescens, Clonostachys Cylindrospora, Coelomycetes Sp., Epicoccum Nigrum and E. Purpurascens
A simple aromatic flavipin (4 8), produced by Chaetomium globosum, could inhibit in vitro egg hatch and juvenile mobility of Meloidogyne incognita, and could also inhibit the hatch of Heterodera glycines (Nitao et al. 2002). Flavipin (4 8) was also found in Epicoccum nigrum (Burge et al. 1976) and E. purpurascens (Brown et al. 1987). Cladobotrin I (49) exhibited nematicidal activity towards Meloidogyne incognita with an LD50 at 100 µg mL−1, and it was isolated from Cladobotryum rubrobrunnescens (Wagner et al. 1998). A 14-membered macrodiolide clonostachydiol (5 0) was isolated from the Clonostachys cylindrospora (Grabley et al. 1993). Its synthesis in vitro has been achieved (Rao et al. 1995). A dose of 2.5 mg kg−1 subcutaneously administered to sheep artificially infected with the nematode Haemunchus cortortus caused 80 to 90 % reduction of nematode (Grabley et al. 1993). Five nematicidal compounds preussomerin C (5 1), preussomerin D (5 2), preussomerin E (5 3), (4RS)4,8-dihydroxy-3,4-dihydronaphthalen-1(2H)-one (5 4) and 4,6,8-trihydroxy-3,4-dihydronaphthalen-1(2H)-one (5 5) were isolated from an aquatic fungus Coelomycetes sp. YMF 1.01029 (Zhou et al. 2009).

Nematicidal Metabolites from Cochliobolus Heterostrophus, C. Miyabeanus, Helminthosporium Leersii and H. Sativum
Four ophiobolane-type sesterterpenes including ophiobolin M (5 6), 6-epiophiobolin M (5 7), ophiobolin C (5 8) and 6-epiophiobolin C (59) have been isolated from Cochliobolus heterostrophus (Tsipouras et al. 1996). Ophiobolin C (58) was first obtained from Helminthosporium species (Cutler et al. 1984) and it was the most active compound among these compounds with an LD50 value of 5 µM against Caenorhabditis elegans. These compounds are non-competitive inhibitors of ivermectin binding to membranes prepared from C. elegans (Tsipouras et al. 1996). A quinone cochlioquinone A (6 0) has been isolated from Cochliobolus miyabeanus, Helminthosporium leersii and H. sativum (Barrow and Murphy 1972; Schaeffer et al. 1990). The ED50 of cochlioquinone A (6 0) against Caenorhabditis elegans was 135 µM (Snook et al. 1998). Cochlioquinone A (6 0) may have a similar mode of action as that of the widely used avermectin because the compound is a competitive inhibitor of [3H]ivermectin and both can bind to the cell membrane of Caenorhabditis elegans.

Nematicidal Metabolites from Coronophora Gregaria and Cylindrocarpon Olidum
A highly methylated polyketide MK7924 (61) was isolated from the culture broth of Coronophora gregaria L2495 and the compound exhibited significant nematicidal activity against Caenorhabditis elegans at 100 µg mL−1 (Kumazawa et al. 2003). There were significant structural differences between MK7924 (61) and other known anthelmintic agents. Therefore, MK7924 (61) could be developed as a promising new type of anthelmintic. Two nematicidal compounds cannabiorcichromenic acid (62) and its 8-chloro derivative (63) were isolated from Cylindrocarpon olidum (Quaghebeur et al. 1994). The mixture of these two compounds could kill 50 % of Heterorhabditis nematodes at 20 µg mL−1 (Quaghebeur et al. 1994).

Nematicidal Metabolites from Daldinia Concentrica, Emericellopsis Poonensis and E. Synnematicola
Two naphthalenes 1-methoxy-8-hydroxynaphthalene (64) and 1,8-dimethoxynaphthalene (65) were isolated from Daldinia concentrica (Dasenbrock 1994) and both were active against C. elegans with LD50 values at 10 µg mL−1 and 25 µg mL−1 respectively. However, these two compounds were only weakly active against Meloidogyne incognita (Anke et al. 1995). The N-terminally acetylated lipophilic linear polypeptide antiamoebin I (66) had been obtained from fungal species Emericellopsis poonensis and E. synnematicola, which showed activity against helminthes (Thirumalachur 1968; Pandey et al. 1977). The structure of antiamoebin I (66) was determined by several spectral methods including X-ray crystallography (Brückner et al. 1980; Snook et al. 1998).

Nematicidal Metabolites from Fusarium Roseum, Trichothecium Roseum, Fusarium Sp. and Geotrichum Sp.
Trichothecolone (67) was obtained from Fusarium roseum and Trichothecium roseum, which had weakly activity against the nematode Anguillula aceti (Freeman et al. 1959; BačÍkovÁ et al. 1965; Konishi et al. 2003). Two cyclodepsipeptides, enniatin A (68) and enniatin B (69), were isolated from the culture broth of Fusarium spp. (Tomoda et al. 1992), and both were weakly active against Meloidogyne incognita (Mayer 1995). An endophytic fungus Geotrichum sp. AL4 was isolated from the leaf of Azadirachta indica. Two new metabolites, 1-((2R,4S,5S)-2-chloro-4-methyl-1,3-oxazinan-5-yl)ethanone (70) and 1-((2R,4S,5R)-2-chloro-4-methyl-1,3-oxazinan-5-yl)ethanone (71) as well as one known compound 2’,4’-dihydroxyacetophenone (72) were isolated from this strain. The three compounds exhibited nematicidal activity against nematodes Bursaphelenchus xylophilus and Panagrellus redivivus (Li et al. 2007).

Nematicidal Metabolites from Gliocladium Roseum, G. Virens and Gymnoascus Reesii
A series of diketopiperazine compounds were isolated from the solid-substrate fermentation cultures of Gliocladium roseum (Dong et al. 2005, 2006). These compounds include gliocladine A (73), B (74), C (75), D (76), E (77), verticillin A (78), 11’-deoxyverticillin A (79), Sch52900 (80), Sch52901 (81) and glioclasine (82). The compounds showed nematicidal activities against Caenorhabditis elegans and Panagrellus redivivus. However, they showed little activity against Bursaphelenchus xylophilus. Compared to the other compounds in this group, glioclasine (82) showed the strongest activities against Bursaphelenchus xylophilus, Caenorhabditis elegans and Panagrellus redivivus with LD50 values at 15, 50 and 200 µg mL−1, respectively (Dong et al. 2005). A sterol, viridian (83) was obtained from Gliocladium virens and some strains of Trichoderma (Blight et al. 1968), and it has been found to possess weak activity against Anguillula aceti (BačÍkovÁ et al. 1965). A nematicidal metabolite (3E,5E)-2,5-dihydroxy-2,7-dihydrooxepine-3-carboxylic anhydride (84) was isolated based on bioassay-guided fractionation from the extracts of the fungus Gymnoascus reesii, which showed activity against M. incoginta (Liu et al. 2011).

Nematicidal Metabolites from Hirsutella Thompsonii Var. Synnematosa, Nigrospora Sphaerica, Verticillium Chlamydosporium and Hypomyces Sp.
A nematicidal compound phomalactone (85) was obtained by bioassay-directed fractionation from Verticillium chlamydosporium (Khambay et al. 2000). The mortality of Meloidogyne incognita reached 84 % in 96 h when the concentration of phomalactone (85) was 500 mg L−1 (Khambay et al. 2000). This compound has been found in other fungi, e.g the entomopathogenic fungi Hirsutella thompsonii var. synnematosa (Krasnoff and Gupta 1994) and Nigrospora sphaerica (Kim et al. 2001). Two photosensitive compounds hypocrellin A (86) and elsinochrome A (87) were isolated from Hypomyces sp. (Dong et al. 2001). These two compounds were able to kill 50 % of the nematode Bursaphelenchus xylophilus within 18 h at concentrations of 50 µg mL−1 for hypocrellin A (86), and 15 µg mL−1 for elsinochrome A (87) (Dong et al. 2001).

Nematicidal Metabolites from Anamophic Fungi Strains D1084 and PF1022
Two novel depsipeptides bursaphelocides A (88) and B (89) were isolated from an unidentified anamorph strain, D1084. These two compounds were active against B. xylophilus at a dose of 100 µg per ball using the “cotton ball on the fungal mat method” (Kawazu et al. 1993). Another unidentified anamorph strain PF1022 produced a novel cyclodepsipeptide PF1022A (90) showed potent anthelmintic activity against Ascaridia galli (Sasaki et al. 1992). Importantly, no toxic effect was observed to the host animals. The efficacy of compound PF1022A (90) against anthelmintic-resistant nematodes in sheep and cattle was investigated and the result confirmed that PF1022A (90) was fully effective against these parasite nematode populations (Samson-Himmelstjerna et al. 2005).

Nematicidal Metabolites from Lachnum Papyraceum
Two new compounds lachnumfuran A (91) and lachnumlactone A (92) were obtained from Lachnum papyraceum (Shan et al. 1996). The two compounds had relatively weak activities against Caenorhabditis elegans with ND90 dosages at 100 and 50 µg mL−1 respectively (Shan et al. 1996). During the investigations of the influences of CaBr2 on the biosynthesis of chlorinated secondary metabolites in Lachnum papyraceum, six isocoumarin derivatives, 6-hydroxymellein (93), 4-chloro-6-hydroxymellein (94), 4-bromo-6-hydroxymellein (95), 6-methoxymellein (96), 4-chloro-6-methoxymellein (97) and 4-chloro-6,7-dihydroxymellein (98) were obtained. Among them, compounds 4-chloro-6-hydroxymellein (94), 4-bromo-6-hydroxymellein (95), 6-methoxymellein (96) and 4-chloro-6,7-dihydroxymellein (98) were isolated for the first time from a natural source (Stadler et al. 1995a, b). These isocoumarin derivatives showed only weak nematicidal effects and the ND90 values of these compounds against Caenorhabditis elegans were all within the region of 100 µg mL−1 (Stadler et al. 1995a).
In additional, a series of mycorrhizins, mycorrhizin A (99), chloromycorrhizin A (100) and (1’-E)-dechloromycorrhizin A (101) were commonly found in normal fermentations of the fungus (Stadler and Anke 1993a). However, in fermentations in media containing a large amount of CaBr2, additional mycorrhizins could be found. These included two brominated derivatives mycorrhizin B1 (102) and mycorrhizin B2 (103) as well as (1’Z)-dechloromycorrhizin A (104) (Stadler et al. 1995c, d, e; Shan et al. 1996). These mycorrhizins were all toxic towards Caenorhabditis elegans but were only weakly active against Meloidogyne incognita. Among these mycorrhizins, mycorrhizin A (99) showed the highest activity against Caenorhabditis elegans with an LD50 at 1 µg mL−1. Based on structural and functional comparisons, it was suggested that chlorine substitutions in the side chains could increase their biological activities, whereas chlorine substitutions within the ring systems seem to weaken their activities (Stadler et al. 1995a). The brominated mycorrhizins showed weaker activities than their chlorinated analogues. However, these differences were not statistically significant (Stadler et al. 1995c). Several minor metabolites, papyracons A (105), B (106), C (107) and D (108), 6-O-methylpapyraceum B (109) and 6-O-methylpapyraceum C (110) have also been isolated from Lachnum papyraceum and they are all weakly active against Caenorhabditis elegans (Stadler et al. 1995c, d, e; Shan et al. 1996). In addition, four isoepoxydon compounds were isolated from Lachnum papyraceum (Stadler and Anke 1993a, b; Stadler et al. 1995c, e). These compounds were lachnumon (111), lachnumon A (112), lachnumon B1 (113) and lachnumon B2 (114). Lachnumon (111) and lachnumon A (112) had similar activities against Caenorhabditis elegans with an LD50 at 25 µg mL−1. Their activities against Meloidogyne incognita were weak, with an LD50 exceeding 100 µg mL−1 for both (Stadler and Anke 1993a, b). The LD90values of lachnumon B1 (113) and lachnumon B2 (114) against Caenorhabditis elegans were 25 µg mL−1 and 50 µg mL−1, respectively. Their activities against Meloidogyne incognita were similar to those of compounds lachnumon (111) and lachnumon A (112) (Stadler et al. 1995c, e). 6-hydroxymellein (93) has also obtained from other taxa including Discula spp. (Venkatasubbaiah and Chilton 1991) and Myxotrichum stipitatum (Kimura et al. 2002). In addition, mycorrhizin A (99) and chloromycorrhizin A (100) have been isolated from a mycorrhizal fungus Monotropa hypopitys (Trofast and Wickberg 1977; Trofast 1978).

Nematicidal Metabolites from Melanconium Betulimum, Mycosphaerella Lethalis and Phomopsis Phaseoli
3-hydroxypropionic acid (3-HPA) (115) was isolated as the main nematicide from the submerged culture of fungus Phomopsis phaseoli originally found on a tropical tree. This compound has also been found from Melanconium betulimum associated with Betula pendula and B. pubescens (Schwarz et al. 2004). The compound showed selective nematicidal activity against Meloidogyne incognita with an LD50 value of 12.5–15 µg mL−1, and against Caenorhabditis elegans with an LD50 value about five times lower (Schwarz et al. 2004). A 9-lactide decane compound lethaloxin (116) isolated from Mycosphaerella lethalis (Arnone et al. 1993), which was proven capable of killing C. elegans with an LD50 at 25 µg mL−1 (Stadler 1993).

Nematicidal Metabolites from Nectria Radicola and Neobulgaria Pura
A macrolide radicicol (117) produced by Nectria radicicola was a highly cytotoxic antiprotozoal and antineoplastic agent (Mirrington et al. 1964). This compound has also been isolated from other fungal species such as Monosporium bonorden, Penicillium luteo-aurantium (Nozawa and Nakajima 1979) and Chaetomium chiversii (Kithsiri Wijeratne et al. 2006). Its two dialkoxy derivatives radicicol B (118) and radicicol C (119) also possessed nematicidal activities against an unidentified soil nematode with an LD50 value at 200 µg mL−1 (Stadler 1993). Compound 14-epicochlioquinone B (120) was isolated as a platelet aggregation inhibitor from the ascomycete Neobulgaria pura (Lorenzen et al. 1994). This compound had a strong nematicidal activity against C. elegans with LD50 value at 10 µg mL−1 (Anke et al. 1995). However, it was approximately 10 times less active against Meloidogyne incognita (Anke et al. 1995).

Nematicidal Metabolites from Oidiodendron Sp. and Ophioceras Dolichostomum
Two compounds including 4-hydroxyphenylacetic acid (4-HPA) (121) and oidiolactone D (122), were isolated from cultures of Oidiodendron sp.. The two compounds showed nematicidal activities against Pratylenchus penetrans and Bursaphelenchus xylophilus (Ohtani et al. 2011). Isoamericanoic acid A (123) and caffeic acid (124) were isolated from the freshwater taxon Ophioceras dolichostomum YMF1.00988. The LD50 values of the two compounds against Bursaphelenchus xylophilus were 133.7 and 46.8 µg mL−1 respectively (Dong et al. 2010).

Nematicidal Metabolites from Paecilomyces Lilacinus and Trichoderma Longibrachiatum
The common acetic acid (125) has been isolated from culture filtrates of Paecilomyces lilacinus and Trichoderma longibrachiatum (Djian et al. 1991; Park et al. 2004). Acetic acid (125) has been shown to have selective nematicidal activities against certain nematodes (Djian et al. 1991). P. lilacinus showed effective treatment on against root-knot nematode on tomato plants under greenhouse conditions (El-Din et al. 2012).
Nematicidal Metabolites from Paecilomyces Spp.
A new macrocyclic lactone derivative paeciloxazine (126) with the pyrrolobenzoxazine skeleton was isolated from the culture broth of Paecilomyces sp. BAUA3058 (Kanai et al. 2004). Biological assay showed that the compound was active against Rhabditis pseudoelongata at 50 µg mL−1. Nematicidal compounds cerebroside A (127) and B (128) were isolated from another strain of Paecilomyces (Zhang et al. 2010). A new nematicidal compound 4-(4’-carboxy-2’-ethyl-hydroxypenty)-5,6-dihydro-6-methyl-cyclobuta[b]pyridine-3,6-dicarboxylic acid (129) was identified from Paecilomyces sp. YMF1.01761. The LD50 value of the compound within 48 h against Panagrellus redivivus was 50.86 mg L−1, Meloidogyne incognita was 47.1 mg L−1, and Bursaphelenchus xylophilus was 167.7 mg L−1 (Liu et al. 2009).

Nematicidal Metabolites from Paraniesslia Spp.
A new compound 3,5-dicarboxyaldehyde-4-hydroxy-acetophenone (130) was obtained from the freshwater fungus Paraniesslia sp. 83. This compound had a nematicidal activity against Bursaphelenchus xylophilus with an LD50 value at 200 ppm in 24 h (Dong 2005). Two sphingolipids including a new (2S,2’R,3R,3’E,4E,8E)-1-O-(β-D-glucopyranosyl)-3-hydroxyl-2-[N-2’-hydroxyl-3’-eicosadecenoyl]amino-9-methyl-4,8-octadecadiene (131) and a known (2S,2’R,3R,3’E,4E,8E)-1-O-(β-D-glucopyranosyl)-3-hydroxyl-2-[N-2’-hydroxyl-3’-oct adecenoyl]amino-9-methyl-4,8-octadecadiene (132) were isolated from another strain of Paraniesslia sp. YMF1.01400. Both compounds showed moderately nematicidal activities against Bursaphelenchus xylophilus (Dong et al. 2005).

Nematicidal Metabolites from Penicillium Bilaiae, P. Charlesii and P. Paraherquei
A new acetylenic nematicidal compound penipratynolene (133) was obtained from the culture filtrate of the fungus Penicillium bilaiae (Nakahara et al. 2004). The compound showed nematicidal activity against the root-lesion nematode Pratylenchus penetrans, capable of killing 77 % of the nematode at a concentration of 300 mg L−1 (Alfaro et al. 2003). It was suggested that the alkyne carbons are likely to play an important role in the nematicidal activities of this group of compounds (Kimura et al. 1981; Mori et al. 1982). Two known compounds, 6-methoxy-carbonylpicolinic acid (134) and 2,6-pyridinedicarboxylic acid (135), were also obtained from the culture filtrate of the strain (Nakahara et al. 2004). By a bioassay at 300 mg L−1, both the two compounds showed nematicidal activities with a mortality of 52 % and 98 % respectively against Pratylenchus penetrans (Nakahara et al. 2004). Nakahara et al. (2004) suggested that the carboxy groups in the compounds are likely to play important roles in their nematicidal activities. The oxindole alkaloid paraherquamide (136) was originally isolated from Penicillium paraherquei and its structure was determined by X-ray diffraction analysis (Yamazaki et al. 1981). Subsequently, paraherquamide (136) and its novel analogs paraherquamides B (137), C (138), D (139), E (140), F (141), G (142) were obtained from another species of Penicillium charlesii (ATCC 20841) (Ondeyka et al. 1990; Liesch and Wichmann 1990). All seven metabolites possessed nematicidal activities against Caenorhabditis elegans with LD50 values in the range of 2.5–160 µg mL−1. Among them, paraherquamide (136) was the most potent with an LD50 value of 2.5 µg mL−1 (Ondeyka et al. 1990; Blanchflower et al. 1991).

Nematicidal Metabolites from Penicillium Roqueforti and P. Simplicissimum
Three new alkaloids marcfortine A (143), B (144), and C (145) were obtained from the mycelium of Penicillium roqueforti (Polondky et al. 1980; Prangé et al. 1981). The chemical structures of marcfortine A (143) and C (145) were established by X-ray analysis. These three compounds possessed potent anthelmithic properties against plant-parasitic and animal-endoparasitic nematodes. A new alkaloid, peniprequinolone (146), together with the known compounds penigequinolones A (147), B (148) and 3-methoxy-4,6-dihydroxy-4-(4’-methoxyphenyl) quinolinone (149) were isolated from the liquid culture of Penicillium cf. simplicissimum (Kusano et al. 2000). The three known compounds were first isolated from other species of the genus Penicillium (Kimura et al. 1996; Hayashi et al. 1997). These compounds were active against the nematode Pratylenchus penetrans at the killing rates of 82.4 %, 69.2 % and 57.7 % respectively at the concentration of 1000 mg L−1. These results indicated that either a phenolic hydroxyl group at C-5 or a tetrahydropyran ring in these compounds might be responsible for their nematicidal activities against Pratylenchus penetrans (Kusano et al. 2000).

Nematicidal Metabolites from Penicillium Sp.
Paraherquamide (136) and its seven novel analogues VM55594 (150), VM54158 (151), VM54159 (152), VM55595 (153), VM55596 (154), VM55597 (155) and VM55599 (156) were isolated from a strain of Penicillium, IMI 332995 (Blanchflower et al. 1991, 1993). The nematicidal activities of compounds paraherquamide (136), VM55594 (150), VM54158 (151), VM54159 (152) were assayed against both Haemunchus contortus larvae and Trichjostrongylus colubriformis adults in vitro. Paraherquamide (136) and VM54159 (152) were more active than compounds VM55594 (150) and VM54158 (151), with MIC50 values of 31.2 and 25.6 µg mL−1 against Haemunchus contortus for paraherquamide (136) and VM54159 (152) respectively. In addition, paraherquamide (136) and VM54159 (152) could cause 99.5 % and 100 % reductions in faecal egg counts of the nematode Trichjostrongylus colubriformis at 4 mg kg−1. The group of 14-de-hydroxy in paraherquamide (136) and VM54159 (152) was more potent than their corresponding the group of 14-hydroxy in analogues VM55594 (150) and VM54158 (151) (Blanchflower et al. 1991). Compound VM55596 (154) was the first N-oxide member in the paraherquamide family and it was found capable of eliminating 94 % faecal eggs of Trichjostrongylus colubriformis when dosed at 2 mg kg−1 (Blanchflower et al. 1993).

Nematicidal Metabolites from Pochonia Chlamydosporia
Two nematicidal aurovertin compounds aurovertins F (157) and D (158) were isolated from Pochonia chlamydosporia. The LD50 value of the two compounds against Panagrellus redivivus was 88.6 and 41.7 µg mL−1 respectively (Niu et al. 2010). Two new azaphilone metabolites pseudohalonectrin A (159) and B (160) were produced by the aquatic fungus P. adversaria YMF 1.01019 (Dong et al. 2006). These two compounds possessed nematicidal activities against the pine wood nematode Bursaphelenchus xylophilus (Dong et al. 2006).

Nematicidal Metabolites from Trichoderma Spp.
A nematicidal sesquiterpene trichodermin (161) was isolated from ethyl acetate extract of Trichoderma sp. YMF1.02647. The compound could kill more than 95 % both Panagrellus redivivus and Caenorhabditis elegans in 72 h at 0.4 g L−1 (Yang et al. 2010). Trichodermin (161) had been isolated from several species of Trichoderma including T. viride, T. harzianum, T. longibrachiatum and T. reesei, and other taxa such as Stachybotrys cylindrospora and Memnoniella echinata (Godtfredsen and Vangedal 1964; Watts et al. 1988; Nielsen et al. 1998; Reino et al. 2008). The volatile organic compound 6-pentyl-2H-pyran-2-one (162) was isolated from Trichoderma sp. YMF 1.00416. Nematicidal activity assays showed that the compound could kill > 85 % of Panagrellus redivivus, Caenorhabditis elegans, and Bursaphelenchus xylophilus in 48 h at 200 mg L−1 in a 2 mL vial (Yang et al. 2012).
Nematicidal Metabolites from Talaromyces Thermophiles
A novel class of potent nematocidal thermolides A-D was isolated from a thermophilic fungus T. thermophilus. Thermolides A (163) and B (164) showed the strongest activities against Meloidogyne incognita, Bursaphelenchus xylophilus and Panagrellus redivivus with LC50 values ranging from 0.5–1.0 µg/mL, similar to those of avermectins. Thermolide C (165) displayed moderate activity, and weak inhibitory effect on the worms was observed for thermolide D (166) (Guo et al. 2012).

Nematicidal Metabolites from Unidentified Ascomycete
5-pentyl-2-furaldehyde (167) was isolated as the principal nematicide from an unidentified ascomycete belonging to the Dermateaceae (Anke et al. 1995). In addition, it has been found in other taxa such as a basidiomycete Irpex lacteus (Hayashi et al. 1981) and an unidentified fungal strain Kyu-W63 (Koitabashi et al. 2004). 5-pentyl-2-furaldehyde (167) was one of the few metabolites with nematicidal activity found in both ascomycetes and basidiomycetes. This compound was active against Caenorhabditis elegans with LD50 at 75 µg mL−1, against Meloidogyne incognita with LD50 at 60 µg mL−1 and against Aphelenchoides besseyi with LD90 at 200 µg mL−1 (Anke et al. 1995; Hayashi et al. 1981). Compounds ymf 1029 A (168), B (169), C (170), D (171), E (172), preussomerin C (173), D (174), (4RS)- 4,8-dihydroxy-3,4-dihydronaphthalen-1(2H)-one (175) and 4,6,8-trihydroxy-3,4-dihydronaphthalen-1(2H)-one (176) were isolated from an unidentified freshwater fungus YMF 1.01029. These compounds had various nematicidal activities against Bursaphelenchus xylophilus (Dong et al. 2008). From the cultures of the ascomycete A111-95, four compounds 5-(2E)-2-buten-1-ylidene-3-(1E)-1-propen-1-yl-2(5H)-furanone (177), pregaliellalactone (178), and the mixture of 5(R)-(1E)-1,3-butadien-1-yl-3-(1E)-1-propen-1-yl-2(5H)-furanone (179) and 5(R)-(3-buten-1-yl)dihydro-3-vinyldihy-2(3H)-furanone (180) with nematicidal activity towards Caenorhabditis elegans and Meloidogyne incognita were obtained (Köpcke et al. 2002). Compound 5-(2E)-2-buten-1-ylidene-3-(1E)-1-propen-1-yl-2(5H)-furanone (177) was also obtained from the basidiomycete Galiella rufa (Hautzel and Anke 1990).
Nematode-Toxic Basidiomycetes and Their Nematicidal Metabolites
Thorn and Barron (1984) reported that ten species of gilled fungi could attack and consume nematodes and considered that five species of Pleurotus could release a potent toxin which completely inactivated nematodes prior to penetration. Later, Barron and Thorn (1987) reported the details of Pleurotus used to attack its nematode victims and figured Pleurotus ostreatus produced tiny droplets of toxin from minute spathulate secetory areas and the toxin trans-2-decenedioic acid was isolated from P. ostreatus (Kwok et al. 1992) which inhibited 95 % Panagrellus redivivus at 300 ppm in 1 hour and was postulated to be identical with ostreatin. Subsequently, six nematicidal compounds were isolated from Pleurotus pulmonarius, and one of them was a new compound (Stadler et al. 1994c). Up to now, 23 species of Pleurotus have been reported to have nematicidal activity. Nematicidal acticity had been considered as one of characters of the genus Pleurotus (Hibbett and Thorn 1994). It is interesting that some edible mushrooms have such nematicidal activity. In addition, Poria cocos, a traditional Chinese medicine, had been also shown to possess nematicidal activity (Li et al. 2005).
Omphalotin A and its derivatives with potent and selective nematicidal activity were produced by the basidiomycete Omphalotus olearius (Mayer et al. 1997; Stener et al. 1997; Büchel et al. 1998). Omphalotin A and its derivatives had similar nematicidal activity with commercially available nematicide ivermectin.
Conversion of secondary metabolites as a response to injury in fruit bodies in basidiomycetes formed extracts with high nematicidal activity (Table 7.3) (Stadler and Sterner 1998). Tricholoma terreum and in all tested Lepista species, the nematicidal activity increased in response to injury, and Tricholoma terreum produced linoleic acid and S-coriolic acid while Lepista spp. produced free linoleic acid, along with unidentified metabolites (Stadler and Sterner 1998). Melanoleuca cognate, M. melaleuca, Laccaria amethystine and Marasmius wynnei produced fatty acids upon fruit body injury (Stadler and Sterner 1998). These findings reflect the presence of chemical defence systems in mushrooms, which is mediated by enzymatic conversions activated by physical injury.
Up to now, about 77 genera of basidiomycetes containing more than 160 species have been reported to possess nematicidal activity by producing active components. The nematode-toxic basidiomycete and their nematicidal compounds are listed in Table 7.3.

Nematicidal Metabolites from Calocybe Gambosa, Cheimonophyllum Candidissimum and Pycnoporus Sanguineus
The alkaloid phenoxazone (181) was isolated from the mycelial cultures of Calocybe gambosa and fruiting bodies of Pycnoporus sanguineus (Schlunegger et al. 1976; Gill 1994). This compound showed nematicidal activity against Meloidogyne incognita (LD50: 50 µg mL−1) (Mayer 1995). Six new bisabolane sesquiterpenes cheimonophyllons A (182), B (183), C (184), D (185), E (186) and cheimonophyllal (187) were obtained from the submerged cultures of Cheimonophyllum candidissimum TA 8644. These compounds were active against Caenorhabditis elegans with LD50 between 10 and 100 µg mL−1 (Stadler et al. 1994a, b). In a further study, compound 1,2-dihydroxymintlactone (188) was isolated from the same fungus (Stadler et al. 1995). 1,2-Dihydroxymintlactone (188) was a new menithol monoterpene and possessed nematicidal activity. The LD50 of 1,2-dihydroxymintlactone (188) against Caenorhabditis elegans was 25 µg mL−1 This was the first compound in the p-menthane group reported from a basidiomycete (Stadler et al. 1995).

Nematicidal Metabolites from Coprinus Comatus and C. Xanthothrix
Coprinus comatus had been proven to be active against several nematodes (Luo et al. 2007; Chen et al. 2010). A new furan 5-hydroxy-3-(hydroxymethyl)-5-methylfuran-2(5H)-one (189), two known furan compounds 5-methylfuran-3-carboxylic acid (190) and 5-hydroxy-3,5-dimethylfuran-2(5H)-one (191), as well as three benzofurans including a new 4,6-dihydroxyisobenzofuran-1,3-dione (192) and known 4,6-dimethoxyisobenzofuran-1(3H)-one (193), and 4,6-dihydroxybenzofuran-3(2H)-one (194), together with 3-formyl-2,5-dihydroxybenzyl acetate (195) were all obtained from Coprinus comatus (Luo et al. 2007). All compounds had nematicidal activities against Panagrellus redivivus and Meloidogyne arenaria at 400 ppm. The LD50 values of 5-methylfuran-3-carboxylic acid (190) and 5-hydroxy-3,5-dimethylfuran-2(5H)-one (191) were 100 ppm at 12 h (Luo et al. 2007). Coprinus xanthothrix produced three nematicidal compounds including a new compound xanthothone (196) and two known compounds 7,8,11-drimanetriol (19 7) and 2-(1H-pyrrol-1-yl)ethanol (19 8) (Liu et al. 2008). The LD50 of these compounds against Panagrellus redivivus and Meloidogyne arenaria was 125–250 ppm (Liu et al. 2008).

Nematicidal Metabolites from Dichomitus Squalens and Hericium Coralloides
A new aromadendrane, 2β,13-dihydroxyledol (19 9) was isolated from the solid mycelial cultures of Dichomitus squalens and this compound exhibited potent activity against Bursaphelenchus xylophilus with LC50 at 35.6 µg mL−1 (Huang et al. 2004). A nematicidal fatty acid mixture containing linoleic acid (7), oleic acid (200), and palmitic acid (20 1) were obtained from the culture of Hericium coralloides. This mixture showed a nematicidal activity against Caenorhabditis elegans (Xiang and Feng 2001). These fatty acids have also obtained from several other taxa.

Nematicidal Metabolites from Irpex Lacteus
A new aromatic compound methyl 3-p-anisoloxypropionate (20 2) and a new furan compound 5-(4-pentenyl)-2-furaldehyde (20 3) were isolated from Irpex lacteus. The LD50 value of the two compounds against A. besseyi was 25 µg mL−1 and 50 µg mL−1 respectively (Hayashi et al. 1981). In addition, 5-pentyl-2-furaldehyde (167) was also isolated from Irpex lacteus (Hayashi et al. 1981). The compound was obtained from an unidentified ascomycete (Anke et al. 1995) and an unidentified fungal strain Kyu-W63 (Koitabashi et al. 2004).

Nematicidal Metabolites from Lactarius Mitissimus, L. Vellereus and Russula Cuprea
Three furan sesquiterpenoids lactarorufin A (20 4), lactarorufin B (20 5) and furantriol (20 6) were isolated from Lactarius mitissimus and the all three compounds showed nematicidal activities against Caenorhabditis elegans with LD50 values at around 100 µg mL−1 (Daniewski et al. 1990; Stadler and Sterner 1998). Marasmane sesquiterpene isovelleral (20 7) could be found in injured fruiting bodies of the mushroom Lactarius vellereus. This compound was considered a key component of the chemical defense system against nematodes in this mushroom species (Sterner et al. 1985; Hansson et al. 1995). Isovelleral (2 0 7 showed nematicidal activity against Meloidogyne incognita with LD30 at 100 µg mL−1 and against Caenorhabditis elegans with LD50 at 50 µg mL−1 (Mayer 1995). Isovelleral (2 0 7) has also been found in injured fruiting bodies of other mushrooms such as Russula cuprea (Clerkyjzio and Sterner 1997).

Nematicidal Metabolites from Lachnella Villosa, Marasmius Conigenus and Peniophora Laeta
Sesquiterpene marasmic acid (20 8) was reported to have a weak activity against M. incognita by Mayer (1995). Marasmic acid (20 8) was originally isolated from Marasmius conigenus (Kavanagh et al. 1949). Subsequently, marasmic acid (20 8) was obtained from several other basidiomycetes including Lachnella villosa, Lachnella sp. and Peniophora laeta (Kupka et al. 1983; Sterner et al. 1985).
Nematicidal Metabolites from Leucopaxillus Albissimus Var. Paradoxus form Albiformis, Limacella Illinita and Mycena Sp.
A novel alkaloid 2-aminoquinoline (20 9) was isolated from the fruiting bodies of Leucopaxillus albissimus var. paradoxus form albiformis (Pfister 1988). At a concentration of 50 µg mL−1, 2-aminoquinoline (20 9) caused 50 % motility, 74 % viability, and 52 % cast formation reductions in the nematode Nippostrongylus braziliensis (Pfister 1988). A new compound illinitone A (2 10) was obtained from fermentations of Limacella illinita, which exhibited nematicidal activity on C. elegans with IC50 at 25 µg mL−1 (Gruhn et al. 2007). Mycenon (21 1) is a chlorinated benzoquinone derivative isolated from the culture broth of a basidiomycete, Mycena sp. TA 87202 (Hautzel et al. 1990). It was shown to be active against Caenorhabditis elegans, with an LD50 at 50 µg mL−1 (Stadler 1993).

Nematicidal Metabolites from Omphalotus Olearius
Peptidal compounds omphalotin A (2 1 2) and its derivatives omphalotin B (21 3), C (21 4) and D (21 5) were obtained from Omphalotus olearius. All compounds possessed strong nematicidal activities against nematodes (Mayer et al. 1997; Stener et al. 1997; Büchel et al. 1998; Anke et al. 1999). Omphalotin A (2 1 2), a cyclic dodecapeptide, was highly toxic (LD90: 0.76 µM) towards Meloidogyne incognita. However, it was approximately 50 times less active against Caenorhabditis elegans (LD90: 38 µM) (Stener et al. 1997; Büchel et al. 1998). The corresponding LD90 values for the commercially available nematicide ivermectin were 4.6 µM and 0.46 µM respectively against Meloidogyne incognita and Caenorhabditis elegans. Omphalotin A (2 1 2) lacks any antimicrobial and phytotoxic activities, and contains only weak cytotoxic activity, making it a potentially useful nematicide. The three derivatives omphalotin B (2 1 3), C (2 1 4) and D (2 1 5) all possessed nematicidal activities similar to that of omphalotin A (Anke et al. 1999). Although the yield of these active compounds is low, the strong nematicidal activity showed it is possible to find new natural nematicidals from products of fungi.

Nematicidal Metabolites from Pleurotus Ferulae, P. Ostreatus and P. Pulmonarius
Pleurotus ferulae was shown to be active against Bursaphelenchus xylophilus and Panagrellus redivivus (Li et al. 2001, 2007) and two nematicidal compounds cheimonophyllon E (21 6) and 5-hydroxymethyl-furancarbaldehyde (21 7) were isolated from the taxon. Pleurotus ostreatus was also reported to be active against nematodes (Thorn and Barron 1984, 1987; Kwok et al. 1992; Stadler et al. 1994a). Trans-2-decenedioic acid (21 8) was isolated from P. ostreatus as the principal nematicide (Kwok et al. 1992). The compound could immobilize 95 % of the nematode Panagrellus redivivus at a concentration of 300 ppm (Kwok et al. 1992). Four aromatics p-anisaldehyde (21 9), p-anisyl alcohol (2 20), 1-(4-methoxyphenyl)-1,2-propanediol (22 1) and 2-hydroxy-(4’-methoxy)-propiophenone (22 2) were isolated from Pleurotus pulmonarius (Stadler et al. 1994a). The LD50 values of these compounds against Caenorhabditis elegans were all similar, at about 100 ppm (Stadler et al. 1994a). Extracts of the mushroom Pleurotus pulmonarius contained a nematicidal fatty acid S-coriolic acid (22 3). This fatty acid could kill the nematode Caenorhabditis elegans with an LD50 value at 10 ppm (Koitabashi et al. 2004). In addition, linoleic acid (7) was also found from the fungus (Stadler et al. 1994).

Nematicidal Metabolites from Polyporus Sulphureus and Poria Cocos
The cyclic depsipeptide beauvericin (3 8) was active against Meloidogyne incognita, Caenorhabditis elegans and Bursaphelenchus xylophilus. This peptide beauvericin (39) has also been isolated from several ascomycetes, but also was isolated from the basidiomycete Polyporus sulphureus (Deol et al. 1978). Polyporus cocos is a widely used traditional medicinal fungus, and produces a novel alkyne 2,4,6-triacetylenic octane diacid (22 4) found capable of killing 83.9 % of Meloidogyne arenaria and 73.4 % of Panagrellus redivivus at 500 ppm within 12 h (Li et al. 2005).
Nematicidal metabolites from Stereum spp.
Stereum sp. 8954 produced two new aromatics, 3,5-dihydroxy-4-(3-methyl-but-2-enyl)-benzene-1,2-dicarbaldehyde (22 5) and butyl 2,4-dihydroxy-6-methylbenzoate (22 6). 3,5-Dihydroxy-4-(3-methyl-but-2-enyl)-benzene-1,2-dicarbaldehyde (22 5) could kill about 90 % of Panagrellus redivivus at 100 ppm in 12 h, while butyl 2,4-dihydroxy-6-methylbenzoate (2 2 6) was less active, capable of killing about 50 % of the same nematode at 200 ppm in 24 h (Li et al. 2006). Five cadinane sesquiterpenoids, named stereumin A (22 7), B (22 8), C (2 2 9), D (2 30) and E (2 3 1) were isolated from the culture broth of the fungal strain Stereum sp. CCTCC AF 207024. The five compounds showed nematicidal activities against the nematode P. redivivus at 400 mg L−1. Stereumin C (2 29) and stereumin D (2 30) killed 84.4 % and 94.9 % of P. redivivus respectively in 48 h (Li et al. 2008).
Conclusions
More than 200 nematicidal compounds have been obtained from fungi, and their diversified structures mainly belong to alkaloids, quinones, isoepoxydons, pyrans, furans, peptides, macrolides, terpenoids, fatty acids, diketopiperazines, aphthalenes, simple aromatics and other kinds of compounds. Among these nematicidal compounds, about 60 % are new natural isolates, which implies that searching for new compounds by screening with different models is an efficient method. Secondary metabolites in fungi are abundant, e.g., 24 compounds including 15 new isolates with nematicidal activities have been isolated from the cultures of Lachnum papyraceum and elucidated to be isoepoxydon, isocoumarin, mycorrhizin and furan compounds (Stadler and Anke 1993a, b; Stadler et al. 1995a, b, c, d, e; Shan et al. 1996), and six new sesquiterpenes and a novel monoterpene possessing nematicidal activity are fungal metabolites isolated from cultures of the basidiomycete Cheimonophyllum candidissimum (Stadler et al. 1995). Fungi are therefore a major source of biologically active natural products.
Many attempts have been made to find potent nematicidal substances. Ivermectin isolated from actinomycete is a commercially available nematicide up to now, but no major commercial product based on nematode-toxic fungi and the compounds isolated from fungi have been developed at present. Sharma (1994) reported that broth cultures of Pleurotus sajor-caju can immobilize the mushroom nematode in Agaricus bisporus.Xiang et al (2000) reported on the effects of Pleurotus ostreatus on the peanut root-knot nematode Meloidogyne arenaria in a greenhouse. The experiment showed that Pleurotus ostreatus could markedly lower Meloidogyne arenaria infection numbers and peanut root knot disease was also reduced by 87–94 %. The key factor affecting control effectiveness was the application time of Pleurotus ostreatus in the soil (Xiang and Feng 2000). The potential of oyster mushrooms to attack and kill Heterodera schachtii was studied, and the result showed some mushrooms could significantly control the nematode (Palizi et al. 2009). Omphalotin A, B, C and D isolated from Omphalotus olearius had similar nematicidal activity to the commercially available nematicide ivermectin (Mayer et al. 1997; Stener et al. 1997; Büchel et al. 1998; Anke et al. 1999). It is necessary to search for nematode-toxic fungi and their active compounds to exploit novel nematicides.
References
Abraham, W. R., & Arfmann, H. A. (1992). Penicillium camemberti a new source of brefeldin A. Planta Medica, 58, 484.
Adams, K. B., Wu, M. T., & Salunkhe, D. K. (1976). Effects of gamma radiation on growth and patulin production of Penicillium expansum and Penicillium patulim. Environmental and Experimental Botany, 16, 189–193.
Alfaro, M. C., Urios, A., González, M. C., Moya, P., & Blanco, M. (2003). Screening for metabolites from Penicillium novae-zeelandiae displaying radical-scavenging activity and oxidative mutagenicity: Isolation of gentisyl alcohol. Mutation Research, 539, 187–194.
Anderson, M. G., Jarman, T. B., & Rickards, R. W. (1995). Structures and absolute configurations of Antibiotics of the oligosporon group from the nematode-trapping fungus Arthrobotrys oligospora. Journal of antibiotics, 48(5), 391–397.
Anke, H., Kolthoum, I., Zähner, H., & Laatsch, H. (1980a). Metabolic products of microorganisms. The anthraquinones of the Aspergillus glaucus group. I. Occurrence, isolation, identification and antimicrobial activity. Archives of Microbiology, 126, 223–230.
Anke, H., Kolthoum, I., & Laatsch, H. (1980b). Metabolic products of microorganisms. The anthraquinones of the Aspergillus glaucus group. II. Biological activity. Archives of Microbiology, 126, 231–236.
Anke, H., Stadler, M., Mayer, A., & Sterner, O. (1995). Secondary metabolites with nematicidal and antimicrobial activity from nematophagous fungi and Ascomycetes. Canadian Journal of Botany, 73(Suppl. 1), 932–939.
Anke, M., Michael, K., Birgit, H., Olov, S., & Heidrun, A. (1999). In-vitro and in-vivo nematicidal activities of the cyclic dodecapeptide omphalotin A. Pesticide Science, 55, 27–30.
Arai, K., Rawlings, B. J., Yoshizawa, Y., & Vederas, J. C. (1989). Biosynthesis of antibiobic A26771B by Penicillium turbatum and dehydrocurvularin by Alternaria cinerariae: Comparison of stereochemistry of polykide and fatty acid enoyl thiol ester reductases. Journal of the American Chemical Society, 111, 3391–3399.
Arnone, A., Assante, G., Montorsi, M., Nasini, G., & Ragg, E. (1993). Secondary mould metabolites. XLIII: Isolation and structure determination of lethaloxin, a fungal macrolide from Mycosphaerella lethalis. Gazzetta Chimica Italiana, 123, 71–73.
BačÍkovÁ, D., Betina, V., & Nemec, P. (1965). Antihelminthic activity of antibiotics. Nature, 206, 1371–1372.
Banks, R. M., Blanchflower, S. E., Everett, J. R., Manger, B. R., & Reading, C. (1997). Novel anthelmintic metabolites from an Aspergillus species; the Aspergillimides. Journal of Antibiotics, 50, 840–846.
Barron, G. L., & Thorn, R. G. (1987). Destruction of nematodes by species of Pleurotus. Canadian Journal of Botany, 65, 774–778.
Barrow, K. D., & Murphy, W. S. (1972). The structure of alboleersin and luteoleesin; the identity of luteoleersin with cochlioquinone A. Journal of the Chemical Society Perkin Transactions, 1, 2837–2839.
Beecham, A. F., Fridrichsons, J., & Mathieson, A. M. (1966). The structure and absolute configuration of gliotoxon and the absolute configuration of sporidesmin. Tetrahedron Letters, 27, 3131–3138.
Bernardini, M., Carilli, A., Pacioni, G., & Santurbano, B. (1975). Isolation of beauvericin from Pacilomyces fumoso-roseus. Phytochemistry, 14, 1865.
Blanchflower, S. E., Banks, R. M., Evertt, J. R., Manger, B. R., & Reading, C. (1991). New paraherquamide antibiotics with anthelmintic activity. Journal of Antibiotics, 44, 492–497.
Blanchflower, A. E., Banks, R. M., Everett, J. R., & Reading, C. (1993). Further novel metabolites of the paraherquamide family. Journal of Antibiotics, 46, 1355–1363.
Blight, M. M., Coppen, J. J. W., & Grove, J. F. (1968). The biogenesis, from mevalonic acid of the steroidal antifungal metabolite viridian. Chemical Communications, 18, 1117–1118.
Bodo, B., Rebuffat, S., Hajji, M. E., & Davoust, D. (1985). Structures of trichorzianine A IIIc, an antifungal peptide from Trichoderma harzianum. Journal of American Chemical Society, 107, 6011–6017.
Brown, A. E., Finlay, R., & Ward, J. S. (1987). Antifungal compounds produced by Epicoccum purpurascens against soil-borne plant pathogenic fungi. Soil Biology & Biochemistry, 19, 657–664.
Brückner, H., Nicholson, G. J., & Jung, G. (1980). Gas chromatographic determination of the configuration of isovaline in antiamoebin, samarosporin (emerimicin IV), stilbellin, suzukacillins and trichotoxins. Chromatographia, 13, 209–213.
Büchel, E., Martini, U., Mayer, A., Anke, H., & Sterner, O. (1998). Omphalotins B, C and D, nematicidal cyclopeptides from Omphalotus olearius. Absolute configuration of omphalotin A. Tetrahedron, 54, 5345–5352.
Burge, W. R., Buckley, L. J., Sullivan, J. D., McGrattan, C. J., & Ikawa, M. (1976). Isolation and biological activity of the pigments of the mold Epicoccum nigrum. Journal of Agricultural and Food Chemistry, 24, 555–559.
Chen, L. J., Chen, Y., Zhang, G. D., & Duan, Y. X. (2010). Nematicidal activity of extraction and fermentation filtrate of Basidiomycetes collected in liaoning province, China. Chinese. Journal of Biological Control, 26, 467–473.
Clerkyjzio, M., & Sterner, O. (1997). Conversion of velutinal esters in the fruit bodies of Russula cuprea. Phytochemistry, 45, 1569–1572.
Cutler, H. G., Crumley, F. G., Cox, R. H., Springer, J. P., Arrendale, R. F., Cole, R. J., & Cole, P. D. (1984). Ophiobolins G and H: New fungal metabolites from a novel source, Aspergillus ustus. Journal of Agricultural and Food Chemistry, 32, 768–772.
Daniewski, W. M., Gumulka, M., & Kibicki, P. (1990). Furantriol, a lactarane sesquiterpene from Lactarius mitissimus. Phytochemistry, 29, 527–529.
Dasenbrock, J. (1994). Isolierung und Strukturaufklärung neuer Wirkstoffe aus Höheren Pilzen. Ph.D. thesis, Department of chemistry, University of Bonn, Bonn, Germany.
Deol, B. S., Ridley, D. D., & Singh, P. (1978). Isolation of cyclodepsipeptides from plant pathogenic fungi. Australian Journal of Chemistry, 31, 1397–1399.
Djian, C., Pijarouvski, L., Ponchet, M., & Arpin, N. (1991). Acetic acid, a selective nematicidal metabolite from culture filtrate of Paecilomyces lilacinus (Thom) Samsan and Trichoderma longibrachiatum Rifai. Nematologica, 37, 101–102.
Dombrink-Kurtzman, M. A., & Blackburn, J. A. (2005). Evaluation of several culture media for production of patulin by Penicillium species. International Journal of Food Microbiology, 98, 241–248.
Dong, J. Y. (2005). Ph.D. thesis, Laboratory for conservation and utilization of bio-resource, Yunnan University, Kunming, China.
Dong, J. Y., Mo, M. H., Chen, J. H., Li, Q. Y., & Zhang, K. Q. (2000). The nematicidal stability of Lampteromyces japonicus. Journal of Yunnan University, 22, 365–368.
Dong, J. Y., Zhang, K. Q., Zhao, Z. X., Liu, W. Z., & Li, Q. Y. (2001). Nematicidal activity of perylenequinones photosensitive compounds. Mycosystema, 20, 515–519.
Dong, J. Y., Zhao, Z. X., Cai, L., Liu, S. Q., Zhang, H. R., Duan, M., & Zhang, K. Q. (2003). Nematicidal effect of freshwater fungal cultures against the pine-wood nematode, Bursaphelenchus xylophilus. Fungal Diversity, 15, 123–133.
Dong, J. Y., He, H. P., Shen, Y. M., & Zhang, K. Q. (2005a). Nematicidal Epipolysulfanyldioxopiperazines from Gliocladium roseum. Journal of Natural Products, 68, 1510–1513.
Dong, J. Y., Li, R., He, H. P., & Zhang, K. Q. (2005b). Nematicidal sphingolipids from the freshwater fungus Paraniesslia sp. YMF1.01400. European Journal of Lipid Science and Technology, 107, 779–785.
Dong, J. Y., Zhou, W., Li, L., Li, G. H., Liu, Y. J., & Zhang, K. Q. (2006a). A new Epidithiodioxopiperazine Metabolite Isolated from Gliocladium roseum YMF1.00133. Chinese Chemical Letters, 17, 922–924.
Dong, J. Y., Zhou, Y. P., Li, R., Zhou, W., Zhu, Y. H., Huang, R., & Zhang, K. Q. (2006b). Newnematicidal azaphilones from the aquatic fungus Pseudohalonectria adversaria YMF1.01019. FEMS Microbiology Letters, 264, 65–69.
Dong, J. Y., Zhu, Y. H., Song, H. C., Li, R., He, H. P., Liu, H. Y., Huang, R., Zhou, Y. P., Wang, L., & Zhang, K. Q. (2007). Nematicidal Resorcylides from the Aquatic Fungus Caryospora callicarpa YMF1.01026. Journal of Chemical Ecology, 33, 1115–1126.
Dong, J. Y., Song, H. C., Li, J. H., Tang, Y. S., Sun, R., Wang, L., Zhou, Y. P., Wang, L. M., Shen, K. Z., Wang, C. R., & Zhang, K. Q. (2008). Ymf 1029A E, Preussomerin Analogues from the Fresh-Water-Derived Fungus YMF 1.01029. Journal of Natural Products, 71, 952–956.
Dong, J. Y., Wang, L., Song, H. C., Wang, L. M., Shen, K. Z., Sun, R., Li, G. H., Li, L., & Zhang, K. Q. (2010). Ophiocerol, a novel macrocylic neolignan from the aquatic fungus Ophioceras dolichostomum YMF1.00988. Natural Products Research, 24, 1004–1012.
Dornberger, K., Ihn, W., Ritzau, M., Gräfe, U., Schlegel, B., Fleck, W. F., & Metzger, J. W. (1995). Chrysospermins, new peptaibol antibiotics from Apiocrea chrysosperma Ap101. Journal of Antibiotics, 48, 977–989.
Ekaterini, R., Lawrence, A. L., & Neussa, G. (2008). Muscodor albus, a potential biocontrol agent against plant-parasitic nematodes of economically important vegetable crops in Washington State, USA. Biological Control, 45, 380–385.
El-Din, H. K. M., Allam, A., & Tag, B. A. (2012). Nematicidal activity of some biopesticide agents and microorganisms against root-knot nematode on tomato plants under greenhouse conditions. Journal of Plant Protection Research, 52, 47–52.
Fardos, A. M. B. (2009). Efficacy of some Trichoderma species in the control of Rotylenchulus reniformis and Meloidogyne javanica. Archives of Phytopathology and Plant Protection, 42, 361–369.
Freeman, G. G., Gill, J. E., & Waring, W. S. (1959). The structure of trichothecin and its hydrolysis products. Journal of the Chemical Society, (0), 1105–1132.
Fukuda, T., Arai, M., Yamaguchi, Y., Masuma, R., Tomoda, H., & Ōmura, S. (2004). New beauvericins, potentiators of antifungal miconazole activity, produced by Beauveria sp. FKI-1366. Journal of Antibiotics, 57, 110–116.
Ghisalberti, E. L., & Rowland, C. V. (1997). 6-Chlorode-hydrocurvularin, a new metabolite from Cochliobolus spicifer. Journal of Natural Products, 56, 2175–2177.
Gill, M. (1994). Pigments of fungi (Macromycetes). Natural Product Reports, 11, 67–90.
Giuma, A. Y., & Cooke, R. C. (1971). Nematotoxin production by Nematoctonus haptocladus and N. concurrens. Transactions of the British Mycological Society, 56, 89–94.
Giuma, A. Y., Hackett, A. M., & Cooke, R. C. (1973). Thermostable nematotoxins produced by germinaling conidia of some endozoic fungi. Transactions of the British Mycological Society, 60, 49–56.
Godtfredsen, W. O., & Vangedal, S. (1964). Trichodermin, a new antibiotic related to trichothecin. Proceedings of the Chemical Society, 6, 188–189.
Grabley, S., Hammann, P., Thiericke, R., Wink, J., Philipps, S., & Zeeck, A. (1993). Secondary metabolites by chemical screening. 21 Clonostachydiol, A novel anthelmintic macrodiolide from the fungus Clonostachys cylindrospora. Journal of Antibiotics, 46, 343–345.
Gruhn, N., Schoettler, S., Sterner, O., & Anke, T. (2007). Biologically active metabolites from the basidiomycete Limacella illinita (Fr.) Murr. Zeitschrift fur Naturforschung—Section C. Journal of Biosciences, 62, 808–812.
Guo, J. P., Zhu, C. Y., Zhang, C. P., Chu, Y. S., Wang, Y. L., Zhang, J. X., Wu, D. K., Zhang, K. Q., & Niu, X. M. (2012). Thermolides, potent nematocidal PKS-NRPS hybrid metabolites from thermophilic fungus Talaromyces thermophiles. Journal of the American Chemical Society, 134, 20306–20309.
Hamill, R. L., Higgens, C. E., Boaz, N. E., & Gorman, M. (1969). The structure of beauvericin, a new depsipeptide antibiotic toxic to Artemia salina. Tetrahedron Letters, 49, 4255–4258.
Hansson, T., Sterner, O., & Strid, K. (1995). Chemotaxonomic evidence for a division of Lactarius vellereus and L. bertillonii as different species. Phytochemistry, 39, 363–365.
Harvey (2000). Strategies for discovering drugs from previously unexplored natural products. Drug Discovery Today, 7, 94–300.
Hautzel, R., & Anke, H. (1990). Screening of basidiomycetes and ascomycetes for plant growth regulating substances. Introduction of the gibberellic acid induced de-novo synthesis of hydrolytic enzymes in embryoless seeds of Triticum aestivum as test system. Zeitschrift fur Naturforschung—Section C.Journal of Biosciences, 45, 68–73.
Hautzel, R., Anke, H., & Sheldrick, W. S. (1990). Mycenon, a new metabolite from a Mycena species ta 87202 (Basidiomycetes) as an inhibitor of isocitrate lyase. Journal of Antibiotics, 43, 1240–1244.
Hayashi, M., Wada, K., & Munakata, K. (1981). New nematicidal metabolites from a fungus, Irepex lacteus. Agricultural and Biological Chemistry, 45, 1527–1529.
Hayashi, H., Nakatani, T., Inoue, Y., Nakayama, M., & Nozaki, H. (1997). New dihydroquinolinone toxic to Artemia salina produced by Penicillium sp. NTC-47. Bioscience Biotechnology and. Biochemistry, 61, 914–916.
Hayashi, A., Fujioka, S., Nukina, M., Kawano, T., Shimada, A., & Kimura, Y. (2007). Fumiquinones A and B, nematicidal quinones produced by Aspergillus fumigatus. Bioscience Biotechnology and. Biochemistry, 71, 1697–1702.
Haydock, P. P. J., Woods, S. R., Grove, I. G., & Hare, M. C. (2006). Chemical control of nematodes. In R. N. Perry & M. Moens (Eds.), Plant Nematology (pp. 392–410). Wallingford: CAB Interna-tional.
He, J., Wijeratne, E. M., Bashyal, B. P., Zhan, J., Seliga, C. J., Liu, M. X., Pierson, E. E., Pierson, L. S., VanEtten, H. D., & Gunatilaka, A. A. (2004). Cytotoxic and other metabolites of Aspergillus inhabiting the rhizosphere of sonoran desert plants. Journal of Natural Products, 67, 1985–1991.
Hibbett, D. S., & Thorn, R. G. (1994). Nematode-trapping in Pleurotus tuberregium. Mycologia, 86, 696–699.
Huang, Z. L., Dan, Y., Huang, Y. C., Lin, L. D., Li, T. H., Ye, W. H., & Wei, X. Y. (2004). Sesquiterpenes from the mycelial cultures of Dichomitus squalens. Journal of Natural Products, 67, 2121–2123.
Hyeon, S., Ozaki, A., Suzuki, A., & Tamura, S. (1976). Isolation of αβ-dehydrocurvularin and β-hydroxycurvularin from Alternaria tomato as sporulation-suppressing factors. Agricultural and Biological Chemistry, 40, 1663–1664.
Kanai, Y., Fujimaki, T., Kochi, S., Konno, H., Kanazawa, S., & Tokumasu, S. (2004). Paeciloxazine, a novel nematicidal antibiolic from Paecilomyces sp. Journal of Antibiotics, 57, 24–28.
Kang, S. W., & Kim, S. W. (2004). New antifungal activity of penicillic acid against Phytophthora species. Biotechnology letters, 26, 695–698.
Kavanagh, F., Hervey, A., & Robbins, W. J. (1949). Antibiotic substances from basidiomycetes. Proceedings of the National Academy of Sciences, 35, 343–349.
Kawazu, K., Murakami, T., Ono, Y., Kanzaki, H., Kobayashi, A., Mikawa, T., & Yoshikawa, N. (1993). Isolation and characterization of two novel nematicidal depsipeptides from an imperfect fungus, strain D1084. Bioscience Biotechnology and Biochemistry, 57, 98–101.
Kennedy, N., & Tampion, J. (1978). A nematotoxin from Nematotonus robustus. Transantions of the British Mycological Society, 70, 140–141.
Khambay, B. P. S., Bourne, J. M., Cameron, S., Kerry, B. R., & Zaki, M. J. (2000). A nematicidal metabolite from Verticillium chlamydosporium. Pest Management Science, 56, 1098–1099.
Khan, S. T., & Kgan, T. A. (1992). Effect of culture filtrates of soil fungi on the hatching and mortality of root knot nematode (Meloidogyne incognita). Current Nematology, 3, 53–60.
Khan, M. R., & Haque, Z. (2011). Soil application of Pseudomonas fluorescens and Trichoderma harzianum reduces root-knot nematode, Meloidogyne incognita, on tobacco. Phytopathologia Mediterranea, 50, 257–266.
Kim, H. L., & Kochevar, J. (1995). Isolation of brefeldin A. General Pharmacology, 26, 363–364.
Kim, J. C., Choi, G. J., Park, J. H., Kim, H. T., & Cho, K. Y. (2001). Activity against plant pathogenic fungi of phomalactone isolated from Nigrospora sphaerica. Pest Management Science, 57, 554–559.
Kimura, Y., Mori, M., Suzuki, A., & Kobayashi, A. (1981). Isolation and identification of two nematicidal substances from roots of Erigeron philadelphicus L. and nematicidal activities of their related compounds. Agricultural and Biological Chemistry, 45, 2915–2917.
Kimura, Y., Kusano, M., Koshino, H., Uzawa, J., Fujioka, S., & Tani, K. (1996a). Penigequinolones A and B, pollen-growth inhibitors produced by Penicillium sp. No. 410. Tetrahedron Letters, 37, 4961–4964.
Kimura, Y., Nakahara, S., & Fujioka, S. (1996b). Aspyrone, a nematicidal compound isolated from the fungus, Aspergillus melleus. Bioscience Biotechnology and. Biochemistry, 60, 1375–1376.
Kimura, Y., Shimada, A., Kusano, M., Yoshii, K., Morita, A., Nishibe, M., Fujioka, S., & Kawano, T. (2002). Myxostiolide, myxostiol, and clavatoic acid, plant growth regulators from the fungus Myxotrichum stipitatum. Journal of Natural Products, 65, 621–623.
Kimura, Y., Tani, S., Hayashi, A., Ohtani, K., Fujioka, S., Kawano, T., & Shimada, A. (2007). Nematicidal activity of 5-hydroxymethyl-2-furoic acid against plant-parasitic nematodes. Zeitschrift Fur Naturforschung C, 62, 234–238.
Kind, R., Zeeck, A., Grabley, S., Thiericke, R., & Zerlin, M. (1996). Secondery metabolites by chemical screening. 30. Helmidiol, a new m acrodiolide from Alternaria alternaya. Journal of Natural Products, 59, 539–540.
Kithsiri Wijeratne, E. M., Paranagama Priyani, A., & Leslie Gunatilaka, A. A. (2006). Five new isocoumarins from Sonoran desert plant-associated fungal strains Paraphaeosphaeria quadriseptata and Chaetomium chiversii. Tetrahedron, 62, 8439–8446.
Kobayashi, A., Hino, T., Yata, S., Itoh, T. J., Sato, H., & Kawazu, K. (1988). Unique spindle poisons. Curvularin and its derivatives, isolated from Penicillium species. Agricultural and Biological Chemistry, 52, 3119–3123.
Koitabashi, M., Kajitani, Y., & Hirashima, K. (2004). Antifungal substances produced by fungal strain Kyu-W63 from wheat leaf and its taxonomic position. Journal of General Plant Pathology, 70, 124–130.
Konishi, K., Iida, A., Kaneko, M., Tomioka, k, Tokuda, H., Nishino, H., & Kumeda, Y. (2003). Cancer preventive potential of trichothecenes from Trichothecium roseum. Bioorganic & Medicinal Chemistry, 11, 2511–2518.
Köpcke, B., Johansson, M., Sterner, O., & Anke, H. (2002). Biologically active secondary metabolites from the ascomycete A111-95. 1. Production, isolation and biological activities. Journal of Antibiotics, 55, 36–40.
Krasnoff, S. B., & Gupta, S. (1994). Identification of the antibiotic phomalactone from the entomopathogenic fungus Hirsutella thompsonii var. synnematosa. Journal of Chemical Ecology, 20, 293–302.
Kumazawa, S., Kanda, M., Utagawa, M., Chiba, N., Ohtani, H., & Mikawa, T. (2003). MK7924, a novel metabolite with nematocidal activity from Coronophora gregaria. Journal of Antibiotics, 56, 652–654.
Kupka, J., Anke, T., Mizumoto, K., Giannetti, B. M., & Steglich, W. (1983). Antibiotics from Basidiomycetes. XVII The effect of marasmic acid on nucleic acid metabolism. Journal of Antibiotics, 36, 155–160.
Kusano, M., Koshino, H., Uzawa, J., Fujioka, S., Kawano, T., & Kimura, Y. (2000). Nematicidal alkaloids and related compounds produced by the fungus Penicillium cf. Simplicissimum. Bioscience Biotechnology and. Biochemistry, 64, 2559–2568.
Kusano, M., Nakagami, K., Fujioka, S., Kawano, T., Shimada, A., & Kimura, Y. (2003). β,γ; -dehydrocurvularin and related compounds as nematicides of Pratylenchus penetrans from the Fungus Aspergillus sp. Bioscience Biotechnology and. Biochemistry, 67, 1413–1416.
Kwok, O. C. H., Plattner, R., Weisleder, D., & Wicklow, D. T. (1992). A namatical toxin from Pleurotus ostreatus NRRL 3526. Journal of Chemical Ecology, 18, 127–136.
Lai, S., Shizuri, Y., Yamamura, S., Kawai, K., Terada, Y., & Furukawa, H. (1989). Novel curvularin-type metabolites of a hybrid strain ME 0005 derived from Penicillium citreo-viride B. IFO and 4692. Tetrahedron Letters, 30, 2241–2244.
Lai, S., Shizuri, Y., Yamamura, S., Kawai, K., Terada, Y., & Furukawa, H. (1990). New metabolites of two hybrid strains ME 0004 and 0005 derived from Penicillium citreo-viride B. IFO 6200 and 4692. Chemistry Letters, 19, 589–592.
Li, Y. Z. (2005). Researches on the screening of nematophagous Basidiomycetes and their effects on nematodes. Master thesis. Laboratory of Plant Pathology, College of Agriculture, Guizhou University, Guiyang.
Li, G. H., Dong, J. Y., Mo, M. H., & Zhang, K. Q. (2001). Nematicidal activity of nematophagous Pleurotus and allied fungi to Panagrellus redivivus. Chinese. Journal of Biological Control, 17, 26–29.
Li, G. H., Shen, Y. M., & Zhang, K. Q. (2005). Nematicidal activity and chemical component of Poria cocos. The Journal of Microbiology, 43, 17–20.
Li, G. H., Li, L., Duan, M., & Zhang, K. Q. (2006). The Chemical Constituents of the Fungus Stereum sp. Chemistry and Biodiversity, 3, 210–216.
Li, G. H., Wang, X. B., Zheng, L. J., Li, L., Huang, R., & Zhang, K. Q. (2007a). Nematicidal metabolites from the fungus Pleurotus ferulae Lenzi. Annals of Microbiology, 57, 527–529.
Li, G. H., Yu, Z. F., Li, X., Wang, X. B., Zheng, L. J., & Zhang, K. Q. (2007b). Nematicidal metabolites produced by the endophytic fungus Geotrichum sp. AL4. Chemistry and Biodiversity, 4, 1520–1524.
Li, G. H., Duan, M., Yu, Z. F., Li, L., Dong, J. Y., Wang, X. B., Guo, J. W., Huang, R., Wang, M., & Zhang, K. Q. (2008). Stereumin A-E, sesquiterpenoids from the fungus Stereum sp. CCTCC AF 207024. Phytochemistry, 69, 1439–1445.
Liesch, J. M., & Wichmann, C. F. (1990). Novel antinematodal and antiparasitic agents from Penicillium Charlesii. II. Structure determination of paraherquamides B, C, D, E, F, and G. Journal of Antibiotics, 43, 1380–1386.
Liu, Y. J. (2005). Ph.D. thesis, Laboratory for conservation and utilization of bio-resource, Yunnan University, Kunming, China.
Liu, Y. J., Liu, Y., & Zhang, K. Q. (2008). Xanthothone E, a new nematicidal N-compound from Coprinus xanthothrix. Chemistry of Natural Compounds, 44, 161–162.
Liu, Y. J., Zhai, C. Y., Liu, Y., & Zhang, K. Q. (2009). Nematicidal activity of Paecilomyces spp. and isolation of a novel active compound. The Journal of Microbiology, 47, 248–252.
Liu, J. H., Wang, L., Qiu, J. Y., Jiang, L., Yan, J. Y., Liu, T., Liu, W. C., & Duan, Y. X. (2011). Nematicidal activity of gymnoascus reesii against meloidogyne incognita. African Journal of Microbiology Research, 5, 2715–2719.
Lopez-Diaz, T. M., & Flannigan, B. (1997). Production of patulin and cytochalasin E by Aspergillus clavatus during malting of barley and wheat. International Journal of Food Microbiology, 35, 129–136.
Lorenzen, K., Anke, T., Anders, U., Hindermayr, H., & Hansske, F. (1994). 14-epidihydrocochlioquinone B, and 14-epocochliqquinone B, antibiotics from fermentation of the ascomycete Neobulgaria pura: Structure elucidation and effects on platelet aggregation. Zertschrift Fur Naturforschung C, 49C, 313–320.
Luo, H., Liu, Y. J., Fang, L., Li, X., Tang, N. H., & Zhang, K. Q. (2007). Coprinus comatus Damages Nematode Cuticles Mechanically with Spiny Balls and Produces Potent Toxins. Applied and Environmental Microbiology, 73, 3916–3923.
Martínez-Luis, S., González, M. C., Ulloa, M., & Mata, R. (2005). Phytotoxins from the fungus Malbranchea aurantiaca. Phytochemistry, 66, 1012–1016.
Mayer, A. (1995). Ph.D. thesis, University of Kaiserslautern, Kaiserslauten.
Mayer, A., Anke, H., & Sterner, O. (1997). Omphalotin, a new cycle peptide with potent nematicidal activity from Omphalotus olearius. I. Fermentation and Biological activity. Natural Product Letters, 10, 25–32.
Meira, B. E., Edna, S., & Yitzhak, S. (2006). Nematicidal activity of Chrysanthemum coronarium. European Journal of Plant Pathology, 114, 427–433.
Meng, Q., Shi, X., Meng, F., Feng, X., & Sun, J. (2012). Isolation of an Acremonium sp. from a screening of 52 seawater fungal isolates and preliminary characterization of its growth conditions and nematicidal activity. Biotechnology Letters, 34, 1847–1850.
Metzger, J., Schlegel, B., Fleck, W. P., Dornberger, K., Ihn, W., Schade, W., & Gräfe, U. J. (1994). Canadian patent Appl. No. 2,115,966 (18.02.94).
Meyer, S. L. F., Huettel, R. N., Liu, X. Z., Humber, R. A., Juba, A., & Nitao, J. K. (2004). Activity of fungal culture filtrates against soybean cyst nematode and root-knot nematode egg hatch and juvenile motility. Nematology, 6, 23–32.
Mirrington, R. N., Ritchie, E., Shoppee, C. W., Taylor, W. C., & Sternhell, C. (1964). The constitution of radicicol. Tetrahedron Letters, 7, 365–370.
Mo, M. H., Li, G. H., Dong, J. Y., & Zhang, K. Q. (2000). Lampteromyces japonicus- A new nematophagous fungi. Mycosystema, 19, 529–533.
Mori, M., Hyeon, S., Kimura, Y., & Suzuki, A. (1982). The nematicidal activity of acetylene compounds. Agricultural and Biological Chemistry, 46, 309–311.
Moulé, Y., & Hatey, F. (1977). Mechanism of the in vitro inhibition of transcription by patulin, a mycotoxin from Byssochlamys nivea. FEBS Letters, 74, 121–125.
Munro, H. D., Musgrave, O. C., & Templeton, R. (1967). Curvularin. Part V. The compound C16H18O5, αβ-dehydrocurvularin. Journal of the Chemical Society C, 947–948.
Nakahara, S., Kusano, M., Fujioka, S., Shimada, A., & Kimura, Y. (2004). Penipratynolene, a novel nematicide from Penicillium bilaiae Chalabuda. Bioscience Biotechnology and. Biochemistry, 68, 257–259.
Nielsen, K. F., Hansen, M. O., Larsen, T. O., & Thrane, U. (1998). Production of trichothecene mycotoxins on water damaged gypsum boards in Danish buildings. International Biodeterioration & Biodegradation, 42, 1–7.
Nitao, J. K. B., Meyer, S. L. F., Oliver, J. E., Schmidt, W. F., & Chitwood, D. J. (2002). Isolation of flavipin, a fungus compound antagonistic to plant-parasitic nematodes. Nematology, 4, 55–63.
Niu, X. M., Wang, Y. L., Chu, Y. S., Xue, H. X., Li, N., Wei, L. X., Mo, M. H., & Zhang, K. Q. (2010). Nematodetoxic aurovertin-type metabolites from a root-knot nematode parasitic fungus Pochonia chlamydosporia. Journal of Agricultural and Food Chemistry, 58, 828–834.
Nozawa, K., & Nakajima, S. (1979). Isolation of radicicol from Penicillium luteo-aurantium, and meleagrin, a new metabolite, from Penicillium meleagrinum. Journal of Natural Products, 42, 374–377.
Ohtani, K., Fujioka, S., Kawano, T., Shimada, A., & Kimura, Y. (2011). Nematicidal activities of 4-hydroxyphenylacetic acid and oidiolactone D produced by the fungus Oidiodendron sp. Zeitschrift fur Naturforschung—Section C. Journal of Biosciences, 66, 31–34.
Ōmura, S., Miyadera, H., Ui, H., Shiomi, K., Yamaguchi, Y., Masuma, R., Nagamitsu, T., Takano, D., Sunazuka, T., Harder, A., Kölbl, H., Namikoshi, M., Miyoshi, H., Sakamoto, K., & Kita, K. (2001). An anthelmintic compound, nafuredin, shows selective inhabition of complex I in helminth mitochondria. Proceedings of the National Academy of Sciences USA, 98, 60–62.
Ondeyka, J. G., Goegelman, R. T., Schaeffer, J. M., Kelemen, L., & Zitano, L. (1990). Novel antinematodal and antiparasitic agents from Penicillium Charlesii. I. Fermentation, isolation and biological activity. Journal of Antibiotics, 53, 1375–1379.
Palizi, P., Goltapeh, E. M., Pourjam, E., & Safaie, N. (2009). Potential of oyster mushrooms for the biocontrol of sugar beet nematode (Heterodera schachtii). Journal of Plant Protection Research, 49, 27–33.
Pandey, R. C., Meng, H., Cook, J. C., & Rinehart, K. L. (1977). Structure of antiamoebin I from high resolution field desorption and gas chromatographic mass spectrometry studies. Journal of American Chemical Society, 99, 5203–5205.
Park, J. O., Hargreaves, J. R., Cole, A. L. J., Ghisalberti, E. L., Gams, W., & Sivasithamparam, K. (2001). Cuticular disruption and mortality of Caenorhabditis elegans exposed to culture filtrate of Byssochlamys nivea westling. Nematology, 3, 355–363.
Park, J. O., Hargreaves, J. R., McConville, E. J., Stirling, G. R., Ghisalberti, E. L., & Sivasithamparam, K. (2004). Production of leucinostatins and nematicidal activity of Australian isolates of Paecilomyces lilacinus (Thom) Samson. Letters in Applied Microbiology, 38, 271–276.
Pfister, J. R. (1988). Isolation and bioactivity of 2-aminoquinoline from Leucopaxillus albissimus. Journal of Natural Products, 51, 969–970.
Polondky, J., Merrien, M. N., Prangé, T., Pascard, C., & Moreau, S. (1980). Isolation and structure (X-ray analysis) of marcfortine A, a new alkaloid from Penicillium roqueforti. Journal of the Chemical Society—Chemical Communications, 13, 601–602.
Prangé, T., Billion, M. A., Vuilhorgne, M., Pascard, C., Polonsky, J., & Moreau, S. (1981). Structures of marcfortine B and C (X-ray analysis), alkaloids from Penicillium Roqueforti. Tetrahedron Letters, 22, 1977–1980.
Quaghebeur, K., Coosemans, J., Toppet, S., & Compernolle, F. (1994). Cannabiorci- and 8-chlorocannabiorcichromenic acid as fungal antagonists from Cylindrocarpon Olidum. Phytochemistry, 37, 159–161.
Radwan, M. A., Farrag, S. A. A., Abu-Elamayem, M. M., & Ahmed, N. S. (2012). Biological control of the root-knot nematode, Meloidogyne incognita on tomato using bioproducts of microbial origin (2012). Applied Soil Ecology, 56, 58–62.
Rao, A. V. R., Murthy, V. S., & Sharma, G. V. M. (1995). The first synthesis and determination of absolute stereochemistry of clonostachydiol-Part II. Tetrahedron Letters, 36, 143–146.
Regaieg, H., Ciancio, A., Raouani, N. H., Grasso, G., & Rosso, L. (2010). Effects of culture filtrates from the nematophagous fungus Verticillium leptobactrum on viability of the root-knot nematode Meloidogyne incognita. World. Journal of Microbiology and Biotechnology, 26, 2285–2289.
Reimerdes, E. H., Engel, G., & Behnert, J. (1975). Investigation on the production of mycotoxins and quantitative evaluation. I The production of penicillic acid by Penicillium cyclopium. Journal of Chromatography, 110, 361–368.
Reino, J. L., Guerrero, R. F., Hernández-Galán, R., & Collado, I. G. (2008). Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochemistry Reviews, 7, 89–123.
Robertson, A. P., Clark, C. L., Burans, T. A., Thompson, D. P., & Geary, T. G. (2002). Paraherquamide and 2-deoxy-paraherquamide distinguish cholinergic receptor subtypes in Ascaris Muscke. The Journal of Pharmacology and Experimental Therapeufics, 302(3), 853–860.
Reyes-Estebanez, M., Herrera-Parra, E., Cristóbal-Alejo, J., Heredia-Abarca, G., Canto-Canché, B., Medina-Baizabal, I., & Gamboa-Angulo, M. (2011). Antimicrobial and nematicidal screening of anamorphic fungi isolated from plant debris of tropical areas in Mexico. African Journal of Microbiology Research, 5, 1083–1089.
Robeson, D. J., & Strobel, G. A. (1981). αβ-dehydrocurvularin and curvularin from Alternaria cinerariae. Zeitschrift fur Naturforschung C, 36c, 1081–1083.
Robeson, D. J., & Strobel, G. A. (1985). The identification of a major phytotoxic component from Alternaria macrospore as αβ-dehydrocurvularin. Journal of Natural Products, 48, 139–141.
Ruanpanun, P., Tangchitsomkid, N., Hyde, K. D., & Lumyong, S. (2010). Actinomycetes and fungi isolated from plant-parasitic nematode infested soils: Screening of the effective biocontrol potential, indole-3-acetic acid and siderophore production. World Journal of Microbiology and Biotechnology, 26, 1569–1578.
Samson-Himmelstjerna, G. V., Harder, A., Sangster, N. C., & Coles, G. C. (2005). Efficacy of two cyclooctadepsipeptides, PF1022A and emodepside, against anthelmintic-resistant nematodes in sheep and cattle. Parasitology, 130, 343–347.
Sankaranarayanan, C., Hussaini, S. S., Kumar, P. S., & Prasad, R. D. (1997). Nematicidal effect of fungal filtrates against root-knot nematodes. Journal of Biological Control, 11, 37–41.
Sasaki, T., Takagi, M., Yaguchi, T., Miyadoh, S., Okada, T., & Koyama, M. (1992). A new anthelmintic cyclodepsipeptide, PF1022A. Journal of Antibiotics, 45, 692–697.
Schaeffer, J. M., Frazier, E. G., Bergstrom, A. R., Williamson, J. M., Liesch, J. M., & Goetz, M. A. (1990). Cochlioquinone A, a nematocidal agent which competes for specific [3H] ivermectin binding sites. Journal of Antibiotics, 43, 1179–1182.
Schlunegger, U. P., Kuchen, A., & Clemencon, H. (1976). Mycelium products in higher fungi. I. phenoxazine derivatives in Calocybe gambosa. Helvetica Chimica Acta, 59, 1383–1388.
Schwarz, M., Köpcke, B., Weber, R. W. S., Sterner, O., & Anke, H. (2004). 3-Hydroxypropionic acid as a nematicidal principle in endophytic fungi. Phytochemistry, 65, 2239–2245.
Shan, R., Stadler, M., Sterner, O., & Anke, H. (1996). New metabolites with nematicidal and antimicrobial activities from the ascomycete Lachnum papyraceum (Karst.) Karst. VIII. Isolation, structure determination and biological activities of minor metabolites structurally related to mycorrhizin A. Journal of Antibiotics, 49, 447–452.
Sharma, V. P. (1994). Potential of Pleurotus sajor-caju for biocontrol of Aphelenchoides camposticola in Agaricus bisporus cultivation. Mushroom Research, 3, 15–20.
Shimada, A., Fujioka, S., Koshino, H., & Kimura, Y. (2010) Nematicidal activity of beauvericin produced by the fungus Fusarium bulbicola. Zeitschrift fur Naturforschung C, 65, 207–210.
Shiomi, K., Ui, H., Suzuki, H., Hatano, H., Nagamitsu, T., Takano, D., Miyadera, H., Yamashita, T., Kita, K., Miyoshi, H., Harder, A., Tomoda, H., & Ōmura, S. (2005). A γ-lactone form nafuredin, nafuredin-γ; also inhabits helminth complex I. Journal of Antibiotics, 58, 50–55.
Singh, S. B., Smith, J. L., Sabnis, G. S., Dombrowski, A. W., Schaeffer, J. M., Goetz, M. A., & Bills, G. F. (1991). Structure and conformation of opiophiobolin K and 6- epiophiobolin K from Aspergillus ustus as a nematocidal Agent. Tetrahedron, 47, 6931–6938.
Singleton, V. L., Bohonos, N., & Ullstrup, A. J. (1958). Decumbin, a new compound from a species of Penicillium. Nature, 181, 1072–1073.
Snook, C. F., Woolley, G. A., Oliva, G., Pattabhi, V., Wood, S. F., Blundell, T. L., & Wallace, B. A. (1998). The structure and function of ntiamoebin I, a proline-rich membrane-active polypeptide. Structure, 6, 783–792.
Stadler, M. (1993). PhD thesis, University of Kaiserslautern, Kaiserslautem, Germany.
Stadler, M., & Anke, H. (1993a). Lachnumon and lachnumol A, new metabolites with nematicidal and antimicrobial activities from the ascomycete Lachnum papyraceum (Karst.) Karst. I. Producing organism, fermentation, isolation and biological activities. Journal of Antibiotics, 46, 961–967.
Stadler, M., & Anke, H. (1993b). Lachnumon and lachnumol A, new metabolites with nematicidal and antimicrobial activities from the ascomycete Lachnum papyraceum (Karst.) Karst. II. Structural elucidation. Journal of Antibiotics, 46, 968–971.
Stadler, M., & Sterner, O. (1998). Production of bioactive secondary metabolities in the fruit bodies of macrofungi as a response to injury. Phytochemistry, 49, 1013–1019.
Stadler, M., Sterner, O., & Anke, H. (1993). New biologically active compounds from the nematode-trappingfungus Arthrobotrys oligospora Fresen. Zeitschrift fur Naturforschung C, 48c, 843–850.
Stadler, M., Anke, H., & Sterner, O. (1994a). Six new antimicrobial and nematicidal bisadolanes from the Basidiomycete Cheimonophyllum candidissimum. Tetrahedron, 50, 12649–12654.
Stadler, M., Anke, H., & Sterner, O. (1994b). New nematicidal and antimicrobial compounds from the Basidiomycete Cheimonophyllum candidissimum (Berk & Curt) Sing, I. Producing organism, fermentation, isolation, and biological activaties. Journal of Antibiotics, 47, 1284–1289.
Stadler, M., Mayer, A., Anke, H., & Sterner, O. (1994). Fatty acids and other compounds with nematicidal activity from cultures of basidiomycetes. Planta Medica, 60, 128–132.
Stadler, M., Anke, H., & Sterner, O. (1995a). Metabolites with nematicidal and antimicrobial activities from the ascomycete Lachnum papyraceum (Karst.) Karst. III. Production of novel isocoumarin derlvatives, isolation and biological activities. Journal of Antibiotics, 48, 261–266.
Stadler, M., Anke, H., & Sterner, O. (1995b). New metabolites with nematicidal and antimicrobial activities from the ascomycete Lachnum papyraceum (Karst.) Karst. IV. Structural elucidation of novel isocoumarin dervatives. Journal of Antibiotics, 48, 267–273.
Stadler, M., Anke, H., & Sterner, O. (1995c). Metabolites with nematicidal and antimicrobial activities from the ascomycete Lachnum papyraceum (Karst.) Karst. V. Production, isolation and biological activities of bromine-contaning mycorrhizin and lachnumon derivatives and four additional new bioactive metabolites. Journal of Antibiotics, 48, 149–153.
Stadler, M., Anke, H., Shan, R., & Sterner, O. (1995d). New metabolites with nematicidal and antimicrobial activities from the ascomycete Lachnum papyraceum (Karst.) Karst. VI. Structure determination of non-halogenated metabolites structurally related to mycorrhizin A. Journal of Antibiotics, 48, 154–157.
Stadler, M., Anke, H., Shan, R., & Sterner, O. (1995e). New metabolites with nematicidal and antimicrobial activities from the Ascomycete Lachnum papyraceum (Karst.) Karst. VII. Structure determination of brominated Lachnumom and mycorrhizin A derivatives. Journal of Antibiotics, 48, 158–161.
Stadler, M., Anke, H., Dekermendjian, K., Reiss, R., Sterner, O., & Witt, R. (1995f). Novel bioactive azaphilones from fruit bodies and mycelial cultures of the Ascomycete Bulgaria inquinans. Natural Products Letters, 7, 7–14.
Stadler, M., Fouron, J. Y., Sterner, O., & Anke, H. (1995g). 1,2-Dihydroxymintlactone, a new nematicidal monoterpene isolated from the Basidiomycete Cheimonophyllum candidissimum (Berk & Curt) Sing. Zeitschrift fur Naturforschung C, 50c, 473–475.
Sterner, O., Bergman, R., Kihlberg, J., & Wickberg, B. (1985). The sesquiterpenes of Lactarius Vellereus and their role in a proposed chemical defense system. Journal of Natural Products, 48, 279–288.
Stener, O., Etzel, W., & Mayer, A. (1997). Omphalotin, a new cycle peptide with potent nematicidal activity from Omphalotus olearius. II. Structure elucidation. Natural Products Letters, 10, 33–38.
Sun, J. H. (1997). The control of growth and reproduce to Bursaphelenchus xylophilus by fungal cultures. Acta Scientiarum Naturalium University Nankaiensis, 30, 82–85.
Sun, J., Wang, H., Lu, F., Du, L., & Wang, G. (2008). The efficacy of nematicidal strain Syncephalastrum racemosum. Annals of Microbiology, 58, 369–373.
Suzuki, Y., Tanaka, H., Aoki, H., & Tamura, T. (1970). Ascotoxin (decumbin), a metabolite of Ascochyta imperfecta. Agricultural and Biological Chemistry, 34, 395–413.
Tarbell, D. S., Carman, R. M., Chapman, D. D., Huffman, K. R., & McCorkinadale, N. J. (1960). The structure of Fumagillin. Journal of the American Chemical Society, 82, 1005–1007.
Thirumalachur, M. J. (1968). Antiamoebin, a new antiprotozoalantihelmintic antibiotic. Part I. Production and biological studies. Hindustan Antibiotics Bulletin, 10, 287–289.
Thorn, R. G., & Barron, G. L. (1984). Carnivorous mushrooms. Science, 224, 76–78.
Tomoda, H., Nishida, H., Huang, X. H., Masuma, R., Kim, Y. K., & Ōmura, S. (1992). New cyclodepsipeptides, enniatins D, E and F produced by Fusarium sp. FO-1305. Journal of Antibiotics, 45, 1207–1215.
Trofast, J., & Wickberg, B. (1977). Mycorrhizin A and chloromycorrhizin A, two antibiotics from mycorrhizal fungus of Monotrapa hypopitys L. Tetrahedron, 33, 875–879.
Trofast, J. (1978). Chloromycorrhizinol A, a furochroman from an isolate of the roots of Monotropa hypopitys. Phytochemistry, 17, 1359–1361.
Tsipouras, A., Adefarati, A. A., Tkacz, J. S., Frazier, E. G., Rohrer, S. P., Birzin, E., Rosegay, A., Zink, D. L., Goetz, M. A., Singh, S. B., & Schaeffer, J. M. (1996). Ophiobolin M and analogues, noncompetitive inhabitors of ivermectin binding with nematocidal activity. Bioorganic & Medicinal Chemistry, 4, 531–536.
Tzean, S. S., & Liou, J. Y. (1993). Nematophagous resupinate basidiomycetous fungi. Ecology and Epidemiology, 83, 1015–1020.
Ui, H., Shiomi, K., Yamaguchi, Y., Masuma, R., Nagamitus, T., Takano, D., Sunazuka, T., Namikoshi, M., & Ōmura, S. (2001). Nafuredin, a novel inhibitor of NADH-fumarate reductase, produced by Aspergillus niger FT-0554. Journal of Antibiotics, 54, 234–238.
Van Dessel, P., Coyne, D., Dubois, T., De Waele, D., & Franco, J. (2011). In vitro nematicidal effect of endophytic Fusarium oxysporum against Radopholus similis, Pratylenchus goodeyi and Helicotylenchus multicinctus. Nematropica, 41, 154–160.
Venkatasubbaiah, P., & Chilton, W. S. (1991). Toxins produced by the dogwood anthracnose fungus Discula sp. Journal of Natural Products, 54, 1293–1297.
Verdejo-Lucas, S., Viera, A., Stchigel, A. M., & Sorribas, F. J. (2009). Screening culture filtrates of fungi for activity against Tylenchulus semipenetrans. Spanish Journal of Agricultural Research, 7, 896–904.
Vurro, M., Evidente, A., Andolfi, A., Zonno, M. C., Giordano, F., & Motta, A. (1998). Brefeldin A and α,β-dehydrocurvularin, two phytotoxins from Alternaria zinniae, a biocontrol agent of Xanthium occidentale. Plant Science, 138, 67–79.
Wagner, C., Anke, H., & Sterner, O. (1998). Rubrobramide, a cytotoxic and phytotoxic metabolite from Cladobotryum rubrobrunnescens. Journal of Natural Products, 61, 501–502.
Wang, J. F., Huang, Y. J., Fang, M. J., Zhang, Y. J., Zheng, Z. H., Zhao, Y. F., & Su, W. J. (2002). Brefeldin A, a cytotoxin produced by Paecilomyces sp. and Aspergillus clavatus isolated from Taxus mairei and Torreya grandis. FEMS Immunology and Medical Microbiology, 34, 51–57.
Watts, R., Dahiya, J., Chandhary, K., & Tauro, P. (1988). Isolation and characterization of a new antifungal metabolite of Trichoderma reesei. Plant and Soil, 107, 81–84.
Wei, L. X., Zhang, H. X., Tan, J. L., Chu, Y. S., Li, N., Xue, H. X., Wang, Y. L., Niu, X. M., Zhang, Y., & Zhang, K. Q. (2011). Arthrobotrisins A-C, oligosporons from the nematode-trapping fungus Arthrobotrys oligospora. Journal of Natural Products, 74, 1526–1530.
William, A. A., Rudong, S., Latchezar, S. T., & Leonard, J. H. (1998). Sesquiterpenes from the nematicidal fungus Clitocybula oculus. Phytochemistry, 49, 589–592.
Willis, A. J., & Thomas, M. B. (1998). Biocontrol-risky but necessary? Trends in Ecology & Evolution, 13, 325–329.
Wirth, J. C., Gilmore, T. E., & Noval, J. J. (1956). Penicillic acid, the antibiotic responsible for the activity of culture filtrate of a strain of Penicillium martensii. Archives of Biochemistry and Biophysics, 63, 452–453.
Xiang, H. Q., & Feng, Z. X. (2001). The nematicidal toxicity of the fruits bodies of Pleurotus ostreatus. Journal of Shenyang Agricultural University, 32, 173–175.
Xu, L., Wang, J., Zhao, J., Li, P., Shan, T., Wang, J., Li, X., & Zhou, L. (2010). Beauvericin from the endophytic fungus, Fusarium redolens, isolated from Dioscorea zingiberensis and its antibacterial activity. Natural Product Communications, 5, 811–814.
Yamazaki, M., Okuyama, E., Kobayashi, M., & Inoue, H. (1981). The structure of paraherquamide, a toxic metabolite from Penicillium Paraherquei. Tetrahedron Letters, 22, 135–136.
Yan, X. N., Sikora, R. A., & Zheng, J. W. (2010). Activities of seven fungal endophytes from cucumber plants to the second stage juveniles of meloidogyne incognita. Chinese Journal of Biological Control, 26, 181–185.
Yang, Z. S. (2008). Screening of nematicidal activities of Trichoderma spp. and the isolation and identification of active compounds. Master thesis, laboratory for conservation and utilization of bio-resource, Yunnan University, Kunming, China.
Yang, Z. S., Li, G. H., Zhao, P. J., Zheng, X., Luo, S. L., Li, L., Niu, X. M., & Zhang, K. Q. (2010). Nematicidal activity of Trichoderma spp. and isolationof an active compound. World Journal of Microbiology and Biotechnology, 26, 2297–2302.
Yang, Z. S., Yu, Z. F., Lei, L. P., Xia, Z. Y., Shao, L., Zhang, K. Q., & Li, G. H. (2012). Nematicidal effect of volatiles produced by Trichoderma sp. Journal of Asia-Pacific Entomology, 15, 647–650.
Yao, T., Liang, Z. Q., Mo, M. H., & Wang, C. Y. (2006). Nematicidal activity of Paecilomyces isolates from Yunnan province. Chinese Journal of Biological Control, 22, 226–229.
Yuan, Y. P., Xiang, M. M., Xi, P. G., & Jiang, Z. D. (2010). Screening of Nematicidal isolates from the Endophytic Fungi of Quisqualis indica and optimization of culture conditions for the isolate. Chinese Journal of Biological Control, 26, 474–479.
Zhang, Y. (2004). Master thesis, TianJin University of Science and Technology, TianJin, China.
Zhang, J. P., & Zhao, B. G. (2003). Influences of fungi isolated from dead pine trees ans other wood decaying fungi on the population growth of Bursaphelenchus xylophilus. Journal of Fujian College of Forestry, 23, 245–248.
Zhang, Y. G., Yuan, W. P., Xia, X. Q., Liu, X., Meng, X. M., Wang, X. J., Zhang, J. S., & Liu, C. H. (2010). Isolation and identification of the nematicidal secondary metabolites from one strain of entomogenous fungi. Chinese Journal of Pesticide Science, 12, 225–228.
Zhao, Z. X. (2004). Master thesis, laboratory for conservation and utilization of bio-resource, Yunnan University, Kunming, China.
Zhou, Y. P., Shen, K. Z., Dong, J. Y., Wang, L. M., Wang, C. R., Wang, L., & Zhang, K. Q. (2009). Nematicidal metabolites of the aquatic fungus Coelomycetes sp. YMF1.01029. Chinese Journal of Antibiotics, 34, 74–78.
Zhu, Y. H., Dong, J. Y., Wang, L., Li, L., He, H. P., Liu, H. Y., & Zhang, K. Q. (2008). Screening and isolation of antinematodal metabolites against Bursaphelenchus xylophilus produced by fungi. Annals of Microbiology, 58, 375–380.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Mushroom Research Foundation
About this chapter
Cite this chapter
Li, GH., Zhang, KQ. (2014). Nematode-Toxic Fungi and their Nematicidal Metabolites. In: Zhang, KQ., Hyde, K. (eds) Nematode-Trapping Fungi. Fungal Diversity Research Series, vol 23. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-8730-7_7
Download citation
DOI: https://doi.org/10.1007/978-94-017-8730-7_7
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-8729-1
Online ISBN: 978-94-017-8730-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)